Abstract
The Alzheimer's Disease Neuroimaging Initiative (ADNI) Private Partners Scientific Board (PPSB) encompasses members from industry, biotechnology, diagnostic, and non‐profit organizations that have until recently been managed by the Foundation for the National Institutes of Health (FNIH) and provided financial and scientific support to ADNI programs. In this article, we review some of the major activities undertaken by the PPSB, focusing on those supporting the most recently completed National Institute on Aging grant, ADNI3, and the impact it has had on streamlining biomarker discovery and validation in Alzheimer's disease. We also provide a perspective on the gaps that may be filled with future PPSB activities as part of ADNI4 and beyond.
Highlights
The Private Partners Scientific board (PPSB) continues to play a key role in enabling several Alzheimer's Disease Neuroimaging Initiative (ADNI) activities.
PPSB working groups have led landscape assessments to provide valuable feedback on new technologies, platforms, and methods that may be taken up by ADNI in current or future iterations.
Keywords: Alzheimer's disease clinical trials, Alzheimer's Disease Neuroimaging Initiative, biomarkers, neuroimaging
1. BACKGROUND
The Alzheimer's Disease Neuroimaging Initiative (ADNI) is a multi‐site, multi‐year, observational study, launched in 2004 by the National Institute on Aging (NIA) to develop and validate a broad range of biomarkers that will aid in facilitating successful clinical trials for the treatment of Alzheimer's disease (AD). 1 ADNI, led by its founder and Principal Investigator Michael Weiner, has successfully investigated neuroimaging and biofluid biomarkers, enabling in vivo AD diagnosis, and fundamentally transforming AD clinical trials.
Building upon the successes of the initial 5‐year study (ADNI 1), the 2‐year extension (ADNI‐GO), and the competitive renewal (ADNI 2), ADNI 3 was launched in 2016 to collect imaging, fluid, and digital biomarkers from cognitively normal (CN) participants as well as from patients clinically diagnosed with either mild cognitive impairment (MCI) due to AD or AD dementia across 59 sites. A major change from ADNI 2 was the addition of longitudinal tau positron emission tomography (PET) with flortaucipir on all participants. During the conduct of ADNI 3, an emphasis on enrolling participants from underrepresented populations was added. As in previous ADNI studies, the relationships among imaging, fluid, and digital biomarkers with established cognitive measures was of particular interest. Importantly, ADNI continues to share all participant‐level data, including multiple clinical and biological measures ranging from imaging to other phenotypic and molecular data, with all qualified researchers who request the data. All data are available without embargo at USC.LONI.ADNI. In addition, ADNI biofluid data (plasma, serum, and cerebrospinal fluid), genetics, and cells are also available on request through the ADNI website.
1.1. ADNI public–private partnership, PPSB, and role of the Foundation for the National Institutes of Health
One key reason for ADNI's considerable success has been its unique structure as a public–private partnership. The Foundation for the National Institutes of Health (FNIH) played a crucial role in the success of ADNI, both by securing funding from private sector partners to supplement NIA's investment of federal dollars and by convening and managing the ADNI Private Partner Scientific Board (PPSB).
The FNIH is an independent not‐for‐profit organization established by the US Congress in 1990 to support the mission of the National Institutes of Health (NIH). Serving as a neutral and independent convenor, at NIA's request, the FNIH enabled the private sector partners to engage with each other and with ADNI, bringing together diverse entities, including industry, non‐profit organizations, and advocacy groups to complement and enhance the program.
The private funding that the FNIH secured, more than $65 million in total, was critical to ADNI's success. So too was its management and coordination of the PPSB for almost two decades, providing an unbiased, neutral forum for collaboration and information sharing. The careful involvement of private partners and their knowledge has been crucial to ADNI's accomplishments, and the PPSB has provided the ADNI leadership with valuable recommendations, suggestions, and feedback.
Many of the organizations that supported ADNI through the FNIH and the PPSB also design and implement clinical trials for investigational drugs. This enabled the PPSB to provide highly relevant scientific input and expertise that have contributed to ADNI's overall success. 2 , 3 In addition to funding, PPSB members have provided important in‐kind intellectual input, including ideas for additional data to be obtained and for further data analyses. For example, PPSB members were able to review and comment on the ADNI grant applications and on protocol design. Most importantly, the PPSB provides a pre‐competitive venue for private for‐profit and non‐profit organizations to collaborate and share scientific perspectives and logistical approaches. Furthermore, the PPSB has independently undertaken and, through FNIH, funded add‐on projects (as described below) that were not originally included in the parent grant. These projects have added significant value to ADNI and helped to advance the understanding of AD.
The PPSB is organized to reflect the core areas that are driven by the ADNI governance (Figure 1). In addition to having a liaison to each of the ADNI cores, the PPSB has established working groups (WGs) that were managed by the FNIH. The liaisons to the ADNI core are members from the PPSB who volunteer to represent the PPSB at the ADNI core meetings; provide updates to the PPSB; and. in some cases, lead meetings of the PPSB WGs. This was constructed for efficiency so that one or more individual representatives to each core could attend the regular ADNI core meetings, understand topics that may require strategic inputs, and socialize this information back to the larger ADNI PPSB for discussion. The PPSB WGs are made up of PPSB members interested in attending the PPSB WG meetings, depending on the agenda. Like the larger PPSB organization, the individual WGs described consist of a combination of members from industry, biotechnology, diagnostic, and non‐profit organizations. In this review, we summarize the major PPSB contributions to ADNI 3.
FIGURE 1.
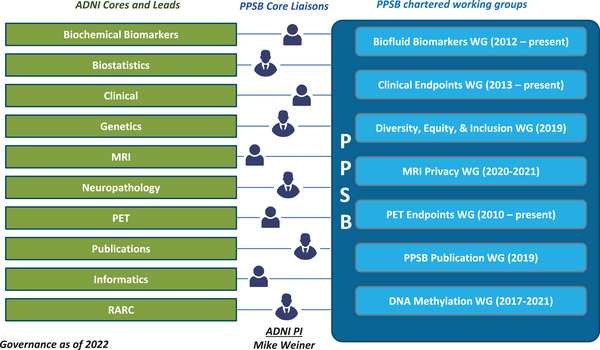
ADNI 3 Core denoted in green (left) and PPSB working groups denoted in blue (right) with year of establishment of working groups indicated. ADNI, Alzheimer's Disease Neuroimaging Initiative; MRI, magnetic resonance imaging; PET, positron emission tomography; PPSB, Private Partners Scientific Board; RARC, Resource Allocation Review Committee; WG, working group.
2. CLINICAL ENDPOINTS WORKING GROUP ACTIVITIES
In the early 2010s, with the advent of pre‐dementia clinical trials, there was an interest across several pharmaceutical companies to develop better endpoints, and the PPSB formed the Clinical Endpoints Working Group (CEWG). 2 The CEWG worked in close collaboration with Paul Aisen and Ron Petersen, chairs of the ADNI clinical core, and has undertaken several activities as part of ADNI 3, which are summarized below.
RESEARCH IN CONTEXT
Systematic review: In this review, the authors summarize the work that has been done by the Private Partners Scientific Board (PPSB) as part of the Alzheimer's Disease Neuroimaging Initiative (ADNI). The review describes the work that has been done across several PPSB working groups (WG), including the Clinical Endpoints WG, PET Endpoints WG, MRI Core Assessment and Privacy WG, and Fluid Biomarkers WG as part of ADNI 2/ADNI 3.
Interpretation: The PPSB has had a significant impact on bringing new assays to validation (e.g., Elecsys cerebrospinal fluid assays) as well as in identifying novel technologies to be deployed (e.g., Early Frames PET) and in data generation (e.g., DNA methylation data) within ADNI.
Future directions: Looking forward to ADNI 4 and beyond, the PPSB undertook due diligence for both digital biomarkers as well as biofluid biomarker assays in collaboration with the ADNI cores. Additionally, the PPSB has established the DEI WG, which will work in collaboration with the ADNI Engagement core to provide guidance from the industry perspective.
2.1. Remote self‐administered cognitive testing—Cogstate Computerized Brief Battery
ADNI 2 included extensive cognitive tests, such as the Boston Naming Test, Category Fluency (Animals), Clock Drawing, Weschler Memory Scale II, Logical Memory immediate and delayed recall, Montreal Cognitive Assessment 4 (MoCA), Rey Auditory Verbal Learning and Trail Making (RAVLT), 5 in addition to the more standard Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS‐Cog) 6 and Mini‐Mental State Examination (MMSE) 7 assessments. All of these assessments required significant annual in‐clinic testing that was eventually updated to occur once every 2 years for CN subjects. Due to the extensive clinical commitment required by this testing, ADNI 2 leadership requested that the CEWG provide insights on the implementation of remote computerized testing. After a comprehensive review of all available digital cognitive tests, the Cogstate Brief Battery (CBB) was selected and added as an optional protocol addendum in ADNI 2: “Cogstate Brief Battery (CBB) in the Alzheimer's Disease Neuroimaging 2 (ADNI 2).” 8 One hundred participants consented to longitudinal CBB testing both in clinic and at home as part of this optional pilot study, adding a digital biomarker to the imaging and fluid biomarkers in ADNI 1 and ADNI‐GO.
The CBB had been previously deployed in the Australian Imaging Biomarkers and Lifestyle study (AIBL) 9 and was later redesigned to be administered in either a clinical setting or remotely at home. In addition, this redesign implemented a new comprehensive self‐training module administered prior to the approximately 10‐ to 15‐minute cognitive test. After the CBB addendum, ADNI 2 was no longer enrolling new participants in the study except for those individuals classified as MCI or CN, who spoke English, and had Internet access. The participants were trained in the clinic by a trained CBB administrator, then instructed to complete another CBB session at home from an Internet‐connected computer within 14 days. Another at‐home or remote CBB session was performed 6 months after the initial CBB in‐clinic visit; after this session, the MCI patients completed the test annually while CN patients completed the test every 18 to 24 months. Throughout 6 years of ADNI 3, participants were repeatedly retrained on completing the CBB remotely to ensure validity.
The results of the ADNI 2 CBB pilot study have been presented at scientific meetings and published elsewhere. 10 The CBB was included as an integral part of ADNI 3 and has been conducted at each in‐clinic visit and also remotely between clinic visits for all the participants in the MCI and CN cohorts. As of March 14, 2023, approximately 643 participants completed baseline in‐clinic CBB assessments with 121 at the 1‐year in‐clinic for the cognitively impaired (CI) population and 60 CI/ 208 total at the 2‐year time point. More than 3000 unsupervised CBB assessments were conducted remotely as part of ADNI 3. In addition, many ADNI 2 participants that were tested with the CBB have rolled into the ADNI 3 resulting in longitudinal data over a greater time period. CBB was one of the first widely used digital biomarkers; however, in a separate study using participants from the Mayo Clinic Study of Aging (MCSA), a lower than anticipated sensitivity of the CBB in the MCI population 11 was observed, and for that reason the CBB was not included in ADNI 4. Data from the CBB from ADNI 3 have not yet been analyzed.
2.2. Financial capacity instrument
Detection of subtle impairment in activities of daily living (ADL) is a critical factor in the selection of subjects for clinical trials in the early phases of AD, and the ability to detect changes in these measures is required for the demonstration of the efficacy of any therapeutic intervention. 12 Performance‐based ADL measures are more advantageous than self‐report because they are quantifiable, repeatable, precise, and not dependent on the preservation of the patient's insight, which is often impaired as the disease progresses. The ability to manage one's finances is among one of the first ADLs to show a decline in AD, 13 and a validated performance‐based measure of financial management capacity would be highly desirable as a measure for clinical trials.
In 2015 the PPSB CEWG identified the Financial Capacity Instrument‐Short Form (FCI‐SF) 14 as a candidate performance‐based ADL measure for ADNI 3. PPSB selected the FCI‐SF as the most advanced performance‐based functional assessment, with preliminary data arguing for its sensitivity in discriminating CI from CN individuals. The FCI‐SF is a 15‐minute assessment, comprising 37 performance items that evaluate four constructs: monetary calculation, financial conceptual knowledge, use of a checkbook/register, and use of a bank statement. There are five domain scores (Mental Calculation, Financial Conceptual Knowledge, Single Checkbook/Register Task, Complex Checkbook/Register Task, Using Bank Statement) and a total score. Six additional processing speed variables capture time to completion for four of the performance items. The test has a manual and a well‐operationalized scoring system and requires a trained administrator. The FCI‐SF was derived from the FCI long form using those items associated with 1‐year progression to AD‐type dementia in a group of amnestic MCI patients.
Implementation of the FCI‐SF in the multicenter ADNI study represented a challenge as all previous experience with this tool came from single‐center studies. A site training and certification protocol was developed, consisting of individual FCI‐SF kits and test forms, a dedicated FCI‐SF web portal, and a training webinar with an embedded training video. Initial data collected by each site was monitored for quality to ensure appropriate test administration and scoring.
The FCI‐SF was successfully implemented in ADNI 3. As of January 1, 2022, 997 ADNI participants had been administered the FCI‐SF, including 558 who were cognitively normal at most recent evaluation, 334 with amnestic MCI, 101 with dementia, and 4 whose status was not available. A total of 2008 assessments have been performed; 626 participants have had at least one follow‐up, and some have received as many as four follow‐ups, up to 4.5 years after baseline.
Three cross‐sectional studies, based on the initial administration of the FCI‐SF in ADNI, have been published to date. In 440 participants, including 179 CN and 261 with MCI or dementia, 15 the following four factors explained 46% of the variance in FCI‐SF scores: (1) basic monetary knowledge and calculation skills, (2) financial judgment, (3) financial conceptual knowledge, and (4) financial procedural knowledge. These factors might be useful to guide further development and clinical use of the FCI‐SF.
Tolbert et al. 16 tested the association between the FCI‐SF and 18F‐florbetapir PET measurement in 243 ADNI participants, including 144 cognitively unimpaired, 79 with MCI, and 20 with mild AD dementia. Total and domain scores as well as completion times were worse in MCI and mild AD dementia participants, and across the cognitive spectrum, higher amyloid level was associated with worse FCI‐SF scores after covarying for age, sex, and education, suggesting that financial capacity impairments in MCI and AD are associated with the extent of cortical amyloid deposition. In aging, amyloid beta (Aβ) deposition was associated with the slowed performance of financial tasks. These findings complement earlier observations in cognitively unimpaired participants in the MCSA, 14 which showed worse performance in several domains for those who were amyloid positive on Pittsburgh compound B (11C‐PiB) PET.
These findings were extended by Gonzalez et al. 17 in 670 ADNI participants (410 CN, 199 with MCI, and 61 with AD dementia) who also had 18F‐flortaucipir tau PET. Moderate correlations on linear regression were seen between FCI‐SF total score and 18F‐flortaucipir signal in each of six regions of interest (entorhinal, inferior temporal, dorsolateral prefrontal, and supramarginal cortices as well as precuneus and posterior cingulate), which were independent of age, education, sex, and performance in verbal recall and executive function. The interaction of amyloid and tau PET in four extratemporal regions (supramarginal and dorsolateral prefrontal cortex, posterior cingulate, and precuneus) also predicted lower FCI‐SF scores. Together, the findings from these analyses suggested that impairments in financial capacity across the cognitive spectrum are related globally as well as regionally to key pathology biomarkers of AD. Importantly, these studies indicate that even in CN ADNI participants, amyloid and tau burden and interaction between amyloid and tau are associated with worse results on this performance‐based ADL. This was one of the first performance‐based tools that was used, but was eventually not included in ADNI 4.
3. PET ENDPOINTS WORKING GROUP
The PET Endpoints Working Group (EWG) was established at the beginning of ADNI 2 and provides a forum for PPSB members to discuss aspects of PET imaging relevant to ADNI and clinical trials in AD. Members participate in regular ADNI PET core meetings run by the PET core lead William Jagust, and the EWG has provided an advisory role to the ADNI PET core for key PET‐related issues. Initial outputs of the PET EWG included technical guidance on longitudinal amyloid PET acquisitions in clinical trials. 18 The PET EWG also provided recommendations and assisted the PET core in defining the criteria and process for selection of candidate tau and amyloid PET tracers for use in ADNI 3 and potentially for future ADNI iterations. The EWG also provides a forum for members to propose and organize supplementary funding for additional PET sub‐studies, most notably the “early frames” amyloid PET sub‐study implemented in ADNI 3. Current topics include the standardization and use of visual reading of amyloid and tau PET and adoption of the Centiloid scale for quantitation of amyloid PET.
Amyloid PET scans in clinical trials are typically acquired over a short (20–30 minutes) set of “late frames,” starting approximately 50 to 90 minutes after injection, depending on the tracer. However, most small‐molecule radioligands, including amyloid PET tracers, are characterized by rapid uptake from the vascular compartment into the brain parenchyma in the first few minutes after injection. Several publications have demonstrated that subjects across the AD spectrum exhibit a pattern of hypoperfusion, detected from that “early frames” signal, that is strongly correlated with hypometabolism detected using 18F‐fluorodeoxyglucose (FDG) PET 19 , 20 , 21 , 22 , 23 (Figure 2A). This then enables imaging biomarkers of both amyloid (“late frames”) and perfusion, a marker of neurodegeneration (“early frames”) to be obtained from a single subject visit and radiotracer injection. Moreover, early frames and late frames data may also be combined and fit to a full kinetic model, from which both a vascular to tissue rate constant parameter (K 1) and a target tissue binding potential may be calculated.
FIGURE 2.
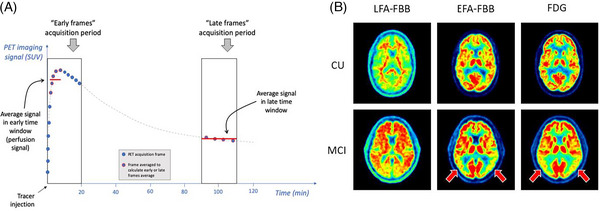
A, Cartoon illustrating the concept of early frames and late frames PET acquisition, for a theoretical time‐activity curve from one region of interest (gray dashed line). Both early and late frame signals are most simply summarized by calculating the average over a given time window, and conventionally expressed as the standardized uptake value ratio (SUVR) by dividing the average value in each voxel or region of interest by the average value in a reference region. Of note, the reference region may be different for early (perfusion) and late (amyloid) frames analyses. The time windows illustrated here correspond to those used in ADNI 3 sub‐study for 18F‐florbetaben. B, Illustrative examples of LFA and EFA perfusion maps from one CU and one MCI participant scanned with FBB, compared to 18F‐FDG PET scans from the same individuals, from the ADNI 3 sub‐study. These examples illustrate similar EFA (perfusion) and [18F]‐FDG (glucose metabolism) profiles in the same participants, including pronounced temporoparietal hypoperfusion and hypometabolism in the MCI case (red arrows). In contrast, the LFA scans reflect amyloid burden and show greater cortical signal in the MCI participant. ADNI, Alzheimer's Disease Neuroimaging Initiative; CU, cognitively unimpaired; EFA, early frames amyloid; FBB, 18F‐florbetaben; FDG, fluorodeoxyglucose; LFA, late frames amyloid; MCI, mild cognitive impairment; PET, positron emission tomography.
A pilot sub‐study to acquire early frames PET data from 18F‐florbetapir PET scans was included in ADNI 2. This comprised cross‐sectional data only but enabled observation of the anticipated close relationship between “early frames”‐based hypoperfusion and hypometabolism from 18F‐FDG PET. 24 However, to be used as an outcome biomarker in clinical trials, the longitudinal measurement characteristics of the early frames amyloid PET signal require characterization.
To fill this gap, the PPSB proposed and funded an ADNI 3 sub‐study which was enabled by the FNIH to start in 2019 in which 100 consenting individuals would be scanned twice, 2 years apart, with an “early frames” PET acquisition as part of their amyloid scan visits. Unlike ADNI 2, both 18F‐florbetapir and 18F‐florbetaben PET scans were to be used for this purpose. In practice, the perfusion PET acquisition comprised a 20‐minute “early frames” acquisition, prior to a subject rest break, and then a “late frames” acquisition for quantification of amyloid burden. A minimum sample size for the sub‐study of 75 CN and 25 MCI was based on annualized change seen over 2 years in ADNI 18F‐FDG PET longitudinal data from CN and MCI groups.
Figure 2B shows examples of “early frames” 18F‐florbetaben acquisitions from ADNI 3. These examples illustrate similar “early frames” and glucose metabolism (18F‐FDG) maps, including pronounced temporoparietal hypoperfusion and hypometabolism in the MCI case. Analyses of the ADNI 3 images suggested that “early frames” 18F‐florbetapir and 18F‐florbetaben regional standardized uptake value ratios (SUVRs) do not significantly differ. 25 Moreover, these results suggest that regional SUVR from “early frames” amyloid scans, regardless of the tracer used, is well correlated with regional SUVR from 18F‐FDG PET scans. This promising data led to a continuation of the longitudinal early frame study into ADNI 4.
4. MRI CORE ASSESSMENT AND PRIVACY WORKING GROUP
The magnetic resonance imaging (MRI) core, headed by Clifford Jack Jr., and its funded investigators are responsible for standardizing the acquisition, quality control, and quantitative analysis of MRI‐based endpoints. A comprehensive assessment of structural, functional, perfusion, and other endpoints can now be derived from a protocol that has evolved significantly since its first instantiation as a largely volumetric protocol for 1.5T scanners in ADNI 1. During ADNI 3, PPSB membership was surveyed for their perspective on the utility of each MRI endpoint in the context of AD clinical trials and drug development. Representatives from ten pharmaceutical companies and three imaging contract research organizations (CROs) completed a questionnaire gauging their interest in acquiring additional data toward each endpoint in future ADNI protocols, and their opinion of how such data are acquired and analyzed. Volumetric MRI was the only sequence for which all pharma and CRO participants agreed continued data collection was a “must have.” Qualitatively, several members echoed that volumetric MRI is essential, both as a reliable indicator of change and in relation to other biomarkers. Diffusion tensor imaging (DTI), fluid‐attenuated inversion recovery (FLAIR), and gradient‐recalled echo (GRE) safety sequences were similarly considered “must have” by most respondents. Interest in the continued acquisition of arterial spin labeling (ASL), quantitative susceptibility mapping (QSM), hippocampal subfields, and vascular pathology was generally moderate, and task‐free functional MRI (fMRI) was the only sequence a member suggested to “exclude” from future protocols. Multi‐compartment modeling and myelin water imaging were suggested as novel endpoints for future development. Most respondents favored abandoning the “basic” fMRI and DTI protocols and restricting future imaging to sites capable of multi‐band, multi‐shell acquisition. There was also general agreement that a single funded lab should be responsible for deriving metrics for each sequence for the ADNI database.
During ADNI 3, the PPSB was alerted to a possible risk to participant privacy after it was demonstrated 26 that tissue information in the volumetric MRI scan could be used to reconstruct features amenable to facial recognition software. ADNI leadership, with the participation of the PPSB, formed a Privacy WG charged with recommending a technical solution to the problem of MRI face de‐identification (FDI) that might be applicable to MR images from ADNI. Such a solution would reduce the risk of participant identification from their MRI scan, be robust to differences in image acquisition and quality, and incur minimal impact on downstream image analysis pipelines. The WG conducted a landscape review, identified a dozen extant approaches to FDI, and consulted with other large consortia. Several candidate approaches were entered into a competitive challenge with scores based on adherence to the policy goals. Recognizing that no single approach is likely to eliminate the risk of facial recognition completely, the WG recommended an algorithmic approach to balance efforts of participant privacy and open science that will be implemented in ADNI going forward.
5. BIOFLUID BIOMARKER WORKING GROUP
The Biofluid Biomarker WG (BBWG) was established during ADNI 2 to serve as a forum for PPSB members to discuss cerebrospinal fluid (CSF) best practices for diagnostic and prognostic intended uses and to align on CSF and biomarker samples and data collection. The BBWG worked hand in hand with the ADNI Biomarker core that was led by Les Shaw and John Trojanowski. In anticipation of ADNI 3 and the need to include an in vitro diagnostics (IVD) platform under development into the Biomarker Core Laboratory activities, the BBWG undertook a due diligence process to identify assays in development by independent companies whose goals were ultimately to achieve regulatory approval. After a thorough evaluation of assays from 10 companies the PPSB recommended to ADNI leadership that the Roche Elecsys immunoassay platform for Aβ1‐42, phosphorylated tau (p‐tau), and total tau (t‐tau) measurements be included in the Biomarker Core laboratory for ADNI 3.
Data from ADNI 2 and ADNI‐GO trial cohorts were used for clinical validation of the Elecsys CSF biomarker cut‐offs, which optimized agreement with amyloid PET visual read. This analysis led to a traditional Food and Drug Administration (FDA) 510(k) clearance of the Elecsys pTau181/Abeta42 ratio on December 8, 202,2 followed by clearance of the Elecsys tTau/Abeta42 CSF ratio on June 5, 2023. The Elecsys AD CSF assay ratios are approved for use in adult patients aged ≥ 55 years who are being evaluated for AD and other causes of cognitive impairment, where a positive or negative ratio result is consistent with a positive or negative amyloid PET scan, respectively. This analysis also supported Roche Elecsys Abeta42, pTau181, and tTau CSF approval in Conformité Européenne (CE)‐mark–accepting countries.
The selection of Roche as the assay provider for inclusion in ADNI‐3 Biomarker Core laboratory moved ADNI out of the pre‐competitive into the post‐competitive space. Several PPSB members were concerned about this change in direction for the use of CSF material and the NIA and ADNI leadership broadened the requirements for access to banked CSF samples. This decision provided the BBWG its first goal for ADNI‐3, to develop a process and evaluate vendors/companies developing commercial IVD tests for access to CSF samples. Under the guidance of the NIA and along with the ADNI Biomarker and Statistical Cores the BBWG developed the Residual CSF Sample program. The NIA would make ADNI CSF banked samples available by expanding the traditional Resource Allocation Review Committee review process to include the following conditions:
All data generated using ADNI CSF would be publicly available and become part of the Laboratory of Neuro Imaging (LONI) database.
Only previously assayed (thawed twice) CSF and not “fresh” CSF aliquots would be used for this purpose.
A transparent, fair process for prioritizing access to such samples would be developed.
5.1. CSF residual sample program
Ten companies were identified and invited to submit letters of intent to receive residual samples for their IVD development program (Figure 3). After review of proposals and the provision of details for running the assays, four companies participated in this program including Meso Scale Diagnostics, Fujirebio, EUROIMMUN, and Saladax/Siemens. In addition to the assays that were run by each company, the Biomarker Core added a set of 20 CSF pools for inclusion into their analytical runs that then would permit comparisons of precision performance across these immunoassays.
FIGURE 3.
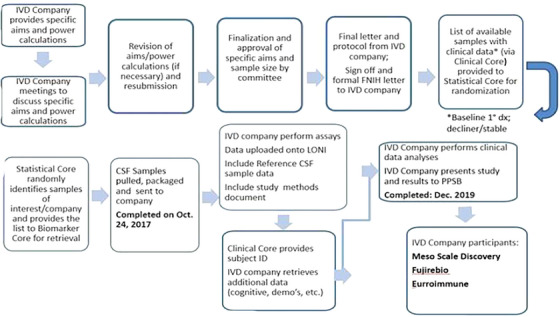
Process of the residual CSF sample protocol review and sample distribution. CSF, cerebrospinal fluid; FNIH, Foundation for the National Institutes of Health; IVD, in vitro diagnostics; LONI, Laboratory of Neuro Imaging; PPSB, Private Partners Scientific Board.
The current status of the IVD program for each of the companies is as follows. Fujirebio used the CSF residual sample program to validate a set cutoff for the Lumipulse G β‐Amyloid Ratio (1‐42/1‐40). The cutoff was established using the Amsterdam Dementia Cohort (ADC) and validated using the ADNI residual CSF samples. The handling procedures of the ADC and ADNI CSF samples differed, and this study also compared the impact of these different procedures on CSF amyloid concentration levels in general and for amyloid PET positive (PET+) and negative (PET–) individuals. The comparison of the amyloid levels across ADC and ADNI showed that Aβ1‐40 differed only modestly between the PET+ and PET– patients. Although there were statistically significant differences in Aβ1‐40 between PET+ and PET– individuals in ADNI and ADC, and between the two data sets, the absolute magnitude of these differences was small. As expected, Aβ1‐42 in the PET+ patients was much lower than those of the PET–, and slightly lower in ADNI overall. The difference in Aβ1‐42 levels between the PET+ and PET– patients did not differ significantly between ADC and ADNI. Also as expected, when the Aβ1‐42/Aβ1‐40 ratio was compared between the PET+ and PET– patients the ratio was much lower in PET+ individuals. However, there was neither an overall difference between the data sets nor an interaction. In sum, the Lumipulse G β‐Amyloid Ratio (1‐42/1‐40) did not require an adjustment factor for handling differences between ADC and ADNI CSF samples.
The validation study was used to support an FDA submission of a de novo application to the Center for Devices and Radiological Health (CDRH) for the Lumipulse G β‐Amyloid Ratio (1‐42/1‐40), which is intended to be used as an alternative to amyloid PET as an aid in the assessment of amyloid pathology for individuals suffering from cognitive complaints. Fujirebio received marketing authorization for their CSF assay “Lumipulse G β‐Amyloid Ratio (1‐42/1‐40)” in May 2022.
EUROIMMUN participated in the ADNI CSF residual sample program in the development of their traditional AD CSF assays (Aβ1‐42, Aβ1‐40, total‐tau, and p‐tau181) for testing on their fully automated, closed, random access chemiluminescence platform (Clinical Laboratory Improvement Amendments [CLIA] approved). Their intention was to get US FDA IVD approval for their assays. As part of their plan, EUROIMMUN performed comparisons with comparable platforms by measuring samples analyzed using Roche immunoassays. To that aim, the correlation between Roche Elecsys β‐amyloid (1‐42) and EUROIMMUN CLIA Beta‐Amyloid 1‐42 (Aβ42) assays were determined. Further, a recalibration of EUROIMMUN's assays with Certified Reference Material (CRM) was undertaken. EUROIMMUN has completed CRM adjustments for enzyme‐linked immunosorbent assay and chemiluminescence platforms for Aβ (1‐42) (R 2 = 0.965, P < 0.0001) using the residual CSF samples from ADNI. This might not be used for FDA submission, because CRMs for Aβ 1‐42 became available after this study was performed. As a consequence, both assays were adjusted toward the CRM.
Meso Scale Diagnostics, LLC used residual CSF samples to demonstrate that the Aβ1‐42/Aβ1‐40 ratio measured with its analytically validated multiplexed assays detects individuals with high 18F F‐florbetapir score with a sensitivity of 85% (95% confidence interval = 78.30% to 90.44%) and specificity of 88% (95% confidence interval = 81.22% to 92.86%) at a threshold of 0.07. The Pearson correlation between the MSD and Roche Aβ42 assays was r = 0.96 (95% confidence interval = 0.949 to 0.971). The study provides a strong foundation for the potential future development of a laboratory‐developed test (LDT) or clinical diagnostic product.
Saladax used residual CSF samples to examine the Aβ42 and Total Tau assays, both analytically validated. They used a heterogenous two‐antibody sandwich format with a magnetic particle solid phase and chemiluminescent detection. Testing was performed on a fully automated Siemens Advia Centaur XPT clinical analyzer over the course of 3 days using one lot of Aβ42 reagents and one lot of Total Tau reagents. Reagents were calibrated on each day of testing, and assay performance was confirmed by testing controls twice daily. In total, 393 ADNI samples were tested of which 373 were individual samples, 10 were ADNI Pool 59 (CN) and 10 were ADNI Pool 56 (AD). Testing was conducted by thawing 50 samples at a time at room temperature for 30 to 60 minutes, vortexing the vials for 8 seconds, and then placing the vials onboard the analyzer for testing (samples were tested directly out of the vials that they were received in from ADNI). Samples were continuously loaded onto the analyzer, and testing was completed for both analytes approximately 30 minutes after a given sample was loaded. Approximately 100 samples were tested n = 1 for Aβ42 and Total Tau each day. The Aβ42 results had a mean of 356 pg/mL, a standard deviation (SD) of 183 pg/mL, and 25th and 75th percentiles of 220 and 461 pg/mL, respectively. The Aβ42 results had a bimodal distribution with a major peak at approximately 217 pg/mL and minor peak at approximately 662 pg/mL. The Total Tau results had a mean of 289 pg/mL, an SD of 146 pg/mL, and 25th and 75th percentiles of 192 and 341 pg/mL, respectively. The Total Tau results resembled a log normal distribution with a peak at approximately 197 pg/mL.
The residual CSF sample program achieved its goals and provided a model for which other biofluid could be evaluated and access stored samples in the pre‐competitive space for companies/entities developing assays.
6. GENETICS AND SYSTEMS BIOLOGY WORKING GROUP
The ADNI Genetics core, led by Andrew Saykin, oversees all genomics activities for ADNI, including the collection of lymphoblastoid cells, genomic DNA, and RNA from enrolled subjects (Figure 4). To gain an understanding of the molecular mechanisms associated with disease progression, several genomics and transcriptomics initiatives have been undertaken by both ADNI investigators and by the PPSB. Apolipoprotein E genotyping, genotyping arrays, and whole genome sequencing have been run on either a subset or all the participants in ADNI, and the data are available on LONI. 27 , 28
FIGURE 4.
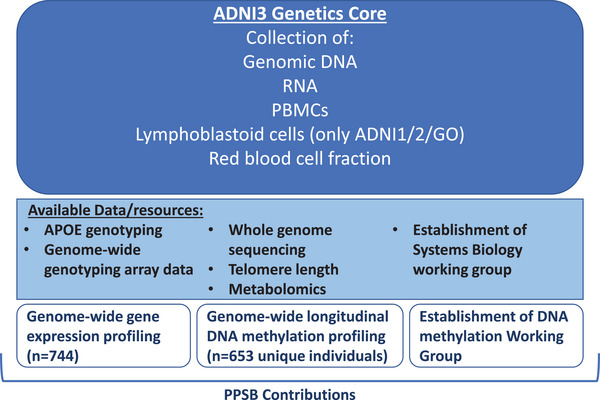
Activities within ADNI 3 Genetics Core: The ADNI3 Genetics Core collects genomic DNA, RNA, PBMCs, and red blood cell fractions. Several datasets have been generated using these ADNI resources (yellow box), and PPSB contributions are highlighted in brown boxes. ADNI, Alzheimer's Disease Neuroimaging Initiative; APOE, apolipoprotein E; PBMC, peripheral blood mononuclear cells.
6.1. Transcriptomic analysis
Genetic variations may impact the expression of genes or proteins, enabling the identification of molecular pathways involved in disease etiology. Several studies in the previous decade had demonstrated that gene expression signatures in blood may help classify AD patients and CN controls and could also predict disease status. However, many of these studies had been done in smaller cohorts with fewer overlaying datasets available. 29
To further evaluate whether differential gene expression changes are associated with disease status, the Genetics core, along with the PPSB, was able to plan a larger scale differential gene expression analysis of peripheral blood RNA. A subset of 811 baseline samples from ADNI 1, ADNI 2, and ADNI‐GO participants that were also included in the whole genome sequencing sub‐study were selected and RNA profiling was performed at the laboratory of a PPSB member. The Affymetrix Human Genome U219 Array (Affymetrix) was used for expression profiling. This dataset has contributed to a better understanding of peripheral blood gene expression in AD, and from association studies with imaging readouts, has helped identify novel genes that may be potential drug targets.
6.2. Epigenomic analysis
Given the advances in the field of epigenetics of neurodegenerative diseases 30 , 31 the PPSB expressed an interest in characterizing peripheral DNA methylation marks within the ADNI cohort. DNA methylation marks are dynamic and can be altered during disease progression or with drug treatment. Peripheral blood DNA methylation changes associated with disease stage or progression will provide a novel non‐invasive method to diagnose and stratify patients in the clinic and will be useful to understand molecular changes associated with disease progression.
To identify differential DNA methylation marks and to potentially validate DNA methylation as a novel peripheral blood‐based biomarker, four PPSB members as well as members of the Genetics core drove the efforts to run DNA methylation analysis on a subset of ADNI subjects. This was a multi‐step process that required a high level of coordination between the Genetics core, the PPSB member companies, and the National Centralized Repository for Alzheimer's Disease (NCRAD). First, samples with complete CSF, imaging, and genomics data were identified by investigators in the Genetics core. A majority of these samples had at least two longitudinal samples per subject. Second, the blinded samples were randomized to account for batches of sample processing and runs. The randomizations also provided a way to include nearly 200 technical replicates, which facilitated testing the robustness of the DNA methylation assay. The samples were then plated out by NCRAD in a randomized order and sent to the laboratory of a PPSB member to be run on Illumina EPIC chips. The generated data were uploaded to LONI and are available for download, and the paper outlining these data has been published. 32 Briefly, genomic loci that were differentially methylated were enriched for genes associated with AD pathology (e.g., BIN1) or that had origins in the brain, which supports the idea of a peripheral blood‐based marker that may serve as a surrogate of brain pathology.
Several follow‐up studies have been published using the DNA methylation data, and these data have been used for validating previous findings as well as for assessing DNA methylation marks associated with specific endophenotypes, including cognitive scores and imaging readouts. 33 , 34
6.3. DNA methylation working group
A WG driven by the PPSB was established to discuss questions of interest in the analysis of the DNA methylation data. This group included representatives from contributing companies and investigators that formed part of the Genetics core. The WG provides a platform for the discussion of analytical methods, and data normalization and quality control analysis were performed within this group. Additionally, the group drives the planning and prioritization of experiments and publications associated with the ADNI DNA methylation dataset. Three publications from this WG that use the methylation dataset have already been accepted, 32 , 33 , 34 and the data are currently being used for additional analyses within the WG.
7. ADNI 3 PPSB LANDSCAPE ASSESSMENT PROCESS
In preparation for the ADNI 4 grant application, ADNI leadership announced a “Request for Proposals (RFP)” concerning new biomarkers. There were a large number of responses including digital biomarkers of cognition, fluid biomarkers, electroencephalogram/event‐related potential measurements and others. The ADNI leadership worked together with the PPSB WGs to assess digital, fluid, and PET biomarkers. PPSB members were surveyed about their experience with different neuroimaging and fluid biomarkers, and an extensive landscape review was completed for each prioritized topic area. The CEWG evaluated digital clinical measures for remote use, the BBWG evaluated blood and CSF assays, and the PET EWG reviewed PET tau tracers. The ADNI 3 MRI Core evaluated MRI defacing software and kept the MRI Privacy Group apprised of their findings. Each WG started by assessing the market for products available and selecting a smaller review team dedicated to meeting regularly to provide insights to the PPSB.
Each review team developed a uniform approach to collect and review topics under the purview of the working group (Figure 5). The teams collected and reviewed supporting published literature, as well as forms, spreadsheets, and presentations provided by organizations that have established biomarker tests. Each organization and company were invited to give 30‐minute presentations of their tests to the respective groups and answer seven standard questions (see Figure 5, green box).
FIGURE 5.
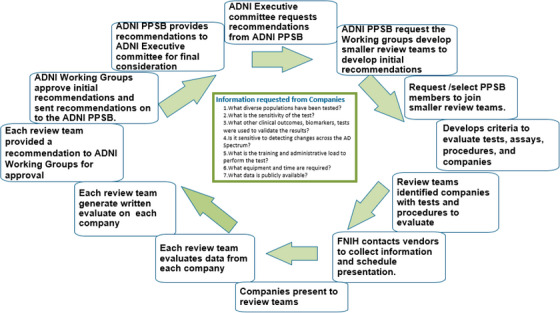
Process of landscape assessment undertaken by PPSB WGs to review and evaluate tests, assays, procedures, and companies. FNIH, Foundation for the National Institutes of Health; PPSB, Private Partners Scientific Board; RARC, Resource Allocation Review Committee; WG, working group.
The review teams presented their recommendations to the WGs. The FNIH moderators helped to coordinate all these recommended presentations and integrated the data into user‐friendly summaries and tables. This information, along with some overarching conclusions, was shared with ADNI PPSB and Core leadership. The planning group for the next phase of ADNI (i.e., ADNI 4) and the Executive Committee made the final determination after the PPSB submitted its final conclusions.
7.1. CEWG landscape assessment
As ADNI has continued investigations over time into the biology, progression, and promising treatments for AD, the aging population has become more comfortable with technology. The efforts advanced by ADNI 3, with the engagement of the PPSB, facilitate the collection of necessary data to allow for better tracking of the AD population and thereby allow us to more fully understand the changes in biology and cognition through the lifespan. Therefore, the CEWG focused on investigating both existing and new testing platforms that can be self‐administered remotely to participants enrolled in potential future ADNI phases. Domains of interest include cognition, behavior, speech and language, and even electrophysiologic assessments. Such assessments are now frequently referred to as digital biomarkers. This process is very similar to previous reviews to identify partners for the other areas of ADNI outlined earlier in this paper. ADNI leadership requested the CEWG review two major categories: remote screening and longitudinal assessments. The screening tool should be an instrument with a demonstrated ability to differentiate CN from MCI. The screening tool should also account for demographic parameters such as age, sex, race, ethnicity, education, and have a high correlation with the cognitive classification and diagnostic standards used in ADNI 3 (which is based on MMSE, 35 Clinical Dementia Rating, 36 and Wechsler Logical Memory Test II–Delayed Story Recall). 37 The longitudinal assessment tools need to show sensitivity to changes over time that correlate with the imaging and CSF biomarker‐confirmed AD population (i.e., AD BM+/MCI) compared to the AD biomarker‐negative normal controls (i.e., AD BM–/CNs).
7.2. BBWG landscape assessment
During ADNI 3, the BBWG in collaboration with the Biomarker core formed the blood‐based biomarker (BBB) Due Diligence Subgroup to formalize evaluation of BBB assays and vendors in preparation for future ADNI studies. This program was undertaken to make a recommendation for BBB to the potential future iteration of ADNI program. Eighteen companies were identified who have active BBB assay development programs ongoing. To date there have been presentations of data from eight companies, both for traditional AD assays such as Aβ40, Aβ42, t‐tau, p‐tau181, p‐tau217 as well as assays such as glial fibrillary acidic protein (GFAP), neurofilament light chain (NfL), and U‐P‐53az, and TAR DNA binding protein (TDP43). This due diligence evaluation program is depicted in Figure 5.
7.3. PET activities: PET tracer selection and visual read for ADNI 4
In addition to individual industry PET imaging responses to the ADNI 4 biomarker RFP, the PET EWG provided substantive input to the ADNI PET core for the selection of additional PET ligands under consideration for ADNI 4. PPSB members were surveyed on interest and preference for additional amyloid and tau tracers, as well as neuroinflammation, synaptic vesicle glycoprotein 2A (SV2A), and other novel tracers. Survey results and subsequent discussion helped to inform the decision to incorporate one additional amyloid tracer (18F‐NAV4694) and two additional tau tracers (18F‐MK‐6240 and 18F‐PI‐2620) into the ADNI 4 design. PET EWG input was also key for the addition of a visual read of all amyloid PET scans into ADNI 4. Visual reads are commonly used as inclusion criteria for industry drug development trials, and help fill a gap noted by the PPSB that prior ADNI PET data provided only quantitative PET analysis. The PET EWG was also instrumental in providing industry expertise and perspective on how to best implement an amyloid visual read in the ADNI 4 study.
8. INCLUSION AND ENGAGEMENT OF UNDERREPRESENTED POPULATIONS IN AD CLINICAL TRIALS
Since its initial launch in 2004, ADNI and all subsequent extensions have reported a lack of representation of participants from ethnoculturally and socioeconomically diverse backgrounds. Without appropriate participation by Black Americans, Latinx Americans, Asian Americans, and Native Americans or other underrepresented populations (URPs) in AD clinical trials and research, it is impossible to get a complete understanding of how racial and ethnic differences may affect the efficacy, safety, and generalizability of potential new treatments and diagnostics. A limitation of ADNI 3 is its lack of ethnic and racial diversity among those enrolled. In 2016 86% of ADNI 3 participants identify as Non‐Hispanic Whites, which does not reflect the demographic distribution of individuals with AD in the United States. 38 , 39 The next iteration of ADNI, led by the core leads Monica Rivera‐Mindt and Ozioma Okonkwo, is committed to enhancing the diversity of its clinical study participants and recognizes the need for proactive approaches and dialogue for successful inclusion and engagement of URPs. The ADNI3 Diversity Taskforce was assembled in 2020 to specifically use a culturally informed, community‐engaged research approach to increase the inclusion and engagement of URPs in ADNI 3. Since the creation of this team, URPs increased from 1.13 to 4.58 URP persons per month by early 2022.
The primary goal of the ADNI 3 PPSB Diversity, Equity, and Inclusion (DEI) WG was to increase the representation of Black and Hispanic participants in ADNI 3. The WG's mission is to leverage private‐sector knowledge and best practices in clinical trial outreach to support efforts to improve the inclusion of racially and ethnically diverse study participants in ADNI 3 through increased community awareness, education, and engagement.
The PPSB DEI WG consists of experts who will provide guidance rooted in empirical evidence, current thinking, and novel approaches based on the team's collective knowledge. The group will monitor real‐time recruitment and make adjustments to their recruitment strategy as the regulatory environment changes, recruitment technologies advance, and other industry expertise continues to develop. We anticipate this engagement to be iterative as the clinical trial landscape continues to evolve.
The WG has acknowledged that recruitment and retention efforts will need to be adaptive to the cultures of the underrepresented populations by considering flexible approaches that are respectful, culturally and linguistically authentic, and sex and age appropriate. Generational differences may challenge recruitment and retention experiences.
To ensure that ADNI 4 reaches the goal of 40% enrollment of URPs, the ADNI Engagement Core will implement recommendations comprehensive culturally informed community‐engaged research approach to (1) promote the recruitment and engagement (e.g., retention, study task completion) of older adults from URPs using culturally informed approaches to represent 50% to 60% of the ADNI 4 cohort; (2) train ADNI site investigators and staff and junior URP investigators in basic, clinical, translational, and Claim, Evidence, Reasoning concepts necessary for success in conducting innovative dementia research; and (3) compare the frequency and etiology of biomarker and cognitive profiles across ethnocultural groups, and examine whether biological, psychological, or sociocultural factors modify these associations. 40 , 41
9. FUTURE PERSPECTIVES OF THE PPSB IN ADNI: CONTRIBUTING TO OPEN DATA SHARING AND CONTINUING TO STRENGTHEN THE PUBLIC–PRIVATE PARTNERSHIP
Since its inception, ADNI has been an open data‐sharing resource, with clinical endpoint, imaging, biomarker, and molecular data becoming available soon after collection with no embargos on data sharing. With this, ADNI pioneered a common, unified platform that facilitates collaborations for researchers in academic, government, non‐profit, and industry settings to identify and validate methods for designing and implementing clinical trials.
Data sharing establishes reproducibility, improves research practices, provides a test bed for new analysis methods, and reduces the cost of science while providing maximum impact of the collected data. 42 ADNI's leadership in data sharing has improved research practices across multiple workflows; for example, ADNI has contributed significantly to image processing pipeline validation, provided a normative dataset for both longitudinal and cross‐sectional analysis, and generated a baseline for method optimization. 43 The extensive sharing of ADNI data also facilitates the development of new analysis methods. ADNI data have been used by companies to model cognitive decline in an effort to optimize readouts from clinical trials, and this may provide insights into biomarkers that may help stage disease. 44 Given the rich phenotyping data available, ADNI continues to be a resource for testing novel pipelines and methods, leading to the establishment of resources to compare various clinical tools and biomarkers across subgroups. ADNI data have been successfully used for understanding the association of genetic factors defined by polygenic risk scores with plasma biomarkers, specifically, the p‐tau181 marker status. 45 Data sharing also provides a platform to evaluate new tools, such as tau PET staging schemes using scans acquired in ADNI 2. 46
With the advent of blood‐based biomarkers that provides low cost, minimally invasive methods to assay for protein biomarkers of AD pathology to identify Aβ status, ADNI has provided samples to PPSB members for validating several of the assays measuring Aβ, tau, and p‐tau species in the blood. 47 , 48 The establishment and validation of these blood‐based biomarkers in a racially and ethnically diverse ADNI cohort will enhance the knowledge needed for recruitment of specific cohorts for clinical trials, including prodromal AD and preclinical AD, as well as to understand the risk of disease progression in an individual. ADNI data have been used extensively in simulations of clinical trials, specifically for enrollment considerations, as well as for power analyses across multiple data types. 49 The availability of such a database with opportunities for subject selection on the basis of multiple factors lowers AD clinical trial costs and duration.
ADNI's structure has proven to be a valuable example of how public–private partnerships may be harnessed effectively, with private partner involvement in financial and advisory capacities since the inception of the study 50 providing an example for other public–private partnerships, including Parkinson's Progression Markers Initiative (PPMI), 51 and Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK‐TBI). 52
An enduring value of the PPSB has been the opportunity for PPSB partners to develop collaborative WGs and fund additional add‐on studies to support and extend ADNI core activities, and the most recent activities are described above. ADNI 2 saw the initiation of the PET EWG, the CEWG, and the BBWG with an initial focus on technical guidance and support of each of the core groups. During ADNI 2, a Database WG was formed, dedicated to common interests and issues for sponsors such as reconciliation of the clinical database with standards such as Clinical Data Interchange Standards Consortium (CDISC), creation of a common relational database, and annotation of endpoints in the database. This facilitated efforts within and between pharmaceutical partners on disease modeling including head‐to‐head comparisons of models with different cognitive endpoints and biomarker correlates. ADNI also benefited from these database efforts and developed a proposal to investigate whether item response theory or Rasch models could offer greater sensitivity for detection of change in cognition over traditional scoring methods.
During ADNI 3, PPSB sponsors continued to share work on disease modeling using ADNI data at their monthly meetings. The FNIH helped mobilize PPSB partners to propose ideas that would accelerate active collaboration and open sharing of analyses, which have been critical elements of success for PPSB partners. As the ADNI 4 PPSB transitions to management by the Alzheimer's Association, the coalescing of ideas around a common goal for the PPSB will be important to maintain as ADNI evolves and will be essential for the long‐term sustainability of ADNI.
CONFLICT OF INTEREST STATEMENT
B.A., E.A., A.T., C.J.W., L.W., and J.W.R. have no conflicts to report. R.B. and A.J.S. are employees of Takeda Pharmaceuticals. S.D.S. is an employee of Eisai. T.D. is an employee of Cognition Therapeutics. G.K. is an employee of F. Hoffman La‐Roche. V.L. is an employee of Lundbeck. G.P.N. and M.E.S. are employees of Janssen Research & Dev. K.R. and A.V. are employees of Abbvie. D.S. is an employee of Clario. S.S. is an employee of Eli Lilly. E.S. has served as a consultant with Biogen Inc., Cogstate Ltd., Cortexyme Inc., Partner Therapeutics Inc., Pinteon Therapeutics Inc., Vaccinex Inc., Acumen Pharmaceuticals Inc., Gates Ventures LLC, Hoffman La Roche Ltd. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to acknowledge the invaluable input and support provided by our colleagues and collaborators Maria C. Carrillo (Alzheimer's Association), Rebecca Edelmayer (Alzheimer's Association), Michael Wiener (ADNI Principal Investigator–UCSF), John Hsaio (National Institute on Aging), Laurie Ryan (National Institute on Aging), Tolga Turan (Abbvie), Archana Iyer (Abbvie), Fedik Rahimov (Abbvie), Miguel Mendoza (Roche), Martin Guess (Roche), Laura Lenzo (Roche), Fujirebio, EUROIMMUN, Meso Scale Diagnostics, and Saladax/Siemens. ELECSYS is a trademark of Roche. We are grateful for the leadership of the late Dr. John Trojanowski as the co‐lead of the ADNI biofluid biomarker core and for helping the biofluid biomarker working group achieve its goals. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Saladax/Siemens; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California on the website USC.LONI.ADNI. AbbVie participated in the interpretation of data, review, and approval of the publication. There is no relevant funding to report for this work.
Albala B, Appelmans E, Burress R, et al. The Alzheimer's Disease Neuroimaging Initiative and the role and contributions of the Private Partners Scientific Board (PPSB). Alzheimer's Dement. 2024;20:695–708. 10.1002/alz.13483
REFERENCES
- 1. Alzheimer's Disease Neuroimaging Initiative . About Alzheimer's disease neuroimaging initiative (ADNI). Accessed on 2017. https://AdniLoniUscEdu/about/#gov‐Container n.d
- 2. Liu E, Luthman J, Cedarbaum JM, et al. Perspective: the Alzheimer's Disease Neuroimaging Initiative and the role and contributions of the Private Partner Scientific Board (PPSB). Alzheimer's Dement. 2015;11:840‐849. doi: 10.1016/j.jalz.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 3. Schmidt ME, Siemers E, Snyder PJ, Potter WZ, Cole P, Soares H. The Alzheimer's disease neuroimaging initiative: perspectives of the industry scientific advisory board. Alzheimer's Dement. 2010;6:286‐290. doi: 10.1016/j.jalz.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 4. Smith T, Gildeh N, Holmes C. The montreal cognitive assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007;52:329‐332. [DOI] [PubMed] [Google Scholar]
- 5. Bean J. Rey auditory verbal learning test. encycl. Clin Neuropsychol. 2011:2174‐2175. [Google Scholar]
- 6. Kueper JK, Speechley M, Montero‐odasso M. The Alzheimer ’ s disease assessment scale – cognitive subscale (ADAS‐Cog): modifications and responsiveness in pre‐dementia populations . A narrative review. J Alzheimer's Dis. 2018;63:423‐444. doi: 10.3233/JAD-170991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:11. [DOI] [PubMed] [Google Scholar]
- 8. Weiner MW, Alzheimer's disease Neuroimaging Initiative 2 Protocol. 2015. [DOI] [PMC free article] [PubMed]
- 9. Lim YY, Ellis KA, Harrington K, et al. Use of the CogState Brief Battery in the assessment of Alzheimer's disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012;34:345‐358. doi: 10.1080/13803395.2011.643227 [DOI] [PubMed] [Google Scholar]
- 10. Edgar CJ, Siemers E, Maruff P, Petersen RC, Aisen PS. Pilot evaluation of the unsupervised, at‐home cogstate brief battery in ADNI‐2. J Alzheimer's Dis. 2021;83:915‐925. doi: 10.3233/JAD-210201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alden EC, Pudumjee SB, Lundt ES, et al. Diagnostic accuracy of the cogstate brief battery for prevalent MCI and prodromal AD (MCI A+T+) in a population‐based sample. Alzheimer's Dement. 2021;17:584‐594. doi: 10.1002/alz.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Services USD of H and H . Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products. 2019.
- 13. Marson DC, Sawrie SM, Snyder S, et al. Assessing financial capacity in patients with Alzheimer disease. Arch Neurol. 2000;57:877‐884. [DOI] [PubMed] [Google Scholar]
- 14. Marson D. Investigating functional impairment in preclinical Alzheimer's disease. J Prev Alzheimer's Dis. 2016;2:4‐6. doi: 10.14283/jpad.2015.44.Investigating [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerstenecker A, Triebel K, Eakin A, Martin R, Marson D. Exploring the factor structure of financial capacity in cognitively normal and impaired older adults. Clin Gerontol. 2019;41:33‐41. doi: 10.1080/07317115.2017.1387211.Exploring [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tolbert S, Liu Y, Hellegers C, et al. Financial management skills in aging, MCI and dementia: cross sectional relationship to 18F‐florbetapir PET cortical β‐amyloid deposition. J Prev Alzheimer's Dis. 2019;6:274‐282. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez C, Tommasi NS, Briggs D, et al. Financial capacity and regional cerebral tau in cognitively normal older adults, mild cognitive impairment, and Alzheimer's disease dementia. J Alzheimer's Dis. 2021;79:1133‐1142. doi: 10.3233/JAD-201122.Financial [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt ME, Chiao P, Klein G, et al. The influence of biological and technical factors on quantitative analysis of amyloid PET : points to consider and recommendations for controlling variability in longitudinal data. Alzheimer's Dement. 2015;11:1050‐1068. doi: 10.1016/j.jalz.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 19. Meyer PT, Hellwig S, Amtage F, et al. Dual‐biomarker imaging of regional cerebral amyloid load and neuronal activity in dementia with PET and 11 C‐labeled pittsburgh compound B. J Nucl Med. 2011;52:393‐400. doi: 10.2967/jnumed.110.083683 [DOI] [PubMed] [Google Scholar]
- 20. Hsiao I‐T, Huang C‐C, Hsieh C‐J, et al. Correlation of early‐phase 18F‐florbetapir (AV‐45/Amyvid) PET images to FDG images: preliminary studies. Eur J Nucl Med Mol Imaging. 2012;39:613‐620. doi: 10.1007/s00259-011-2051-2 [DOI] [PubMed] [Google Scholar]
- 21. Yoon H, Sahn B, Hyang J, et al. NeuroImage : clinical Dual‐phase 18 F‐florbetaben PET provides cerebral perfusion proxy along with beta‐amyloid burden in Alzheimer ’ s disease. NeuroImage Clin. 2021;31:102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matthews DC, Strother SC, Lukic AS, Andrews RD, Wernick MN, Schmidt ME. Measurement of neurodegeneration using a multivariate early frame amyloid PET classifier. Transl Res Clin Interv. 2022;8:1‐12. doi: 10.1002/trc2.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen YJ, Rosario BL, Mowrey W, et al. Relative 11 C‐PiB delivery as a proxy of relative CBF: quantitative evaluation using single‐session 15 O‐water and 11 C‐PiB PET. J Nucl Med. 2015:1199‐1205. doi: 10.2967/jnumed.114.152405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Devous MD. Employing early uptake data from F18‐ Florbetapir scans as an estimate of regional cerebral blood flow: comparison to F18‐FDG. Alzheimer's Dement. 2014(Alzheimer). doi: 10.1016/j.jalz.2014.05.190 [DOI] [Google Scholar]
- 25. Myoraku A, Klein G, Landau S, Tosun D. Regional uptakes from early‐frame amyloid PET and 18F‐FDG PET scans are comparable independent of disease state. Eur J Hybrid Imaging. 2022;6:2. doi: 10.1186/s41824-021-00123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwarz CG, Kremers WK, Wiste HJ, et al. NeuroImage Changing the face of neuroimaging research : comparing a new MRI de‐facing technique with popular alternatives. Neuroimage. 2021;231:117845. doi: 10.1016/j.neuroimage.2021.117845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramanan VK, Nho K, Shen L, et al. FASTKD2 is associated with memory and hippocampal structure in older adults. Mol Psychiatry. 2015;20:1197‐1204. doi: 10.1038/mp.2014.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saykin AJ, Shen L, Foroud TM, et al. Alzheimer's disease neuroimaging initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimer's Dement. 2010;6:265‐273. doi: 10.1016/j.jalz.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Booij BB, Lindahl T, Wetterberg P, et al. A gene expression pattern in blood for the early detection of Alzheimer’ s disease. J Alzheimer's Dis. 2011;23:109‐119. doi: 10.3233/JAD-2010-101518 [DOI] [PubMed] [Google Scholar]
- 30. Lunnon K, Smith R, Hannon E, et al. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer's disease. Nat Neurosci. 2014;17:1164‐1170. doi: 10.1038/nn.3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jager PL De, Srivastava G, Lunnon K, et al. Alzheimer ’ s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Publ Gr. 2014;17:1156‐1163. doi: 10.1038/nn.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vasanthakumar A, Davis JW, Idler K, et al. Harnessing peripheral DNA methylation differences in the Alzheimer ’ s Disease Neuroimaging Initiative (ADNI) to reveal novel biomarkers of disease. Clin Epigenetics. 2020;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li QS, Vasanthakumar A, Davis JW, et al. Association of peripheral blood DNA methylation level with Alzheimer ’ s disease progression. Clin Epigenetics. 2021:1‐16. doi: 10.1186/s13148-021-01179-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim BH, Vasanthakumar A, Li QS, et al. Integrative analysis of DNA methylation and gene expression identifies genes associated with biological aging in Alzheimer's disease. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2022;14:1‐13. doi: 10.1002/dad2.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tombaugh TN, Mclntyre NJ. The mini‐mental state examination. Prog Geriatr. 1992;40:922‐935. [DOI] [PubMed] [Google Scholar]
- 36. Pasternak E, Smith G. Cognitive and neuropsychological examination of the elderly. 1st ed. Elsevier B.V.; 2019. doi: 10.1016/B978-0-12-804766-8.00006-6 [DOI] [PubMed] [Google Scholar]
- 37. Montgomery V, Harris K, Stabler A, Lu LH. Effects of delay duration on the WMS logical memory performance of older adults with probable Alzheimer ’ s disease, probable vascular dementia, and normal cognition. Arch Clin Neuropsychol. 2017;32:375‐380. doi: 10.1093/arclin/acx005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lennon JC, Resch ZJ, Aita SL, et al. Black and White individuals differ in dementia prevalence, risk factors, and symptomatic presentation. Alzheimer's Dement. 2022:1461‐1471. doi: 10.1002/alz.12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aging NI on . Data shows racial disparities in Alzheimer's disease diagnosis between Black and White research study participants. 2021.
- 40. Aging NI on . Together we make the difference: National strategy for recruitment and participation in Alzheimer's and related dementias clinical research. 2019. [DOI] [PMC free article] [PubMed]
- 41. Weiner MW, Albala B, Jack CR Jr., et al. Increasing participant diversity in AD research : plans for digital screening, blood testing, and a community‐engaged approach in the Alzheimer ’ s Disease Neuroimaging Initiative 4. Alzheimer's Dement. 2023;19:307‐317. doi: 10.1002/alz.12797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poldrack RA, Gorgolewski KJ. Making big data open : data sharing in neuroimaging. Nat Neurosci. 2014;17:1510. doi: 10.1038/nn.3818 [DOI] [PubMed] [Google Scholar]
- 43. Wyman BT, Harvey DJ, Crawford K, et al. Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimer's Dement. 2014;9:332‐337. doi: 10.1016/j.jalz.2012.06.004.Standardization [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Raket LL. Statistical disease progression modeling in Alzheimer disease. Front Big Data. 2020;3:1‐18. doi: 10.3389/fdata.2020.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zettergren A, Lord J, Ashton NJ, et al. Association between polygenic risk score of Alzheimer's disease and plasma phosphorylated tau in individuals from the Alzheimer's disease neuroimaging initiative. Alzheimer's Res Ther. 2021;13:1‐10. doi: 10.1186/s13195-020-00754-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schwarz AJ, Shcherbinin S, Slieker LJ, et al. Topographic staging of tau positron emission tomography images. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2018;10:221‐231. doi: 10.1016/j.dadm.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goudey B, Fung BJ, Schieber C, Disease A. A blood‐based signature of cerebrospinal fluid A β 1 – 42 status. Sci Rep. 2019;9:4163:1‐12. doi: 10.1038/s41598-018-37149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tosun D, Veitch D, Aisen P, et al. Detection of b ‐amyloid positivity in Alzheimer ’ s disease neuroimaging initiative participants with demographics, cognition, MRI and plasma biomarkers. Brain Commun. 2021;3:fcab008. doi: 10.1093/braincomms/fcab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cullen NC, Zetterberg H, Insel PS, et al. Comparing progression biomarkers in clinical trials of early Alzheimer's disease. Ann Clin Transl Neurol. 2020;7:1661‐1673. doi: 10.1002/acn3.51158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jones‐Davis D, Buckholtz N. The impact of ADNI: what role do public‐private partnerships have in pushing the boundaries of clinical and basic science research on Alzheimer's disease? Alzheimer's Dement. 2015;11:860‐864. doi: 10.1016/j.jalz.2015.05.006.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marek K, Jennings D, Lasch S, et al. The Parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95:629‐635. doi: 10.1016/j.pneurobio.2011.09.005.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huie JR, Mondello S, Lindsell CJ, et al. Biomarkers for traumatic brain injury: data standards and statistical considerations. J Neurotrauma. 2021;38:2514‐2529. doi: 10.1089/neu.2019.6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


