Abstract
Background:
The etiology of hypertrophic cardiomyopathy (HCM) in the young is highly varied. Ventricular preexcitation (preexcitation) is well recognized, yet little is known regarding the specificity for any etiology, and the characteristics of the responsible accessory pathways (APs).
Methods:
Retrospective cohort study of patients <21 years of age with HCM/preexcitation from 2000–2022. Etiology of HCM was defined as isolated HCM, storage disorder, metabolic disease, or genetic syndrome. Atrioventricular APs (true APs) were distinguished from fasciculoventricular fibers (FVF) using standard invasive EP study (EPS) criteria. APs were defined as high-risk if any of the following were <250 ms: shortest preexcited RR interval in AF, shortest paced preexcited cycle length, or anterograde AP effective refractory period.
Results:
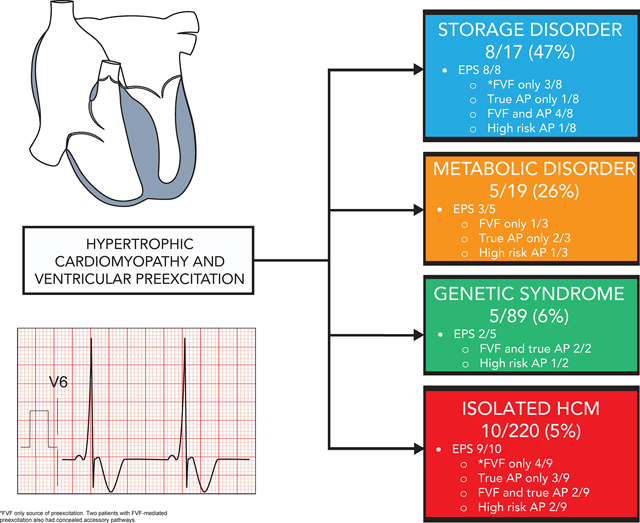
We identified 345 patients with HCM and 28 (8%) had preexcitation (isolated HCM 10/220, storage disorder 8/17, metabolic disease 5/19, genetic syndrome 5/89). Six (21%) had clinical AF (1 with SPRRI <250 ms). Twenty-two underwent EPS which identified 23 true APs and 16 FVFs. Preexcitation was exclusively FVF-mediated in 8 (36%) patients. Five (23%) patients had APs with high-risk conduction properties (including ≥1 patient in each etiologic group). Multiple APs were seen in 8 (36%) and AP plus FVF in 10 (45%). Ablation was acutely successful in 13/14 patients with recurrence in 3. One procedure was complicated by CHB after ablation of a high-risk midseptal AP. There were significant differences in QRS amplitude and delta wave amplitude between groups. There were no surface ECG features which differentiated APs from FVFs.
Conclusions:
Young patients with HCM and preexcitation have a high likelihood of underlying storage disease or metabolic disease. Non-isolated HCM should be suspected in young patients with very large QRS and delta wave amplitudes. Surface ECG is not adequate to discriminate preexcitation from a benign FVF from that secondary to potentially life-threatening APs.
Keywords: Hypertrophic cardiomyopathy, accessory pathway, Wolff-Parkinson-White syndrome, fasciculoventricular fiber
Graphical Abstract
Introduction
Ventricular preexcitation (preexcitation) has long been known to occur at increased frequency in patients with hypertrophic cardiomyopathy (HCM)1,2. This appears to be true in patients with sarcomeric/non-sarcomeric HCM not related to an underlying syndrome (isolated HCM) and in patients with several distinct systemic disorders which result in an HCM phenotype. These include storage diseases such as PRKAG2 cardiomyopathy3–5 and LAMP2-mediated Danon disease6–9 as well as metabolic disease including cardioskeletal mitochondrial myopathies10–13. It is crucial to identify patients with these phenocopies as their prognosis and management differ from patients with isolated HCM.
Preexcitation can be caused by fasciculoventricular fibers (FVFs) originating distal to the atrioventricular (AV) node or by true AV accessory pathways (AP) spanning the AV junction. While FVFs are considered benign and do not predispose to sudden cardiac death (SCD) or participate in AV reciprocating tachycardia (AVRT), true AP can participate in AVRT and, in some cases, predispose to SCD secondary to rapid conduction of atrial fibrillation (AF) and degeneration to ventricular fibrillation14. Furthermore, it is well known that both preexcitation and HCM predispose to the development of AF15,16. While the relationship between preexcitation and HCM is established, data are sparse regarding the relative frequency of preexcitation in different etiologies of HCM expressed in young people, the electrophysiological properties of the responsible APs, and risk for life-threatening arrhythmia.
Methods
We performed a single-center retrospective cohort study of patients aged <21 years with both HCM and preexcitation with a clinical encounter at Boston Children’s Hospital between January 1, 2000 and October 31, 2022. This study was approved by our Institutional Review Board with a waiver of informed consent and the data supporting the findings of this study are available upon request from the corresponding author. Preexcitation was defined as the presence of a PR interval less than the lower limit of normal for age with a delta wave. HCM was defined according to the 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy17:
Maximal left ventricular wall thickness z-score ≥2.5 in children without a family history of HCM and no pathogenic or likely pathogenic (P/LP) variant in a gene known to cause HCM
Maximal left ventricular wall thickness z-score >2 in a child with a P/LP variant in a gene known to cause HCM or a positive family history and no hemodynamic explanation for left ventricular hypertrophy.
The etiology of HCM was classified into one of the following mutually exclusive categories:
Storage disease including PRKAG2 cardiomyopathy and Danon disease (LAMP2) if patients had a P/LP variant in either of these genes or histopathologic evidence of storage disease on endomyocardial biopsy.
Metabolic disease if patients had a P/LP variant in a gene associated with known metabolic disease including those associated with mitochondrial myopathy or a clear phenotype indicative of metabolic disease.
Genetic syndrome associated with HCM if patients had a clear syndromic phenotype with extracardiac manifestations without features suggestive of storage or metabolic disease.
Isolated HCM if patients had a P/LP genetic variant in a gene encoding a sarcomeric protein known to be associated with HCM or HCM in the absence of features suggestive of an underlying systemic disorder.
Clinical data on all patients in our internal database of children with HCM was examined to determine the frequency of preexcitation within each etiologic group. Demographic and clinical data collected included age at diagnosis of preexcitation and age at diagnosis of HCM as well as sex assigned at birth. A history of clinical AF and supraventricular tachycardia (SVT) was recorded, and in those with documented AF the shortest preexcited RR interval (SPRRI) was recorded. Additional data collected included family history of preexcitation and/or HCM. Heart transplantation or death during the study period was also recorded.
ECG data were abstracted from the first available 12-lead ECG with preexcitation and included PR interval, QRS duration, QRS amplitude (average of V1 and V6), V6 R-wave and S-wave V1 amplitude, and delta wave amplitude (DWA) 40 ms after delta wave onset (highest amplitude in any limb lead). R-wave amplitude in V6 and S-wave amplitude in V1 were normalized (amplitude divided by age-specific upper limit of normal from our institutional database) to account for differences in age.
In patients who underwent invasive electrophysiology study (EPS) data including the cause of preexcitation (classified as FVF if the HV interval was <35 ms and did not change with decremental atrial extrastimulus testing, or true AP if the HV was <35 ms and the HV interval decreased with decremental atrial extrastimulus testing18) as well as number and location of APs were collected. APs with anterograde conduction were defined as high risk if any of the following characteristics were met at baseline (i.e. not during isoproterenol infusion): SPRRI during AF <250 ms, shortest paced preexcited cycle length (SPPCL) during atrial pacing <250 ms, or anterograde AP effective refractory period <250 ms. Use of isoproterenol and AP characteristics during isoproterenol infusion were also collected.
Kruskal-Wallis tests were used to evaluate variability in continuous variables between etiologic groups while Fisher’s exact test was used for binary variables. Given the small size of our cohort, post-hoc analyses were not performed. Comparison was made of ECG characteristics in patients with preexcitation exclusively due to an FVF to those with preexcitation secondary to a true AP using Fisher’s exact test for binary variables and the Wilcoxon rank-sum test for continuous variables. ECG preexcitation patterns were analyzed using the Arruda algorithm19 to estimate the likely AP location and compared to the invasively determined location. The Arruda algorithm was classified as correct if it correctly predicted the location of at least one AP with anterograde conduction.
Results
We identified 28 patients with preexcitation from a total of 345 HCM patients (8%). The frequency of preexcitation differed significantly between HCM etiologies [isolated HCM 10/220 (5%), storage disease 8/17 (47%), metabolic disease 5/19 (26%), genetic syndrome 5/89 (6%), p <0.001]. Clinical characteristics are summarized in table 1. There were significant differences between groups at age of both preexcitation and HCM diagnosis, and between groups with regards to average QRS amplitude, DWA, and normalized V6 R-wave amplitude. Clinical and ECG characteristics are summarized by HCM etiologic group in table 2.
Table 1.
Clinical characteristics.
| Patients | 28 |
| Male sex | 15 (52) |
| Age at preexcitation diagnosis (years) | 9.5 (4.2–13.8) |
| Age at HCM diagnosis (years) | 11.2 (0.9–14.4) |
| Concomitant VPe & HCM diagnosis | 14 (48) |
| Etiology of HCM | |
| Storage disease | 8 (29) |
| Metabolic disease | 5 (18) |
| Genetic syndrome | 5 (18) |
| Isolated HCM | 10 (36) |
| History of reentrant supraventricular tachycardia | 6 (21) |
| History of atrial fibrillation | 6 (21) |
| History of syncope | 2 (7) |
Data expressed n (%) or median (IQR)
HCM = hypertrophic cardiomyopathy
Table 2.
Clinical, ECG, and invasive electrophysiology study characteristics by HCM etiologic group.
| Overall (n = 28) |
Storage disorder (n = 8) |
Metabolic disease (n = 5) |
Genetic syndrome (n = 5) |
Isolated (n = 10) |
p-value | |
|---|---|---|---|---|---|---|
| Clinical Characteristics | ||||||
| Age at preexcitation diagnosis (years) | 9.5 (0.0–17.2) | 14.6 (7.2–17.2) | 3.4 (0.5–10.7) | 1.3 (0.0–12.9) | 11.6 (1.7–17.0) | 0.008 |
| Age at HCM diagnosis (years) | 11.2 (0.0–19.3) | 13.4 (0.0–17.2) | 0.3 (0.0–5.7) | 11.0 (0.0–14.4) | 13.0 (0.9–19.3) | 0.048 |
| Male sex | 15 (52) | 2 (25) | 4 (80) | 2 (40) | 7 (70) | 0.188 |
| Family history of preexcitation | 7 (25) | 2 (25) | 3 (60) | 0 (0) | 2 (20) | 0.222 |
| Family history of HCM | 8 (29) | 2 (25) | 1 (20) | 2 (40) | 3 (30) | 1.000 |
| *Atrial fibrillation | 6 (21) | 4 (50) | 0 (0) | 0 (0) | 2 (20) | 0.126 |
| *Reentrant SVT | 6 (21) | 1 (13) | 1 (20) | 1 (20) | 3 (30) | 0.916 |
| Syncope | 2 (7) | 0 (0) | 1 (20) | 0 (0) | 1 (10) | 0.499 |
| ECG Characteristics | ||||||
| PR interval | 93 (55–145) | 95 (80–116) | 82 (55–90) | 88 (80–108) | 107 (65–145) | 0.121 |
| QRS duration | 110 (85–192) | 120 (95–192) | 108 (100–140) | 100 (85–108) | 110 (96–160) | 0.057 |
| DWA 40 ms after delta onset (mm) | 4.6 (1.1–22.5) | 8.2 (4.1–22.5) | 5.4 (2.8–11.5) | 4.8 (2.5–7.3) | 3.3 (1.1–15.3) | 0.013 |
| QRS ampltiude (average V1 and V6, mm) | 28.3 (10.6–79.9) | 42.3 (22.8–79.9) | 18.3 (14.4–47.0) | 28.8 (11.6–47.5) | 25.7 (10.6–34.2) | 0.049 |
| V6 R-wave amplitude (mm) | 25.2 (4.4–65.0) | 39.7 (11.6–65.0) | 16.4 (4.4–46.3) | 28.8 (11.0–44.0) | 17.5 (11.4–35.1) | 0.062 |
| Normalized V6 R-wave amplitude | 1.0 (0.3–2.5) | 1.6 (0.5–2.5) | 0.6 (0.3–2.0) | 1.1 (0.5–1.8) | 0.7 (0.4–1.5) | 0.046 |
| V1 S-wave amplitude (mm) | 20.7 (0.0–75.3) | 31.8 (0.0–75.3) | 18.4 (5.9–40.9) | 9.2 (2.6–30.5) | 20.3 (0.0–30.5) | 0.194 |
| Normalized V1 S-wave amplitude | 1.0 (0.0–3.1) | 1.3 (0.0–3.1) | 0.8 (0.2–2.0) | 0.4 (0.1–1.3) | 0.9 (0.0–1.4) | 0.237 |
| EPS Characteristics | Overall (n = 22) |
Storage disorder (n = 8) |
Metabolic disease (n = 3) |
Genetic syndrome (n = 2) |
Isolated (n = 9) |
|
| Patients with AP with anterograde conduction | 14 (64) | 5 (63) | 2 (66) | 2 (100) | 5 (66) | 0.912 |
| Patients with multiple true APs | 8 (36) | 2 (25) | 1 (33) | 0 (0) | 5 (56) | 0.470 |
| Patients with high risk true AP | 5 (23) | 1 (13) | 1 (33) | 1 (50) | 2 (22) | 0.653 |
| Patients with FVF | 16 (73) | 7 (88) | 1 (33) | 2 (100) | 6 (66) | 0.307 |
| Patients with FVF-mediated preexcitation | 8 (36) | 3 (38) | 1 (33) | 0 (0) | 4 (44) | 0.912 |
Data expressed n (%) or median (range); n = number of patients
One patient had both SVT and AF
AF = atrial fibrillation, AP = accessory pathway, DWA = delta wave amplitude, EPS = electrophysiology study, HCM = hypertrophic cardiomyopathy, SVT = supraventricular tachycardia.
We constructed receiver operating characteristic curves to explore the relationships between average QRS amplitude, DWA, and etiology of HCM. The area under the curve (AUC) for the relationship between average QRS amplitude and storage disease was 0.83. Youden’s J-statistic was maximized at a threshold value of ≥33.5 mm which was 87.5% sensitive and 85.0% specific for the presence of storage disease (figure 1A). The AUC for the relationship between DWA and non-isolated HCM was 0.81 and Youden’s J-statistic was maximized at a threshold of ≥4.8 mm which was 72.2% sensitive and 90.0% specific for non-isolated HCM (figure 1B).
Figure 1.
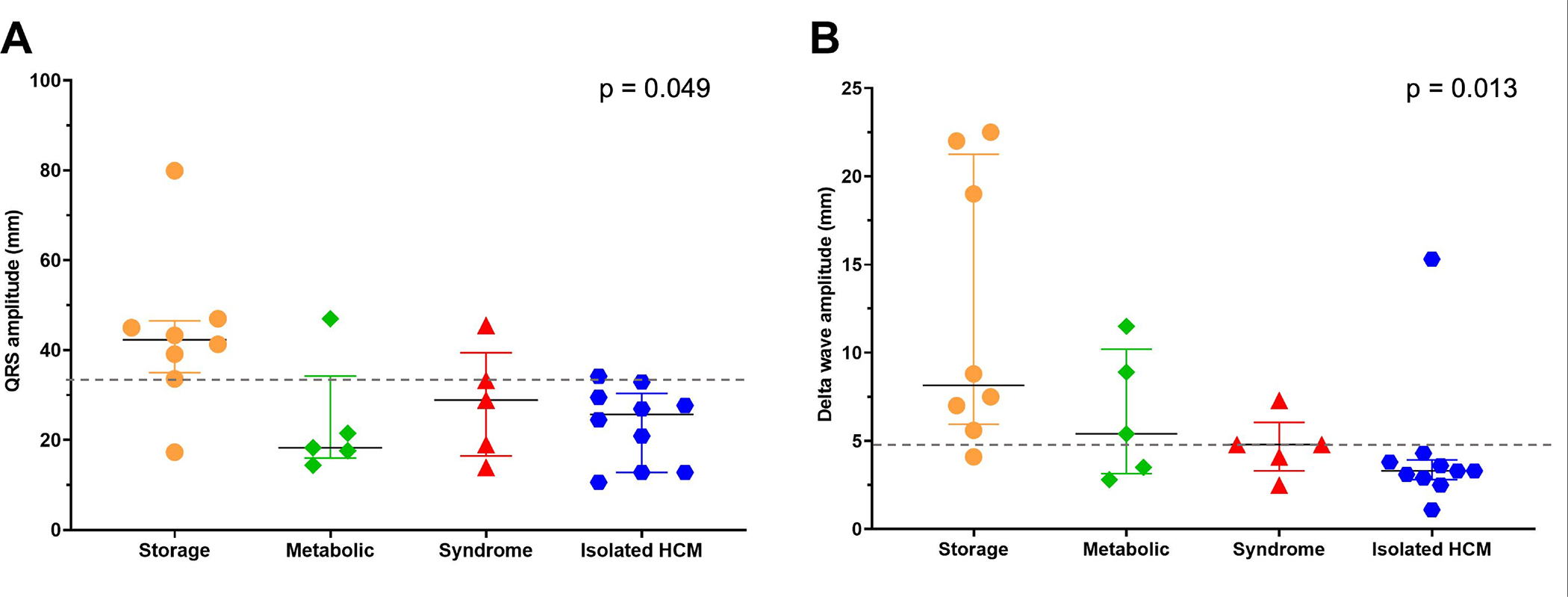
ECG features which aid in differentiation between HCM etiologic groups: A) QRS amplitude v. HCM etiologic group: an average QRS amplitude ≥33.5 mm (dashed line) is 87.5% sensitive and 85.0% specific for an underlying storage disorder. B) Delta wave amplitude v. HCM etiologic group: a DWA amplitude ≥4.8 (dashed line) mm is 72.2% sensitive and 90.0% specific for non-isolated HCM. P-values determined using Kruskall-Wallis test.
Preexcited AF was seen in 6 patients during the study period, including at presentation in 2. In 4 patients preexcitation was secondary to an FVF and the average ventricular rate during preexcited AF was <150 bpm. In 2 patients, anterograde conduction during preexcited AF was, at least in part, over an AP with more rapid conduction: one patient with Danon disease and a SPRRI 315 ms presented in congestive heart failure and one patient with isolated HCM had a SPRRI of 210 ms; figure 2A). Two patients presented with reentrant SVT and two patients had syncope. There were no cases of cardiac arrest or sudden death.
Figure 2.
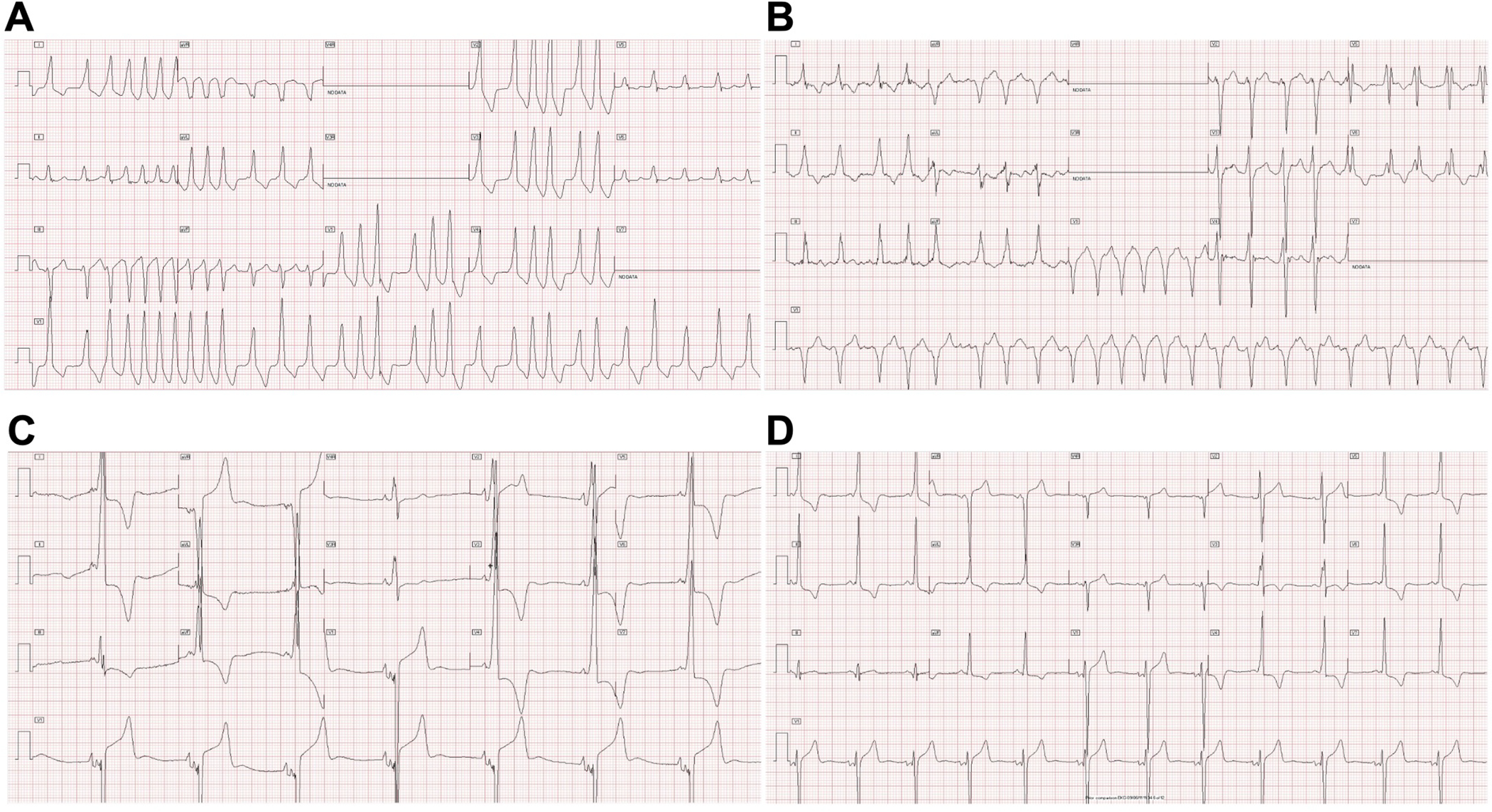
ECG examples: A) Rapidly conducted preexcited atrial fibrillation (SPRRI 210 ms) in a patient with isolated HCM. B) Preexcited atrial fibrillation (SPRRI 315 ms) in a patient with Danon disease and an FVF. C) Preexcitation with massive QRS and delta wave amplitudes in a patient with PRKAG2 cardiomyopathy. D) Preexcitation in a patient with isolated HCM with more modest QRS and delta wave amplitudes.
Twenty-two patients underwent invasive EPS. A total of 39 total AP (including both FVF and true AP) were identified – 23 true AP (including 5 with retrograde only conduction) and 16 FVF. In 8 patients preexcitation was secondary to an FVF only, and in 2 of these patients there was at least 1 additional concealed AP with retrograde only conduction (all retrograde only APs were along the left AV groove). EPS data are summarized by HCM etiologic group in table 2. EPS indications and patient-level EPS data are presented in table 3. AP location and nature are depicted for each etiologic group in figure 3. Five patients had AP with high-risk characteristics at baseline including at least one patient from each etiologic category. AP were characterized during isoproterenol infusion in 6 (27%) patients. Anterograde AP conduction was rapid during isoproterenol infusion (AP ERP, SPRRI, or SPPCL <250 ms) in 2 of these patients, though both of these AP met high-risk criteria under baseline conditions. Radiofrequency/cryoablation was performed in 14 patients targeting 19 true AP, with acute success in 13 (93%). A subsequent recurrence of AP conduction was seen in 3 (23%) patients. No FVF were ablated. One patient with isolated HCM who presented with rapidly conducted preexcited AF was found to have midseptal and right lateral true APs with anterograde-only conduction as well as an FVF and experienced complete AV block as a result of radiofrequency ablation of the midseptal AP. There were no other procedural complications.
Table 3.
Invasive EP study indications and AP characteristics by patient.
| Patient number | HCM etiology | Age at EPS (years) | Indication for EPS | AP nature‡/location | High risk AP |
|---|---|---|---|---|---|
| 1 | Isolated | 13.8 | Preexcited AF | 1) FVF 2) right lateral 3) midseptal* |
Yes |
| 2 | Genetic syndrome | 12.9 | Asymptomatic preexcitation | 1) FVF 2) left anterior |
No |
| 3 | Isolated | 13.6 | SVT | 1) right anterior 2) anteroseptal |
No |
| 4 | Storage disorder | 14.6 | SVT, preexcited AF | 1) FVF 2) right lateral |
No |
| 5 | Isolated | 10.0 | SVT | Left lateral | No |
| 6 | Genetic syndrome | 6.5 | Asymptomatic preexcitation | 1) FVF 2) right posteroseptal* |
Yes |
| 7 | Metabolic disease | 12.1 | SVT | 1) right lateral* 2) midseptal 3) right posteroseptal |
Yes |
| 8 | Isolated | 12.6 | SVT | 1) FVF 2) left posteroseptal† 3) left lateral† |
No |
| 9 | Storage disorder | 16.8 | Preexcited AF | 1) FVF 2) right anterior |
No |
| 10 | Metabolic disease | 7.0 | Syncope | Right posteroseptal | No |
| 11 | Storage disorder | 12.6 | Asymptomatic preexcitation | 1) FVF 2) midseptal* |
Yes |
| 12 | Isolated | 15.4 | Asymptomatic preexcitation | Anteroseptal | No |
| 13 | Storage disorder | 11.0 | Syncope | Anteroseptal | No |
| 14 | Storage disorder | 7.5 | Asymptomatic preexcitation | 1) FVF 2) right lateral |
No |
| 15 | Storage disorder | 17.6 | Preexcited AF | FVF | No |
| 16 | Metabolic disease | 11.5 | Asymptomatic preexcitation | FVF | No |
| 17 | Isolated | 16.8 | Preexcited AF | FVF | No |
| 18 | Isolated | 3.8 | Asymptomatic preexcitation | 1) FVF 2) left posterior* 3) left posterior† |
Yes |
| 19 | Isolated | 19.6 | Asymptomatic preexcitation | FVF | No |
| 20 | Isolated | 16.9 | Palpitations | FVF | No |
| 21 | Storage disorder | 16.4 | Preexcited AF | FVF | No |
| 22 | Storage disorder | 10.4 | Asymptomatic preexcitation | 1) FVF 2) left posteroseptal† 3) left lateral† |
No |
high risk AP
retrograde only AP
true atrioventricular AP with anterograde conduction unless otherwise noted
HCM = hypertrophic cardiomyopathy, EPS = electrophysiology study, SVT = supraventricular tachycardia, AF = atrial fibrillation, FVF = fasciculoventricular fiber, AP = accessory pathway
Figure 3.
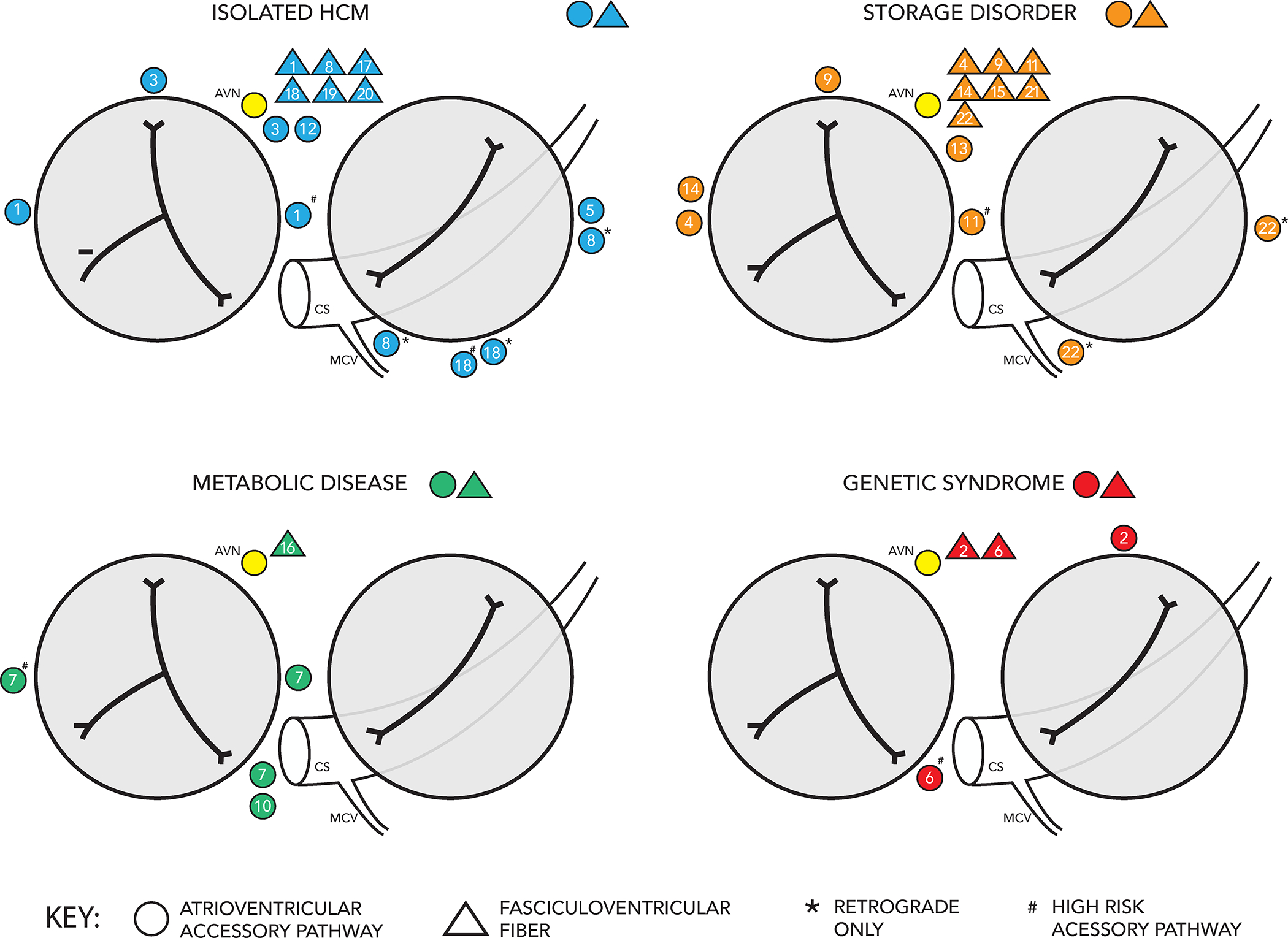
Location and nature of APs in 22 patients who underwent invasive electrophysiology study organized by HCM etiologic group.
Two patients with storage disease who underwent true AP ablation subsequently developed QRS widening during follow up and repeat EPS were performed due to concern for AP recurrence. However, preexcitation was confirmed to be secondary to known FVF in both cases and the QRS widening was attributed to evolving cardiac conduction disease and resultant intraventricular conduction delay.
In patients who underwent invasive EPS, the Arruda algorithm19 correctly predicted the location of an AP with anterograde conduction in only 11 (50%) patients. Notably 10 (45%) patients had >1 source of preexcitation, though this did not appear to impact the accuracy of the Arruda algorithm (correct in 5/12 (42%) with one AP with anterograde conduction and 6/10 (60%) with more than one AP with anterograde conduction, p = 0.67). In the group of patients whose preexcitation was exclusively secondary to an FVF, the Arruda algorithm localized the surface ECG preexcitation pattern to a site outside the anteroseptal region in 3 of 6 cases (right anterior/anterolateral in 2 cases and left lateral/anterolateral in 1 case). There were no clinical or ECG characteristics which differentiated patients with preexcitation secondary to an FVF versus those with true AP-mediated preexcitation (table 4).
Table 4.
Characteristics of patients with ventricular preexcitation exclusively due to FVF conduction versus patients with at least one true AP with anterograde conduction.
| FVF (n = 8) | True anterograde AP (n= 14) | p-value | |
|---|---|---|---|
| Age at preexcitation diagnosis (years) | 15.0 (1.7–17.2) | 11.6 (0.5–16.7) | 0.172 |
| Age at HCM diagnosis (years) | 14.1 (0.3–19.3) | 12.5 (0.3–16.7) | 0.633 |
| Male sex | 4 (50) | 8 (57) | 1.000 |
| PR interval (ms) | 103 (80–125) | 95 (65–145) | 0.560 |
| QRS duration (ms) | 115 (96–125) | 113 (95–192) | 0.946 |
| DWA 40 ms after delta onset (mm) | 4.1 (3.3–15.3) | 4.9 (1.1–22.5) | 0.838 |
| Average QRS amplitude (mm) | 29.9 (14.4–47.0) | 31.4 (10.6–79.9) | 0.811 |
| V6 R-wave amplitude (mm) | 30.6 (12.8–39.3) | 28.1 (11.4–65.0) | 0.918 |
| Normalized V6 R-wave amplitude | 1.2 (0.5–1.6) | 1.1 (0.4–2.5) | 0.891 |
| V1 S-wave amplitude (mm) | 22.6 (5.9–41.8) | 27.0 (0.0–75.3) | 0.891 |
| Normalized V1 S-wave amplitude | 1.1 (0.2–2.0) | 1.1 (0.0–3.1) | 0.946 |
| Storage disease | 3 (38) | 5 (26) | 1.000 |
| Metabolic disease | 1 (13) | 2 (14) | 1.000 |
| Syndromic | 0 (0) | 2 (14) | 0.491 |
| Isolated HCM | 4 (50) | 5 (38) | ref |
| Supraventricular tachycardia | 0 (0) | 5 (36) | 0.115 |
| Atrial fibrillation | 3 (38) | 3 (21) | 0.624 |
data expressed as n (%) or median (range)
HCM = hypertrophic cardiomyopathy, DWA = delta wave amplitude, FVF = fasciculoventricular fiber, AP = accessory pathway
Over the course of a median follow up of 5.1 years (range 0.0–13.4 years), 1 patient with a genetic syndromic etiology of HCM died from a non-cardiac cause. Three patients (2 patients with Danon disease and 1 with a mitochondrial myopathy) underwent heart transplantation during follow up.
Discussion
In this cohort of young patients with HCM and comorbid preexcitation, we make a number of important observations regarding the underlying etiology of HCM and AP characteristics which include the following:
The frequency with which preexcitation is comorbid with HCM differs markedly between HCM etiologic groups.
Evaluation of QRS and delta wave amplitude may be useful for differentiation between underlying etiologies of HCM.
Surface electrocardiography cannot reliably distinguish between preexcitation secondary to a benign FVF and that due to a potentially life-threatening true AP in this population.
Young patients with HCM and preexcitation have a high frequency of both multiple true AP and high-risk AP as well as frequent coexistence of FVF and true AP.
Although isolated disease is the most common etiology of pediatric HCM, only 5% of these patients had associated preexcitation in our cohort. This contrasts strikingly with the approximately 50% of patients with HCM secondary to storage disease and 25% of children with HCM secondary to metabolic disease who had comorbid preexcitation. These findings are consistent with prior reports of relatively rare preexcitation in isolated HCM cohorts1 and high rates of preexcitation in patients with storage disease7,20–22. Thus, identification of preexcitation in a young patient with HCM should raise suspicion for an underlying storage or metabolic disease. While evidence of an underlying systemic disorder may be obvious in some patients with dysmorphic features, anomalies in other visceral organs, musculoskeletal weakness, or developmental delay, others may have no extracardiac features. This is particularly true of patients with PRKAG2 cardiomyopathy as most patients do not have extracardiac manifestations aside from mild skeletal myopathy in a minority23, and females with Danon disease in whom extracardiac manifestations occur in less than 40% and may be quite subtle including transaminitis, mild/sub-clinical skeletal myopathy, and visual disturbances24.
While previous work has described large QRS amplitudes in patients with preexcitation and PRKAG2 cardiomyopathy23, ours is the first to quantify and directly compare ECG characteristics between different etiologic groups in young patients with HCM and preexcitation. We found DWA ≥4.8 mm was 90% specific for non-isolated HCM and average QRS amplitude ≥33.5 mm demonstrated approximately 85% sensitivity and specificity for storage disease. Representative ECGs from patients with storage disease and isolated HCM are shown in figure 2 (C&D). The only patient with storage disease who had an average QRS amplitude <33.5 mm and DWA <4.8 mm was an adolescent male with Danon disease whose cardiac phenotype had progressed to a hypokinetic stage with congestive heart failure at the time of presentation. He had significant fibrotic myocardial replacement apparent on cardiac magnetic resonance imaging explaining his diminished QRS and delta wave amplitudes. Additionally, younger age at presentation was more common in patients with metabolic or genetic syndromic etiologies. That said, infantile presentation does not exclude isolated HCM or storage disease and remarkably one previously described patient with PRKAG2 cardiomyopathy in our cohort was noted to have cardiac hypertrophy in utero25. Furthermore, while we did not have any patients with Pompe disease (glycogen storage disease type II) in our cohort, neonatal presentation with HCM and preexcitation is described26.
Identifying the etiology of HCM in patients with HCM and preexcitation is crucial for several reasons. Genetic syndromes, storage disorders, and metabolic disease often involve disease processes in other organ systems that require identification and appropriate management. The cardiac phenotype is also varied across different etiologic groups. Both Danon disease and PRKAG2 cardiomyopathy are associated with an increased likelihood of atrial arrhythmias as well as cardiac conduction disease and early systolic dysfunction leading to congestive heart failure20,22–24,27. Etiology-specific disease-modifying therapy is presently available for conditions such as Pompe disease28 and gene therapy for other conditions such as Danon disease is under investigation29. Thus, early identification of patients who may benefit from these therapies is important.
FVFs are APs which arise distal to the AV node and typically connect the bundle of His to the ventricular myocardium in the paraHisian/anteroseptal region18,30. FVFs are not thought to predispose to life-threatening rapidly conducted preexcited AF or participate in SVT, though sudden death in a patient with PRKAG2 cardiomyopathy and FVF-mediated preexcitation with documented rapidly conducted preexcited AF has been described, possibly secondary to enhanced AV nodal conduction3. Previous work in individuals without HCM has demonstrated FVF-mediated preexcitation can be differentiated from that secondary to a true AP by a longer PR interval, shorter QRS duration, and smaller DWA31,32. While O’Leary et al found DWA >5 mm was 96% specific for true AP-mediated preexcitation and DWA <2 mm was 96% specific for FVF-mediated preexcitation in a cohort of children without structural heart disease, in our cohort 3 of 8 patients with FVF-mediated preexcitation had DWA >5 mm and the only patient with DWA <2 mm had true AP-mediated preexcitation. Additionally, we found no difference in PR interval or QRS duration between patients with FVF and true AP-mediated preexcitation and, concordant with a prior report in patients with Danon disease6, no ECG characteristic reliably differentiated these two groups. Furthermore, while preexcitation secondary to FVF conduction typically results in a surface ECG pattern suggestive of an anteroseptal location30, 50% of patients in our cohort with FVF-mediated preexcitation had a preexcitation pattern inconsistent with an anteroseptal location using the Arruda algorithm19, which performed poorly overall in our cohort. The limited utility of the Arruda algorithm in our cohort is consistent with a prior report which found the Arruda algorithm and multiple additional AP localization algorithms to be of limited utility in pediatric patients33. Administration of intravenous adenosine has previously been shown to be useful in distinguishing between FVF and true AP-mediated preexcitation32, however other authors have reported up to 20% of true APs are adenosine sensitive34 and AV nodal conduction may be relatively adenosine insensitive in patients with PRKAG2 cardiomyopathy3. Thus, we conclude invasive EPS is required to definitively characterize preexcitation in patients with HCM.
Previous studies have provided detailed characterization of APs in patients with Danon disease6 and PRKAG2 cardiomyopathy3 and the present study expands upon these findings. Darden et al reported on a cohort of patients with Danon disease including 9 patients with preexcitation who underwent invasive EPS6. Concordant with our findings, they described several patients with multiple true AP, the coexistence of FVF and true AP, and the presence of true AP with high-risk anterograde conduction characteristics in two patients. While Sternick et al previously reported on a cohort of PRKAG2 cardiomyopathy patients with exclusively FVF-mediated preexcitation with no co-occurrence of FVF and true AP3, others have reported preexcitation secondary to true AP in this population21,35–37. Here we describe 3 patients with PRKAG2 cardiomyopathy and true AP -- 1 patient with both an FVF and true AP anterograde conduction, 1 patient with exclusively true AP-mediated preexcitation, and an additional patient with FVF-mediated preexcitation as well as two true AP with retrograde-only conduction. Darden et al additionally reported a patient with Danon disease who underwent successful AP ablation and subsequently developed QRS widening and PR prolongation 4 years later6. We observed a similar phenomenon in 2 patients with PRKAG2 cardiomyopathy both of whom underwent repeat EPS which ruled out true AP-mediated preexcitation, suggesting distal conduction system disease or intraventricular conduction delay as the etiology of QRS widening.
While preexcitation has long been described in a minority of patients with isolated HCM1,2, reports including detailed AP characterization are sparse and lack true APs38. Notable findings from this subset of our cohort include the finding of true AP with high-risk anterograde conduction in 2 of 10 patients, frequent coexistence of FVFs and true AP, multiple true AP in several patients, and true AP located in both septal as well as both right and left free wall locations. Additionally, two patients in this group had documented AF including one patient who presented with rapid preexcited AF (figure 2A), highlighting the potential for life-threatening arrhythmias in these patients. Our study additionally characterizes preexcitation in a small group of patients with HCM associated with metabolic and genetic syndromes in whom previous reports are sparse39. As with the other etiologic groups described in the present study, notable findings in these groups include patients with high-risk true AP, multiple true AP, and the coexistence of FVF and true AP in multiple patients.
Our study was limited by its small size as only 22 included patients underwent invasive EPS over the 22-year study period. It is further limited by its retrospective nature and is subject to the biases inherent to this sort of study design. Additionally, our institution is a referral center for pediatric HCM, and our patient population may not be generalizable.
In summary, the presence of preexcitation in a young patient with HCM should raise suspicion for underlying storage, metabolic, or syndromic disease and identifying these etiologies has significant implications for prognosis and management of these patients and their families. Very large QRS and delta wave amplitudes should further heighten the suspicion for an underlying storage disease. Invasive EPS is required for characterization of preexcitation in this population as surface ECG characteristics do not reliably distinguish between preexcitation secondary to benign FVF and potentially life-threatening true AP in this population with a high incidence of high-risk true AP, multiple true AP, and coexistence of FVF and true AP.
What is Known:
A subset of patients with hypertrophic cardiomyopathy (HCM) have associated ventricular preexcitation (preexcitation) which can be due to atrioventricular accessory pathways (AP) or benign fasciculoventricular fibers (FVF).
What this Study Adds:
In patients with HCM, the prevalence of preexcitation is 10 times higher in patients and underlying storage disease and 5 times higher in patients with underlying metabolic disease than in patients with isolated HCM.
Atrioventricular AP with high-risk characteristics, multiple AP, and the coexistence of AP and FVF can be found in patients with HCM secondary to underlying storage disease, metabolic disease, genetic syndromes, and in patients with isolated HCM.
No surface ECG characteristic reliably discriminates between potentially life-threatening atrioventricular AP-mediated preexcitation and benign FVF-mediated preexcitation in patients with HCM.
Sources of Funding:
RP was supported by the National Heart, Lung, and Blood Institute training grant T32HL007572.
Nonstandard Abbreviations and Acronyms:
- preexcitation
Ventricular preexcitation
- HCM
hypertrophic cardiomyopathy
- FVF
fasciculoventricular fiber
- AP
accessory pathway
- AV
atrioventricular
- SCD
sudden cardiac death
- AVRT
atrioventricular reciprocating tachycardia
- AF
atrial fibrillation
- P/LP
pathogenic or likely pathogenic
- SVT
supraventricular tachycardia
- SPRRI
shortest preexcited RR interval
- DWA
delta wave amplitude
- EPS
electrophysiology study
- SPPCL
shortest paced preexcited cycle length
Footnotes
Disclosures: None.
References:
- 1.Frank S, Braunwald E. Idiopathic hypertrophic subaortic stenosis. Clinical analysis of 126 patients with emphasis on the natural history. Circulation. 1968; 37:759–88. [DOI] [PubMed] [Google Scholar]
- 2.Perosio AM, Suarez LD, Bunster AM, Locreille A, Apkarian OA, Vallazza MA, Foye R. Pre-excitation syndrome and hypertrophic cardiomyopathy. J Electrocardiol. 1983;16:29–40. [DOI] [PubMed] [Google Scholar]
- 3.Sternick EB, Oliva A, Gerken LM, Magalhães L, Scarpelli R, Correia FS, Rego S, Santana O, Brugada R, Wellens HJ. Clinical, electrocardiographic, and electrophysiologic characteristics of patients with a fasciculoventricular pathway: the role of PRKAG2 mutation. Heart Rhythm. 2011;8:58–64. [DOI] [PubMed] [Google Scholar]
- 4.Ahamed H, Balegadde AV, Menon S, Menon R, Ramachandran A, Mathew N, Natarajan KU, Nair IR, Kannan R, Shankar M, et al. Phenotypic expression and clinical outcomes in a South Asian PRKAG2 cardiomyopathy cohort. Sci Rep. 2020;10:20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porto AG, Brun F, Severini GM, Losurdo P, Fabris E, Taylor MRG, Mestroni L, Sinagra G. Clinical Spectrum of PRKAG2 Syndrome. Circ Arrhythm Electrophysiol. 2016;9:e003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darden D, Hsu JC, Tzou WS, von Alvensleben JC, Brooks M, Hoffmayer KS, Brambatti M, Sauer WH, Feld GK, Adler E. Fasciculoventricular and atrioventricular accessory pathways in patients with Danon disease and preexcitation: A multicenter experience. Heart Rhythm. 2021;18:1194–1202. [DOI] [PubMed] [Google Scholar]
- 7.Jhaveri S, Herber J, Zahka K, Boyle GJ, Saarel EV, Aziz PF. Arrhythmias and fasciculoventricular pathways in patients with Danon disease: a single center experience. J Cardiovasc Electrophysiol 2019;30:1932–1938. [DOI] [PubMed] [Google Scholar]
- 8.López-Sainz Á, Salazar-Mendiguchía J, García-Álvarez A, Campuzano Larrea O, López-Garrido MÁ, García-Guereta L, Fuentes Cañamero ME, Climent Payá V, Peña-Peña ML, Zorio-Grima E, et al. Clinical Findings and Prognosis of Danon Disease. An Analysis of the Spanish Multicenter Danon Registry. Rev Esp Cardiol (Engl Ed). 2019;72:479–486. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wang F, Chen X, Liang Y, Deng H, Liao H, Rao F, Wei W, Zhang Q, Zhang B, et al. Fasciculoventricular Pathways Responsible for Ventricular Preexcitation in Patients With Danon Disease. Circ Arrhythm Electrophysiol. 2018;11:e006704. [DOI] [PubMed] [Google Scholar]
- 10.Brecht M, Richardson M, Taranath A, Grist S, Thorburn D, Bratkovic D. Leigh Syndrome Caused by the MT-ND5 m.13513G>A Mutation: A Case Presenting with WPW-Like Conduction Defect, Cardiomyopathy, Hypertension and Hyponatraemia. JIMD Rep. 2015;19:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malfatti E, Laforêt P, Jardel C, Stojkovic T, Behin A, Eymard B, Lombès A, Benmalek A, Bécane HM, Berber N, et al. High risk of severe cardiac adverse events in patients with mitochondrial m.3243A>G mutation. Neurology. 2013;80:100–105. [DOI] [PubMed] [Google Scholar]
- 12.Wahbi K, Larue S, Jardel C, Meune C, Stojkovic T, Ziegler F, Lombès A, Eymard B, Duboc D, Laforêt P. Cardiac involvement is frequent in patients with the m.8344A>G mutation of mitochondrial DNA. Neurology. 2010;74:674–677. [DOI] [PubMed] [Google Scholar]
- 13.Limongelli G, Tome-Esteban M, Dejthevaporn C, Rahman S, Hanna MG, Elliott PM. Prevalence and natural history of heart disease in adults with primary mitochondrial respiratory chain disease. Eur J Heart Fail. 2010;12:114–121. [DOI] [PubMed] [Google Scholar]
- 14.Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301:1080–1085. [DOI] [PubMed] [Google Scholar]
- 15.Campbell RW, Smith RA, Gallagher JJ, Pritchett EL, Wallace AG. Atrial fibrillation in the preexcitation syndrome. Am J Cardiol. 1977;40:514–520. [DOI] [PubMed] [Google Scholar]
- 16.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104:2517–2524. [DOI] [PubMed] [Google Scholar]
- 17.Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:3022–3055. [DOI] [PubMed] [Google Scholar]
- 18.Josephson ME. Preexcitation syndrome. Clinical Electrophysiology. 1993:311–416. [Google Scholar]
- 19.Arruda MS, McClelland JH, Wang X, Beckman KJ, Widman LE, Gonzalez MD, Nakagawa H, Lazzara R, Jackman WM. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 1998;9:2–12. [DOI] [PubMed] [Google Scholar]
- 20.Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, Ahmad F, Lozado R, Shah G, Fananapazir L, Bachinski LL, Roberts R. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344:1823–1831. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Sainz A, Dominguez F, Lopes LR, Ochoa JP, Barriales-Villa R, Climent V, Linschoten M, Tiron C, Chiriatti C, Marques N, et al. European Genetic Cardiomyopathies Initiative Investigators. Clinical Features and Natural History of PRKAG2 Variant Cardiac Glycogenosis. J Am Coll Cardiol. 2020;76:186–197. [DOI] [PubMed] [Google Scholar]
- 22.Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy RT, Mogensen J, McGarry K, Bahl A, Evans A, Osman E, Syrris P, Gorman G, Farrell M, Holton JL, et al. Adenosine monophosphate-activated protein kinase disease mimicks hypertrophic cardiomyopathy and Wolff-Parkinson-White syndrome: natural history. J Am Coll Cardiol. 2005;45:922–930. [DOI] [PubMed] [Google Scholar]
- 24.Lotan D, Salazar-Mendiguchía J, Mogensen J, Rathore F, Anastasakis A, Kaski J, Garcia-Pavia P, Olivotto I, Charron P, Biagini E, et al. Clinical Profile of Cardiac Involvement in Danon Disease: A Multicenter European Registry. Circ Genom Precis Med. 2020;13:e003117. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Gray A, Hardie DG, Uzun A, Shaw S, Padbury J, Phornphutkul C, Tseng YT. A novel, de novo mutation in the PRKAG2 gene: infantile-onset phenotype and the signaling pathway involved. Am J Physiol Heart Circ Physiol. 2017;313:H283–H292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulkley BH, Hutchins GM. Pompe’s disease presenting as hypertrophic myocardiopathy with Wolff-Parkinson-White syndrome. Am Heart J. 1978;96:246–252. [DOI] [PubMed] [Google Scholar]
- 27.Sternick EB, Oliva A, Magalhães LP, Gerken LM, Hong K, Santana O, Brugada P, Brugada J, Brugada R. Familial pseudo-Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 2006;17:724–732. [DOI] [PubMed] [Google Scholar]
- 28.Chien YH, Hwu WL, Lee NC. Pompe disease: early diagnosis and early treatment make a difference. Pediatr Neonatol. 2013;54:219–227. [DOI] [PubMed] [Google Scholar]
- 29.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2023. Feb 8. Identifier NCT03882437, Gene Therapy for Male Patients With Danon Disease (DD) Using RP-A501; AAV9.LAMP2B. Available from: https://clinicaltrials.gov/ct2/show/NCT03882437 [Google Scholar]
- 30.Lev M, Fox SM, Bharati S, Greenfield JC, Rosen KM, Pick A. Mahaim and James fibers as a basis for a unique variety of ventricular preexcitation. Am J Cardiol. 1975;36:880–888. [DOI] [PubMed] [Google Scholar]
- 31.O’Leary ET, Dewitt ES, Mah DY, Gauvreau K, Walsh EP, Bezzerides VJ. Differentiation of fasciculoventricular fibers from anteroseptal accessory pathways using the surface electrocardiogram. Heart Rhythm. 2019;16:1072–1079. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Nakamura Y, Yoshida S, Yoshida Y, Shintaku H. Differentiating fasciculoventricular pathway from Wolff-Parkinson-White syndrome by electrocardiography. Heart Rhythm. 2014;11:686–690. [DOI] [PubMed] [Google Scholar]
- 33.Wren C, Vogel M, Lord S, Abrams D, Bourke J, Rees P, Rosenthal E. Accuracy of algorithms to predict accessory pathway location in children with Wolff-Parkinson-White syndrome. Heart. 2012;98:202–206. [DOI] [PubMed] [Google Scholar]
- 34.Fishberger SB, Saul JP, Triedman JK, Epstein MR, Walsh EP. Use of adenosine-sensitive nondecremental accessory pathways in assessing the results of radiofrequency catheter ablation. Am J Cardiol. 1995;75:1278–1281. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal V, Dobrolet N, Fishberger S, Zablah J, Jayakar P, Ammous Z. PRKAG2 mutation: An easily missed cardiac specific non-lysosomal glycogenosis. Ann Pediatr Cardiol. 2015;8:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sri A, Daubeney P, Prasad S, Baksi J, Kinali M, Voges I. A Case Series on Cardiac and Skeletal Involvement in Two Families with PRKAG2 Mutations. Case Rep Pediatr. 2019;7640140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang KQ, Lu CX, Zhang Y, Yang YK, Li JC, Lan T, Meng X, Fan P, Tian T, Wang LP, et al. A novel PRKAG2 mutation in a Chinese family with cardiac hypertrophy and ventricular pre-excitation. Sci Rep. 2017;7:2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalra V, Akrawinthawong K, Kalra M, Jain R. Familial Hypertrophic Cardiomyopathy With Fasciculoventricular Accessory Pathway. JACC Case Rep. 2022;4:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SB, Weng WC, Lee NC, Hwu WL, Fan PC, Lee WT. Mutation of mitochondrial DNA G13513A presenting with Leigh syndrome, Wolff-Parkinson-White syndrome and cardiomyopathy. Pediatr Neonatol. 2008;49:145–149. [DOI] [PubMed] [Google Scholar]


