Abstract
Background:
Smoking is a well-established risk factor for MS; however, it is not known whether its effect on disease risk varies by race/ethnicity.
Methods:
We conducted a nested case-control study among US military personnel who have serum samples stored at the Department of Defense Serum Repository. We measured serum cotinine levels, a marker of tobacco smoke exposure, in 157 Black and 23 White individuals who developed MS during follow-up. Controls were randomly selected and matched to each case by age, sex, race/ethnicity, dates of sample collection, and branch of military service.
Results:
Smoking was not associated with an increased risk of MS in Black people (RR: 1.08, 95% CI: 0.63–1.85). The results remained similar in analyses restricted to smoking status at baseline, to samples collected 5 years before symptom onset, and using different cut-off levels in cotinine to define smoking status. Smoking was not statistically significantly associated with MS risk in White people, but the point estimate was similar to what has previously been reported in other studies (RR: 1.85, 95% CI: 0.56–6.16).
Conclusions:
Smoking was not associated with MS risk in Black people. Given the consistent association between smoking and MS risk in predominantly White populations, this may suggest that the association between smoking and MS varies by race/ethnicity.
Keywords: multiple sclerosis, smoking, cotinine, epidemiology
1. INTRODUCTION
Multiple sclerosis (MS), an inflammatory disorder of the central nervous system, affects more than 2.8 million people world wide and is an important cause of neurological disability in young adults.1 Over the last five decades, the incidence of MS has been relatively stable in White people, but recent studies have reported an increased incidence in Black people.2–4 In a longitudinal study assessing trends in MS incidence by race and ethnicity over time, there was a steady increase in MS risk in both Black men and Black women across a 60-year period, and in the most recent assessment, MS risk was higher in Black people than in White people, the opposite of what was reported in the earliest assessment.3 It is unclear what underlies this increase in incidence among Black people, but where it has occurred over a relatively short period of time, changes in exposure to environmental risk factors may play a role.
Smoking is one environmental risk factor that has consistently been associated with a 50% increase in MS risk.5 Further, it has been estimated that smoking avoidance could prevent 8% of all MS cases.6 However, this evidence is built on studies including predominantly White populations; thus, it remains unclear whether the association varies by race and ethnicity, and may potentially help explain the increase in MS incidence among Blacks.
To address this, we conducted a study aiming to evaluate whether smoking was associated with an increased MS risk in a racially diverse and well-characterized cohort of young adults.
2. MATERIAL AND METHODS
2.1. Study design and population
This is a nested case-control study in a cohort comprised of more than 10 million active-duty US military personnel with at least one serum sample stored at the Department of Defense Serum Repository. Samples are collected from active-duty personnel at entry into the military and, on average, every 2 years thereafter. All samples are stored at −30ºC and linked with the Defense Medical Surveillance System, which provides individual-level demographic and health data.7, 8
2.2. Standard protocol approvals, registrations, and patient consent
The research protocol was approved by the institutional review boards of the Harvard T. H. Chan School of Public Health and the Uniformed Services University of the Health Sciences, both of which waived the informed consent for the use of existing biological samples and medical records data.
2.3. Case and control ascertainment
We identified individuals who received a diagnosis of MS while being on active duty as previously described.8 Briefly, medical records were reviewed, and an MS diagnosis was confirmed if made by a neurologist in patients with a clinical history of 2 or more relapses and evidence of lesions on MRI. Controls were randomly selected using risk-set sampling (i.e., the controls were at risk of becoming a case at the time the case they were matched to developed MS) and matched to each case by age (±1 year), sex, race and ethnicity (non-Hispanic White and non-Hispanic Black), dates of sample collection (±30 days), and branch of military service (Army, Air Force, Navy, or Marines). Information on race and ethnicity was provided by the Armed Forces Health Surveillance Division, based on categories defined by the Department of Defense. The sample size was determined based on available funding, with priority given to assessments of samples from Black individuals.
2.4. Laboratory Analyses
We measured the nicotine metabolite cotinine, a marker of recent tobacco exposure,9 in pre-symptomatic samples by enzyme-linked immunosorbent assay (Calbiotech Inc. Spring Valley, CA). Cut-off values in serum cotinine used to classify individuals as current smokers ranged from 3 to 20 ng/mL in previous studies, yielding a sensitivity of 73.2% to 98.8% and a specificity of 78.7% to 99%.10 To minimize the risk of misclassification of smoking status, we used 25ng/mL as the cut-off in our main analyses, as done in previous analyses.11 Individuals with cotinine levels above 25 ng/mL in any pre-symptomatic samples were defined as ever-smokers. In addition, a dose-response for smoking intensity prior to MS was defined by three levels according to the number and time points in which samples have reached the 25ng/mL threshold: never smokers (no samples), previous smokers (earliest sample only), and current smokers (earliest and latest samples). Serum 25 hydroxyvitamin D levels and EBV VCA IgG seropositivity were measured at the earliest sample, as previously described.8, 12
2.5. Statistical analyses
We used conditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between smoking status, as defined by cotinine levels, and MS risk, stratified by race. Since controls were selected using risk-set sampling, the ORs are unbiased estimates of rate ratios (RRs).13 As these analyses are conducted within strata of case-control sets, they are adjusted by matching factors (age, sex, race and ethnicity, dates of sample collection, and branch of military service) by design. We first compared ever-smokers to never-smokers (model 1) and then further categorized smoking status into previous (cotinine >25ng/mL in the first and <25ng/mL in the last sample) or current smokers (cotinine >25ng/mL in both samples) (model 2). In further analyses, we also additionally adjusted for serum 25-hydroxyvitamin D levels in quartiles. To test for interaction between smoking and MS risk by race/ethnicity on the multiplicative scale, we included a product term between ever smoking and race/ethnicity in a logistic regression model adjusted by the matching factors. Further, to test for interaction on the additive scale, we estimated the relative excess risk due to interaction (RERI) based on the delta method.14
We conducted sensitivity analyses using a lower (13 ng/mL) cotinine threshold to account for possible differential metabolic rates of cotinine in Black people15, 16 and estimated the average cotinine levels at all time points to reduce the possible influence of random variation in individual measurements and evaluated their association with MS risk using 25 ng/mL as cut-off (model 3; average levels above or below cut-off). We also evaluated whether a lower cut-off in cotinine levels to define non-smokers (10 ng/mL vs. 25 ng/mL), which is less likely to misclassify individuals as non-smokers, affected the results (model 4) and tested whether smoking status at baseline, which could be a marker of prolonged exposure since the samples were collected at an earlier age, were associated with MS risk (model 5). To further evaluate
the robustness of the results, we restricted baseline cotinine measurements to samples collected more than 2 years and 5 years before symptom onset to minimize the risk of confounding by preclinical disease activity (reverse causation), such as changes in smoking behavior that might have occurred during the prodromal phase of the disease.
We used median imputation for missing values of 25-hydroxyvitamin D and cotinine in subjects with at least one measured level. In total, cotinine levels were imputed for 4 individuals (three Black individuals and one White individual). All analyses were conducted in R version 4.3 (the R Foundation) using the survival package. The figure was made using the ggplot2 package. The alpha level was set at .05 and all tests were 2-sided.
2.6. Data availability
The data that support the findings of this study are not publicly available due to restricted access, but the statistical code used can be found at the Harvard Dataverse,17 and further information is available from the corresponding author upon reasonable request.
3. RESULTS
Descriptive characteristics were similar in cases and controls, as expected from the matching procedure (Table 1). Minor differences between Black cases and controls are related to the different number of controls in matched sets (129 sets were matched 1:1, while 28 sets were matched 1:2). There was a higher proportion of females among Black people than among White people.
Table 1:
Descriptive table of baseline demographic characteristics of study population by MS diagnosis
| non-Hispanic Black |
non-Hispanic White |
|||
|---|---|---|---|---|
| Characteristics | Cases (n=157) | Controls (n=185)* | Cases (n=23) | Controls (n=46) |
| Sex - n (%) | ||||
| Male | 80 (50.96) | 91 (49.19) | 19 (82.61) | 38 (82.61) |
| Female | 77 (49.04) | 94 (50.81) | 4 (17.39) | 8 (17.39) |
| Age, years - median (IQR) | ||||
| First serum sample collection | 19 (18–23) | 20 (18–23) | 22 (20–28) | 21.5 (20–28) |
| Second serum sample collection** | 28 (23–33) | 28 (23–33) | 28 (25–34) | 28 (25–34) |
| MS onset | 28 (23–33) | NA | 28 (24–35) | NA |
| Baseline vitamin D levels, nmol/L – median (IQR) | 45 (33–64) | 48 (37–63) | 72 (63–89) | 82 (68–97) |
| Baseline EBV VCA IgG seropositivity – n (%) | 153 (98.08) | 180 (98.90) | 22 (95.65) | 42 (91.30) |
129 cases were matched on 1:1 basis whereas 28 cases on a 1:2
Individuals with 2 pre-symptomatic samples – unavailable for 12 Black cases and 17 controls, 9 White cases and 18 controls
Abbreviations: EBV: Epstein-Barr Virus, IQR: Inter Quartile Range, NA: not applicable
At baseline, 24/185 (13%) of the Black controls had cotinine levels >25ng/ml, while 23/157 (15%) of those who developed MS were smokers (Figure 1). In comparison, smokers comprised 14/46 (30%) of the White controls and 8/23 (35%) of the White cases. During follow-up, we observed an increase in the proportion of Black smokers in both cases (32/145 [22%]) and controls (35/168 [21%]). In contrast, there was a decrease in White smokers among controls (7/28 [25%]) and an increase among cases (7/14 [50%]).
Figure 1:
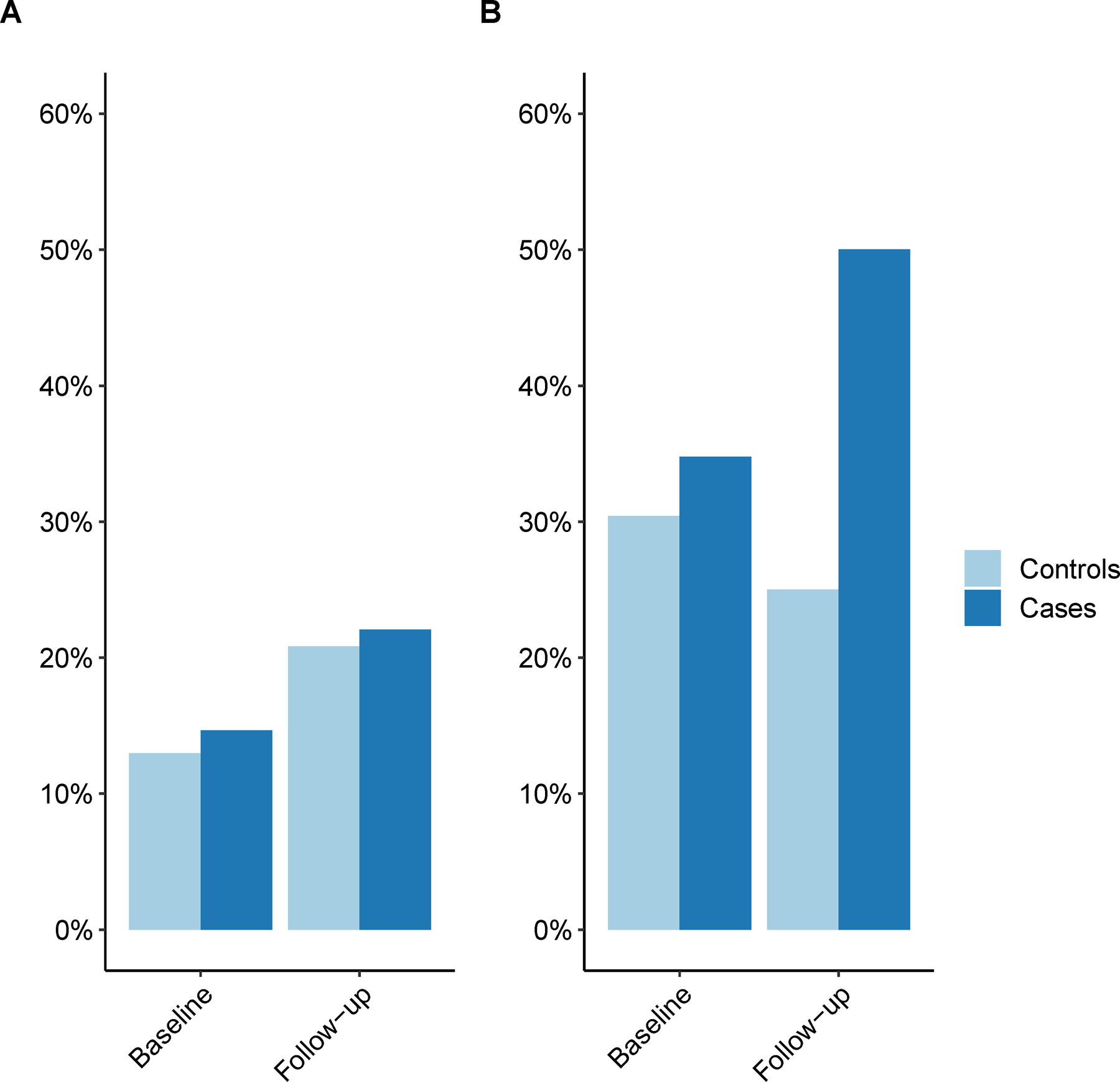
Proportion of smokers in cases and controls using a 25ng/mL cotinine threshold. A. Black people, B. White people
We found no association between pre-symptomatic smoking status and MS risk in Black people (RR: 1.08, 95% CI: 0.63–1.85, p=0.78) (Table 2; model 1). The results were consistent when further adjusted for 25-hydroxyvitamin D levels (RR: 1.09, 95% CI: 0.63–1.90, p=0.75) and lowering the cotinine threshold to 13ng/mL (RR: 0.93, 95% CI: 0.55–1.57, p=0.79). Further, the results remained similar in analyses of average cotinine levels, both when using 25 ng/mL (model 3) and 10 ng/mL (model 4) as the cut-off in cotinine levels to classify individuals as non-smokers and when restricting to baseline cotinine measurements to define smoking status (Table2; model 5). Consistent results were obtained after further restricting baseline cotinine measurements to samples collected 2 years (RR: 1.47, 95% CI: 0.73–2.98, p=0.29) and 5 years (RR: 1.2, 95% CI: 0.48–2.98, p=0.70) before MS onset.
Table 2:
Association between pre-symptomatic smoking status and MS diagnosis
| non-Hispanic Black |
non-Hispanic White |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | RR (95% CI) | p-value | Cases | Controls | RR (95% CI) | p-value | |
| Model 1 | ||||||||
| Ever smoker | 157 | 185 | 1.08 (0.63–1.85) | 0.78 | 23 | 46 | 1.85 (0.56 – 6.16) | 0.31 |
| Model 2 | ||||||||
| Previous smoker vs Never smoker | 131 | 149 | 1.67 (0.37 – 7.64) | 0.51 | 11 | 26 | NA | NA |
| Current smoker vs Never Smoker | 1.19 (0.55 – 2.55) | 0.66 | 2.41 (0.41 – 14.02) | 0.33 | ||||
| Model 3 | ||||||||
| Average serum cotinine (>25ng/mL vs ≤25ng/mL) | 157 | 185 | 0.99 (0.57 – 1.70) | 0.96 | 23 | 46 | 1.77 (0.67 – 4.66) | 0.25 |
| Model 4 | ||||||||
| Average serum cotinine (>25ng/mL vs <10ng/mL) | 151 | 177 | 0.89 (0.51–1.58) | 0.70 | 22 | 43 | 1.73 (0.62–4.82) | 0.30 |
| Model 5 | ||||||||
| Baseline smoker | 157 | 185 | 1.13 (0.60 – 2.16) | 0.70 | 23 | 46 | 1.24 (0.41 – 3.73) | 0.71 |
Abbreviations: CI: Confidence Interval, RR: Rate Ratio, NA: not applicable.
Individuals with cotinine levels above 25 ng/mL in any pre-symptomatic samples were defined as ever-smokers. Additional levels according to the time points in which samples that have reached the 25ng/mL threshold: never smokers (no samples), previous smokers (earliest sample only), and current smokers (earliest and latest samples). Baseline smokers have reached the 25ng/mL threshold at their earliest sample, but no conditions were applied for follow-up samples.
Models were adjusted by matching variables (age, sex, race and ethnicity, dates of sample collection, and branch of military service) by design.
Among White people, smoking was not associated with a statistically significantly increased MS risk, although the point estimate was similar to what has previously been observed in other studies (RR: 1.85, 95% CI: 0.56–6.16, p=0.31; Table 2). In the analysis comparing current smokers to never smokers, the RR was even higher (RR: 2.41, 95% CI: 0.41–14.02, p=0.33), but the estimate was not statistically significant (Table 2; model 2). The point estimates remained similar in the remaining models (Table 2; models 3, 4, and 5). We did not find a significant interaction between race and smoking status, neither when testing for interaction on the multiplicative (p=0.47) nor the additive (p=0.75) scale.
4. DISCUSSION
In this study, we did not find an association between smoking and MS risk in Black people. The results remained similar when adjusting for potential confounders, when considering different time intervals to MS onset, and using different cut-offs in cotinine levels to define smoking status. While the modest sample size affected the statistical power to detect a statistically significant association between smoking and MS risk in our study, we would have expected to observe a point estimate closer to what has previously been reported in White people if a true effect was present.5, 18 Thus, our results may be consistent with a differential effect of smoking on MS risk in Black and White people.
While the association between smoking and MS risk was first reported almost 60 years ago and later has been found in numerous investigations, largely in White populations, the underlying mechanisms by which smoking contributes to causing the disease remain unknown.19, 20 However, the well-established association between tobacco use and the risk of other systemic autoimmune disorders, such as rheumatoid arthritis, systemic lupus erythematosus, and Crohn’s disease, supports a direct biological effect on the immune system.6, 21 A variety of mechanisms have been proposed and include direct NF-IκB kinase inhibition, reduction of histone acetylation, and Toll-like receptor activation secondary to oxidative stress.22 In the experimental autoimmune encephalomyelitis model, the lungs have been implicated as an activation site for myelin basic protein reactive T cells enabling their migration to the CNS.23 Although speculative, it is possible that pro-inflammatory changes in the lung parenchyma due to cigarette smoking may increase the likelihood of autoreactive T cell activation.22
It is unclear what underlies the seemingly differential effect of smoking in Black and White people in our study. Serum cotinine levels have been reported to be higher per cigarette smoked in Black than in White people, possibly due to slower clearance and higher intake per cigarette;15 still, nicotine itself, which cotinine is a metabolite of, does not seem to increase MS risk and may even be neuroprotective.24 Further, the metabolism, and therefore also likely the adverse effects, of other substances in tobacco may be independent of nicotine metabolism.
Our study has several strengths. The nested design minimizes selection bias, a usual concern in case-control studies. Additionally, as all samples were handled in a similar manner, with matched case-control sets measured in the same assay run, and analyzed blindly, measurement bias is unlikely. We also used serum cotinine as an objective measure of smoking, which avoids relying on individuals’ reporting of a socially undesirable habit that may be prone to underreporting. Our study also has some limitations. First, the modest sample size affected the power of our analyses and the stability of the point estimates, thus resulting in wide confidence intervals that are compatible with an adverse effect of smoking. Still, the fact that approximately the same number of MS cases and control among Black people were defined as smokers is more supportive of the hypothesis that smoking does not affect MS risk among Black people than the alternative hypothesis that smoking increases MS risk among Black people. Second, the limited number of White individuals with cotinine measurements also likely affected our power to test for interactions between races and smoking. Third, since cotinine is a measure of recent tobacco smoking, we may have missed previous smokers or individuals who only occasionally smoke, thus misclassifying smoking status in some individuals. Lastly, we cannot exclude that our results may be affected by residual or unmeasured confounding.
In this study, smoking was not associated with MS risk in Black people. Given the consistent association between smoking and MS risk in previous studies that predominantly included White individuals, our results may be consistent with a differential effect of smoking on MS risk in Black and White people. Further studies, with larger sample sizes, are needed to confirm these findings.
Highlights.
Smoking is a well-established risk factor for multiple sclerosis
Smoking was not associated with multiple sclerosis risk in Black people
This may suggest that the association between smoking and MS varies by race/ethnicity
Funding
This study was supported by National Institute of Neurologic Disorders and Stroke (R01 NS103891) awarded to Dr. Ascherio.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: V.A. Schoeps, D.W. Niebuhr, X. Peng, J.D. Mancuso, and K. Bjornevik have nothing to disclose. M. Cortese reports having received a speaking honorarium from Roche. K.L. Munger is an employee of Biogen and holds stock in the company. A. Ascherio reports having received speaking honoraria from Biogen, GlaxoSmithKline, Merck, Moderna, Prada Foundation, Roche, and Web MD.
Disclaimer
The views expressed in this article are those of the authors and do not reflect official policy or position of the Uniformed Services University of the Health Sciences or the US Department of Defense.
Foot note
The abstract of this paper was presented at the 38th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 26–28 Oct 2022, Amsterdam, NL.
References
- 1.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler 2020; 26: 1816–1821. 2020/11/12. DOI: 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer-Gould A, Brara SM, Beaber BE and Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 2013; 80: 1734–1739. 2013/05/08. DOI: 10.1212/WNL.0b013e3182918cc2. [DOI] [PubMed] [Google Scholar]
- 3.Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain : a journal of neurology 2012; 135: 1778–1785. 2012/05/26. DOI: 10.1093/brain/aws099. [DOI] [PubMed] [Google Scholar]
- 4.Wallin MT, Culpepper WJ, Nichols E, et al. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology 2019; 18: 269–285. DOI: 10.1016/s1474-4422(18)30443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handel AE, Williamson AJ, Disanto G, et al. Smoking and multiple sclerosis: an updated meta-analysis. PLoS One 2011; 6: e16149. 2011/01/21. DOI: 10.1371/journal.pone.0016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascherio A and Munger KL. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention-An Update. Semin Neurol 2016; 36: 103–114. 2016/04/27. DOI: 10.1055/s-0036-1579693. [DOI] [PubMed] [Google Scholar]
- 7.Russell KL. The Department of Defense Serum Repository (DoDSR): A Study of Questions. Mil Med 2015; 180: 1–2. 2015/10/09. DOI: 10.7205/MILMED-D-15-00099. [DOI] [PubMed] [Google Scholar]
- 8.Levin LI, Munger KL, Rubertone MV, et al. Temporal Relationship Between Elevation of Epstein-Barr Virus Antibody Titers and Initial Onset of Neurological Symptoms in Multiple Sclerosis. JAMA 2005; 293: 2496–2500. DOI: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- 9.Vine MF, Hulka BS, Margolin BH, et al. Cotinine concentrations in semen, urine, and blood of smokers and nonsmokers. Am J Public Health 1993; 83: 1335–1338. 1993/09/01. DOI: 10.2105/ajph.83.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S Overview of Cotinine Cutoff Values for Smoking Status Classification. Int J Environ Res Public Health 2016; 13 2016/12/17. DOI: 10.3390/ijerph13121236. [DOI] [PMC free article] [PubMed]
- 11.Cortese M, Munger KL, Martinez-Lapiscina EH, et al. Vitamin D, smoking, EBV, and long-term cognitive performance in MS: 11-year follow-up of BENEFIT. Neurology 2020; 94: e1950–e1960. 2020/04/18. DOI: 10.1212/WNL.0000000000009371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama 2006; 296: 2832–2838. DOI: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 13.Knol MJ, Vandenbroucke JP, Scott P and Egger M. What do case-control studies estimate? Survey of methods and assumptions in published case-control research. Am J Epidemiol 2008; 168: 1073–1081. 2008/09/17. DOI: 10.1093/aje/kwn217. [DOI] [PubMed] [Google Scholar]
- 14.Mathur MB and VanderWeele TJ. R Function for Additive Interaction Measures. Epidemiology 2018; 29: e5–e6. 2017/09/14. DOI: 10.1097/EDE.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-Stable EJ, Herrera B, Jacob P, 3rd and Benowitz NL. Nicotine metabolism and intake in black and white smokers. Jama 1998; 280: 152–156. 1998/07/21. DOI: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 16.Tompkins EL, Beltran TA and Bedno SA. Differentiating between smokers and nonsmokers using serum cotinine. Biomark Med 2019; 13: 1025–1033. 2019/08/07. DOI: 10.2217/bmm-2019-0027. [DOI] [PubMed] [Google Scholar]
- 17.Schoeps V Statistical code for: Smoking and Multiple Sclerosis risk in Black people: a nested case-control study. Ed.: Harvard Dataverse, 2023. doi: 10.7910/DVN/E2ZN7B [DOI] [PMC free article] [PubMed]
- 18.Degelman ML and Herman KM. Smoking and multiple sclerosis: A systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult Scler Relat Disord 2017; 17: 207–216. 2017/10/23. DOI: 10.1016/j.msard.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers 2018; 4: 43. 2018/11/10. DOI: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 20.Antonovsky A, Leibowitz U, Smith HA, et al. Epidemiologic Study of Multiple Sclerosis in Israel: I. An Overall Review of Methods and Findings. Archives of Neurology 1965; 13: 183–193. DOI: 10.1001/archneur.1965.00470020073010. [DOI] [PubMed] [Google Scholar]
- 21.Perricone C, Versini M, Ben-Ami D, et al. Smoke and autoimmunity: The fire behind the disease. Autoimmun Rev 2016; 15: 354–374. 2016/01/17. DOI: 10.1016/j.autrev.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Alrouji M, Manouchehrinia A, Gran B and Constantinescu CS. Effects of cigarette smoke on immunity, neuroinflammation and multiple sclerosis. J Neuroimmunol 2019; 329: 24–34. 2018/10/27. DOI: 10.1016/j.jneuroim.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Odoardi F, Sie C, Streyl K, et al. T cells become licensed in the lung to enter the central nervous system. Nature 2012; 488: 675–679. 2012/08/24. DOI: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 24.Hedstrom AK, Hillert J, Olsson T and Alfredsson L. Nicotine might have a protective effect in the etiology of multiple sclerosis. Mult Scler 2013; 19: 1009–1013. 2013/01/16. DOI: 10.1177/1352458512471879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to restricted access, but the statistical code used can be found at the Harvard Dataverse,17 and further information is available from the corresponding author upon reasonable request.


