Abstract
Objective:
Growing evidence suggests that environmental heat stress negatively influences fetal growth and pregnancy outcomes. However, few studies have examined the impact of heat stress on pregnancy outcomes in low-resource settings. We combined data from a large multi-country maternal-child health registry and meteorological data to assess the impacts of heat stress.
Design:
Retrospective cohort study.
Setting:
Three sites based in South Asia as part of the Global Network for Women’s and Children’s Health research in India (Belagavi and Nagpur) and Pakistan (Thatta).
Population:
Data from women enrolled between 2014 – 2020 in the Global Network’s Maternal Newborn Health Registry (MNHR), a prospective, population-based registry of pregnancies were utilized.
Methods:
A total of 126,273 pregnant women were included in this analysis. Daily maximal air temperatures (Tmax) were acquired from local meteorological records. Associations between averages of daily maximal temperatures for each trimester and main outcomes were analyzed using modified Poisson regression approach.
Main outcomes measures:
Incidence of stillbirth, preterm birth, low-birth weight (<2,500 g) or evidence of pregnancy hypertension or pre-eclampsia.
Results:
In the overall cohort, risk of preterm birth was positively associated with greater temperature in the second trimester (RR 1.05, CI 1.02 −1.07, p = 0.0002). Among individual sites, the risk of PTB was greatest in Nagpur (RR 1.07, CI 1.03 −1.11, p = 0.0005) and associated with second trimester temperature. The overall risk of LBW was associated with ambient temperature in second trimester (RR 1.02, CI 1.01 – 1.04, p = 0.01). The risk for LBW was associated with first trimester heat in Thatta and with second trimester heat in Nagpur. Finally, the overall risk of gestational hypertensive disease was associated with greater temperature in the third trimester among all sites (RR 1.07, CI 1.02 – 1.12, p = 0.005) and particularly significant for Nagpur (RR 1.13, CI 1.05 – 1.23, p = 0.002). These findings highlight the increased risk of detrimental obstetric and neonatal outcomes with greater temperature.
Conclusion:
In a multi-country, community-based study, greater risk of adverse outcomes was observed with increasing temperature. The study highlights the need for deeper understanding of covarying factors and intervention strategies, especially in regions where high temperatures are common.
Keywords: climate change, heat stress, pregnancy, low-birth weight, pre-eclampsia, stillbirth
Tweetable abstract:
Excess heat in pregnancy is associated with greater risk of preterm birth and low-birth weight.
INTRODUCTION
The impacts of climate change on human health are rapidly increasing and manifesting in every corner of the globe. Increasing heat stress and extreme heat events are one of the hallmarks of climate change1. January 2023 marked the 527th consecutive month with temperatures above the 20th-century average with global surface temperature 1.57°C above the average2. Emerging evidence provides strong support linking multiple climate change-associated-exposures (heat exposure, air quality) to health outcomes in pregnancy3. Importantly, the health burdens due to heat stress are unevenly distributed. Women and young children, especially in resource-limited settings are at high risk due to the double-burden of extant health disparities and poor resilience to heat stress4–6. Thus, studies examining the impacts of heat stress in pregnancy in resource-limited settings are acutely needed.
Higher average air temperatures lead to more frequent periods of extremely hot weather. Climate models indicate that in most representative concentration pathway (RCP) scenarios, the frequency and duration of extreme heat events is likely to increase5 7 8. Along with greater heat events, the health impacts are also projected to rise. Children born in 2020 are estimated to experience 2 to 7-fold increased risk of extreme weather events, relative to those born in 1960, underscoring the greater risk for heat waves among other events9. UNICEF estimates that currently around 559 million children are exposed to high heatwave frequency globally. By 2050, virtually every child on earth (>2 billion children) is forecast to face more frequent heatwaves10. While heat excursions are a concern globally, the south Asian subcontinent is especially vulnerable11. Unprecedented heat waves in the summer of 2022 in the south Asian subcontinent were clearly aided by global heating due to climate change based on work from the World Weather Attribution Initiative. Over 1 billion people in India and Pakistan were potentially exposed to ambient temperatures over >40°C, with recorded temperatures reaching 50°C (120°F) in Jacobabad, Pakistan.
Heat stress during pregnancy has a range of negative effects on both the mother and developing fetus3, 6, 12. Greater temperatures have been associated with increased risk of preterm birth, stillbirth, shorter gestational length and smaller birth weight and length. Results from a recent systematic review and meta-analysis which included seventy studies from around the world support these conclusions. However, only a quarter of these studies originated in low or middle-income countries (LMIC). Thus, more granular information on the impacts of high heat during pregnancy from LMIC are needed to better understand the global burden of disease in pregnancy from climate-related heat.
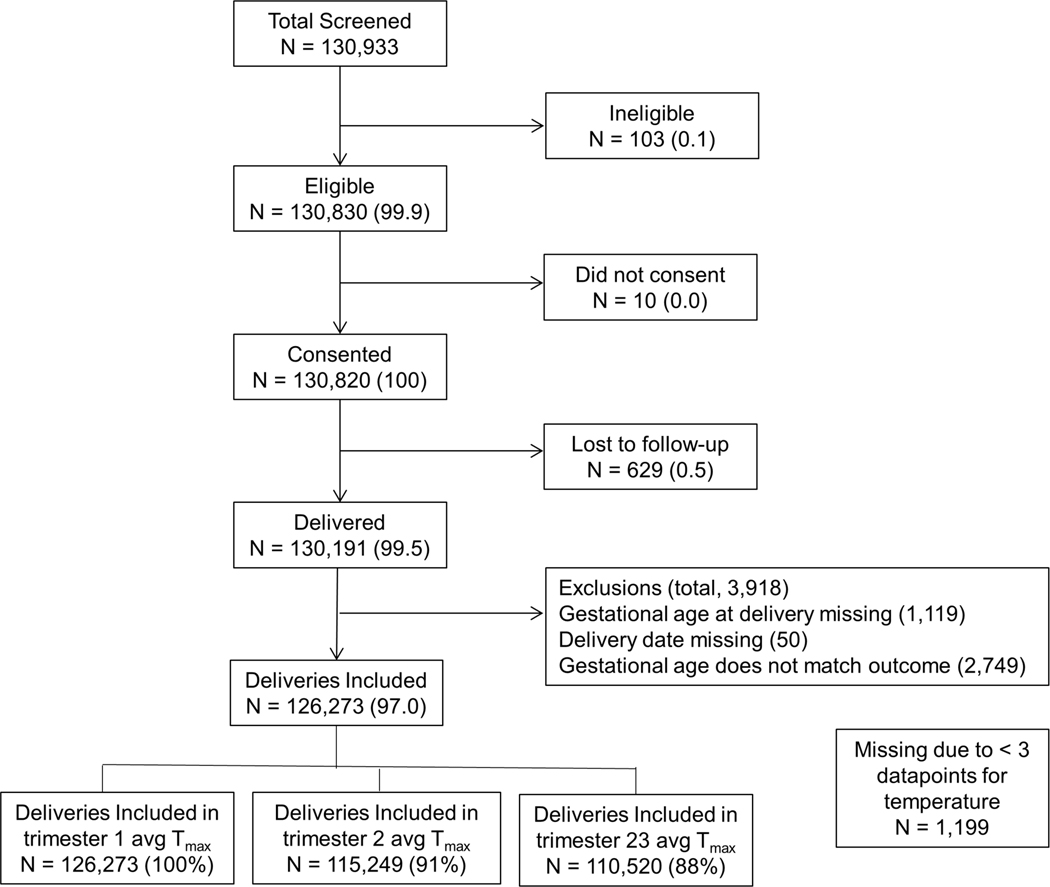
In the present study, we examined associations between ambient temperature maximums during pregnancy trimesters and obstetric and neonatal outcomes using a retrospective cohort. We leveraged data collected as part of a multisite registry of pregnant women and their infants (Maternal Newborn Health Registry, MNHR)13, 14 along with air temperature data from meteorological records collected at regional weather stations (Figure 1). This analysis focused on four outcomes: incidence of stillbirth, preterm birth, low birth weight (<2,500 g) and pregnancy hypertension or pre-eclampsia at three sites based in South Asia (India and Pakistan). The sites were chosen based on occurrence of high temperatures and a high burden of low birthweight and neonatal morbidity. Two of the sites in India (Nagpur, Maharashtra and Belagavi, Karnataka) showed geographic and climatic variation, with Nagpur having hotter summers. The site in Pakistan is in Thatta (Sindh province), Pakistan, situated west of the river Indus and is a semi-arid region. Thatta has a subtropical climate and experiences very hot summers and cold winters. Temperatures in the hot months frequently rise above 35°C at all sites with Nagpur and Thatta showing the highest temperatures between May and August. Given the rural/semi-rural nature of the locations, with little access to air-conditioning, exposure to high temperatures was likely widely prevalent. The analysis derived health outcome information from the MNHR which is an ongoing prospective population-based registry of pregnant women, fetuses and neonates receiving care in defined catchment areas at the sites13, 14. This analysis examined the relationship of heat stress to specific pregnancy outcomes in this cohort.
Figure 1.
Participant flow diagram showing selection of the analytical cohort.
METHODS
Study data were collected as part of the MNHR13, 14. The MNHR is conducted within the Eunice Kennedy Shriver NICHD Global Network of Women and Children’s Health Research (Global Network). The Global Network conducts both interventional as well as observational studies addressing pregnancy and child outcomes15. The Global Network supports development of local research capacity and infrastructure and conducts clinical trials in resource-limited countries. Currently there are eight sites within the Global Network distributed in Asia, Africa and Central America. The MNHR was developed in 2008 to gather vital statistics and accurate incidence of births, stillbirths, neonatal deaths and measures of obstetric and neonatal care with the goal of documenting maternal child health outcomes and assessing interventions to improve outcomes. At each site the target population is women who reside in or receive healthcare in a specified group of communities (clusters). Each cluster is defined by a geographical area where mothers receive primary perinatal care from designated healthcare facilities. There are currently between 8 and 10 clusters at each site and an estimated 300 – 500 deliveries per year within each cluster, although the specific number of deliveries varies by cluster. More detailed information about the Global Network and individual sites has been published previously13. For the current analysis, data from 2014 to 2020 were utilized for three south Asian sites (Belagavi and Nagpur, India; and Thatta, Pakistan). The sites were chosen for their comparable climate patterns and routinely high annual temperatures.
Study population and procedures.
The analysis included data from 126,273 pregnant women recruited between 2014 and 2020. Since the MNHR is a population-based registry, all pregnant women and their newborns who were residents of the study clusters were eligible to participate in the MNHR. Research staff at each site use a variety of surveillance methods to identify pregnant women as early as possible, including community sensitization activities, review of local hospital and clinic logs and mobile phone-based and in-person household surveys. Data were formally collected at three time-points, at enrollment during pregnancy, within 72 h of delivery and at 42-days post-partum. In addition to medical data, information on socio-economic, demographic, health care characteristics and pregnancy outcomes were also collected. Standardized methods and definitions were utilized across the sites. Gestational age was estimated using ultrasound, last menstrual period (LMP), or clinical data such as physical examination, and other available information when LMP is unknown. An algorithm, based on recommendations from the American College of Obstetrics and Gynecology, was used to assign the gestational age and estimated delivery date for the study [21]. Birth weight was recorded within 48 h of delivery using weighing scales provided by the study. Measurements of the infants born alive were consistently obtained (near 98% of all live births). However, when birth weight was not obtained, weight was estimated by trained research personnel to distinguish infants weighing less than 2500 g. Clinical conditions were recorded by research personnel, using the WHO definitions, whenever possible [22] including incidence of preterm birth and gestational hypertension or pre-eclampsia. Other major outcomes included stillbirth (fetal demise after 20 weeks gestation and prior to delivery), neonatal death (death at < 28 days), and maternal mortality (death of mother during pregnancy or up to 6-weeks postpartum). These standardized definitions were used to collect the data across sites, with a manual of operations and training materials used to reinforce the definitions across study sites [9].
Derivation of ambient heat exposure.
Daily maximum air temperature for the duration between 2014 – 2020 were acquired from the closest automated surface observation systems using the Global Surface Summary of the Day (GSODR) package. GSODR is a set of data mining tools that facilitates finding, transfer and formatting of meteorological data (National Centers for Environmental Information)16. For each site, multiple stations were included, and daily temperature data were averaged when multiple data points were present. In general, variation between stations was low. For each participant, gestational length was subtracted from the date of birth to calculate the date of conception. From these date, the average daily maximum temperature (avg Tmax) for each trimester (90-day window) was calculated. These are referred to as avg Tmax for trimesters 1, 2 and 3, respectively. For third trimester association analysis, only participants with a minimum of 3 days in the third trimester were included in cases of birth that occurred early in the third trimester.
Statistical Analysis.
All statistical analysis were carried out in R (version 4.01)17 and SAS (SAS v.9.4, Cary NC). Descriptive data are expressed as count (percent) for categorical variables or mean ± SD for continuous variables. The main exposure variable was the average maximal daily temperature for each trimester. Independent associations between trimester specific heat exposure and four outcome variables were assessed: incidence of preterm birth (PTB), gestational hypertension or severe pre-eclampsia, low birth weight (<2,500 g, LBW) and stillbirth (SB). All outcomes were dichotomous categorical variables. Analysis was carried out using a modified Poisson regression approach. Relative risks with corresponding 95% CI and p-values obtained from modified Poisson approach with a sandwich estimator for each categorical outcome and 5°C change in trimester average daily maximum temperatures. Models include site and site by outcome interaction. Site-specific relative risks were derived from the site by outcome interaction. A nominal p-value of p < 0.004 was considered statistically significant based on a conservative Bonferroni correction for 4 outcomes (α/n or 0.05/12 = 0.004)
RESULTS
The analytical cohort included a total of 126,273 pregnant women who were part of the registry from 2014– 2020. The participant flow is presented in Figure 1. Descriptive characteristics of the cohort are shown in Table 1. Participants were balanced across the three sites (34% from Belagavi, 34% from Nagpur and 32% from Thatta). Approximately 91% of women in the cohort were between 20 to 35 years of age and 36% were nulliparous. Consistent with prevalent chronic malnutrition in this setting, 32% of women had a BMI < 18.5 and low hemoglobin values (~80% with a Hb < 10.9 g/dl). Gestational length for the majority of pregnancies (~88%) was > 28 weeks. Among the overall cohort, 3% of mothers showed evidence of HTN or severe pre-eclampsia (Table 2). The stillbirth rate was 2.99% and the incidence of preterm birth was 16.4%. Overall, 22.2% of infants were LBW (< 2,500 g).
Table 1:
Maternal Characteristics by Site
| Characteristics | Overall | Belagavi | Nagpur | Pakistan |
|---|---|---|---|---|
| Pregnant women, n | 126,273 | 43,334 | 42,666 | 40,273 |
| Maternal age, n (%) | 126,271 | 43,334 | 42,665 | 40,272 |
| < 20 | 8,204 (6.5) | 5,299 (12.2) | 1,087 (2.5) | 1,818 (4.5) |
| 20–35 | 115,250 (91.3) | 37,821 (87.3) | 41,326 (96.9) | 36,103 (89.6) |
| > 35 | 2,817 (2.2) | 214 (0.5) | 252 (0.6) | 2,351 (5.8) |
| Maternal education, n (%) | 126,244 | 43,331 | 42,642 | 40,271 |
| No formal schooling | 38,959 (30.9) | 4,664 (10.8) | 1,081 (2.5) | 33,214 (82.5) |
| Primary or secondary | 75,757 (60.0) | 34,320 (79.2) | 34,890 (81.8) | 6,547 (16.3) |
| University + | 11,528 (9.1) | 4,347 (10.0) | 6,671 (15.6) | 510 (1.3) |
| Parity, n (%) | 124,894 | 43,333 | 42,640 | 38,921 |
| 0 | 44,404 (35.6) | 15,366 (35.5) | 21,384 (50.2) | 7,654 (19.7) |
| 1–2 | 59,112 (47.3) | 24,813 (57.3) | 20,401 (47.8) | 13,898 (35.7) |
| 3 + | 21,378 (17.1) | 3,154 (7.3) | 855 (2.0) | 17,369 (44.6) |
| Multiple birth, n (%) | 1,044 (0.9) | 290 (0.8) | 343 (0.9) | 411 (1.1) |
| Body mass index (BMI) measured (Kg/m2), n (%) | 126,168 | 43,305 | 42,648 | 40,215 |
| < 18.5 | 40,721 (32.3) | 12,962 (29.9) | 16,447 (38.6) | 11,312 (28.1) |
| 18.5–25 | 74,599 (59.1) | 26,614 (61.5) | 23,689 (55.5) | 24,296 (60.4) |
| ≥ 25 | 10,848 (8.6) | 3,729 (8.6) | 2,512 (5.9) | 4,607 (11.5) |
| Hemoglobin, n (%) | 111,811 | 43,306 | 42,626 | 25,879 |
| Very low (< 7 g/dl) | 1,767 (1.6) | 203 (0.5) | 71 (0.2) | 1,493 (5.8) |
| Low (7.0–10.9 g/dl) | 87,206 (78.0) | 31,357 (72.4) | 36,596 (85.9) | 19,253 (74.4) |
| Normal (11.0–12.9 g/dl) | 20,728 (18.5) | 10,357 (23.9) | 5,736 (13.5) | 4,635 (17.9) |
| High (≥ 13 g/dl) | 2,110 (1.9) | 1,389 (3.2) | 223 (0.5) | 498 (1.9) |
| Delivery mode, n (%) | 126,273 | 43,334 | 42,666 | 40,273 |
| Vaginal | 85,006 (67.3) | 27,212 (62.8) | 26,579 (62.3) | 31,215 (77.5) |
| Vaginal, assisted | 546 (0.4) | 261 (0.6) | 98 (0.2) | 187 (0.5) |
| C-section | 27,220 (21.6) | 8,554 (19.7) | 13,118 (30.7) | 5,548 (13.8) |
| Miscarriage/MTP | 13,501 (10.7) | 7,307 (16.9) | 2,871 (6.7) | 3,323 (8.3) |
| Gestational age at delivery, n (%) | 126,273 | 43,334 | 42,666 | 40,273 |
| < 14 weeks (Trimester 1) | 10,313 (8.2) | 5,949 (13.7) | 1,987 (4.7) | 2,377 (5.9) |
| 14–28 weeks (Trimester 2) | 4,952 (3.9) | 1,875 (4.3) | 1,308 (3.1) | 1,769 (4.4) |
| > 28 weeks (Trimester 3) | 111,008 (87.9) | 35,510 (81.9) | 39,371 (92.3) | 36,127 (89.7) |
| Trimester 1 average daily maximum temperature available (for all deliveries), n (%) | 126,273 (100.0) | 43,334 (100.0) | 42,666 (100.0) | 40,273 (100.0) |
| Trimester 2 average daily maximum temperature (for deliveries after 14 weeks), n (%)1 | 115,249 (91.3) | 37,065 (85.5) | 40,511 (94.9) | 37,673 (93.5) |
| Missing due to < 3 datapoints, n | 711 | 320 | 168 | 223 |
| Trimester 3 average daily maximum temperature (for deliveries after 28 weeks), n (%)1 | 110,520 (87.5) | 35,420 (81.7) | 39,238 (92.0) | 35,862 (89.0) |
| Missing due to < 3 datapoints, n | 488 | 90 | 133 | 265 |
Missing trimester average daily maximum temperature if a minimum of 3 datapoints were not available to calculate the value.
Table 2.
Maternal and Infant Outcomes
| Outcomes | Overall | Belagavi | Nagpur | Pakistan |
|---|---|---|---|---|
| Trimester 1 average daily maximum temperature available (Overall) | ||||
| Pregnant women, n | 126,273 | 43,334 | 42,666 | 40,273 |
| Evidence of hypertensive disease/severe pre-eclampsia/eclampsia, n (%) | 3,750 (3.0) | 1,559 (3.6) | 1,094 (2.6) | 1,097 (2.7) |
| Infants, n | 127,366 | 43,624 | 43,020 | 40,722 |
| Stillbirth, n (rate/1000) | 3,401 (29.9) | 816 (22.5) | 830 (20.7) | 1,755 (47.0) |
| Preterm birth, n (%) | 18,618 (16.4) | 4,038 (11.1) | 4,504 (11.2) | 10,076 (27.0) |
| Low birth weight (< 2,500g), n (%) | 25,215 (22.2) | 8,005 (22.1) | 8,108 (20.3) | 9,102 (24.5) |
| Trimester 2 average daily maximum temperature available | ||||
| Pregnant women, n | 115,249 | 37,065 | 40,511 | 37,673 |
| Evidence of hypertensive disease/severe pre-eclampsia/eclampsia, n (%) | 3,744 (3.2) | 1,556 (4.2) | 1,094 (2.7) | 1,094 (2.9) |
| Infants, n | 116,322 | 37,355 | 40,865 | 38,102 |
| Stillbirth, n (rate/1000) | 3,401 (29.9) | 816 (22.5) | 830 (20.7) | 1,755 (47.0) |
| Preterm birth, n (%) | 18,618 (16.4) | 4,038 (11.1) | 4,504 (11.2) | 10,076 (27.0) |
| Low birth weight (< 2,500g), n (%) | 25,215 (22.2) | 8,005 (22.1) | 8,108 (20.3) | 9,102 (24.5) |
| Trimester 3 average daily maximum temperature available | ||||
| Pregnant women, n | 110,520 | 35,420 | 39,238 | 35,862 |
| Evidence of hypertensive disease/severe pre-eclampsia/eclampsia, n (%) | 3,609 (3.3) | 1,505 (4.2) | 1,068 (2.7) | 1,036 (2.9) |
| Infants, n | 111,487 | 35,682 | 39,567 | 36,238 |
| Stillbirth, n (rate/1000) | 2,327 (20.9) | 448 (12.6) | 596 (15.1) | 1,283 (35.4) |
| Preterm birth, n (%) | 16,596 (14.9) | 3,449 (9.7) | 4,079 (10.3) | 9,068 (25.0) |
| Low birth weight (< 2,500g), n (%) | 23,573 (21.2) | 7,451 (20.9) | 7,775 (19.7) | 8,347 (23.0) |
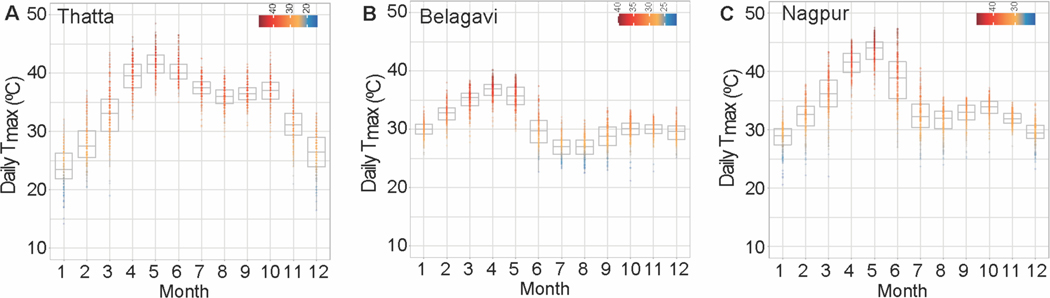
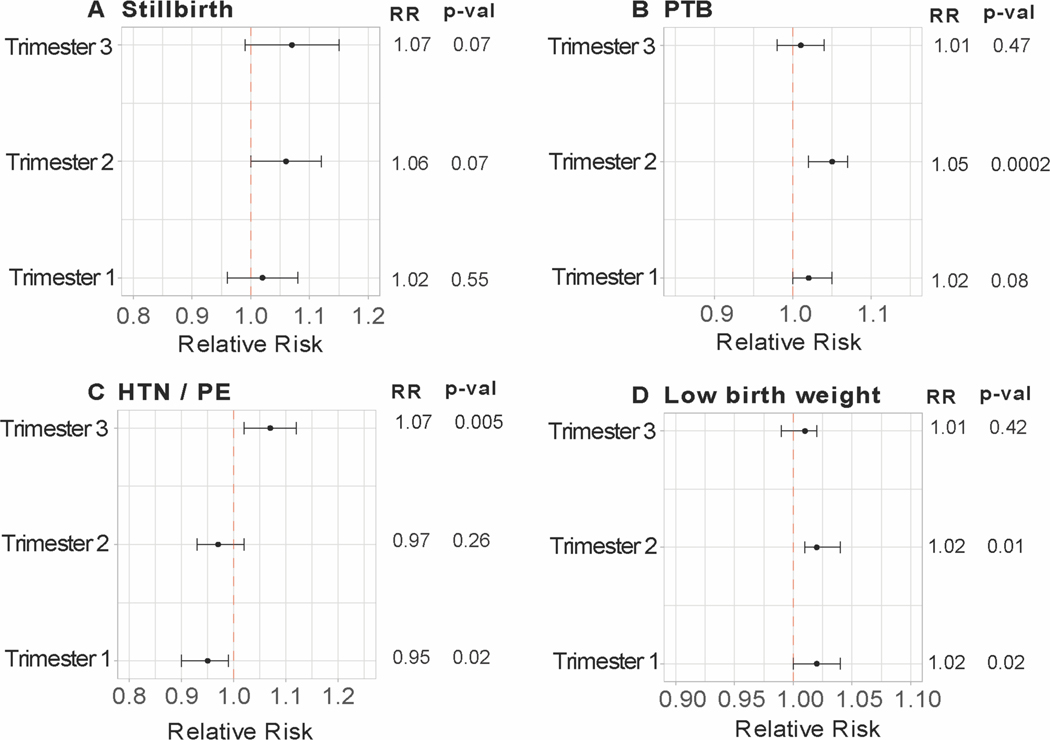
Average maximal temperatures by month for each site are presented in Figure 2. Temperatures in Thatta, Pakistan were highest in the months between April and June (>40°C). Likewise, both Nagpur and Belagavi sites in India also showed high temperatures during these months (Figure 2B–C). Independent associations between heat exposure in each trimester and four outcome variables were assessed via modified Poisson regression for the full cohort, accounting for site as well as including a site by outcome interaction term. Results are described as relative risks associated with a 5°C increase in the trimester average daily Tmax. In the full cohort, the relative risks for stillbirth were not significantly changed with increasing temperature in any trimester. In the second and third trimester, relative risks of SB were 1.06 (CI 1.0 −1.12, p = 0.07) and 1.07 (CI 0.99 −1.15, p = 0.07) (Figure 3A). Site specific analysis for stillbirth was consistent with this finding but indicated much greater relative risk for Belagavi (RR 1.14, CI 1.0 – 1.30, p = 0.05 and RR 1.15, CI 0.97 – 1.36, p = 0.11 in second and third trimesters, respectively) (Figure S1) but did not reach the a priori established p-value cutoff.
Figure 2.
Average daily maximal temperatures for summarized by month for the study duration for the three study sites.
Figure 3.
Relative risks for the incidence of (A) stillbirth; (B) preterm birth; (C) gestational hypertensive disease; and (D) low-birth weight for the combined cohort. Relative risks with corresponding 95% CI and p-values obtained from modified Poisson approach with a sandwich estimator for each categorical outcome and 5-degree Celsius change in trimester average daily maximum temperatures. Models include site and site by outcome interaction. Site-specific relative risks were derived from the site by outcome interaction and are presented in supplementary figures.
The risk of PTB was significantly associated with maximal temperature in the second trimester (RR 1.05, CI 1.02 – 1.07, p = 0.0002) and to a smaller degree with first trimester heat (RR 1.02, CI 1.0 – 1.05, p = 0.08) with all sites combined (Figure 3B). For individual sites, PTB was associated with second trimester heat exposure in Nagpur (RR 1.07, CI 1.03 – 1.11, p = 0.0005), and with first trimester heat exposure in Thatta (RR 1.04, CI 1.01 – 1.06, p = 0.003) (Figure S2). Incidence of HTN/severe PE showed an association with increasing temperature in the third trimester (RR 1.07, CI 1.02 – 1.12, p = 0.005) (Figure 3C). At the individual site level, this association was only significant in Nagpur with a larger effect size (RR 1.13, CI 1.05 – 1.23, p = 0.002). The Belagavi site showed associations of HTN/severe PE with second trimester heat but were above the established significance level (RR 1.11, CI 1.01 – 1.21, p = 0.03) (Figure S3). In the full cohort, incidence of LBW was positively associated with greater heat exposure in the first (RR 1.02, CI 1.0 – 1.04, p = 0.02) and second trimesters (RR 1.02, CI 1.01 – 1.04, p = 0.01) (Figure 3D). These effects were above the significance threshold. In site-specific analysis, LBW incidence was positively associated with heat exposure in the first trimester (RR 1.03, CI 1.01 – 1.06, p = 0.009) in Thatta, while LBW was strongly associated with second trimester heat in Nagpur (RR 1.07, CI 1.04 – 1.10, p < 0.0001)(Figure S4).
DISCUSSION
Main Findings
In this report, we leveraged data from a multi-year community-based health registry to reveal associations between chronic pregnancy-wide exposure to heat and key outcomes for mother and child. The main findings suggest that greater temperatures in pregnancy were associated with increased risk of severe pre-eclampsia, PTB, and low birth weight. These risks were associated with heat exposure in specific pregnancy windows and differed by site which may point to other environmental and physiological factors not studied in this report. Climate change is increasing the frequency, duration, and intensity of heat waves across the globe. A confluence of climate, geography, high population density and occupational exposures places regions in south Asia at particularly high risk to excess heat stress11. In addition to greater mortality, hot weather increases risk of cardiorespiratory diseases, mental health issues, adverse pregnancy outcomes and burdens on the healthcare systems of communities18. Severe heat waves and associated loss of human and livestock have been noted in south Asia over the last two decades. Analysis by the World Weather Attribution group indicated that the disastrous heat wave in 2022 over parts of India and Pakistan was 30-times more likely due to climate change. In combination with prevalent nutritional issues in women of childbearing age, risks to pregnant women and neonates are likely magnified with increasing annual temperatures across the region 19.
Interpretation
Our findings are broadly consistent with previous studies and recent meta-analysis. Most large studies have been conducted in high-income countries and noted greater risk of PTB with increasing ambient temperature. A case-crossover analysis of preterm births in northern California indicated a 11.6% (4.1–19.7) increase in overall PTB per 5.6°C increased temperature20. Analysis of medical records from 12 sites across the continental United States that included 223,375 singleton deliveries also indicated that both acute and chronic ambient temperature extremes increased PTB risk21. Another analysis which included 16 counties in California and 58,000 preterm birth showed 8.6% (6.0 – 11.3) higher risk of PTB for a 5.6°C increase in the weekly average apparent temperature during warm season12. A recent meta-analysis of 70 studies also examined the impact of high temperatures on preterm birth, low birthweight, and stillbirth22. Summary meta-analysis of 6 studies showed a 16% higher risk of preterm birth during heatwave days compared to on non-heatwave days and 1.05 greater odds of PTB for each 1°C increase in temperature. The summary measure of associations between exposure to higher temperatures during a trimester or all of gestation was an odds ratio of 1.14 (95% CI, 1.11 – 1.16). These estimates are in the same direction, albeit larger than those observed in the current study. Of note, no study included in the meta-analysis examining associations with PTB was conducted in LMICs.
The risk of stillbirth in the context of heat exposure has also been examined primarily in high-income countries (Australia, United States, Canada and China). Evidence from a meta-analysis of eight studies suggests that risk of stillbirth increased by 1.05 per 1°C and by 3.39-fold when temperature effects were examined over pregnancy22. A recent report from Ghana utilized district level heat exposure data for 5.9 million births (including 90,000 stillbirths). This analysis also found greater risk of stillbirth with higher-moderate heat stress (75th to 90th percentiles of UTCI values)23. Our study is the first to examine this association in India and Pakistan. While the increased risk of stillbirth with average maximal temperature in the first trimester did not reach statistical significance (p = 0.07), the magnitude was comparable to previous reports. A large proportion of stillbirths among our sites are associated with intrauterine hypoxia and maternal-fetal vascular malperfusion24. Given that heat stress also has potential to alter uterine blood supply and be associated with placenta function25, 26, greater evaluation of stillbirth outcomes is necessary.
Pregnancy is a uniquely vulnerable period for hot ambient conditions. Extreme heat taxes the thermoregulatory system in pregnancy. Heat stress during pregnancy can cause dehydration and counterregulatory hormonal changes such as antidiuretic hormone and oxytocin release which can reduce uterine blood flow and alter fetal metabolism27. In animal studies, heat stress in pregnancy is also known to impact placental development associated with endothelial dysfunction, inflammation and oxidative stress that contribute to placental insufficiency. Thus, greater heat exposure in pregnancy is consistent with increased risk of gestational hypertension and pre-eclampsia. Fewer studies have examined the association between gestational hypertensive disease and heat stress, and none have examined this in south Asia. A large study using a national cohort of ~2 million pregnant women in China found increased risk of gestational hypertension and preeclampsia with higher temperatures in the first half of pregnancy (up to 20 weeks of gestation) but protection with higher temperatures in the preconception period28. Another report from Johannesburg, South Africa indicated greatest association between ambient temperature in the first 3–4 weeks of pregnancy and gestational hypertensive disease, consistent with the effect of heat on early placental development29. A previous report which examined placental gene expression at term also observed global changes in mRNA expression consistent with placental insufficiency with heat stress in early pregnancy25. However, there were major differences in the analytical methodologies, population studied and range of temperature values between the studies that could contribute to discrepancies in findings. Our studies indicated a consistent increase in gestational hypertensive disease risk with increasing temperature in the third trimester within the Indian sites (~11–13% increased risk) even given the variation in temperature exposures.
Strengths and Limitations
The present study’s strengths include a multi-site community-based registry, where women were enrolled without any preconditions. Thus, findings are more likely representative of effects on women in the catchment area. The study also included data over multiple years representing a period when several heat waves and extreme heat events occurred in this region. The registry sites are primarily in rural and semi-rural areas of India and Pakistan. The study population was balanced across sites. The study also contributes to a knowledge-gap about the effects of heat in LMICs. Most previous studies have examined acute effects of excess heat on PTB and stillbirths using case-control crossover designs. The present study analyzed discreet exposure windows in pregnancy and broadly evaluated the long-term exposure to heat stress. Our study also has limitations. The present analysis only utilized daily maximal air temperature as the exposure to ambient heat stress. More physiologically relevant heat exposure measures such as the Universal Thermal Climate Index (UTCI) which take relative humidity and wind speed into account were not utilized. However, since individual level data for exposure were not available, calculation of UTCI may be less meaningful in this context. We also did not calculate the acute impact of heat stress on outcomes or account for correlation of temperatures between trimesters. This might be more relevant to outcomes such as stillbirth and are worthy of deeper analysis using case-cohort designs and lag non-linear models. We also did not incorporate additional environmental variables such as air quality metrics and other measures (home environment, occupational heat exposure) which could amplify or diminish heat responses. However, given the retrospective nature of the analysis some of this information was not available. Lastly, we did not account for regional differences in acclimation. One approach would be to define site-specific temperature extremes based on temperature distributions for each site. However, the focus of the current work was to examine associations through the continuum of temperature values.
Conclusions
The study provides additional evidence to support detrimental influences of heat exposure during pregnancy on important obstetric and neonatal outcomes. The findings suggest that in a low-resource setting, the incidence of pregnancy hypertension/severe pre-eclampsia, preterm birth and low-birth weight are increased in association with ambient heat exposure. These consequences may be inter-related and primary drivers of these outcomes may lie in vascular and placental dysfunction associated with heat stress. Finally given the negative effects on fetal growth, greater heat stress in south Asia may portend an intergenerational legacy of climate-change associated health impacts.
Supplementary Material
ACKNOWLEDGEMENTS
We are thankful for the contributions of the study participants and research teams in India and Pakistan. Funding was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (E.M.M). The funding agency had no role in the design of the present study, nor with collection, analysis, and interpretation of data or in writing the manuscript.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Shindell D, Zhang Y, Scott M, Ru M, Stark K, Ebi KL. The Effects of Heat Exposure on Human Mortality Throughout the United States. Geohealth. 2020. Apr;4(4):e2019GH000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NOAA. Global Climate Report January 2023. National Centers for Environmental Information 2023 [cited 2023 January 2023]; Available from: [Google Scholar]
- 3.Kuehn L, McCormick S. Heat Exposure and Maternal Health in the Face of Climate Change. Int J Environ Res Public Health. 2017. Jul 29;14(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haines A, Ebi K. The Imperative for Climate Action to Protect Health. N Engl J Med. 2019. Jan 17;380(3):263–73. [DOI] [PubMed] [Google Scholar]
- 5.Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Belesova K, Boykoff M, et al. The 2019. report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019 Nov 16;394(10211):1836–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekkar B, Pacheco S, Basu R, DeNicola N. Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA Netw Open. 2020. Jun 1;3(6):e208243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkins-Kirkpatrick SE, Lewis SC. Increasing trends in regional heatwaves. Nat Commun. 2020. Jul 3;11(1):3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer EM, Sippel S, Knutti R. Increasing probability of record-shattering climate extremes. Nat Clim Change. 2021. Aug;11(8):689-+. [Google Scholar]
- 9.Thiery W, Lange S, Rogelj J, Schleussner CF, Gudmundsson L, Seneviratne SI, et al. Intergenerational inequities in exposure to climate extremes. Science. 2021. Oct 8;374(6564):158–60. [DOI] [PubMed] [Google Scholar]
- 10.UNICEF. The coldest year of the rest of their lives. 2022. [Google Scholar]
- 11.Dimitrova A, Ingole V, Basagana X, Ranzani O, Mila C, Ballester J, et al. Association between ambient temperature and heat waves with mortality in South Asia: Systematic review and meta-analysis. Environ Int. 2021. Jan;146:106170. [DOI] [PubMed] [Google Scholar]
- 12.Basu R, Malig B, Ostro B. High ambient temperature and the risk of preterm delivery. Am J Epidemiol. 2010. Nov 15;172(10):1108–17. [DOI] [PubMed] [Google Scholar]
- 13.McClure EM, Garces AL, Hibberd PL, Moore JL, Goudar SS, Saleem S, et al. The Global Network Maternal Newborn Health Registry: a multi-country, community-based registry of pregnancy outcomes. Reprod Health. 2020. Nov 30;17(Suppl 2):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bose CL, Bauserman M, Goldenberg RL, Goudar SS, McClure EM, Pasha O, et al. The Global Network Maternal Newborn Health Registry: a multi-national, community-based registry of pregnancy outcomes. Reprod Health. 2015;12 Suppl 2(Suppl 2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koso-Thomas M, McClure EM, Global Network for Ws, Children’s Health Research I. The Global Network for Women’s and Children’s Health Research: A model of capacity-building research. Semin Fetal Neonatal Med. 2015. Oct;20(5):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparks AH HT, Nelson A. GSODR: Global Summary Daily Weather Data in R. Journal of Open Source Software. 2017;2(10):177. [Google Scholar]
- 17.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2021. [Google Scholar]
- 18.Ebi KL, Capon A, Berry P, Broderick C, de Dear R, Havenith G, et al. Hot weather and heat extremes: health risks. Lancet. 2021. Aug 21;398(10301):698–708. [DOI] [PubMed] [Google Scholar]
- 19.Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JR, et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet. 2019. Feb 23;393(10173):791–846. [DOI] [PubMed] [Google Scholar]
- 20.Basu R, Chen H, Li DK, Avalos LA. The impact of maternal factors on the association between temperature and preterm delivery. Environ Res. 2017. Apr;154:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha S, Liu D, Zhu Y, Kim SS, Sherman S, Mendola P. Ambient Temperature and Early Delivery of Singleton Pregnancies. Environ Health Perspect. 2017. Mar;125(3):453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chersich MF, Pham MD, Areal A, Haghighi MM, Manyuchi A, Swift CP, et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ. 2020. Nov 4;371:m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyadanu SD, Tessema GA, Mullins B, Kumi-Boateng B, Ofosu AA, Pereira G. Prenatal exposure to long-term heat stress and stillbirth in Ghana: A within-space time-series analysis. Environ Res. 2023. Apr 1;222:115385. [DOI] [PubMed] [Google Scholar]
- 24.McClure EM, Saleem S, Goudar SS, Tikmani SS, Dhaded SM, Hwang K, et al. The causes of stillbirths in south Asia: results from a prospective study in India and Pakistan (PURPOSe). Lancet Glob Health. 2022. Jul;10(7):e970–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankar K, Ali SA, Ruebel ML, Jessani S, Borengasser SJ, Gilley SP, et al. Maternal nutritional status modifies heat-associated growth restriction in women with chronic malnutrition. PNAS Nexus. 2023. Jan;2(1):pgac309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonell A, Sonko B, Badjie J, Samateh T, Saidy T, Sosseh F, et al. Environmental heat stress on maternal physiology and fetal blood flow in pregnant subsistence farmers in The Gambia, west Africa: an observational cohort study. Lancet Planet Health. 2022. Dec;6(12):e968–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels L, Nakstad B, Roos N, Bonell A, Chersich M, Havenith G, et al. Physiological mechanisms of the impact of heat during pregnancy and the clinical implications: review of the evidence from an expert group meeting. Int J Biometeorol. 2022. May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong T, Chen P, Mu Y, Li X, Di B, Li J, et al. Association between ambient temperature and hypertensive disorders in pregnancy in China. Nat Commun. 2020. Jun 10;11(1):2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Part C, le Roux J, Chersich M, Sawry S, Filippi V, Roos N, et al. Ambient temperature during pregnancy and risk of maternal hypertensive disorders: A time-to-event study in Johannesburg, South Africa. Environ Res. 2022. Sep;212(Pt D):113596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.