Abstract
Objective:
Medication for opioid use disorder (MOUD) improves treatment retention and reduces illicit opioid use. A-CHESS is an evidence-based smartphone intervention shown to improve addiction-related behaviors. We tested the efficacy of MOUD-alone versus MOUD plus A-CHESS to determine whether the combination further improved outcomes.
Methods:
In an unblinded parallel-group randomized controlled trial, 414 participants recruited from outpatient programs were assigned 1:1 to receive MOUD-only or MOUD+A-CHESS for 16 months and followed an additional 8 months. All participants were on methadone, buprenorphine, or injectable naltrexone. The primary outcome was abstinence from illicit opioid use; secondary outcomes were treatment retention, health services use, other substance use, and quality of life; moderators were MOUD type, gender, loneliness, pain severity, and withdrawal symptoms severity. Data sources were surveys comprising multiple validated scales and urine screens every 4 months.
Results:
There was no difference in abstinence between participants using MOUD+A-CHESS versus MOUD-only over time (OR=1.10, 95% CI 0.90–1.33, p=.35). However, abstinence was moderated by withdrawal symptoms severity (OR 0.95, 95% Cl 0.91–1.00, p=.047) and MOUD type (OR 0.57, 95% Cl 0.34–0.97; p=.039). Among participants without withdrawal symptoms, abstinence increased more for those receiving MOUD+A-CHESS versus MOUD-only (OR 1.30, 95% Cl 1.01–1.67, p=.039). Among participants taking methadone, MOUD+A-CHESS patients were more likely to be abstinent over time (b= 0.28, SE=0.09, p=.003) than MOUD-only patients (b= 0.06, SE=0.08, p=.48), although the two groups were not significantly different from each other (∆b=0.22, SE=0.11, p=.053). MOUD+A-CHESS was also associated with greater meeting attendance (OR 1.25, 95% Cl 1.05–1.49, p=.014) and decreased emergency department and urgent care use (OR 0.88, 95% Cl 0.78–0.99, p=.034).
Conclusions:
Overall, MOUD+A-CHESS did not improve abstinence relative to MOUD alone. However, MOUD+A-CHESS may provide benefits for subsets of patients and may impact treatment utilization.
Keywords: mHealth, addiction, opioids, opioid use disorder, medications for opioid use disorder, MOUD, methadone, buprenorphine, smartphone
INTRODUCTION
Background
The incidence of opioid use disorder (OUD) has risen steeply in recent decades, with devastating consequences for patients, families, and communities. In 2020, an estimated 3 million Americans had OUDs (1), an increase of about 50% in 10 years (2). U.S. emergency department visits related to the nonmedical use of opioids reached 285,000 in 2020 (3), and 68,630 deaths resulted from opioid overdose (4). The Centers for Disease Control and Prevention estimates that in 2021 the number of opioid-related deaths surpassed 80,000, an increase of 17% in a single year.(5)
For those with OUDs, access to treatment is a challenge, with only about 10% of patients who need treatment receiving it.(6) Worse, treatment often fails. Following detoxification from opioid dependence, relapse rates are high (7); even after inpatient treatment the majority of patients relapse within a year.(8) In the U.S., three medications for opioid use disorder (MOUD) are approved for treatment: methadone, buprenorphine, and naltrexone.(9) Along with other supportive services, such as peer support, MOUD has been shown to increase rates of recovery from OUD.(9) However, most patients who receive MOUD treatment do not achieve long-term, stable abstinence.(10) While reductions in use and mortality risk are desirable real-world patient outcomes, abstinence is an FDA-recommended clinical outcome to evaluate treatments for substance use disorders including opioids.(11,12) It is a stable indicator of longer-term outcomes (13), it can be biologically confirmed via urine drug screening, and it facilitates the use of intent-to-treat analyses that include all participants randomized to treatment.
The randomized clinical trial (RCT) described here assessed the extent to which MOUD effectiveness might be improved by A-CHESS, the Addiction-treatment version of the Comprehensive Health Enhancement Support System. A-CHESS is an evidence-based smartphone intervention designed to assist recovery from substance use disorders (SUDs) with a suite of motivational, social support, and coping tools. A large (N=349) randomized controlled trial previously found that A-CHESS decreased risky drinking days and enhanced long-term abstinence among people with alcohol use disorder leaving residential treatment, one-third of whom reported illicit opioid use.(14) Related field tests in drug courts (15), Federally Qualified Health Centers (16), and among women in Appalachia (17) also found positive outcomes for alcohol and opioid use.
Study Objectives
In the current trial, we assessed the potential of A-CHESS to improve long-term outcomes of MOUD among participants with OUD. The primary hypothesis was that participants receiving MOUD plus A-CHESS would achieve a higher probability of abstinence from illicit opioid use (i.e., no days of illicit use) than participants receiving MOUD alone. Our secondary hypotheses were that those assigned to MOUD+A-CHESS would show less use of other illicit substances, higher quality of life, greater retention in opioid treatment, and lower health services use compared to MOUD-only. We tested MOUD type, gender, withdrawal symptoms severity, pain severity, and loneliness as moderators to the impact of MOUD+A-CHESS versus MOUD-only. The study variables were pre-specified in the protocol.(18) 1
Additional secondary hypotheses related to human immunodeficiency virus and hepatitis C virus are addressed elsewhere.(19) We also tested A-CHESS use and communication style patterns as predictors of outcomes, and will report these findings separately.
METHODS
Trial Design
In this nonblinded parallel-group randomized controlled trial, 414 participants with OUD were assigned 1:1 to receive either MOUD+A-CHESS or MOUD-only for 16 months and were followed for an additional 8 months post-intervention.
Participants
Participants were eligible if they were currently on MOUD; were 18 or older; met DSM-5 criteria for OUD of at least moderate severity (4 or higher) in the last 12 months; had no acute medical problems requiring immediate inpatient treatment; had no history of psychotic disorders; were willing to participate; could provide two verified contacts as locators, if necessary; could read and write in English; agreed to share health-related data with primary care clinicians; and were, at study intake, abstinent from illicit opioids for at least 1 week and no longer than 4 months.
Patients were recruited from outpatient detoxification and treatment programs at two sites in Massachusetts and one in Wisconsin. Potential participants were identified by a site staff person and asked if they were interested in learning about the study. If yes, the UW study coordinator or site coordinator provided a detailed overview, including participant responsibilities and confidentiality protections. Interested participants then gave written consent and completed a baseline survey. Race/ethnicity information was collected via self-identification. Participants also self-identified gender as male or female or could decline to respond; we did not ask for biological sex. Last, participants were randomized to receive MOUD-only or MOUD+A-CHESS.
Interventions
MOUD-only.
Participants in the control arm received methadone, buprenorphine, or injectable naltrexone and treatment as usual at each site. This could include a recovery plan, behavioral interventions such as group counseling, and sessions with a substance use counselor. Sequence and duration of medication and behavioral interventions varied by patient.
MOUD+A-CHESS.
Participants in the experimental arm received A-CHESS for 16 months along with their MOUD. As described previously (18), A-CHESS services are based on self-determination theory constructs of intrinsic motivation, social support, and coping competence (20) to address numerous determinants and antecedents of relapse. For a complete description of app features, see the online supplement, including Figure S1.
MOUD+A-CHESS participants who did not have an Android smartphone were given one loaded with the app, along with a data plan for the 16-month intervention period. Participants who already had a compatible Android smartphone had A-CHESS installed and were compensated more for each survey they completed. We provided up to one replacement phone, if needed. If participants lost a second phone, we offered to load A-CHESS onto an appropriate smartphone they obtained. Data plans were terminated after 16 months, but participants could continue to access A-CHESS via other connectivity.
The UW or site coordinator trained participants to use A-CHESS and customize it with, for example, sources of support, high-risk locations to avoid, and recovery motivations. App content was refreshed monthly with healthy activities, local AA/NA meetings, and clinic schedules for group sessions. Participants demonstrated they could use A-CHESS before leaving training.
Study Variables and Measures
For assessing outcomes and other variables, participants were asked to complete phone surveys (~30 minutes) with the UW study coordinator at baseline and months 4, 8, 12, and 16, and post-intervention at months 20 and 24. Survey measures used for quantitative data collection are described below. In addition, results from urine screens were recorded at baseline and all subsequent surveys, if possible. The study logic is presented in Figure 1.
Figure 1.
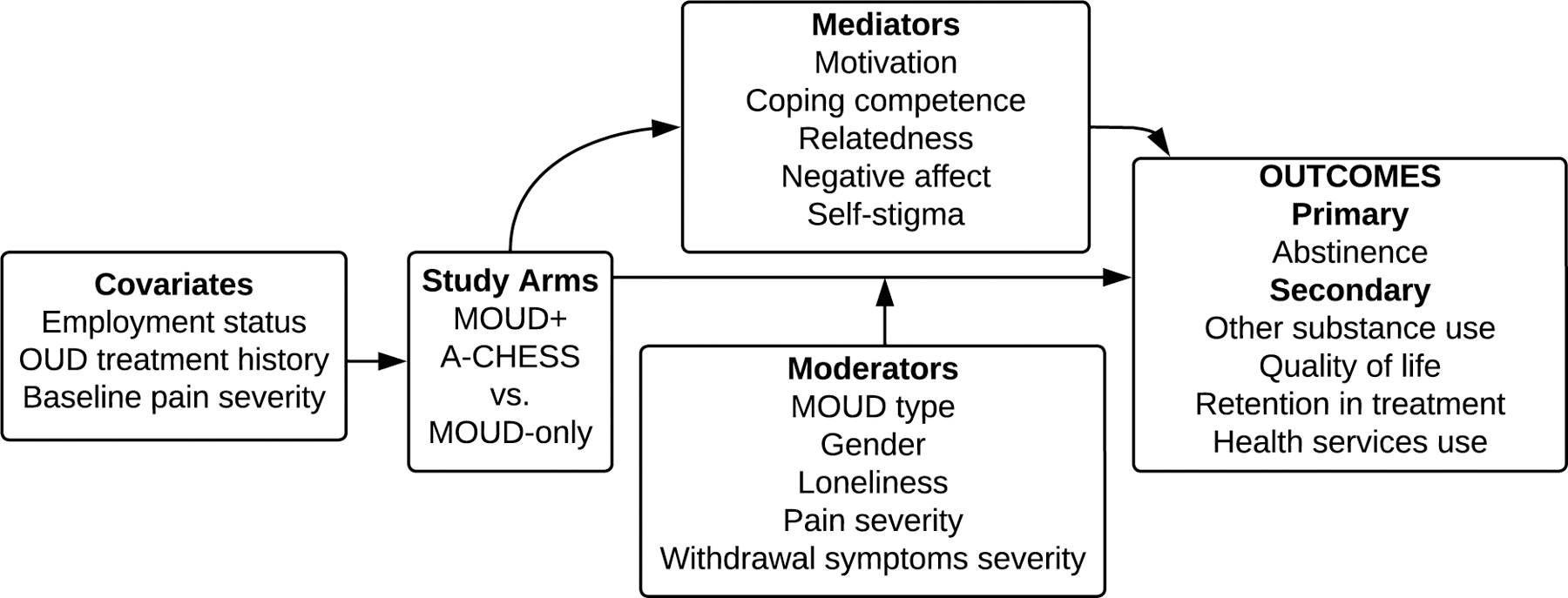
Study logic
Primary outcome.
Self-reported abstinence from illicit opioid use was documented for the 30-day period preceding each survey with a variant of the widely-used timeline follow-back (21), with illicit opioid use separated from other substances. Urine drug screens were used only if administered between 30 days before and 1 day after the survey to align with a survey question about past 30 days of illicit opioid use. Results from urine screens were used to validate self-reported information. Inconsistency between screens and self-reports did not affect participants’ ability to continue in the study, but if participants reported abstinence (i.e., 0 days of use) while a screen was positive, their status was changed to non-abstinent.
Secondary outcomes.
For retention in treatment, participants reported whether they were staying on MOUD at each timepoint over the 24 months. In addition, they reported engagement with other forms of treatment outside their clinic facility: meetings attendance (NA/AA, 12 step, smart recovery), outpatient treatment, residential treatment, and therapy/counseling. Each of these variables was analyzed separately. Patients completed a 30-day timeline follow-back at each survey to document other nonprescribed drug and alcohol use as well as health services use (overnight hospitalizations, emergency room and urgent care visits, visits with any other providers; all variables analyzed separately) during the past 4 months. The Satisfaction with Life Scale (22) was used to assess quality of life.
Moderation.
Analyses examined whether effects differed by gender, MOUD type, loneliness (Brief UCLA Loneliness Scale; 23), pain severity, and withdrawal symptoms severity. Severity variables were self-reported on a scale of 1 (“not at all severe”) to 10 (“very severe”). SUD severity, a planned moderator, was dropped from the model because DSM-5 values were not consistently documented during the clinic intake process.
Mediation.
Self-determination theory constructs were assessed as follows: for motivation, the Treatment Self-Regulation Questionnaire (24); for coping competence, the revised Drug-Taking Confidence Questionnaire (25); and for relatedness, the McTavish Bonding Scale (26). The Positive and Negative Affect Scale (27,28) measured negative affect, and the self-devaluation subscale of the Substance Abuse Self-Stigma Scale (29) measured self-stigma.
Covariates.
Potential covariates tested included sociodemographic variables (gender, age, race/ethnicity, education, housing status, employment status, marital status); historical factors (age at regular use of opioids, past OUD treatment, mental health disorder diagnosis); and pain severity (30).
Sample Size Determination and Power
We proposed recruiting 440 patients, anticipating 35% attrition over time, to produce a final N of 286. The final N was calculated to provide approximately 82% power to detect a standardized mean difference of 0.35 (a small to medium-sized effect) between study arms in a linear model with up to six covariates, using an alpha of .05. Power was calculated based on formulae from Cohen (31) that are implemented in the pwr package in R (32). Assumed attrition rates were calculated based on data from our recruitment sites.
Randomization
The project director used a computer-generated allocation sequence to randomize participants to MOUD+A-CHESS versus MOUD-only in a 1:1 ratio using a block design stratified by gender, site, and MOUD type. Block size was 16. The project director informed the site coordinator of group assignment by email, identifying participants by identification number only. The site coordinator enrolled participants into their arm and, when participants were assigned to MOUD+A-CHESS, provided training in system use. Staff were blinded at baseline, before randomization, but as is generally the case with trials of mHealth for SUDs (33) blinding was not possible once participants did (or did not) receive the technology.
Statistical Methods
Outcomes were analyzed with mixed-effects models (glmmTMB() from the glmmTMB package) implemented in R statistical software. These models account for correlated measurements within participants, use all available data (allowing for intent-to-treat rather than only complete-case analysis), and provide unbiased estimates when data are missing at random.(34) Each model included a random effect for participant and study timepoint, as well as fixed effects for timepoint, arm, and arm-by-timepoint interaction. Timepoint was treated as a continuous variable. Models predicting illicit opioid abstinence used a binary distribution with a logit link. We also included covariates marginally related (p<0.2) to illicit opioid use. For our primary outcome, effects are described as significant if p<.05. Secondary analyses (e.g., tests of moderation, alternative outcomes) should be considered exploratory with their unadjusted p-values interpreted in that context.
Each moderator was examined in separate models. Models assessing moderation by type of MOUD allowed type to vary over time based on participant self-report. Moderation was tested in two ways: methadone versus buprenorphine (participants receiving naltrexone or no MOUD were set to “missing” in this model), and methadone versus all other MOUD types (including no MOUD). This was done due to the small sample sizes of MOUDs other than methadone and buprenorphine (see Table S1 in the online supplement). In models with withdrawal symptoms severity as a continuous moderator, withdrawal and illicit opioid use were assessed concurrently, but a causal relationship could not be established because withdrawal questions referred to symptoms experienced over the preceding 4 months while questions about illicit opioid use referred to past 30 days only. All follow-up analyses were pulled from the fitted model. A region-of-significance analysis was performed by adjusting the centering of the variables to find the values of withdrawal for which a significant interaction between arm and timepoint was observed. Both withdrawal severity and MOUD type were time-varying moderators. Simple slopes analyses were conducted by applying the “emtrends()” function from the emmeans package in R to the fitted model.
Ethics and Registration
The study was approved by the UW–Madison Health Sciences Institutional Review Board and the Western Institutional Review Board, and is registered at ClinicalTrials.gov (NCT02712034).
RESULTS
Participants
A total of 414 participants received one of the two interventions and were included in analyses (see Figure S2 in the online supplement for the CONSORT flow diagram). Recruitment began in April 2016 and ended in May 2018, the 16-month intervention period ended in September 2019, and data collection continued through May 2020.
Table 1 presents participant characteristics at baseline. Most participants identified as white (94.0%) and male (54.8%) and began regular use of illicit opioids at age 24. Employment status (yes/no), treatment history (number of times in treatment to stop using opioids), and baseline pain severity rating were associated with illicit opioid use and included as covariates in adjusted models.
Table 1.
Participant characteristics at baseline
| Characteristic | MOUD-only (N=206) | MOUD+A-CHESS (N=208) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Female | 92 | 44.7 | 95 | 45.7 |
| Racea | ||||
| American Indian | 2 | 1.0 | 1 | <1 |
| American Indian, Black | 0 | 0 | 1 | <1 |
| American Indian, Black, White | 1 | <1 | 1 | <1 |
| American Indian, White | 5 | 2.4 | 2 | 1.4 |
| Asian, Black | 1 | <1 | 0 | 0 |
| Asian, White | 1 | <1 | 0 | 0 |
| Black | 1 | <1 | 0 | 0 |
| Black, White | 6 | 2.9 | 2 | 1.0 |
| White | 189 | 91.7 | 200 | 96.2 |
| Ethnicity Hispanic or Latino | 17 | 8.3 | 21 | 10.1 |
| Highest level of education | ||||
| Less than high school | 62 | 30.1 | 68 | 32.7 |
| High school diploma or GED | 86 | 41.7 | 76 | 36.5 |
| 2-year degree or above | 58 | 28.2 | 64 | 30.8 |
| Pretreatment living arrangement | ||||
| Alone | 34 | 16.5 | 34 | 16.3 |
| With others | 165 | 80.1 | 159 | 76.4 |
| Sober house/ treatment | 6 | 3.0 | 9 | 4.3 |
| Homeless | 1 | <1 | 5 | 2.4 |
| Not currently employed | 161 | 78.2 | 152 | 73.1 |
| Married | 125 | 60.7 | 112 | 53.8 |
| Coping with other mental health problems or issues | 141 | 68.4 | 150 | 72.1 |
| History of treatment for chronic pain | 73 | 35.4 | 66 | 31.7 |
| Mean | SD | Mean | SD | |
| Age (years) | 37.07 | 9.89 | 37.35 | 10.22 |
| Age (years) when began regular use of opioids | 23.86 | 7.55 | 24.32 | 7.74 |
| Number times in treatment for opioids use | 7.17 | 12.27 | 7.19 | 11.37 |
| Pain (0=no pain, 10=very severe) | 3.93 | 2.73 | 3.64 | 2.75 |
1 person in MOUD+A-CHESS did not respond.
Time-stamped A-CHESS usage data (e.g., services selected, pages viewed, message text) were captured in our database. In the first year, participants used A-CHESS an average of 32.3% of days, and in the second year 18.3% of days (see Table S2). Of the original 208 MOUD+A-CHESS participants, 191 (91.8%) were using the app after the first month (30 days), 153 (73.6%) after 6 months (182 days), and 123 (59.1%) after one year (360 days).
Across all participants (N=414), 64.5% completed the 24-month survey. Missed survey rates differed statistically between arms at 4-, 20-, and 24-month surveys. At 4 months, MOUD-only had a 7.4% higher missing rate than MOUD+A-CHESS (χ2 =4.00, p=.045). At 20 and 24 months, MOUD+A-CHESS had 12.2% and 10.8% higher missing survey rates than MOUD-only (χ2s >5.24, ps<.022; see Table S3); this may be attributed to the fact that phone service was no longer provided to MOUD+A-CHESS participants after 16 months, affecting our ability to track and communicate with participants and possibly reducing their motivation to complete surveys.
Because we used linear mixed models, which can handle missing data, all 414 participants (206 MOUD-only, 208 MOUD+A-CHESS) who completed baseline surveys were included in the final analyses. A total of 267 participants (144 MOUD-only, 123 MOUD+A-CHESS) completed the 24-month survey, which was 19 fewer than expected after attrition. All participants were analyzed according to original study arm assignment.
Outcomes and Estimation
Primary outcome.
There was no difference in illicit opioid abstinence between participants in the MOUD+A-CHESS versus MOUD-only arm over time (i.e., arm x timepoint, OR 1.10, 95% CI 0.90–1.33, p=.35; see Table S4 and Figure S3 in the online supplement for estimates by arm over time). An intent-to-treat analysis, where all missing outcomes were re-coded as using illicit opioids, also did not yield a significant difference between study arms over time in illicit opioid abstinence (OR 0.89, 95% Cl 0.74–1.07, p=.22). We did not test mediators because the primary outcome was not significant.
Type of MOUD (methadone vs. buprenorphine, all other MOUD options set to “missing”) moderated the effect of arm over time for illicit opioid abstinence (i.e., MOUD Type x Arm x Timepoint, OR 0.57, 95% Cl 0.34–0.97, p=.039; see Figure 2). Simple slopes analysis for the timepoint effect showed that for those on methadone, the probability of abstinence significantly increased over time for participants in MOUD+A-CHESS (b= 0.28, SE=0.09, p=.003); the probability increased but not significantly for participants on MOUD-only (b= 0.06, SE=0.08, p=.48). These timepoint slopes were not significantly different from each other (∆b= 0.22, SE=0.11, p=.053). For those on buprenorphine, participants on MOUD-only showed a significant increase over time in the probability of abstinence (b= 0.68, SE=0.19, p<.001); the probability also increased for those on MOUD+A-CHESS but not significantly (b= 0.34, SE=0.17, p=.053). These timepoint slopes, too, were not significantly different from each other (∆b= −0.34, SE=0.25, p=.166).
Figure 2.
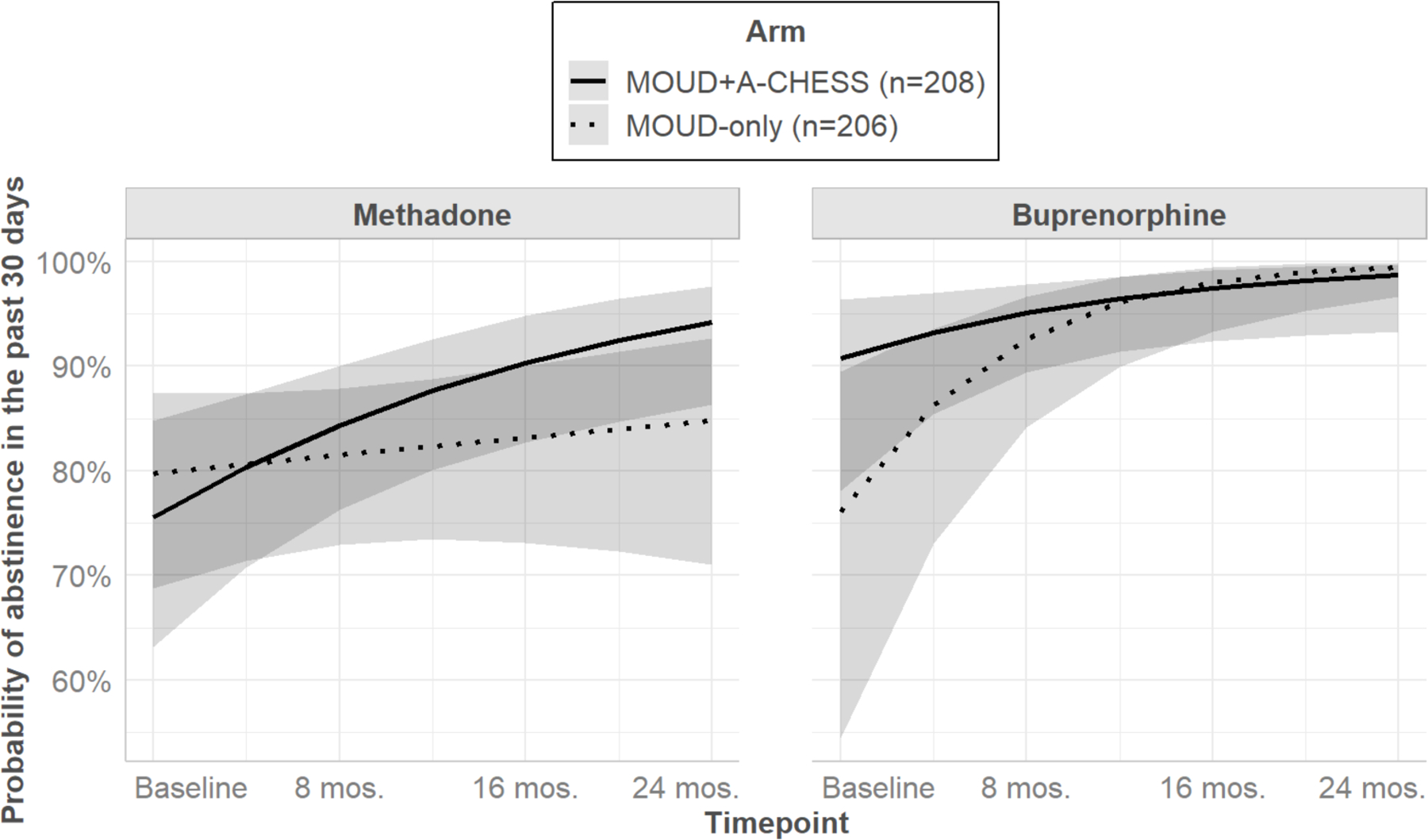
Predicted probabilities of illicit opioid abstinence over time by arm and MOUD type (shaded areas are 95% confidence intervals)
We also observed this moderation effect for illicit opioid abstinence when comparing methadone to all other MOUD types (buprenorphine, naltrexone, and no MOUD) in a more conservative model including the full sample (OR 0.65, 95% Cl 0.43–0.99, p=.044).
Withdrawal symptoms severity also moderated the effect of arm over time for illicit opioid abstinence (i.e., withdrawal x arm x timepoint; OR 0.95, 95% Cl 0.91–1.00, p=.047). A region-of-significance analysis showed that the moderation effect of arm by timepoint for abstinence emerged only for participants reporting no withdrawal symptoms (scored zero) (OR 1.30, 95% Cl 1.01–1.67, p=.039); this effect was nonsignificant for any rating of withdrawal severity (range=1–10) when symptoms were present (Table 2). However, it should be noted that the majority of participants (more than 60%) reported withdrawal scores of zero, which is where we observed the significant arm by timepoint interaction.
Table 2.
Inferential statistics for the withdrawal moderation model’s region-of-significance test (arm x timepoint)
| Withdrawal symptoms severity | Odds ratio (OR) | Withdrawal cumulative percentile a | 95% CI | p-value |
|---|---|---|---|---|
| 0 (no symptoms) | 1.30 | 60.46 | 1.01–1.67 | .039 |
| 1 (symptoms present, “not at all severe”) | 1.24 | 60.87 | 0.99–1.56 | .062 |
| 2 | 1.18 | 62.97 | 0.96–1.46 | .119 |
| 3 | 1.13 | 66.47 | 0.92–1.39 | .25 |
| 4 | 1.08 | 70.61 | 0.87–1.33 | .49 |
| 5 | 1.03 | 75.34 | 0.82–1.29 | .81 |
| 6 | 0.98 | 79.25 | 0.77–1.26 | .88 |
| 7 | 0.94 | 83.39 | 0.71–1.24 | .64 |
| 8 | 0.89 | 88.90 | 0.66–1.22 | .48 |
| 9 | 0.85 | 91.49 | 0.60–1.21 | .37 |
| 10 (symptoms present, “very severe”) | 0.81 | 100.00 | 0.55–1.19 | .29 |
Empirical cumulative percentiles for withdrawal symptoms severity are provided to clarify the positively skewed distribution of scores. See Figure S4 in the online supplement for probability density plots for withdrawal severity at each time point.
Figure 3 displays the moderation effect of withdrawal symptoms severity. Simple slopes analyses are also provided in the figure for the effect of arm over time for the 25th, 50th, and 75th percentiles of severity. As Table 2 and Figure S4 show, withdrawal symptoms were positively skewed (skew=0.96); thus, both the 25th and 50th percentiles were equal to the score of zero.
Figure 3.

Predicted probabilities of illicit opioid abstinence over time by arm and withdrawal symptoms severity (shaded areas are 95% confidence intervals)
Gender, pain severity, and loneliness did not moderate the difference between MOUD+A-CHESS versus MOUD-only over time for the primary outcome (see Table S5 for inferential statistics).
Secondary outcomes.
There was no difference in illicit marijuana, sedative, stimulant, or alcohol use between participants in the MOUD+A-CHESS and MOUD-only arms over time (see Table S6 for inferential statistics), nor did we find significant differences between the MOUD+A-CHESS and MOUD-only arms over time for quality of life.
However, as shown in Figure 4, we found significant arm by timepoint effects for meeting attendance, one of our measures of retention in treatment (OR 1.25, 95% Cl 1.05–1.49, p=.014), and for emergency room/urgent care visits, a measure of health services use (OR 0.88, 95% Cl 0.78–0.99, p=.034). Simple slopes analysis for the timepoint effect showed that participants in the MOUD+A-CHESS arm had slower declines in meeting attendance (b= –0.21, SE=0.07, p=.001) than those on MOUD-only (b= –0.44, SE=0.07, p<.001). Those in the MOUD+A-CHESS arm had fewer emergency/urgent visits over time (b= –0.20, SE=0.05, p<.001) compared to MOUD-only (b= –0.07, SE=0.04, p=.136).
Figure 4.

Predicted probabilities of meeting attendance and emergency room/urgent care visits over time by arm (shaded areas are 95% confidence intervals)
With regard to our other retention-in-treatment variables, we did not find significant differences between the MOUD+A-CHESS and MOUD-only arms over time for staying on MOUD (OR 0.90, 95% Cl 0.75–1.07, p=.22). We also did not find differences in outpatient visits or therapy/counseling, and we were unable to test residential treatment center attendance due to a lack of variability in the data. Among our other measures of health services use (hospitalizations, other provider visits), we also found no significant differences. Each variable was analyzed separately.
DISCUSSION
In our primary analysis including all participants, this study did not find that A-CHESS increased abstinence for people who used illicit opioids or other substances. This null finding indicates that A-CHESS did not benefit the average study participant with regard to our primary outcome. However, other planned analyses suggested possible differences between subsets of MOUD+A-CHESS participants and between arms on certain secondary outcomes: MOUD+A-CHESS was more effective than MOUD-only for participants not experiencing withdrawal symptoms; MOUD type moderated A-CHESS effects such that MOUD+A-CHESS appeared more effective for participants on methadone versus those on buprenorphine; and relative to MOUD-only, MOUD+A-CHESS participants showed increased meeting attendance and fewer emergency room/urgent care visits. Although these tests of moderation and effects on secondary outcomes were specified prior to data analysis, they should be interpreted cautiously and replicated in future studies, given that we conducted a large number of tests.
While there have been promising pilot studies in the last decade (35,36), this is to our knowledge the first large, long-term (24 months) RCT to test effects of a smartphone intervention in combination with MOUD. mHealth in general is a rapidly expanding field, with benefits of accessibility, cost, versatility, and fidelity and with potential to augment treatment and extend the reach of evidence-based interventions.(37) For substance use disorders in particular, mHealth may reduce stigma as well as provide “just in time” intervention because of the portability of smartphones. There are, in fact, countless apps claiming to facilitate recovery available for download—but almost none are regulated or proven.(37,38) In a recent evaluation of 904 free or low-cost apps, only 7 offered evidence-based content.(38) As such, mHealth for illicit substance use is in a “formative stage,”(39) with substantially more clinical research and dissemination effort needed to realize its potential.(37–39) The current study did not find between-group differences for our primary outcome, but it suggests questions to pursue regarding the potential contribution of mHealth for the average patient receiving MOUD.
Relative to mobile apps and eHealth in general,(40,41) A-CHESS usage data indicated high use of the app (91.8% of participants at 1 month, 73.6% at 6 months, 59.1% at 12 months), and yet MOUD+A-CHESS did not increase abstinence relative to MOUD-only. Sustaining engagement is a good start, but research is needed to understand what specific content, services, or design variables are effective in reducing substance use or sustaining abstinence.(39) As described in the online supplement, A-CHESS offers features intended to reduce and distract from cravings, provide peer support, remind patients of reasons to abstain, connect them with clinic support, alert them to real-time risks, provide relevant health news and information, locate support meetings, and more. Future studies should focus on the effectiveness of individual features and on identifying, developing, and testing features and aspects most likely to assist and sustain recovery. It is possible that a future, optimized version of A-CHESS or similar mHealth tools could produce benefits on average not seen in the current trial.
Limitations
Participants in the MOUD+A-CHESS arm were provided with smartphones and internet service; hence, there were incentives to join and continue participation that may limit the generalizability of results to real-world implementation involving one’s own cellphone or data plan. Further impacting generalizability, participants were drawn from treatment centers in areas with little racial or ethnic diversity.
The study also had limitations with regard to examining the moderating effects of MOUD type. We did not have equivalent numbers of participants for each MOUD. At baseline, 300 participants were receiving methadone, 90 were receiving buprenorphine, and 44 were receiving injectable naltrexone. Moreover, a patient’s treatment medication could vary during the study. In addition, few buprenorphine participants had used opioids in the past 30 days at baseline and, by chance, the majority of them were assigned to the MOUD-only arm (14/44) versus MOUD+A-CHESS (8/46). Without a larger and more balanced sample, we cannot determine whether A-CHESS would have had similar effects across all MOUD types.
Finally, participants’ MOUD dosage information was not available. Region-of-significance analyses conducted to clarify the moderating effect of withdrawal symptoms severity suggested that the benefits of A-CHESS were limited to periods when participants reported no withdrawal symptoms. Withdrawal scores were highly positively skewed such that participants spent much of their 24 months in the study free from withdrawal symptoms. A-CHESS appeared to be beneficial during those withdrawal-free periods in which medication dosing was adequate to relieve symptoms and/or acute withdrawal symptoms had subsided. However, detailed data on medication dosing and use of a thorough withdrawal symptom assessment tool (e.g., Amass et al. [42]) could help clarify how and when A-CHESS maximally benefits patients. Future app development and research could involve testing new content within A-CHESS focused on coping with withdrawal symptoms.
Conclusions
Mobile health systems have the potential to be as present in patients’ lives as the symptoms of addiction, offering the promise of help anytime and anywhere. This study aimed to understand whether bundling MOUD with a mobile relapse-prevention system could improve long-term recovery from opioid use disorder. Our results indicate that, on average, adding A-CHESS does not improve abstinence from illicit opioid use. However, the app may help certain patients under certain conditions. In particular, patients appeared more likely to benefit during periods when they were not experiencing withdrawal symptoms, and patients receiving methadone with A-CHESS appeared to benefit more than those receiving other types of MOUD with A-CHESS. Finally, the app appeared to positively impact the use of certain health services. More research to identify effective adjuncts to support those using any MOUD is needed.
Supplementary Material
Table S1. Number of participants who reported using a MOUD at each timepoint
Table S2. Number of days of A-CHESS use by MOUD+A-CHESS participants
Table S3. Differences in survey completion rates over time between MOUD+A-CHESS and MOUD-only arms
Table S4. Number of participants by arm using opioids at each timepoint
Table S5. Inferential statistics for all moderators of abstinence (arm x timepoint x moderator)
Table S6. Inferential statistics for secondary outcomes (arm x timepoint)
Figure S1. A-CHESS home screen
Figure S2. CONSORT flow of participants through the study
Figure S3. Probability of abstinence in the past 30 days for MOUD+A-CHESS and MOUD-only groups over time
Figure S4. Probability density plots for withdrawal symptoms severity at each timepoint (probability range = 0–1; withdrawal symptoms severity rating 0 = no symptoms, 1 = symptoms “not at all severe,” 10 = symptoms “very severe”)
Acknowledgments
This study was funded by the National Institute on Drug Abuse (NIDA), National Institutes of Health (1R01DA040449–01). Simon Goldberg was supported by the National Center for Complementary and Integrative Health (NCCIH), National Institutes of Health (K23AT010879). Neither NIDA nor NCCIH had any role in study design, interpretation of data, or publication of results. The authors wish to express deep gratitude to Laura Reuter at SSTAR for her extraordinary efforts with recruitment, data collection, and participant support and to Lori L. Gustafson PhD for her invaluable review of the analysis and manuscript.
Footnotes
Disclosures
David H. Gustafson Sr. has a small shareholder interest in CHESS Health, a corporation that develops healthcare technology for patients struggling with addiction; this relationship is managed by Dr. Gustafson and the UW–Madison’s Conflict of Interest Committee. In addition, Dr. Gustafson has consulted with Dartmouth-Hitchcock Medical Center on matters of quality improvement. All other authors report no financial relationships or commercial interests.
Trial Registration: ClinicalTrials.gov NCT02712034
The protocol described the primary outcome as days of illicit opioid use. However, this outcome was changed from days of illicit use to any days of illicit use (i.e., abstinence) prior to data collection, based on recommendation of our center’s M.D. for the reasons listed in the Background section.
REFERENCES
- 1.Azadfard M, Huecker MR, Leaming JM. Opioid Addiction. [Updated 2022 Apr 7]. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2022. Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448203/. Accessed December 28, 2022. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13–4795. Rockville, MD: Substance Abuse and Mental Health Services Administration. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUHnationalfindingresults2012/NSDUHnationalfindingresults2012/NSDUHresults2012.htm. Accessed December 28, 2022. [Google Scholar]
- 3.Langabeer JR, Stotts AL, Bobrow BJ, et al. Prevalence and charges of opioid-related visits to U.S. emergency departments. Drug Alcohol Depend 2021;221:108568. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Death rate maps and graphs: Drug overdose deaths remain high Published 2 June 2022. Available from https://www.cdc.gov/drugoverdose/deaths/index.html. Accessed September 27, 2022.
- 5.National Institute on Drug Abuse. Percentage of overdose deaths involving methadone declined between January 2019 and August 2021 Published 13 July 2022. Available from https://nida.nih.gov/news-events/news-releases/2022/07/percentage-of-overdose-deaths-involving-methadone-declined-between-january-2019-august-2021. Accessed September 27, 2022.
- 6.O’Reilly KB. Overdose epidemic: 90% who need substance-use disorder treatment don’t get it American Medical Association, 22 Oct 2019. Available from https://www.ama-assn.org/delivering-care/overdose-epidemic/90-who-need-substance-use-disorder-treatment-don-t-get-it. Accessed December 28, 2022. [Google Scholar]
- 7.Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: A randomized trial of outpatient opioid medically supervised withdrawal with naltrexone versus buprenorphine. Am J Psychiatry 2017;174(5):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat 2013;45(3):302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families (Treatment Improvement Protocol 63) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2021. Available from: https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf. Accessed May 4, 2023. [Google Scholar]
- 10.Zhu Y, Evans EA, Mooney LJ, et al. Correlates of long-term opioid abstinence after randomization to methadone versus buprenorphine/naloxone in a multi-site trial. J Neuroimmune Pharmacol 2018;13(4):488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Drug Evaluation and Research (CDER), Food and Drug Administration, U.S. Department of Health and Human Services. Opioid Use Disorder: Endpoints for Demonstrating Effectiveness of Drugs for Treatment: Guidance for Industry Published October 2020. Available from https://www.fda.gov/media/114948/download. Accessed May 4, 2023.
- 12.Center for Drug Evaluation and Research (CDER), Food and Drug Administration, U.S. Department of Health and Human Services. Alcoholism: Developing Drugs for Treatment: Guidance for Industry Published February 2015. Available from https://www.fda.gov/files/drugs/published/Alcoholism---Developing-Drugs-for-Treatment.pdf. Accessed May 4, 2023.
- 13.Witkiewitz K, Wilson AD, Pearson MR, Hallgren KA, Falk DE, Litten RZ, Kranzler HR, Mann KF, Hasin DS, O’Malley SS, & Anton RF (2017). Temporal Stability of Heavy Drinking Days and Drinking Reductions among Heavy Drinkers in the COMBINE Study. Alcoholism, Clinical and Experimental Research, 41(5), 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafson DH, McTavish FM, Chih MY, et al. A smartphone application to support recovery from alcoholism: A randomized clinical trial. JAMA Psychiatry 2014;71(5):566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson K, Richards S, Chih MY, Moon TJ, Curtis H, Gustafson DH. A pilot test of a mobile app for drug court participants. Subst Abuse 2016;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quanbeck A, Gustafson DH, Marsch LA, et al. Implementing a mobile health system to integrate the treatment of addiction into primary care: a hybrid implementation-effectiveness study. J Med Internet Res 2018;20(1):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston DC, Mathews WD, Maus A, Gustafson DH. Using smartphones to improve treatment retention among impoverished substance-using Appalachian women: A naturalistic study. Subst Abuse 2019;13:1178221819861377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafson DH Sr, Landucci G, McTavish F, et al. The effect of bundling medication-assisted treatment for opioid addiction with mHealth: Study protocol for a randomized clinical trial. Trials 2016;17(1):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochstatter KR, Gustafson DH Sr, Landucci G, et al. Effect of an mHealth intervention on Hepatitis C testing uptake among people with opioid use disorder: randomized controlled trial. JMIR Mhealth Uhealth 2021;9(2):e23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000;55(1):68–78. [DOI] [PubMed] [Google Scholar]
- 21.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend 1996; 42(1):49–54. [DOI] [PubMed] [Google Scholar]
- 22.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction with Life Scale. J Pers Assess 1985;49(1):71–75. [DOI] [PubMed] [Google Scholar]
- 23.Russell DW. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. J Pers Assess 1996;66(1):20–40. [DOI] [PubMed] [Google Scholar]
- 24.Neal DJ, Carey KB. A follow-up psychometric analysis of the Self-Regulation Questionnaire. Psychol Addict Behav 2005;19(4):414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sklar SM, Annis HM, Turner NE. Development and validation of the Drug-Taking Confidence Questionnaire: A measure of coping self-efficacy. Addict Behav 1997;22(5):655–670. [DOI] [PubMed] [Google Scholar]
- 26.Namkoong K, DuBenske LL, Shaw BR, et al. Creating a bond between caregivers online: Effect on caregivers’ coping strategies. J Health Commun 2012;17(2):125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 28.Measelle JR, Stice E, Springer DW. A prospective test of the negative affect model of substance abuse: Moderating effects of social support. Psychol Addict Behav 2006;20(3):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luoma JB, Nobles RH, Drake CE, et al. Self-stigma in substance abuse: Development of a new measure. J Psychopathol Behav Assess 2013;35(2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011;152(10):2399–2404. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Routledge. [Google Scholar]
- 32.pwr: Basic functions for power analysis (version 1.3–0). [Internet] Published March 17, 2020. Available from https://CRAN.R-project.org/package=pwr. Accessed May 5, 2023.
- 33.Bahadoor R, Alexandre JM, Fournet L, et al. Inventory and analysis of controlled trials of mobile phone applications targeting substance use disorders: A systematic review. Front Psychiatry 2021;12:622394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft 2015;67(1):1–48. [Google Scholar]
- 35.Guarino H, Acosta M, Marsch LA, et al. A mixed-methods evaluation of the feasibility, acceptability, and preliminary efficacy of a mobile intervention for methadone maintenance clients. Psychol Addict Behav 2016;30(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodges J, Waselewski M, Harrington W, et al. Six-month outcomes of the HOPE smartphone application designed to support treatment with medications for opioid use disorder and piloted during an early statewide COVID-19 lockdown. Addict Sci Clin Pract 2022;17(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiluk BD, Nich C, Buck MB, et al. Randomized clinical trial of computerized and clinician-delivered CBT in comparison with standard outpatient treatment for substance use disorders: Primary within-treatment and follow-up outcomes. Am J Psychiatry 2018;175(9):853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tofighi B, Chemi C, Ruiz-Valcarcel J, Hein P, Hu L. Smartphone apps targeting alcohol and illicit substance use: Systematic search in commercial app stores and critical content analysis. JMIR Mhealth Uhealth 2019;7(4):e11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shams F, Wong JSH, Nikoo M, et al. Understanding eHealth cognitive behavioral therapy targeting substance use: realist review. J Med Internet Res 2021;23(1):e20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair I Mobile app download statistics & usage statistics (2022). BuildFire. Available from https://buildfire.com/app-statistics/. Accessed December 6, 2022. [Google Scholar]
- 41.Baumel A, Muench F, Edan S, Kane J. Objective user engagement with mental health apps: systematic search and panel-based usage analysis. J Med Internet Res 2019;21(9):e14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amass L, Kamien JB, Mikulich SK. Efficacy of daily and alternate-day dosing regimens with the combination buprenorphine-naloxone tablet. Drug Alcohol Depend 2000;58(1–2):143–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Number of participants who reported using a MOUD at each timepoint
Table S2. Number of days of A-CHESS use by MOUD+A-CHESS participants
Table S3. Differences in survey completion rates over time between MOUD+A-CHESS and MOUD-only arms
Table S4. Number of participants by arm using opioids at each timepoint
Table S5. Inferential statistics for all moderators of abstinence (arm x timepoint x moderator)
Table S6. Inferential statistics for secondary outcomes (arm x timepoint)
Figure S1. A-CHESS home screen
Figure S2. CONSORT flow of participants through the study
Figure S3. Probability of abstinence in the past 30 days for MOUD+A-CHESS and MOUD-only groups over time
Figure S4. Probability density plots for withdrawal symptoms severity at each timepoint (probability range = 0–1; withdrawal symptoms severity rating 0 = no symptoms, 1 = symptoms “not at all severe,” 10 = symptoms “very severe”)


