Abstract
An individual’s nutritional status has a powerful effect on sexual maturation. Puberty onset is delayed in response to chronic energy insufficiency and is advanced under energy abundance. The consequences of altered pubertal timing for human health are profound. Late puberty increases the chances of cardiometabolic, musculoskeletal and neurocognitive disorders, whereas early puberty is associated with increased risks of adult obesity, type 2 diabetes mellitus, cardiovascular diseases and various cancers, such as breast, endometrial and prostate cancer. Kennedy and Mitra’s trailblazing studies, published in 1963 and using experimental models, were the first to demonstrate that nutrition is a key factor in puberty onset. Building on this work, the field has advanced substantially in the past decade, which is largely due to the impressive development of molecular tools for experimentation and population genetics. In this Review, we discuss the latest advances in basic and translational sciences underlying the nutritional and metabolic control of pubertal development, with a focus on perspectives and future directions.
Introduction
Puberty is a major developmental event in the life-course of any individual. Puberty is characterized by an array of somatic, psychological and behavioural changes, which include completion of growth and sexual maturation, as well as developing the ability to reproduce1. Pubertal timing is defined by strong genetic determinants, but is also sensitive to a wide variety of endogenous and environmental cues2.
The main neuroendocrine pillar for pubertal development is the activation of the hypothalamic–pituitary–gonadal (HPG) axis. In this highly hierarchical system, gonadotropin-releasing hormone (GnRH) from the hypothalamus drives the pulsatile secretion of pituitary gonadotropins (luteinizing hormone (LH) and follicle-stimulating hormone), which in turn activate gonadal maturation and the secretion of sex steroids and peptides. Pulses of GnRH are the hypothalamic output of the HPG axis, and these pulses are a reflection of episodic activity of afferent neuronal inputs; in particular a population of kisspeptin neurons that is now understood to be the long-sought ‘pulse generator’3-5. Timed activation of the HPG axis is responsible for sexual and phenotypic maturation at puberty.
In humans, the clinical hallmarks of puberty onset are the initiation of breast development in girls (that is, thelarche) and enlargement of testicular volume, to over 3 ml, in boys (that is, gonadarche). According to normative data, the occurrence of these indices before the age of 8 years in girls or 9 years in boys defines the clinical condition of precocious puberty. By contrast, initiation of breast development or testicular growth that occurs after the age of 13 years or 14 years, respectively, usually denotes delayed puberty.
The need for stored energy to enable reproductive success has been known for centuries, and was based on empirical observations of livestock fertility by farmers. However, it was not until rigorous experimental studies under controlled environmental conditions performed in rats by Kennedy and Mitra6 that the effects of nutrition and body weight were identified as major determinants of pubertal development. By adjusting litter size at birth, and hence the availability of milk for each offspring, the authors created conditions of overnutrition (small litter size) and undernutrition (large litter size) in early postnatal development. They observed that the time of puberty onset was delayed in rats raised in large litters. These rats were also smaller than those in normal size and small litters, leading to the hypothesis that sexual maturation is correlated with body weight, not chronological age.
About a decade later, epidemiological studies showed that conditions of extreme leanness in girls delayed pubertal progression, which suggested there was a role for a critical amount of body adipose tissue within the body weight factor that was required for proper sexual maturation7,8. Following much debate and inconsistencies generated by experimental studies in animal models with distinct reproductive strategies3,9, it is now well accepted that in primates and laboratory mice and rats, sufficient stored energy must be available during pubertal transition for growth and differentiation of reproductive organs and tissues.
Kennedy and Mitra’s seminal studies are highly relevant for human health. In the past five decades, studies from around the world have consistently found earlier ages at puberty onset in girls, as defined by the initiation of breast development10-13. In boys, less definitive evidence suggests a trend towards earlier onset of testicular enlargement13,14. The obesity pandemic has been proposed as one of the determinants for this trend in girls, and possibly also in boys15,16. These trends are concerning, as early puberty has been associated with increased risks of some health issues in adulthood, including obesity, type 2 diabetes mellitus, cardiovascular diseases and various cancers, such as breast, endometrial and prostate cancer, which lead to reduced life expectancy13,17-20.
Disruption of pubertal timing in humans also occurs in conditions of undernutrition, including anorexia nervosa, strenuous exercise, chronic illness and relative energy deficiency, which might lead to cardiometabolic, musculoskeletal and neurocognitive disorders (that is, psychosocial functioning and educational achievements) in adult life21. In addition, relative energy deficiency due to exercise or extreme leanness leads to primary amenorrhoea in college athletes and women22,23.
Undernutrition combined with late puberty was a common phenomenon during the early Industrial Revolution, with onset of menstruation (menarche) typically occurring at 15–16 years of age10,13,14. From the early 19th to the mid-20th century, the average age at menarche reduced by about 4 years, which was probably due to improved nutrition. More recently, however, the decline in age at menarche seems to have slowed down in most developed countries11-13,24,25. Indeed, global data since the 1960s show contrasting findings in menarcheal timing26-29, whereas most studies have consistently documented earlier ages at thelarche over time11,12,14,26,29,30. The conflicting data must be evaluated with caution as socioeconomic traits, ethnicity and genetic differences are also pieces of the same puzzle (Box 1), and the specific cause (or causes) of the potential disruption of the timing and tempo of pubertal development within a population is unclear. Early breast development in girls with obesity could be the result of increased aromatase activity in adipocytes and increased oestrogen production31,32. Whether this effect leads to the activation of the HPG axis is debatable.
Box 1. Effects of ethnicity and genetics on timing of growth and puberty.
One of the many factors contributing to the remarkably wide age range of human puberty onset is ethnicity. For example, in several studies the average age at menarche has been reported to be about 7 months earlier in African American girls and in girls of Pacific descent than in girls of European ancestry212-214. African American girls and girls of Pacific descent generally have a greater average BMI than girls of European ancestry, which suggests that childhood body weight has an effect on the timing of puberty in these groups. Although adiposity contributes to early puberty, other factors also need to be considered. It is important to note that BMI is not an ideal adiposity index for comparisons across ethnicities; for the same BMI, African American children215,216 and children of Pacific descent217 might have less visceral adipose tissue and an earlier muscle growth spurt than other groups. For some ethnicities, early puberty onset might at least in part reflect this earlier muscle growth. Ignoring ethnic differences in body composition can lead to over-simplified assumptions about the relationship between body adipose tissue and puberty timing that fail to incorporate variations in the age at which the childhood growth spurt starts. This observation highlights the need for improved understanding of the role of hormones linked to muscle growth, such as insulin-like growth factor 1 and irisin, in pubertal timing.
Altogether, these observations highlight the association between pubertal timing and body energy reserves, nutritional status and growth, as initially hypothesized by Kennedy and Mitra in 1963. In this Review, we aim to synthesize the vast literature and scientific knowledge produced following their seminal work. We focus on developments over the past two decades in basic and translational sciences, with an emphasis on future directions and the relevance of pubertal timing for human health.
Indicators of energy reserves
In most mammals, one of the main strategies to store energy is the accumulation of lipids in the form of triacylglycerol in adipocytes. High-energy fatty acids are then produced via lipolysis and are released when required33. Adipocytes also secrete hormones that serve as metabolic cues that convey the amount of stored energy available; this information is used during sexual maturation, for differentiation and for growth. Of these hormones, leptin is a key permissive signal for pubertal progression that acts in the brain34,35.
Circulating levels of leptin are closely correlated with the amount of body adipose tissue and the nutritional status of the individual. Synthesis and release of leptin is modulated by a complex network of neurohumoral factors that also influence pubertal development. Of these factors, insulin, growth hormones (GH), gonadal hormones, pro-inflammatory cytokines and the autonomic nervous system are well described35,36.
Absent or dysfunctional leptin signalling disrupts sexual maturation in rodents and humans37,38. Leptin administration to leptin-deficient mice induces puberty, and excess leptin advances puberty onset in wild-type mice38-41. These effects of leptin are attained via actions on subsets of hypothalamic neurons that relay metabolic cues to the neuroendocrine reproductive axis.
The endocrine role of adipose tissue goes well beyond leptin physiology, but the action of other adipokines on pubertal development has yet to be demonstrated. An inhibitory role for adiponectin is suggested by its inverse relationship with leptin levels42. Consistent with this suggestion, total adiponectin levels are reduced in girls with central precocious puberty (CPP) and are negatively correlated with the Tanner stages of sexual maturity rating43. Of note, the opposite trend is documented for high molecular weight adiponectin levels, which are high in CPP and are positively correlated with Tanner stages43. Further work is needed to determine whether these relationships are causal, and, if so, the underlying mechanisms.
Excess energy consumption might directly alter hypothalamic function independently of the hormones present in the circulation. Studies from several laboratories have demonstrated that the hypothalamus has increased levels of ceramide in mouse models of obesity44,45. Ceramides are sensors of fatty acids, which might drive cellular stress and dysfunction when in excess. Using the litter size paradigm pioneered by Kennedy and Mitra6, studies demonstrated that early overfed rats (raised in small litters) with advanced puberty have a high ceramide content in the paraventricular nucleus of the hypothalamus (PVH)46. Blockade of ceramide synthesis in the PVH markedly delays puberty in overweight female rats, which suggests that the overfeeding-induced elevated levels of ceramide in the PVH has a role in pubertal progression46. This finding is particularly relevant as the PVH is a crucial hub in neuroendocrine and autonomic regulation.
Indicators of nutritional status
Breakdown of nutrients into glucose, amino acids and free fatty acids provides signals of nutritional status. Sensors for these various substrates are expressed on cells throughout the body, including cells of reproductive tissues and hypothalamic cells involved in GnRH release47-49, such as kisspeptin neurons and GnRH neurons. In addition, food intake affects the secretion of specific hormones, such as ghrelin, insulin, fibroblast growth factor 21 (FGF21) and glucagon-like peptide 1, that signal states of nutrient deficit or surplus.
Ghrelin is a hunger-associated peptide that is secreted by cells in the gastrointestinal tract50. When ghrelin is administered during the pubertal transition, pubertal maturation is delayed in male rats51, whereas administration of a ghrelin antagonist during pregnancy in mice advances puberty in male and female offspring52. Thus, ghrelin might be a signal of nutritional deficiency that alters pubertal timing. Although the molecular mechanism associated with these effects is not entirely clear, ghrelin might signal energy deficits by increasing the activity of sirtuins in the hypothalamus (such as SIRT1)53, which is discussed in a subsequent section (see section on ‘Epigenetic regulation’) in more detail. Ghrelin can also have opposing effects on pubertal timing, due to its ability to promote GH secretion. Ghrelin administration increases plasma concentrations of GH and accelerated vaginal opening in mice when administered from birth50. The timing and duration of elevations in levels of ghrelin are critical to its effects on pubertal development.
Insulin, which is associated with satiety and abundant energy stores, also has a role in the metabolic control of puberty. Prior to the discovery of insulin, humans with uncontrolled type 1 diabetes mellitus (T1DM) usually exhibited an absence of pubertal development and had hypogonadotropic hypogonadism. The introduction of insulin (from the 1920 onwards) improved fertility, but on average menarche occurred 1.1 years later in girls with T1DM than in those without T1DM and a considerable proportion of the girls with T1DM exhibited primary amenorrhoea into their late teens54. Even into the 1990s, studies found that menarche was delayed by 5 months to 1 year in girls with T1DM who were using one or two daily insulin injections55,56. Modern insulin therapy seems to result in only mild delays in thelarche and menarche57. In boys with T1DM under current treatment paradigms initiation of puberty seems to occur at the usual ages, but these boys might progress through puberty faster than boys without T1DM58. Direct actions of insulin that might underlie these findings have been clarified by animal studies. Insulin receptors are found at all levels of the HPG axis and are required for normally timed puberty and adult fertility in mice59,60. Although circulating levels of insulin correlate positively with body weight and adiposity, mouse studies demonstrate that hyperinsulinaemia without obesity can induce early female puberty61. Importantly, insulin receptors on astrocytes62, rather than on neurons60,63, seem to be the main players in this effect.
FGF21, a liver-derived hormone involved in metabolic regulation, and its co-receptor, β-klotho, can contribute to pubertal maturation in mice and humans by promoting GnRH neurite outgrowth and release64. During starvation, levels of FGF21 increase, which drives ketogenesis and suppresses whole-body growth. Overexpression of FGF21 in mice delays puberty and causes infertility in females. These mice are smaller than wild-type mice, but the percentage of adipose tissue mass and concentrations of leptin and adiponectin are unchanged, which indicates that FGF21 has a role in reproductive maturation and function that is independent of adipokines65. In addition, the anorexigenic neuropeptide, glucagon-like peptide 1, which is produced in the gut following a meal and in the brainstem, might contribute to pubertal onset through stimulation of GnRH and LH release. This effect might involve direct actions on kisspeptin neurons66,67.
In addition to these tissue-derived factors, the gut microbiome might influence puberty by regulating the host’s levels of sex hormone68,69 or by releasing factors that reflect nutrient status into the circulation. High-fat diet feeding reduces the amount of short-chain fatty acids released by the gut microbiome70. Furthermore, early puberty induced by a high-fat diet in rodents can be reversed by administration of short-chain fatty acids71 or probiotics72. Early puberty induced by a high-fat diet can be reproduced by transplanting the microbiota of these mice to mice fed a low-fat diet73,74. Admittedly, however, tests of causality have yet to be performed in humans.
Indicators of musculoskeletal growth
Levels of several hormones that modulate both muscle growth and reproductive function increase markedly during human adolescence, including the anabolic hormones (GH75-77, insulin78 and insulin-like growth factor 1 (IGF1)75,79-81) and the myokines (myostatin and irisin82-85). This increase is transient in the cases of GH and insulin, and only evident in boys in the case of myostatin. Mice with whole-body or brain-specific genetic deletion of the receptors for these hormones remain fertile59,86-88, which indicates that their roles in reproductive maturation must be modulatory rather than necessary. Nevertheless, components of the growth axis might contribute to the wide variation that exists in human pubertal timing.
Pulsatile GH secretion increases at puberty, driving a pronounced and sustained increase in IGF1 levels75,79. Conditional GH receptor knockout from either kisspeptin neurons or the brain has shown that central GH signalling is not required for pubertal timing89. A stronger case exists, however, for IGF1. Individuals with very low levels of IGF1 experience delayed puberty90, whereas girls with precocious puberty have elevated IGF1 levels91,92. Male and female rats treated centrally with IGF1 or IGF1 antibodies exhibit advanced or delayed puberty, respectively93,94. Conditional IGF1 receptor knockout has revealed a requirement for the actions of IGF1 on GnRH neurons for typical pubertal timing in male and female mice87. Furthermore, electrophysiological measurements from brain slices taken from prepubertal and pubertal male mice indicate that IGF1 disinhibits endocannabinoid suppression of GnRH neuronal activity, which increases presynaptic γ-aminobutyric acid (GABA) release to activate GnRH neurons95.
In most mammals, including humans, muscle mass correlates with pubertal timing at least as well as adiposity correlates with pubertal timing96-99. Although myostatin’s role in pubertal growth and sexual maturation has received little attention, one study published in 2022 showed that its levels increase with age and pubertal stage in boys82, presumably to modulate muscle hypertrophy driven by anabolic hormones. Furthermore, sows with a mutation in the gene encoding myostatin exhibit delayed puberty100. Levels of another myokine hormone, irisin, are also elevated in girls with CPP101. Global deletion of the gene encoding irisin, treatment with an irisin receptor antagonist or neuronal irisin receptor knockout all delay female (but not male) puberty in mice88. Further studies on this subject are warranted (Box 1).
Neural effectors in pubertal timing
Although GnRH neurons themselves seem to have a role in the control of pubertal timing and prepubertal growth102, the effect of metabolic cues on puberty is mediated mainly by neural targets upstream of GnRH neurons34,103. In the past decade, the neuronal circuitry and glial components of this tightly controlled developmental programme have been unravelled through collaborative work of laboratories around the world.
The primary order neurons of this network are mostly located in the hypothalamus, where receptors of metabolic cues, such as those for leptin (leptin receptor (LepR)), ghrelin (GH secretagogue receptor), IGF1 (IGF1 receptor) and insulin (insulin receptors), are abundant34. Leptin, an adipokine that is produced by the white adipocytes and secreted in the bloodstream in levels correlated with adipose tissue mass, is relevant for sexual maturation104,105. Due to its critical role, leptin is also the most studied adipokine in timed sexual maturation.
Leptin reaches the brain parenchyma via three independent mechanisms. One mechanism is fenestrated capillaries in circumventricular organs, including the vascular organ of the lamina terminalis and the median eminence. Another mechanism is carrier-mediated or receptor-mediated active transport for targets inside the blood–brain barrier. The final mechanism is active transport of tanycytes along the third ventricle106-108.
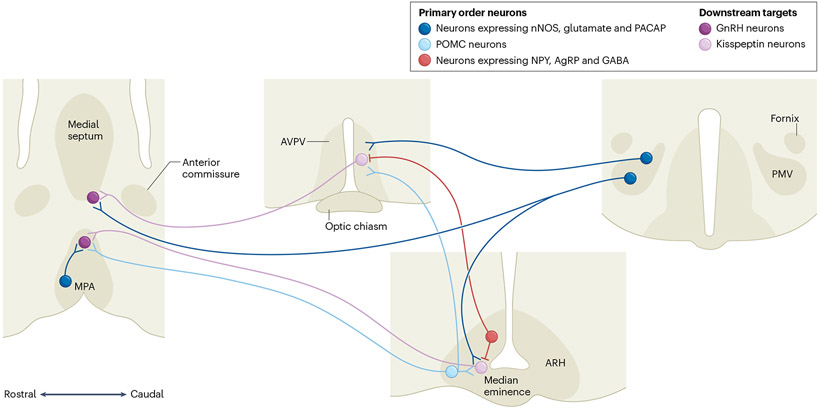
Within the brain, leptin targets the primary order neurons; that is, those expressing the LepR long form (LepRb), which are mostly located in the hypothalamus and brainstem. A dense collection of LepRb is found in the arcuate nucleus (ARH), the ventral premammillary nucleus (PMV) and the medial preoptic area (MPA) (Fig. 1). In the ARH, neurons expressing kisspeptin, neurokinin B and dynorphin (KNDy) are key elements in HPG activation and pubertal progression109-111, and are highly responsive to metabolic and nutritional cues112-114. These responses seem to be attained via direct and/or indirect paths. For example, KNDy neurons are directly affected by metabolic sensors, including mammalian target of rapamycin (mTOR) and AMP-activated kinase (AMPK)47,48. Direct leptin action in KNDy neurons, however, is not necessary for typical pubertal maturation115. In fact, LepRb expression in a subset of KNDy neurons is induced after puberty by as-yet-unidentified mechanisms, which indicates that leptin’s effect on sexual maturation is upstream of KNDy neurons. The main candidate in this regard is GABAergic neurons, which fall into numerous subpopulations, usually grouped by region and the different neuropeptides they produce.
Fig. 1 ∣. Schematic illustration of the neural pathways associated with leptin action in pubertal development in mice.
Primary order neurons (that is, those that respond directly to circulating levels of leptin, expressing leptin receptor long-form (LepRb)) are located in three distinct nuclei. In the arcuate nucleus (ARH), in the medial preoptic area (MPA) and in the ventral premammillary nucleus (PMV). In the ARH, LepRb neurons co-express neuropeptide Y (NPY), agouti-related protein (AgRP) and GABA, or proopiomelanocortin (POMC). In the MPA and PMV, LepRb neurons co-express nitric oxide synthase (nNOS) and glutamate, and specifically for PMV, they also co-express pituitary adenylate cyclase-activating polypeptide (PACAP). The primary order neurons modulate the activity of downstream targets, gonadotropin-releasing hormone (GnRH) and kisspeptin neurons. AVPV, anteroventral paraventricular nucleus. Adapted with permission from ref. 34, American Physiological Society.
A prominent role for leptin-responsive GABAergic neurons has been described by independent laboratories116,117. Among several first-order GABAergic neurons, those expressing neuropeptide Y (NPY) and agouti-related protein (AgRP) are thought to be the main players. The expression of Npy and Agrp increases in conditions of negative energy balance (for example, fasting) or blunted leptin signalling118-120. Re-expression of the gene encoding endogenous LepRb only in AgRP neurons of obese and infertile LepR-null mice restores puberty and deletion of LepRb from AgRP neurons delays pubertal development in female mice121. AgRP neurons directly innervate KNDy neurons, conveying signals of energy deficiency122. Together, these findings indicate that leptin action in AgRP, NPY and GABA neurons comprises a crucial component of pubertal timing via downstream actions in KNDy neurons, potentially via GABAergic neurotransmission122.
AgRP neurons are part of the melanocortin system along with proopiomelanocortin (POMC) neurons, which also express LepRb. Most of the metabolic and reproductive effects of POMC neurons are mediated by the actions of α-melanocyte-stimulating hormone (αMSH) on melanocortin receptors 3 and 4 (MC3R and MC4R)123. POMC neurons located in the ARH are comprised of a mixed group of GABAergic and glutamatergic neurons but, contrary to what was initially thought, deletion of LepRb from POMC neurons does not disrupt puberty onset in mice124,125. The melanocortin system does have a role in adult reproduction, based on rodent studies, but the downstream effectors and the neural circuitry are still under debate. For example, a role for MC4R in sexual maturation has been reported in rats, but not mice, using pharmacological challenges126,127, whereas population and mouse genetics suggest that MC3R is required for typical pubertal timing128,129. Additional studies using conditional and temporal manipulation of melanocortin receptors are necessary to probe these issues in a controlled manner.
Using similar genetic manipulation of LepRb expression in mice, the PMV has been identified as another key hypothalamic site of leptin action in pubertal development130-132. Obese and infertile LepRb-null mice with re-expression of endogenous LepRb in PMV neurons show pubertal development and improved fertility. The PMV neurons are mostly stimulated (depolarized) by acute leptin administration133, and express glutamate, pituitary adenylate cyclase-activating polypeptide (PACAP) and the gaseous neurotransmitter, nitric oxide (NO)132,134,135.
The role of NO in GnRH neuronal firing and secretion is well-established136,137. NO controls the amplitude of the first postnatal activation of the HPG axis, which is called mini-puberty130,138. In addition, mice lacking neuronal NO synthase (nNOS) (encoded by Nos1) and humans with rare mutations in NOS1 are infertile130. Furthermore, global inhibition or deletion of NO production blocks leptin-induced secretion of GnRH and LH139,140. Deletion of LepRb from nNOS cells negatively affects pubertal maturation; however, which primary order neurons are associated with these effects is still under debate141. Leptin administration increases phosphorylation and activity of nNOS in the PMV and MPA134,140. In addition, blockade of nNOS in MPA cells and lesions of PMV neurons disrupt the ability of leptin to restore puberty in leptin-deficient mice131,140. Similarly, deletion of PACAP from LepRb neurons, which are also co-expressed in the PMV, disrupts typical pubertal maturation142. The effects of the PMV and MPA on puberty are attained via direct actions on GnRH neurons, kisspeptin neurons and KNDy neurons132,140,142.
Combined RNA and TRAP sequencing in wild-type and leptin-deficient mice has been used to identify novel candidate genes associated with pubertal transition. This technique enabled the identification of a dynamic change in genes associated with neuropil remodelling in the mediobasal hypothalamus143. In fact, GnRH neurons morphologically and functionally interact with glial cells, including astrocytes and tanycytes144. As an integral part of the GnRH neural network, the glial cells sense, coordinate and relay homeostatic information to GnRH neurons that are necessary for puberty onset144,145. At birth, GnRH neurons already morphologically interact with tanycytic processes, which regulate their periodic access to pituitary portal blood vessels at their termination field in the median eminence146. However, in the preoptic region, GnRH neurons assemble their own glial entourage around their cell bodies during early postnatal development by establishing communication channels with glial progenitors using chemotrophic factors, such as prostaglandin D2147. Once recruited by GnRH neurons, glial cells eventually differentiate into astrocytes that are associated with the neurons throughout life147. This infantile astrogenesis in the vicinity of GnRH neurons is not only required for their correct wiring in the developing hypothalamus147 but also for the modulation of their firing activity via the paracrine activation of erythroblastic oncogene B receptors and the release of potent gliotransmitters, such as prostaglandin E2147-149. Interfering with these early neuron–glia interactions in rats and mice leads to delayed puberty and altered adult oestrous cyclicity and fertility disorders147,150-152. Whether this neuron–glia communication is sensitive to early nutritional stress needs further investigation.
In the hypothalamus, as in other brain areas, astrocytes are not only involved in synaptogenesis and are sophisticated sensors and modulators of neuronal activity153,154, but, along with tanycytes, are also involved in regulating energy metabolism at multiple levels. For example, they detect blood-borne energy signals and shuttle them into the brain155-160 and use connexin-43-mediated cellular networks to efficiently transmit metabolites in certain hypothalamic functional domains161,162. This function is epitomized by the crucial role of insulin in the central control of puberty. As mentioned already, findings in experimental models show that the ability of astrocytes to sense insulin, which is shuttled into the mediobasal hypothalamus by tanycytes or active transporters in endothelial cells160,163, seems to underlie the ability of insulin to advance the onset of puberty in mice62. Whether this phenomenon also occurs in humans needs further investigation.
Similar to the alterations in tanycytic shuttling of ghrelin from the periphery into the hypothalamus caused by early postnatal overfeeding in mice (as a result of rearing in small litter sizes)164, astrocyte morphology, receptor expression and receptor function are also altered by maternal or early life exposure to a high-fat diet165,166. Further studies are needed to unravel the mechanisms by which the metabolic state affects these two glial cell populations, and the implications of these mechanisms for GnRH system regulation and the establishment of fertility.
Molecular pathways engaged by metabolic and nutritional cues
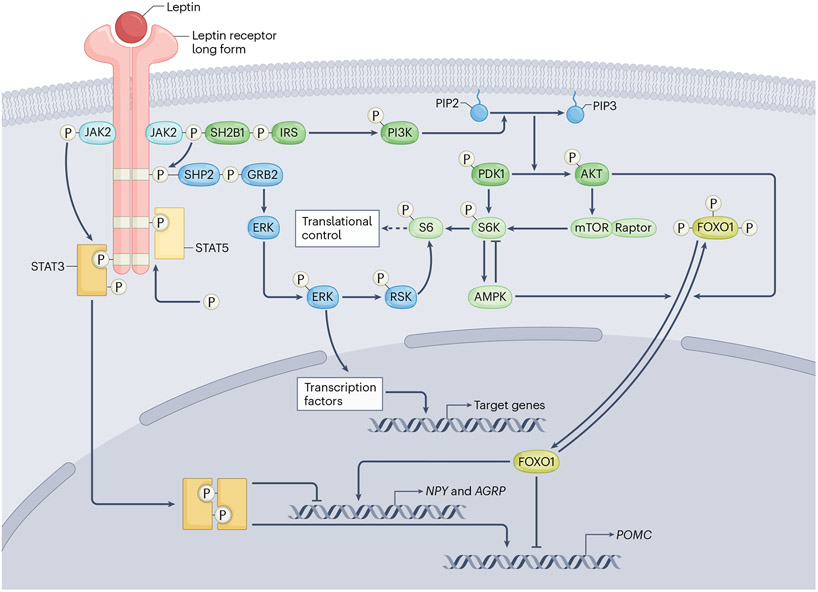
As indicated in previous sections, six decades after Kennedy and Mitra’s ingenious work, it is now clear that diverse signals from stored energy, nutritional status and growth modulate pubertal timing. Although a lot remains to be learned about how these metabolic and nutritional components ultimately calibrate GnRH neurons to regulate puberty onset, several molecular components have been identified, as summarized in this section (Fig. 2).
Fig. 2 ∣. Summary of the major signalling pathways engaged by hypothalamic long form of the leptin receptor in pubertal development.
The leptin receptor long form (LepRb) uses JAK2 to phosphorylate its phosphotyrosine residues, which then serve as binding sites where signalling molecules become phosphorylated and either move into the nucleus to serve as transcription factors or interact with a variety of other transcription factors. The LepRb signalling pathways include STAT, SHP2–ERK1/2 and PI3K–AKT–FOXO1 pathways. AGRP, agouti-related peptide; AMPK, adenosine monophosphate-activated protein kinase; ERK, extracellular signal-regulated kinase; FOXO1, forkhead box protein O1; GRB2, growth factor receptor-bound protein 2; IRS, insulin receptor substrate; JAK2, Janus kinase 2; mTOR, mammalian target of rapamycin NPY, neuropeptide Y; PDK1, phosphatidylinositol-dependent protein kinase 1; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol 4,5-biphosphate; PIP3, phosphatidylinositol 3,4,5-triphosphate; POMC, pro-opiomelanocortin; RSK, p90 ribosomal S6 kinase; S6, ribosomal protein S6; S6K, p70 S6 kinase; SHP2, Src homology-2 domain-containing protein tyrosine phosphatase-2; SH2B1, Src homology-2B adaptor protein 1; STAT, signal transducer and activator of transcript. Adapted from ref. 167, CC-BY 4.0.
Leptin acting upon LepRb recruits discrete intracellular signalling pathways105. Lacking intrinsic tyrosine kinase activity, LepRb uses Janus kinase 2 (JAK2) to phosphorylate three of its highly conserved phosphotyrosine residues. These binding sites enable signalling molecules to become phosphorylated and either move into the nucleus to serve as transcription factors or interact with a variety of other transcription factors167. The importance of these pathways in mediating leptin’s role in pubertal timing is incompletely understood.
Signal transducer and activator of transcription 3 (STAT3) is critical for leptin’s effects on appetite and metabolism, but its role in puberty and adult reproductive function remains unclear. In LepRb–STAT3 knockout mice168,169, or mice with a mutated LepRb that precludes STAT3 phosphorylation170, puberty onset and oestrous cycles are normal despite moderate obesity. By contrast, puberty does not occur in mice with brain-wide STAT3 deletion171, similar to mice with global leptin or LepRb deficiency. One caveat for the LepRb–STAT3 knockout experiments is that the Lepr-Cre mouse line probably expresses Cre recombinase fairly weakly; it is plausible that this weak expression results in partial STAT3 deletion that is sufficient to disrupt metabolic regulation but not pubertal onset. The same Cre driver was used to demonstrate that LepRb–STAT5 signalling is not required for puberty168. Studies using global STAT5 deletion172 or mutation of the STAT5 activation site on LepRb173 have also shown no or minimal reproductive deficits. In addition to JAK–STAT signalling, extracellular signal-regulated kinases 1/2 (ERK1/2) are also recruited by LepRb, which leads to the activation of various transcription factors167, including the adaptor protein SHP2. Neuronal deletion of the ERK1/2–SHP2 pathway leads to subfertility in female mice174, but whether this phenotype is a result of metabolic dysregulation needs investigation.
As with insulin and IGF1 receptor activation, leptin–LepRb binding can also activate the insulin receptor substrate–phosphoinositide 3-kinase (PI3K)–AKT pathway. Downstream of PI3K are mTOR and the transcription factor forkhead box protein 1 (FOXO1)167. Female mice with specific deletion of the PI3K-p110α subunit in cells with LepRb exhibit delayed pubertal development and are insensitive to leptin’s promotion of puberty onset; however, this effect might be secondary to the lean phenotype of these mice175. PI3K–AKT inhibits the activation of FOXO1. Activated FOXO1 stimulates transcription of Agrp and Npy and suppresses transcription of Pomc176-178, which might suppress GnRH activity.
Compelling evidence gathered in preclinical models strongly suggests that key cellular energy and metabolic sensors, operating in kisspeptin neurons and/or their upstream afferents, have a major role in pubertal timing. According to experimental data from female rodent models, these metabolic sensors probably include mTOR and AMPK, which, as mentioned already, operate in discrete hypothalamic neuronal populations to regulate body energy homeostasis, acting in a reciprocal manner179, and can modulate downstream signalling factors of key metabolic hormones, such as FOXO1. Thus, the mTOR signalling pathway, which modulates FOXO1-mediated gene transcription in conditions of energy abundance and leptin sufficiency, has been suggested to suppress feeding. By contrast, activation of this pathway via AMPK in the hypothalamus in conditions of energy and leptin deficiency leads to orexigenic responses and suppressed thermogenesis. In keeping with these roles in energy homeostasis, it has been documented that preserved brain mTOR signalling, which reflects sufficient energy stores and nutritional inputs, is needed for normal pubertal progression and adequate Kiss1 expression in the ARH, as well as for mediating the permissive effects of leptin on pubertal maturation in female rats180. By contrast, pharmacological or virogenetic activation of AMPK signalling in the brain during an energy deficit delays puberty in female rats47. In genetics studies in mice, independent laboratories demonstrated that the role of AMPK as a metabolic sensor in pubertal timing and female cyclicity is attained via actions in kisspeptin neurons47,48.
Epigenetic regulation
Different epigenetic mechanisms, including changes in DNA methylation, histone modifications and microRNAs (miRNAs), which operate mainly in kisspeptin and GnRH neurons, participate in pubertal control, and might contribute to its modulation by nutritional cues (Table 1).
Table 1 ∣.
The major epigenetic mechanisms involved in the control of puberty, and its eventual modulation by nutritional cues
| Epigenetic mechanism | Species | Refs. |
|---|---|---|
| Pubertal changes in DNA methylation and/or hypothalamic expression of ZNF genes | Human, rhesus monkey and rat | 181,205 |
| Polycomb group-mediated transcriptional repression of Kiss1 before puberty | Rat | 181 |
| SIRT1 repression of Kiss1 transcription | Rat | 183 |
| Trithorax group-mediated activation of Kiss1 transcription | Rat | 206 |
| Repression of KDM6B by EED (member of polycomb group) before puberty controls Kiss1 gene expression | Rat | 182 |
| Polycomb group members mediate transgenerational effects of endocrine disruptors | Rat | 207 |
| Variations in the LIN28B locus are associated with age at menarche | Human | 208-211 |
| miR-200, miR-429 and miR-155 contribute to changes in gonadotropin-releasing hormone expression at puberty | Mouse | 186 |
| miRNA biogenesis in kisspeptin neurons is required for completion of female puberty | Mouse | 188 |
| miR-30b represses Mkrn3 expression to modulate female pubertal timing | Rat | 189 |
EED, embryonic ectoderm development protein; KDM6B, lysine demethylase 6B; miRNA, microRNA; SIRT1, sirtuin 1.
A major hub for the epigenetic control of puberty is formed by two groups of transcriptional regulators: the polycomb group and the trithorax group. These factors repress or activate, respectively, Kiss1 expression, mainly in the ARH. Two members of the Polycomb group, namely embryonic ectoderm development protein (EED) and chromobox 7, interact with the Kiss1 promoter. During the prepubertal period, they contribute to the suppression of Kiss1 expression, mainly by inducing a repressive chromatin configuration linked to H3K27me3. Increased methylation of the gene promoters of EED and chromobox 7 during pubertal maturation in female rats leads to reduced levels of these repressors, and hence increased Kiss1 expression181. Prepubertally, EED also represses the expression of lysine demethylase 6B (KDM6B) in female rats, as a complementary mechanism for epigenetic control of puberty182. KDM6B is responsible for erasing trimethylation of histones at the K27 residue, which is a repressive mark. The resulting weakening of EED expression during the pubertal transition enhances KDM6B levels in rats and thereby reduces inhibitory epigenetic action at the Kiss1 promoter182.
Exactly how epigenetic mechanisms might contribute to the nutritional control of puberty is yet to be revealed. As mentioned in a previous section (see section ‘Indicators of nutritional status’), a plausible component of such mechanisms is a metabolic sensor, SIRT1 (ref. 183). SIRT1 is the best-characterized member of the sirtuin family, with pleotropic functions in the control of cell and body metabolism, as well as healthy lifespan. Studies in female rats have shown that SIRT1 recruits EED to the Kiss1 promoter as a means of suppressing Kiss1 expression. Accordingly, hypothalamic SIRT1 content decreases during pubertal maturation183. Moreover, nutritional status modulates SIRT1 levels in kisspeptin neurons in the ARH; levels of SIRT1 increase in conditions of undernutrition and decrease in conditions of early onset obesity (as a result of small litter rearing and subsequent high-fat diet feeding after weaning). Notably, molecular analyses demonstrated that SIRT1 acts as repressor of the Kiss1 promoter, which delays pubertal onset in conditions of undernutrition. By contrast, early obesity advances the physiological removal of SIRT1 from the Kiss1 promoter, a phenomenon that seems to contribute to an acceleration of puberty onset that is linked to overweight in rats183. As it operates as a protein deacetylase, targeting chromatin histones (among others), SIRT1 probably constitutes one of the epigenetic mechanisms that links early nutritional status and pubertal timing.
In addition to DNA methylation and histone modifications, miRNAs also modulate central and peripheral elements of the HPG axis184-186 and might have roles in puberty. Genome-wide association studies (GWAS) point to a major role in puberty for lin-28 homologue B (LIN28B), which is an RNA-binding protein that inhibits maturation of let-7 miRNAs. Functional genomic studies have shown that blockade of canonical miRNA biosynthesis by conditional ablation of Dicer in GnRH neurons prevents completion of pubertal maturation and causes infertility in mice186. In addition, miR-200, miR-429 and miR-155 control the activator switch of GnRH neurons during pubertal maturation186, which, if not properly flipped, can lead to GnRH deficiency later in life, as shown in a trisomic mouse model187. Similarly, specific knockout of Dicer in Kiss1-expressing cells causes central hypogonadism and impairment of pubertal completion in female mice188. Nevertheless, initiation of puberty is observed in both sexes despite congenital ablation of Dicer; male mice lacking Dicer in cells expressing Kiss1 even attain fertility. However, both male and female mice rapidly progress towards hypogonadotropic hypogonadism, apparently due to a marked suppression of Kiss1 transcription caused by enhanced expression of specific Kiss1 repressors, preceding a loss of kisspeptin neurons after 1 month of age. In addition, miR-30b has been suggested to cooperate in pubertal control189 via its capacity to suppress makorin ring finger protein 3 gene (MKRN3) expression, which operates as a pubertal repressor in humans190. Whether these miRNA-related pathways participate in the nutritional control of puberty is yet to be defined and future investigation is warranted.
Population genetics and clinical relevance
Population genetics provides a powerful approach to understanding the determinants of pubertal timing and its links with nutritional status and metabolism. GWAS and, over the past decade, whole-exome studies (WES) avoid the limitations of specifying candidate genes and enable pooling of data from large cohort studies around the world. Early GWAS for pubertal timing, based on recalled age at menarche, identified a substantial shared genetic architecture with BMI and obesity191. Furthermore, Mendelian randomization was used to infer a causal effect of increasing BMI on risk of early menarche192. However, this overlap (the genome-wide genetic correlation between BMI and age at menarche was −0.35, P = 1.6 × 10−72) made it difficult to distinguish between BMI-related and BMI-unrelated age at menarche variants17.
In 2021, a study using both GWAS and WES identified MC3R as a key factor linking the leptin–melanocortin sensing of nutritional status to hypothalamic KNDy neurons129. In the UK Biobank, women who carried rare variants in MC3R that had been experimentally characterized as deleterious, or predicted to be deleterious using in silico techniques, reported age at menarche several months later than non-carriers. These women were also shorter as children and adults and had reduced lean body mass compared with non-carriers. Hypothalamic neuron expression data suggest that MC3R might also link nutritional sensing to GH axis activation129.
Another approach that has gained traction over the past decade is the effective partitioning of GWAS variants related to age at menarche by their associations with early childhood weight gain. As such, it is possible to distinguish between genes and biological pathways that influence puberty timing through early growth and nutrition from those mechanisms that directly trigger sex hormone activation and response193. An observation described in a preprint194 is that even those variants that directly promote earlier age at menarche (without increasing childhood weight) also promote increased adult BMI and obesity risk. It is unclear how earlier puberty timing might promote higher BMI; however, puberty is characterized by marked metabolic changes, including insulin resistance and body adipose tissue gains in girls, and longitudinal studies are needed to explore how these changes differ by age at puberty195. Hence, 60 years after Kennedy and Mitra’s seminal publication6, it seems that the link between metabolism and pubertal development is bidirectional, and this insight has important implications for understanding how sex hormone pathways affect the risks of future metabolic diseases101.
Although population genetics have had an important role in understanding the determinants of pubertal timing and their links with nutritional status and metabolism, studies of individual patients and families with extreme perturbations in body weight and in pubertal timing have led to identification of key players in these processes. In this context, it has been hypothesized that familial CPP might be linked with genetic factors that affect metabolism and body weight. Loss-of-function mutations in MKRN3, a maternally imprinted gene located on human chromosome 15q in the Prader–Willi syndrome critical region, have been identified in families with CPP190. Experimental studies suggest that MKRN3 acts in KNDy neurons to reduce levels of neurokinin B and kisspeptin through both transcriptional and post-transcriptional mechanisms196-198. Although Prader–Willi syndrome is associated with obesity, the roles of MKRN3 in obesity and in the interaction between metabolism and pubertal timing remain unclear. The effects of MKRN3 on pubertal timing seem to be independent of leptin in vivo in mice199, but other metabolic mediators have not yet been formally assessed. Of note, it has been reported that low serum levels of MKRN3 in girls with CPP are reduced further in girls with obesity and CPP200, which suggests that MKRN3 has a metabolic influence.
Genetic defects in another maternally imprinted gene, Delta-like homologue 1 (DLK1), which is located on chromosome 14q, have been identified in association with familial CPP201. DLK1 is a non-canonical ligand of the delta–notch signalling pathway and influences a range of developmental processes, including the differentiation of preadipocytes into mature adipocytes202. The mechanisms by which DLK1 deficiency leads to human CPP remain unknown. Notably, metabolic abnormalities, such as overweight, obesity and insulin resistance, are more prevalent in individuals with CPP that is associated with DLK1 mutations, than in patients who have idiopathic CPP, which suggests that DLK1 is a new factor linking puberty and metabolism203.
Conclusions
The observations of Kennedy and Mitra opened a new era for the understanding of environmental determinants of pubertal timing, with long-reaching implications for human health. Their seminal work not only set the scene for epidemiological studies by Frisch and colleagues on the critical adipose tissue mass hypothesis204, but paved the way for a plethora of experimental, clinical and genetic studies that, over the past half century, have expanded our knowledge on the neural and molecular basis of pubertal development, and its modulation by metabolic cues. Far more complex than initially thought, this interaction is bidirectional, illustrating the dynamic interplay between reproductive and metabolic signals across the lifespan. These interactions are also highly redundant, with multiple convergent factors, which arise from different tissues and operate at multiple levels to finely tune body metabolic status and pubertal timing. The complexity and redundancy of these interactions reflect the critical importance of ensuring sufficient energy stores to enable successful reproduction and infant survival, and of adjusting pubertal timing to internal environmental cues, including the nutritional and hormonal milieu.
Although progress in the field has been astonishing, much is yet to be determined about the molecular mechanisms and neuroendocrine circuits responsible for the fine tuning of puberty by nutritional and metabolic cues, and, even more importantly, of the hierarchy of the multiple signals involved. One intriguing issue is whether similar or divergent mechanisms operate to transduce the effects of energy deficit or excess on pubertal maturation. Although ‘permissive’ signals that are downregulated in conditions of energy deficiency might explain why this deficit leads to pubertal delay, the mechanisms connecting obesity and earlier puberty are less clear and will probably require substantial efforts to be elucidated in the coming years. The specific nutrients or global energy signatures and the specific neuronal populations mediating the effect of early obesity on pubertal timing remain unexplained and future investigation is warranted. From a clinical perspective, challenges remain in distinguishing between pubertal variations associated with metabolic cues (or other systemic or environmental inputs), sex differences and pathological conditions associated with advanced or delayed puberty.
All in all, improved understanding of the links between nutritional status and pubertal timing will generate insights into the clinical and epidemiological associations between childhood nutrition, pubertal timing and risks of metabolic diseases and cancers in later life. These research directions will expand the legacy of Kennedy and Mitra’s seminal work and promise to further fuel the field for many decades to come.
Key points.
In 1963, Kennedy and Mitra published a seminal study in rats demonstrating that body weight is a major determinant of pubertal timing.
An increasing incidence of earlier ages at puberty has been documented; early pubertal timing favours the occurrence of type 2 diabetes mellitus, cardiovascular diseases and certain cancers in adulthood.
Macronutrients and hormones that modulate growth and/or signal adipose tissue mass serve as metabolic cues conveying the nutritional status and stored energy available for sexual maturation, differentiation and growth.
The effect of metabolic cues on puberty is mediated by neural targets upstream of GnRH neurons; considerable progress in defining the neuronal circuitry and glial components has been achieved.
A number of molecular pathways and epigenetic mechanisms have been identified as primary components in the modulation of pubertal timing by hormones and nutritional cues.
Acknowledgements
The authors acknowledge the support of the Royal Society of New Zealand #UOO1706 (G.M.A.), of NIH grants R01HD104418 (J.W.H.), R37HD019938, R01HD082314, R21HD098684 (U.B.K.) R01HD090151, R01HD099084, R01DK133760 (V.M.N.), U54AG062322 (V.M.N. and U.B.K.), R01HD069702, R01HD096324 (C.F.E.), from Agencia Estatal de Investigación, Spain PID2020-118660GB-I00; co-funded with EU funds from FEDER Program (M.T.-S.), from the European Commission, Program Horizon Europe HE-ERC-2022-ADG-101096793 (M.T.-S.), the European Union Horizon 2020 research and innovation programme no. 847941 miniNO (V.P.) and no. 810331 WATCH ERC Synergy (V.P.) and the Medical Research Council unit programmes MC_UU_12015/2, MC_UU_00006/2 (J.R.B.P. and K.K.O.).
Footnotes
Competing interests
J.R.B.P. is an employee of Insmed Innovation UK, holds stock/stock options in Insmed, and receives research funding from GSK. The other authors declare no competing interests.
References
- 1.Argente J. et al. Molecular basis of normal and pathological puberty: from basic mechanisms to clinical implications. Lancet Diabetes Endocrinol. 11, 203–216 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avendano MS, Vazquez MJ & Tena-Sempere M Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum. Reprod. Update 23, 737–763 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Plant TM & Barker-Gibb ML Neurobiological mechanisms of puberty in higher primates. Hum. Reprod. Update 10, 67–77 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Sisk CL & Foster DL The neural basis of puberty and adolescence. Nat. Neurosci 7, 1040–1047 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Tena-Sempere M. The roles of kisspeptins and G protein-coupled receptor-54 in pubertal development. Curr. Opin. Pediatr 18, 442–447 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Kennedy GC & Mitra J Body weight and food intake as initiating factors for puberty in the rat. J. Physiol 166, 408–418 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisch RE Fatness, menarche, and female fertility. Perspect. Biol. Med 28, 611–633 (1985). [DOI] [PubMed] [Google Scholar]
- 8.Frisch RE The right weight: body fat, menarche and fertility. Proc. Nutr. Soc 53, 113–129 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Schneider JE Energy balance and reproduction. Physiol. Behav 81, 289–317 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Kaplowitz P. Pubertal development in girls: secular trends. Curr. Opin. Obstet. Gynecol 18, 487–491 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Biro FM et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 126, e583–e590 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman-Giddens ME et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 99, 505–512 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Burt Solorzano CM & McCartney CR Obesity and the pubertal transition in girls and boys. Reproduction 140, 399–410 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Euling SY et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics 121, S172–S191 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Ahmed ML, Ong KK & Dunger DB Childhood obesity and the timing of puberty. Trends Endocrinol. Metab 20, 237–242 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Reinehr T & Roth CL Is there a causal relationship between obesity and puberty? Lancet Child. Adolesc. Health 3, 44–54 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Day FR, Elks CE, Murray A, Ong KK & Perry JR Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci. Rep 5, 11208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman DS et al. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr. 3, 3 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollis B. et al. Genomic analysis of male puberty timing highlights shared genetic basis with hair colour and lifespan. Nat. Commun 11, 1536 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day FR et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet 49, 834 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J & Chan YM Adult consequences of self-limited delayed puberty. Pediatrics 139, e20163177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welt CK et al. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med 351, 987–997 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Mountjoy M. et al. The IOC consensus statement: beyond the Female Athlete Triad–Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med 48, 491–497 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Papadimitriou A. The evolution of the age at menarche from prehistorical to modern times. J. Pediatr. Adolesc. Gynecol 29, 527–530 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Piras GN et al. The levelling-off of the secular trend of age at menarche among Italian girls. Heliyon 6, e04222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parent AS et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev 24, 668–693 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Jansen EC, Herran OF & Villamor E Trends and correlates of age at menarche in Colombia: results from a nationally representative survey. Econ. Hum. Biol 19, 138–144 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Pereira A, Corvalan C, Merino PM, Leiva V & Mericq V Age at pubertal development in a Hispanic-Latina female population: should the definitions be revisited? J. Pediatr. Adolesc. Gynecol 32, 579–583 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Eckert-Lind C et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analysis. JAMA Pediatr. 174, e195881 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenfield RL, Lipton RB & Drum ML Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics 123, 84–88 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Jasik CB & Lustig RH Adolescent obesity and puberty: the “perfect storm”. Ann. N. Y. Acad. Sci 1135, 265–279 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Dunger DB, Ahmed ML & Ong KK Effects of obesity on growth and puberty. Best. Pract. Res. Clin. Endocrinol. Metab 19, 375–390 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Grabner GF, Xie H, Schweiger M & Zechner R Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat. Metab 3, 1445–1465 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Hill JW & Elias CF Neuroanatomical framework of the metabolic control of reproduction. Physiol. Rev 98, 2349–2380 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans MC, Campbell RE & Anderson GM Physiological regulation of leptin as an integrative signal of reproductive readiness. Curr. Opin. Pharmacol 67, 102321 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Casado ME, Collado-Perez R, Frago LM & Barrios V Recent advances in the knowledge of the mechanisms of leptin physiology and actions in neurological and metabolic pathologies. Int. J. Mol. Sci 24, 1422 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chehab FF, Lim ME & Lu R Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet 12, 318–320 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Farooqi IS Leptin and the onset of puberty: insights from rodent and human genetics. Semin. Reprod. Med 20, 139–144 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Farooqi IS et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med 341, 879–884 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Ahima RS, Dushay J, Flier SN, Prabakaran D & Flier JS Leptin accelerates the onset of puberty in normal female mice. J. Clin. Invest 99, 391–395 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chehab FF, Mounzih K, Lu R & Lim ME Early onset of reproductive function in normal female mice treated with leptin. Science 275, 88–90 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Matsubara M, Maruoka S & Katayose S Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur. J. Endocrinol 147, 173–180 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Sitticharoon C, Sukharomana M, Likitmaskul S, Churintaraphan M & Maikaew P Increased high molecular weight adiponectin, but decreased total adiponectin and kisspeptin, in central precocious puberty compared with aged-matched prepubertal girls. Reprod. Fertil. Dev 29, 2466–2478 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Contreras C et al. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. 9, 366–377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magnan C, Levin BE & Luquet S Brain lipid sensing and the neural control of energy balance. Mol. Cell Endocrinol 418, 3–8 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Heras V. et al. Central ceramide signaling mediates obesity-induced precocious puberty. Cell Metab. 32, 951–966 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Roa J. et al. Metabolic regulation of female puberty via hypothalamic AMPK-kisspeptin signaling. Proc. Natl Acad. Sci. USA 115, E10758–E10767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torsoni MA et al. AMPKα2 in Kiss1 neurons is required for reproductive adaptations to acute metabolic challenges in adult female mice. Endocrinology 157, 4803–4816 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franssen D. et al. AMP-activated protein kinase (AMPK) signaling in GnRH neurons links energy status and reproduction. Metabolism 115, 154460 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Hayashida T. et al. Ghrelin in neonatal rats: distribution in stomach and its possible role. J. Endocrinol 173, 239–245 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Aguilar E, Tena-Sempere M & Pinilla L Role of excitatory amino acids in the control of growth hormone secretion. Endocrine 28, 295–302 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Torres PJ et al. The role of intragestational ghrelin on postnatal development and reproductive programming in mice. Reproduction 156, 331–341 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Velasquez DA et al. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 60, 1177–1185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergqvist N. The gonadal function in female diabetics. Acta Endocrinol. Suppl 19, 1–20 (1954). [PubMed] [Google Scholar]
- 55.Schriock EA, Winter RJ & Traisman HS Diabetes mellitus and its effects on menarche. J. Adolesc. Health Care 5, 101–104 (1984). [DOI] [PubMed] [Google Scholar]
- 56.Kjaer K, Hagen C, Sandø SH & Eshøj O Epidemiology of menarche and menstrual disturbances in an unselected group of women with insulin-dependent diabetes mellitus compared to controls. J. Clin. Endocrinol. Metab 75, 524–529 (1992). [DOI] [PubMed] [Google Scholar]
- 57.Codner E, Merino PM & Tena-Sempere M Female reproduction and type 1 diabetes: from mechanisms to clinical findings. Hum. Reprod. Update 18, 568–585 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Gaete X. et al. Earlier puberty in boys with type 1 diabetes mellitus compared to a simultaneously recruited group of control adolescents. Pediatr. Diabetes 20, 197–201 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Brüning JC et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Evans MC, Hill JW & Anderson GM Role of insulin in the neuroendocrine control of reproduction. J. Neuroendocrinol 33, e12930 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Saleh FL et al. Hyperinsulinemia induces early and dyssynchronous puberty in lean female mice. J. Endocrinol 254, 121–135 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manaserh IH et al. Ablating astrocyte insulin receptors leads to delayed puberty and hypogonadism in mice. PLoS Biol. 17, e3000189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans MC, Rizwan M, Mayer C, Boehm U & Anderson GM Evidence that insulin signalling in gonadotrophin-releasing hormone and kisspeptin neurones does not play an essential role in metabolic regulation of fertility in mice. J. Neuroendocrinol 26, 468–479 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Xu C. et al. KLB, encoding β-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol. Med 9, 1379–1397 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Owen BM et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med 19, 1153–1156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacLusky NJ et al. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 141, 752–762 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Outeirino-Iglesias V, Romani-Perez M, Gonzalez-Matias LC, Vigo E & Mallo F GLP-1 increases preovulatory LH source and the number of mature follicles, as well as synchronizing the onset of puberty in female rats. Endocrinology 156, 4226–4237 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Korpela K. et al. Gut microbiota develop towards an adult profile in a sex-specific manner during puberty. Sci. Rep 11, 23297 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sisk-Hackworth L, Kelley ST & Thackray VG Sex, puberty, and the gut microbiome. Reproduction 165, R61–R74 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ilyes T, Silaghi CN & Craciun AM Diet-related changes of short-chain fatty acids in blood and feces in obesity and metabolic syndrome. Biology 11, 1556 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L. et al. Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats. Front. Endocrinol 13, 1051797 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan X, Shangguan H, Zhang Y, Lin X & Chen R Intervention effect of probiotics on the early onset of puberty induced by daidzein in female mice. Mol. Nutr. Food Res 67, e2200501 (2023). [DOI] [PubMed] [Google Scholar]
- 73.Bo T. et al. Effects of high-fat diet during childhood on precocious puberty and gut microbiota in mice. Front. Microbiol 13, 930747 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M. et al. Microbial reconstitution reverses early female puberty induced by maternal high-fat diet during lactation. Endocrinology 161, bqz041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martha PM Jr. et al. Alterations in the pulsatile properties of circulating growth hormone concentrations during puberty in boys. J. Clin. Endocrinol. Metab 69, 563–570 (1989). [DOI] [PubMed] [Google Scholar]
- 76.Cemeroglu AP, Barkan AL, Kletter GB, Beitins IZ & Foster CM Changes in serum immunoreactive and bioactive growth hormone concentrations in boys with advancing puberty and in response to a 20-hour estradiol infusion. J. Clin. Endocrinol. Metab 82, 2166–2171 (1997). [DOI] [PubMed] [Google Scholar]
- 77.Batch JA & Werther GA Changes in growth hormone concentrations during puberty in adolescents with insulin dependent diabetes. Clin. Endocrinol 36, 411–416 (1992). [DOI] [PubMed] [Google Scholar]
- 78.Sabin MA et al. Insulin and BMI as predictors of adult type 2 diabetes mellitus. Pediatrics 135, 144–151 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Cavarzere P. et al. Growth hormone retesting during puberty: a cohort study. Eur. J. Endocrinol 182, 559–567 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Juul A & Skakkeæk NE Why do normal children have acromegalic levels of IGF-I during puberty? J. Clin. Endocrinol. Metab 104, 2770–2776 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Orçun A, Yildiz Z & Köroğlu Dağdelen L Pediatric reference intervals for free testosterone, 17-OH progesterone, androstenedione, and IGF-1 with chemiluminescence immunoassay. Steroids 186, 109078 (2022). [DOI] [PubMed] [Google Scholar]
- 82.Baumgartner M. et al. Plasma myostatin increases with age in male youth and negatively correlates with vitamin D in severe pediatric obesity. Nutrients 14, 2133 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reinehr T, Elfers C, Lass N & Roth CL Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J. Clin. Endocrinol. Metab 100, 2123–2130 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Li M, Liao B, Zhong J & Lan D Serum irisin levels increase in girls with central precocious puberty not dependent on BMI: a pilot study. Endocr. Connect 11, e220028 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kutlu E. et al. Serum irisin levels in central precocious puberty and its variants. J. Clin. Endocrinol. Metab 106, e247–e254 (2021). [DOI] [PubMed] [Google Scholar]
- 86.McPherron AC, Lawler AM & Lee SJ Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387, 83–90 (1997). [DOI] [PubMed] [Google Scholar]
- 87.DiVall SA et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J. Clin. Invest 120, 2900–2909 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Decourt C, Evans MC, Inglis MA & Anderson GM Central irisin signaling Is required for normal timing of puberty in female mice. Endocrinology 164, bqac208 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bohlen TM et al. Central growth hormone signaling is not required for the timing of puberty. J. Endocrinol 243, 161–173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Savage MO et al. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome). J. Clin. Endocrinol. Metab 77, 1465–1471 (1993). [DOI] [PubMed] [Google Scholar]
- 91.JuuL A. et al. Serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 levels are increased in central precocious puberty: effects of two different treatment regimens with gonadotropin-releasing hormone agonists, without or in combination with an antiandrogen (cyproterone acetate). J. Clin. Endocrinol. Metab 80, 3059–3067 (1995). [DOI] [PubMed] [Google Scholar]
- 92.Baier I, Pereira A, Ferrer P, Iniguez G & Mericq V Higher prepubertal IGF-1 concentrations associate to earlier pubertal tempo in both sexes. Horm. Res. Paediatr 96, 404–411 (2023). [DOI] [PubMed] [Google Scholar]
- 93.Hiney JK, Srivastava V, Nyberg CL, Ojeda SR & Dees WL Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology 137, 3717–3728 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Pazos Fanchez-Franco F, Balsa J, Lopez-Fernandez J, Escalada J & Cacicedo L Regulation of gonadal and somatotropic axis by chronic intraventricular infusion of insulin-like growth factor 1 antibody at the initiation of puberty in male rats. Neuroendocrinology 69, 408–416 (1999). [DOI] [PubMed] [Google Scholar]
- 95.Balint F, Csillag V, Vastagh C, Liposits Z & Farkas I Insulin-like growth factor 1 increases GABAergic neurotransmission to GnRH neurons via suppressing the retrograde tonic endocannabinoid signaling pathway in mice. Neuroendocrinology 111, 1219–1230 (2021). [DOI] [PubMed] [Google Scholar]
- 96.Gemelli IFB, Farias EDS & Spritzer PM Association of body composition and age at menarche in girls and adolescents in the Brazilian Legal Amazon. J. Pediatr 96, 240–246 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosales Nieto CA et al. Selection for superior growth advances the onset of puberty and increases reproductive performance in ewe lambs. Animal 7, 990–997 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Boyne MS et al. Growth, body composition, and the onset of puberty: longitudinal observations in Afro-Caribbean children. J. Clin. Endocrinol. Metab 95, 3194–3200 (2010). [DOI] [PubMed] [Google Scholar]
- 99.de Ridder CM et al. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J. Clin. Endocrinol. Metab 70, 888–893 (1990). [DOI] [PubMed] [Google Scholar]
- 100.Han SZ et al. Reproduction traits of heterozygous myostatin knockout sows crossbred with homozygous myostatin knockout boars. Reprod. Domest. Anim 56, 26–33 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Cheng HL et al. Impact of growth, gonadal hormones, adiposity and the sodium-to-potassium ratio on longitudinal adolescent measures of blood pressure at puberty. J. Hum. Hypertens 37, 835–843 (2023). [DOI] [PubMed] [Google Scholar]
- 102.Vanacker C. et al. Neuropilin-1 expression in GnRH neurons regulates prepubertal weight gain and sexual attraction. EMBO J. 39, e104633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quennell JH et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150, 2805–2812 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elias CF & Purohit D. Leptin signaling and circuits in puberty and fertility. Cell. Mol. life Sci 70, 841–862 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Allison MB & Myers MG Jr. 20 years of leptin: connecting leptin signaling to biological function. J. Endocrinol 223, T25–T35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Balland E. et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 19, 293–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Banks WA The blood-brain barrier as an endocrine tissue. Nat. Rev. Endocrinol 15, 444–455 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Banks WA, Kastin AJ, Huang W, Jaspan JB & Maness LM Leptin enters the brain by a saturable system independent of insulin. Peptides 17, 305–311 (1996). [DOI] [PubMed] [Google Scholar]
- 109.Popa SM, Clifton DK & Steiner RA The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu. Rev. Physiol 70, 213 (2008). [DOI] [PubMed] [Google Scholar]
- 110.Seminara SB & Crowley WF Jr. Kisspeptin and GPR54: discovery of a novel pathway in reproduction. J. Neuroendocrinol 20, 727–731 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pinilla L, Aguilar E, Dieguez C, Millar RP & Tena-Sempere M Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol. Rev 92, 1235–1316 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Comninos AN, Jayasena CN & Dhillo WS The relationship between gut and adipose hormones, and reproduction. Hum. Reprod. Update 20, 153–174 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Manfredi-Lozano M, Roa J & Tena-Sempere M Connecting metabolism and gonadal function: novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front. Neuroendocrinol 48, 37–49 (2018). [DOI] [PubMed] [Google Scholar]
- 114.Navarro VM et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci 29, 11859–11866 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cravo RM et al. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS ONE 8, e58698 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zuure WA, Roberts AL, Quennell JH & Anderson GM Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J. Neurosci 33, 17874–17883 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martin C. et al. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J. Neurosci 34, 6047–6056 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tritos NA, Elmquist JK, Mastaitis JW, Flier JS & Maratos-Flier E Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology 139, 4634–4641 (1998). [DOI] [PubMed] [Google Scholar]
- 119.Mizuno TM et al. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140, 4551–4557 (1999). [DOI] [PubMed] [Google Scholar]
- 120.Cone RD et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord 25, S63–S67 (2001). [DOI] [PubMed] [Google Scholar]
- 121.Egan OK, Inglis MA & Anderson GM Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J. Neurosci 37, 3875–3886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Padilla SL et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl Acad. Sci. USA 114, 2413–2418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ellacott KL & Cone RD The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent. Prog. Horm. Res 59, 395–408 (2004). [DOI] [PubMed] [Google Scholar]
- 124.Balthasar N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004). [DOI] [PubMed] [Google Scholar]
- 125.van de Wall E. et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149, 1773–1785 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hohmann JG et al. Differential role of melanocortins in mediating leptin’s central effects on feeding and reproduction. Am. J. Physiol. Regul. Integr. Comp. Physiol 278, R50–R59 (2000). [DOI] [PubMed] [Google Scholar]
- 127.Manfredi-Lozano M. et al. Defining a novel leptin–melanocortin–kisspeptin pathway involved in the metabolic control of puberty. Mol. Metab 5, 844–857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duckett K. et al. Prevalence of deleterious variants in MC3R in patients with constitutional delay of growth and puberty. J. Clin. Endocrinol. Metab 20, dgad373 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lam BYH et al. MC3R links nutritional state to childhood growth and the timing of puberty. Nature 599, 436–441 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chachlaki K. et al. NOS1 mutations cause hypogonadotropic hypogonadism with sensory and cognitive deficits that can be reversed in infantile mice. Sci. Transl. Med 14, eabh2369 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Donato J Jr et al. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J. Neurosci 29, 5240–5250 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Donato J Jr. et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. Invest 121, 355–368 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Williams KW et al. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. J. Neurosci 31, 13147–13156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Donato J Jr., Frazao R, Fukuda M, Vianna CR & Elias CF Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology 151, 5415–5427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leshan RL et al. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J. Neurosci 29, 3138–3147 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clasadonte J, Poulain P, Beauvillain JC & Prevot V Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology 149, 587–596 (2008). [DOI] [PubMed] [Google Scholar]
- 137.Chachlaki K. et al. Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus. J. Comp. Neurol 525, 3177–3189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Delli V. et al. Male minipuberty involves the gonad-independent activation of preoptic nNOS neurons. Free. Radic. Biol. Med 194, 199–208 (2023). [DOI] [PubMed] [Google Scholar]
- 139.Yu WH, Walczewska A, Karanth S & McCann SM Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology 138, 5055–5058 (1997). [DOI] [PubMed] [Google Scholar]
- 140.Bellefontaine N. et al. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J. Clin. Invest 124, 2550–2559 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE & Myers MG Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat. Med 18, 820–823 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ross RA et al. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. eLife 7, e35960 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han X. et al. Hypothalamic and cell-specific transcriptomes unravel a dynamic neuropil remodeling in leptin-induced and typical pubertal transition in female mice. iScience 23, 101563 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Prevot V. Glial control of neuronal function. Nat. Rev. Endocrinol 18, 195 (2022). [DOI] [PubMed] [Google Scholar]
- 145.Nampoothiri S, Nogueiras R, Schwaninger M & Prevot V Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis. Nat. Metab 4, 813–825 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Prevot V. et al. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev 39, 333–368 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Pellegrino G. et al. GnRH neurons recruit astrocytes in infancy to facilitate network integration and sexual maturation. Nat. Neurosci 24, 1660–1672 (2021). [DOI] [PubMed] [Google Scholar]
- 148.Clasadonte J. et al. Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation. Proc. Natl Acad. Sci. USA 108, 16104–16109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vanacker C, Defazio RA, Sykes CM & Moenter SM A role for glial fibrillary acidic protein (GFAP)-expressing cells in the regulation of gonadotropin-releasing hormone (GnRH) but not arcuate kisspeptin neuron output in male mice. eLife 10, e68205 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prevot V. et al. Normal female sexual development requires neuregulin–erbB receptor signaling in hypothalamic astrocytes. J. Neurosci 23, 230–239 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ma YJ, Junier MP, Costa ME & Ojeda SR Transforming growth factor-α gene expression in the hypothalamus is developmentally regulated and linked to sexual maturation. Neuron 9, 657–670 (1992). [DOI] [PubMed] [Google Scholar]
- 152.Moeller-Gnangra H, Ernst J, Pfeifer M & Heger S ErbB4 point mutation in CU3 inbred rats affects gonadotropin-releasing-hormone neuronal function via compromised neuregulin-stimulated prostaglandin E2 release from astrocytes. Glia 67, 309–320 (2019). [DOI] [PubMed] [Google Scholar]
- 153.Verkhratsky A & Zorec R Astroglial signalling in health and disease. Neurosci. Lett 689, 1–4 (2019). [DOI] [PubMed] [Google Scholar]
- 154.Sloan SA & Barres BA Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol 27, 75–81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pena-Leon V. et al. Prolonged breastfeeding protects from obesity by hypothalamic action of hepatic FGF21. Nat. Metab 4, 901–917 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rodriguez-Cortes B. et al. Suprachiasmatic nucleus-mediated glucose entry into the arcuate nucleus determines the daily rhythm in blood glycemia. Curr. Biol 32, 796–805 (2022). [DOI] [PubMed] [Google Scholar]
- 157.García-Cáceres C. et al. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166, 867–880 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Duquenne M. et al. Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function. Nat. Metab 3, 1071–1090 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Imbernon M. et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab. 34, 1054–1063 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Porniece Kumar M. et al. Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat. Metab 3, 1662–1679 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Clasadonte J, Scemes E, Wang Z, Boison D & Haydon PG Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron 95, 1365–1380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lhomme T. et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J. Clin. Invest 131, e140521 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Banks WA, Owen JB & Erickson MA Insulin in the brain: there and back again. Pharmacol. Ther 136, 82–93 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Collden G. et al. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol. Metab 4, 15–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ogassawara TB et al. Food deprivation in F0 generation and hypercaloric diet in F1 generation reduce F2 generation astrogliosis in several brain areas after immune challenge. Int. J. Dev. Neurosci 64, 29–37 (2018). [DOI] [PubMed] [Google Scholar]
- 166.Contu L, Nizari S, Heath CJ & Hawkes CA Pre- and post-natal high fat feeding differentially affects the structure and integrity of the neurovascular unit of 16-month old male and female mice. Front. Neurosci 13, 1045 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Evans MC, Lord RA & Anderson GM Multiple leptin signalling pathways in the control of metabolism and fertility: a means to different ends? Int. J. Mol. Sci 22, 9210 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singireddy AV, Inglis MA, Zuure WA, Kim JS & Anderson GM Neither signal transducer and activator of transcription 3 (STAT3) or STAT5 signaling pathways are required for leptin’s effects on fertility in mice. Endocrinology 154, 2434–2445 (2013). [DOI] [PubMed] [Google Scholar]
- 169.Piper ML, Unger EK, Myers MG & Xu AW Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol. Endocrinol 22, 751–759 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bates SH et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 (2003). [DOI] [PubMed] [Google Scholar]
- 171.Gao Q. et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl Acad. Sci. USA 101, 4661–4666 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee JY et al. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS ONE 3, e1639 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patterson CM et al. Leptin action via LepR-b Tyr1077 contributes to the control of energy balance and female reproduction. Mol. Metab 1, 61–69 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang SQ et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13, 341–355 (2004). [DOI] [PubMed] [Google Scholar]
- 175.Garcia-Galiano D. et al. PI3Kα inactivation in leptin receptor cells increases leptin sensitivity but disrupts growth and reproduction. JCI Insight 2, e96728 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kitamura T. et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med 12, 534–540 (2006). [DOI] [PubMed] [Google Scholar]
- 177.Yang G. et al. FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1. J. Biol. Chem 284, 3719–3727 (2009). [DOI] [PubMed] [Google Scholar]
- 178.Kim MS et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat. Neurosci 9, 901–906 (2006). [DOI] [PubMed] [Google Scholar]
- 179.Xu J, Ji J & Yan XH Cross-talk between AMPK and mTOR in regulating energy balance. Crit. Rev. Food Sci. Nutr 52, 373–381 (2012). [DOI] [PubMed] [Google Scholar]
- 180.Roa J. et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 150, 5016–5026 (2009). [DOI] [PubMed] [Google Scholar]
- 181.Lomniczi A. et al. Epigenetic control of female puberty. Nat. Neurosci 16, 281–289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wright H, Aylwin CF, Toro CA, Ojeda SR & Lomniczi A Polycomb represses a gene network controlling puberty via modulation of histone demethylase Kdm6b expression. Sci. Rep 11, 1996 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vazquez MJ et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat. Commun 9, 4194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gaytan F. et al. Distinct expression patterns predict differential roles of the miRNA-binding proteins, Lin28 and Lin28b, in the mouse testis: studies during postnatal development and in a model of hypogonadotropic hypogonadism. Endocrinology 154, 1321–1336 (2013). [DOI] [PubMed] [Google Scholar]
- 185.Wang JM & Zhang K Microarray analysis of microRNA expression in bone marrow-derived progenitor cells from mice with type 2 diabetes. Genom. Data 7, 86–87 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Messina A. et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat. Neurosci 19, 835–844 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Manfredi-Lozano M. et al. GnRH replacement rescues cognition in Down syndrome. Science 377, eabq4515 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Roa J et al. Dicer ablation in Kiss1 neurons impairs puberty and fertility preferentially in female mice. Nat. Commun 13, 4663 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Heras V. et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 17, e3000532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Abreu AP et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med 368, 2467–2475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Elks CE et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet 42, 1077–1085 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mumby HS et al. Mendelian randomisation study of childhood BMI and early menarche. J. Obes 2011, 180729 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cousminer DL et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum. Mol. Genet 23, 4452–4464 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Katherine AK et al. Understanding the genetic complexity of puberty timing across the allele frequency spectrum. Preprint at medRxiv www.medrxiv.org/content/10.1101/2023.06.14.23291322v1 (2023). [Google Scholar]
- 195.Kelsey MM & Zeitler PS Insulin resistance of puberty. Curr. Diabetes Rep 16, 64 (2016). [DOI] [PubMed] [Google Scholar]
- 196.Abreu AP et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J. Clin. Invest 130, 4486–4500 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Naule L. et al. MKRN3 inhibits puberty onset via interaction with IGF2BP1 and regulation of hypothalamic plasticity. JCI Insight 8, e164178 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Roberts SA et al. Hypothalamic overexpression of makorin ring finger protein 3 results in delayed puberty in female mice. Endocrinology 163, bqac132 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Roberts SA et al. The peripubertal decline in makorin ring finger protein 3 expression is independent of leptin action. J. Endocr. Soc 4, bvaa059 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Eren SE & Simsek E Comparison of makorin ring finger protein 3 levels between obese and normal weight patients with central precocious puberty. J. Clin. Res. Pediatr. Endocrinol 15, 182–189 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dauber A. et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J. Clin. Endocrinol. Metab 102, 1557–1567 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.da Silva C, Durandt C, Kallmeyer K, Ambele MA & Pepper MS The role of pref-1 during adipogenic differentiation: an overview of suggested mechanisms. Int. J. Mol. Sci 21, 4104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gomes LG et al. DLK1 is a novel link between reproduction and metabolism. J. Clin. Endocrinol. Metab 104, 2112–2120 (2019). [DOI] [PubMed] [Google Scholar]
- 204.Frisch RE & McArthur JW Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 185, 949–951 (1974). [DOI] [PubMed] [Google Scholar]
- 205.Bessa DS et al. Methylome profiling of healthy and central precocious puberty girls. Clin. Epigenetics 10, 146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Toro CA, Wright H, Aylwin CF, Ojeda SR & Lomniczi A Trithorax dependent changes in chromatin landscape at enhancer and promoter regions drive female puberty. Nat. Commun 9, 57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lopez-Rodriguez D. et al. Multi- and transgenerational outcomes of an exposure to a mixture of endocrine-disrupting chemicals (EDCs) on puberty and maternal behavior in the female rat. Env. Health Perspect 129, 87003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Perry JR et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat. Genet 41, 648–650 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.He C. et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat. Genet 41, 724–728 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sulem P. et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat. Genet 41, 734–738 (2009). [DOI] [PubMed] [Google Scholar]
- 211.Ong KK et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat. Genet 41, 729–733 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Osinubi A, Lewis-de los Angeles CP, Poitevien P & Topor LS Are black girls exhibiting puberty earlier? Examining implications of race-based guidelines. Pediatrics 150, e2021055595 (2022). [DOI] [PubMed] [Google Scholar]
- 213.Parnell W, Scragg R, Wilson N, Schaaf D & Fitzgerald E NZ food NZ children. Key results of the 2002 National Children’s Nutrition Survey. Ministry of Health https://www.health.govt.nz/system/files/documents/publications/nzfoodnzchildren.pdf (2003). [Google Scholar]
- 214.Cabrera SM, Bright GM, Frane JW, Blethen SL & Lee PA Age of thelarche and menarche in contemporary US females: a cross-sectional analysis. J. Pediatr. Endocrinol. Metab 27, 47–51 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Staiano AE, Broyles ST, Gupta AK & Katzmarzyk PT Ethnic and sex differences in visceral, subcutaneous, and total body fat in children and adolescents. Obesity 21, 1251–1255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Goran MI et al. Visceral fat in white and African American prepubertal children. Am. J. Clin. Nutr 65, 1703–1708 (1997). [DOI] [PubMed] [Google Scholar]
- 217.Rush EC, Plank LD, Davies PS, Watson P & Wall CR Body composition and physical activity in New Zealand Maori, Pacific and European children aged 5-14 years. Br. J. Nutr 90, 1133–1139 (2003). [DOI] [PubMed] [Google Scholar]