Abstract
Objective:
Human behaviors, thoughts, and emotions are guided by memories of the past. Thus, there can be little doubt that memory plays a fundamental role in the behaviors (e.g., binging), thoughts (e.g., body-image concerns), and emotions (e.g., guilt) that characterize eating disorders (EDs). Although a growing body of research has begun to investigate the role of memory in EDs, this literature is limited in numerous ways and has yet to be integrated into an overarching framework.
Methods:
In the present article, we provide an operational framework for characterizing different domains of memory, briefly review existing ED memory research within this framework, and highlight crucial gaps in the literature.
Results:
We distinguish between three domains of memory—episodic, procedural, and working—which differ based on functional attributes and underlying neural systems. Most recent ED memory research has focused on procedural memory broadly defined (e.g., reinforcement learning), and findings within all three memory domains are highly mixed. Further, few studies have attempted to assess these different domains simultaneously, though most behavior is achieved through coordination and competition between memory systems. We, therefore, offer recommendations for how to move ED research forward within each domain of memory and how to study the interactions between memory systems, using illustrative examples from other areas of basic and clinical research.
Discussion:
A stronger and more integrated understanding of the mechanisms that connect memory of past experiences to present ED behavior may yield more comprehensive theoretical models of EDs that guide novel treatment approaches.
Public Significance:
Memories of previous eating-related experiences may contribute to the onset and maintenance of eating disorders (EDs). However, research on the role of memory in EDs is limited, and distinct domains of ED memory research are rarely connected. We, therefore, offer a framework for organizing, progressing, and integrating ED memory research, to provide a better foundation for improving ED treatment and intervention going forward.
Keywords: anorexia, associative learning, binge-eating, bulimia, eating disorders, episodic memory, learning, memory, procedural memory, working memory
1 ∣. BACK TO THE FUTURE: PROGRESSING MEMORY RESEARCH IN EATING DISORDERS
Eating disorders (EDs), including anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED), are generally characterized by the over or under-consumption of food and disturbances in body image (American Psychiatric Association, 2013; Coffino et al., 2019). EDs are associated with significant psychiatric and medical comorbidity, psychosocial impairment, chronicity, and mortality (Hudson et al., 2007; Smink et al., 2012). As existing interventions for EDs are often unsuccessful in achieving lasting remission (Hagan & Walsh, 2021; Linardon, 2018; Watson & Bulik, 2013), there is an urgent need to clarify the risk and maintenance processes underlying ED onset and maintenance, to improve prevention and treatment approaches. To this end, there has been an explosion of research characterizing the neurocognitive mechanisms that give rise to the cognitive (e.g., attention biases), emotional (e.g., affect regulation difficulties), and behavioral (e.g., decision making) features of EDs. Although it is well-accepted in other fields that each of these features and mechanisms relies heavily on memory processes, research on memory in EDs is relatively limited, and studies evaluating the connections between different facets of memory in EDs are rare. To help bolster research in this area, we outline an operational framework for organizing memory research, briefly summarize existing findings on memory in EDs within this framework, and highlight approaches for addressing important gaps in the literature.
1.1 ∣. Defining memory
Memory refers to a set of mechanisms that allow past experience to guide current behaviors, thoughts, and emotions (Tulving, 1983; Tulving, 1985). For example, memory of the consequences of past behavior motivates us to engage in or avoid those behaviors in the future (Duncan & Shohamy, 2020). Memory is what allows us to maintain a self-image, to construct an ideal self-image, and to compare the two (Conway, 2005). Memory is why we can consciously recollect moments from our past and re-experience the sensations and emotions of those moments in the present (Tulving, 1985). Memory helps guide our attention to the key features of our environment (Sherman & Turk-Browne, 2022) and is what allows us to problem solve, plan, and imagine the future (Schacter et al., 2012).
Memory researchers typically distinguish between different domains of memory based on their functional attributes and underlying neural systems. However, there is no universally accepted framework for making and categorizing such distinctions (Henke, 2010; Howard & Cohen, 2004; Tulving, 2007). To provide organizational clarity in the current article, we operationally distinguish between three domains of memory—episodic, procedural, and working memory (see Figures 1 and 2)—each of which has demonstrated relevance to eating behavior and may hold promise as potential treatment targets (see Table 1). However, it is important to note that although these are common distinctions, we are using the terms broadly, and they do not encapsulate all facets of memory. Finally, a key theme of this article is that these memory systems are highly interactive in guiding behavior, making it important to better understand not only how each memory system influences EDs separately, but also how they do so in concert (Collins & Frank, 2012; Duncan & Shohamy, 2020; Gershman & Daw, 2017a).
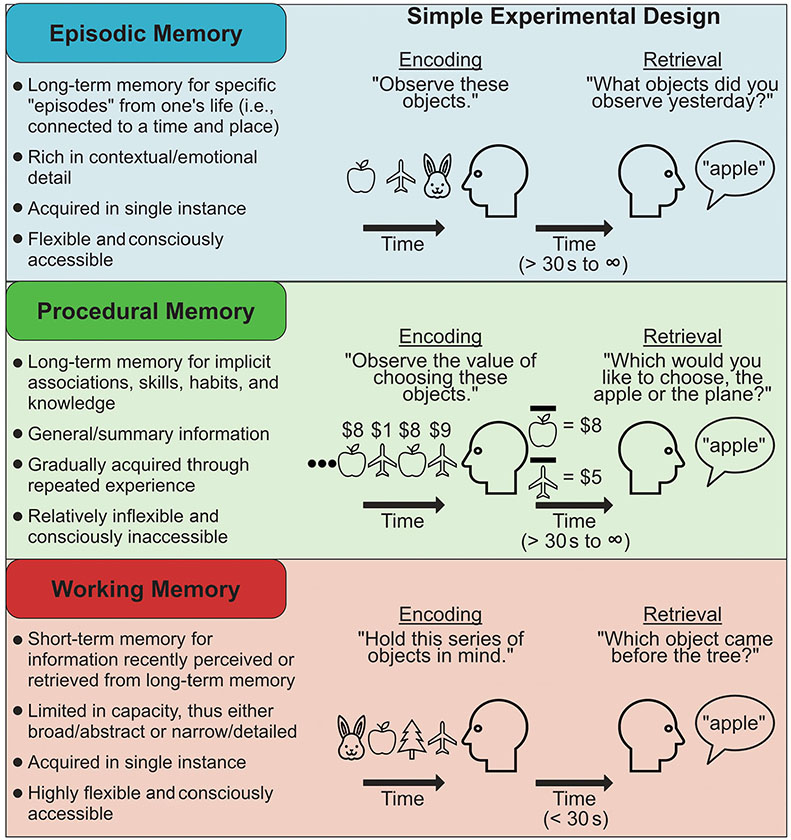
FIGURE 1.
Contrasting three broad domains of memory. Key features (left) and typical methods for testing (right) three domains of memory. This operational framework for distinguishing memory domains is based on differences in functional attributes and underlying neural systems (Henke, 2010; Howard & Cohen, 2004; Tulving, 2007), but its definitions here are broad for the sake of organizational clarity. Note that memory encoding refers to the representation of information so that it has the potential to influence thought and behavior in the future (i.e., memory creation). Memory retrieval refers to the processes that allow previously encoded information to influence thoughts or behavior in the present. In the procedural memory panel, the apple/airplane with a bar over it represents the average (i.e., generalized) value of the item.
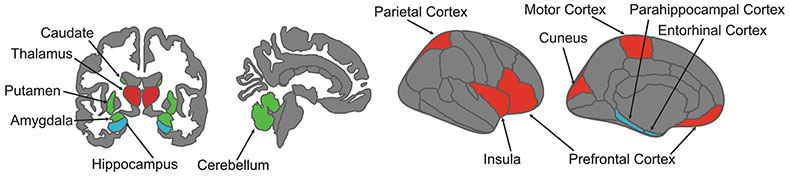
FIGURE 2.
Neuroanatomy of memory domains. A given brain region may contribute to multiple memory systems. However, episodic, procedural, and working memory depend on somewhat anatomically distinct networks (Rottschy et al., 2012; Sherman et al., 2023). Working memory is reliant upon inferior, superior, and medial frontal regions, parietal and insular cortices, and the thalamus (regions in red). Procedural memory function is supported by the dorsal striatum (regions in green), whilst episodic memory relies upon medial temporal lobe structures, including the hippocampus, parahippocampal gyrus, and entorhinal cortex (regions in blue). Given the fairly distinct anatomical systems supporting different types of memory, neuroimaging approaches (e.g., fMRI, MEG, and EEG), may be useful in elucidating the particular memory alterations that exist in EDs, as well as indicating targets of interventions to address these alterations.
TABLE 1.
Hypothetical case examples highlighting likely relationships between memory and eating disorders.
| Case 1 clinical notes | Case 2 clinical notes | Case 3 clinical notes |
|---|---|---|
| Amanda is a 22-year-old female with anorexia nervosa. Amanda continues to recall an idealized view of the onset of her eating disorder (e.g., “when I first started restricting, I felt really great, lost a bunch of weight, and people commented on how good I looked.”) which drives continued attempts to restrict, despite awareness that the behavior is harming her. Additionally, when her therapist prompts her to imagine herself in the future, she struggles to imagine a life either with or without her eating disorder. Therefore, she struggles to access salient, future-focused motivation to change her behavior. | Patrick is a 29-year-old male presenting for treatment of binge-eating disorder. Patrick describes that, 8 years ago, he began binge eating because it helped him relax and experience some pleasure when he was feeling sad or lonely. However, he says that now, binge eating rarely provides him any relief, and usually makes him feel even worse. Despite this, he feels a strong and automatic need to eat whenever he is feeling sad, and this usually turns into a binge-eating episode. | Yvonne is an 18-year-old female with bulimia nervosa. Yvonne explains that when a thought about food gets into her mind, it is difficult to get it out, even when there is no food around. She notes that, as a result, it is often difficult to think about anything else. For example, she describes that her long-term goals seem to fade from her mind, and she has trouble engaging fully with her environment (e.g., when spending time with friends). |
| Memory in Case 1 | Memory in Case 2 | Memory in Case 3 |
| Memory allows recollecting the past and imagining the future. See especially the sections “Episodic Memory” and “Episodic Memory in EDs.” | Memory allows behaviors to become relatively automatic, driven by internal or external cues. See especially the sections “Procedural Memory” and “Procedural Memory in EDs.” | Memory allows actively maintaining and utilizing information in mind to guide attention and behavior. See especially the sections “Working Memory” and “Working Memory in EDs.” |
1.1.1 ∣. Episodic memory
Episodic memory refers to the ability to store and retrieve specific experiences from one's past, typically with rich associative and contextual detail (Tulving, 1985). For example, episodic memory underlies the ability to recall a conversation that you had a month ago, re-experience the words, gestures, and emotions of that conversation, and recollect incidental details, like the outfit you were wearing. Importantly, episodic memories are flexible: they can be re-examined from different perspectives, they can be combined and compared, and they can be distorted, forgotten, and recovered over time (Biderman et al., 2020; Schacter, 2021). The flexibility and richness of episodic memory likely reflect that its primary function is to help us adaptively make decisions, problem solve, and plan for the future, rather than simply to re-experience the past (Schacter et al., 2012). For example, brain areas crucial to episodic memory, such as the hippocampus and other areas of the medial temporal lobe, show similar activity during decision making and thinking about the future as during episodic memory retrieval, suggesting that episodic memories are implicitly sampled and modified to guide behavior (Addis et al., 2007; Bakkour et al., 2019; Wang et al., 2015). Episodic memory, therefore, likely plays a central role in adaptive and maladaptive psychological functioning (Beck & Haigh, 2014; Bornstein & Pickard, 2020; Brewin, 2014; Heller & Bagot, 2020).
1.1.2 ∣. Procedural memory (and learning)
Procedural memory refers to the gradual acquisition of skills, habits, and knowledge1 through repeated experience (Gershman & Daw, 2017b; Henke, 2010; Howard & Cohen, 2004). It is the set of mechanisms that underlie associative learning, or the ability to form implicit associations between stimuli, contexts, behaviors, and/or outcomes (e.g., reward learning and classical conditioning), and it supports the automaticity of behaviors found to reliably achieve a goal (i.e., habit formation). For example, the incrementally learned associations between contextual stimuli (e.g., the sound of a bell), behaviors (e.g., pressing a lever), and outcomes (e.g., the receipt of food) are stored as procedural memories, which then support automatic response tendencies (e.g., pressing a lever when hearing a bell).
Unlike episodic memory, procedural memory generally does not depend on the hippocampus/medial temporal lobe, and instead is largely reliant upon the striatum, amygdala, and cerebellum (Daw & O'Doherty, 2014; Poldrack & Packard, 2003). Furthermore, whereas episodic memory captures unique details of single instances from the past, procedural memory typically reflects summary information generalized across multiple instances. This summary information describes the value (or utility) of a given action performed in a particular state (e.g., a lever press in the presence of a bell) (O'Doherty et al., 2002; Seymour et al., 2004). Since this information is immediately available (i.e., no computation is required), action evaluation is simple, allowing behavior that was adaptive in the past to be repeated automatically. However, because procedural memories are typically formed through direct and repeated experience, they are often slow to be acquired (Lally et al., 2010), and difficult to change (Orbell & Verplanken, 2010), even if they become inaccurate (e.g., pressing the lever no longer leads to a reward) (Tricomi et al., 2009). Thus, while procedural memory is highly advantageous for completing daily routines, overreliance on this system can be maladaptive when circumstances change. Indeed, excessive use of procedural memory and learning systems in lab-based behavioral tasks appears characteristic of several psychiatric disorders, including substance use and obsessive-compulsive disorders (Gillan & Robbins, 2014; Sjoerds et al., 2013; Voon et al., 2015).
1.1.3 ∣. Working memory
Working memory refers to the ability to hold small amounts of information actively in mind over brief intervals, to mentally manipulate this information, and to use it in service of ongoing tasks (Baddeley, 1986; Miller, 1960). As such, working memory forms a key component of the cognitive architecture supporting a range of behaviors, from the short-term retention of various kinds of information (e.g., visual, verbal, and semantic), to more complex cognitive processes including language comprehension (Collette et al., 2000; Martin et al., 1994), mental arithmetic (Logie et al., 1994), learning and problem solving (Cowan, 2014), and the control and guidance of attention (Desimone & Duncan, 1995). For example, to find a friend in a crowded market, details about the friend's appearance, such as their hair color or what they told you they would be wearing, can be retrieved from long-term memory, maintained in an active state in working memory, and used to guide attention. The ability to temporarily hold information in an active state supports behavioral flexibility by allowing behavior to be guided by internally generated plans and goals, rather than fixed habits and learned stimulus–response associations (D'Esposito & Postle, 2015). Although this is often adaptive, it can sometimes contribute to maladaptive outcomes, such as when an inability to keep particular information out of working memory contributes to excessive worry (Stout et al., 2015), depression (Baddeley, 2013), or other forms of distress (Brewin et al., 2010).
1.2 ∣. The current state of research on memory in EDs
Although severe, widespread deficits of neuropsychological function are unlikely to characterize EDs, it has long been suggested that specific memory abnormalities may be a factor in their onset and maintenance (Lena et al., 2004). This potential is highlighted by structural and functional alterations in the neurocircuits underlying memory systems in ED populations (Mele et al., 2020; Walton et al., 2022), as well as research suggesting that the manipulation of memory may be a useful tool for ED prevention (Pennesi & Wade, 2018). Indeed, memory plays a central, though often implicit, role in many models of ED onset and maintenance (Schaefer, Forester, Dvorak, et al., 2023). For example, affect regulation models suggest that previous experiences of mood improvement following ED behaviors (e.g., dietary restriction) contribute to continuation of that behavior in the future (Haynos et al., 2017; Heatherton & Baumeister, 1991). Habit models extend this proposed learning process, positing that when contextual cues (e.g., negative affect) become associated with specific response-outcome contingencies (e.g., mood improvement following dietary restriction) in memory, the presence of these cues can trigger the response behavior even when the behavior no longer results in the desired outcome (Steinglass & Walsh, 2016; Walsh, 2013). Expectancy models more explicitly hypothesize that cognitive summaries of past experiences are stored in memory, which are then used to predict the future outcome of a given behavior (e.g., the expected outcomes associated with pursuing thinness or consuming food) (Hohlstein et al., 1998). Finally, models highlighting the role of inhibitory control in ED pathology assume that alterations (i.e., impairments in binge-type EDs and enhancements in restrictive-type EDs) in the ability to actively maintain and utilize joint representations of the current environment, prior experience, and current goals in memory contribute to the onset/persistence of EDs (Brooks et al., 2017; Dawe & Loxton, 2004; Wierenga et al., 2014). However, the precise memory mechanisms that might contribute to these models are often untested. Moreover, relevant findings from ED research are typically not discussed within a broader learning and memory context, which would allow researchers to easily link or differentiate findings across the field. In the following sections, we, therefore, outline existing ED memory research within each memory domain and then highlight the connections between them.
1.2.1 ∣. Episodic memory in EDs
There is a substantial body of research demonstrating a relationship between episodic memory and eating behavior (Higgs & Spetter, 2018; Seitz et al., 2021). Perhaps the most striking evidence comes from patients with the inability to form episodic memories (due to hippocampal damage), who, without memory for having recently eaten, will continue to eat multiple meals within a short period of time (Rozin et al., 1998). Similarly, animal research has shown that the hippocampus and other brain regions underlying episodic memory play an important role in integrating and filtering internal signals of satiety and hunger, suggesting that these brain regions not only help to create and retrieve memories of previous eating episodes but also serve a gate-keeping function with regard to physiological hunger and satiety cues (Parent et al., 2022). Evidence linking episodic memory to eating behavior also comes from experimental studies in healthy humans. For example, cueing episodic memory retrieval for a recent meal influences subsequent food intake (Collins & Stafford, 2015; Higgs, 2002; Vartanian et al., 2016), as does manipulating the way memory for an eating episode is created or stored (Brunstrom et al., 2012; Higgs & Woodward, 2009; Robinson et al., 2012). Thus, episodic memory appears to play an important role in modulating experiences of hunger and satiety, as well as in eating-related decision making. Despite this, there has been relatively little research examining the relationship between episodic memory and EDs.
Much of the research that has been conducted to date has focused on whether individuals with EDs have general episodic memory deficits (i.e., reduced ability to create, store, and retrieve new information, such as a series of neutral words/images) or biases (e.g., better memory for disorder-relevant stimuli), but results from these studies have been mixed. There is evidence of enhanced memory for disorder-relevant information, such as food or body stimuli (though there are also null or inconsistent findings) (Griffith et al., 2015; Nikendei et al., 2008; Svaldi et al., 2010; Tekcan et al., 2008). Furthermore, one study found that biased episodic memory for negatively valenced information may help explain emotion regulation difficulties in AN (Manuel & Wade, 2013), suggesting that memory biases may underlie key affect regulation deficits in EDs. However, despite intriguing recent findings, tests of general episodic memory deficits have led to highly inconsistent results overall (Eneva et al., 2017; Keeler et al., 2022a; Stedal et al., 2021; Terhoeven et al., 2017; Terhoeven, Faschingbauer, et al., 2023; Weider et al., 2016).
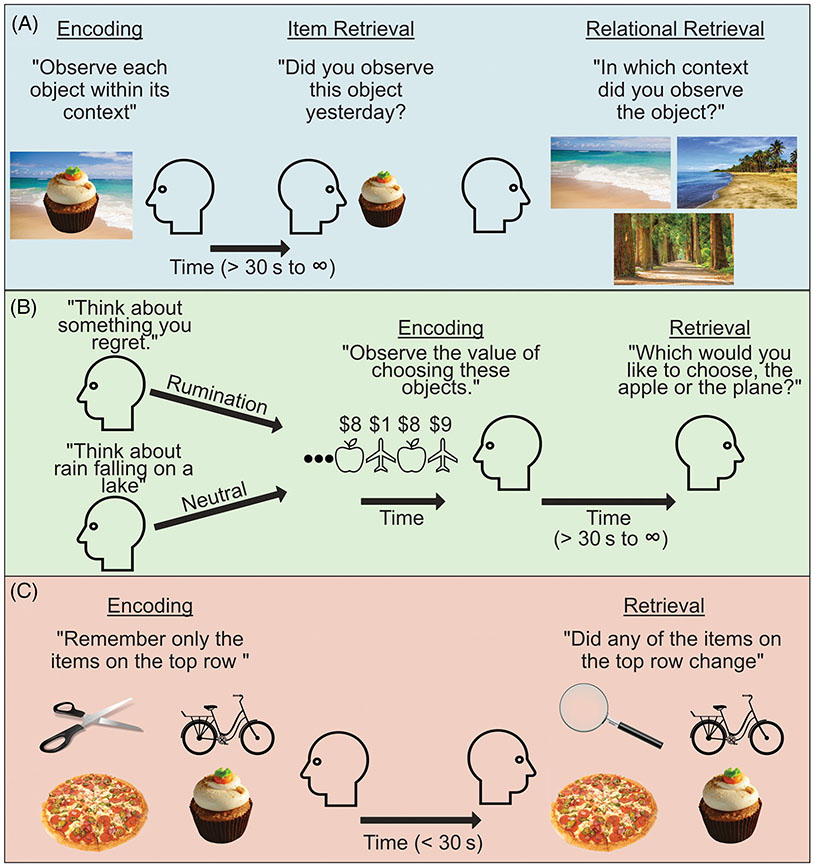
The reason for this inconsistency is likely manifold (e.g., small/heterogenous samples, conflation of memory domains, heterogeneous measures, and stimuli). However, a core issue may be an overreliance on general neuropsychological tests of cognitive function (e.g., from the NIH Toolbox). Although the use of these general memory impairment measures is useful for highlighting potential deficits among ED populations, the intentional broadness and simplicity of these tasks may obscure more nuanced and theoretically meaningful facets of memory. For example, these and other tasks have almost exclusively focused on episodic item memory (i.e., the ability to remember a previously encountered item, such as an object/face/word) rather than on episodic relational2 memory (i.e., the ability to remember the relationships between items or an item and its context, such as the room in which an object was encountered). This distinction between item and relational memory is often crucial in clinically focused memory research (e.g., on schizophrenia, post-traumatic stress disorder, and aging) (Jung & Lee, 2016; Old & Naveh-Benjamin, 2008). For example, a recent study on alcohol use disorder found that individuals who demonstrated more generalized or “gist” level relational memory (see Figure 3a) for the contexts in which alcoholic items were encountered consumed more alcohol over the following month (Goldfarb et al., 2020). In contrast, memory for the alcohol-related items alone was unrelated to drinking behavior. This might reflect that more generalized relational memory (e.g., remembering a beer outside rather than specifically at the beach) could allow a wider variety of contexts to cue memories (and perhaps craving) of alcohol, and in turn, alcohol use. In EDs, more generalized relational memory for food or eating experiences could potentially play a similar role.
FIGURE 3.
Examples for progressing research on each domain of memory separately. (a) Episodic item memory (for the cupcake) may be successful, while relational memory for the context the item was encountered in can be specific (beach on left), general (beach on right), or failed (forest) (Goldfarb et al., 2020). (b) Procedural memory can be influenced by cognitive or affective states. For example, a ruminative state can impair procedural memory and thus potentially impair adaptive behavior (Hitchcock, Fried, & Frank, 2022). (c) Working memory is useful not only when it maintains information important to current goals (items in top row), but also when it successfully filters out information that is irrelevant to current goals (food items on bottom row) (Stout et al., 2015).
Although the studies reviewed above typically assessed memory for arbitrary (but controlled) information (e.g., lists of words/images/movies), another approach is to assess episodic memory for personally meaningful events from the past (e.g., a first date), called autobiographical memory (Williams et al., 2007). Recent research using this approach has consistently found overgeneralized autobiographical memory among individuals with EDs (Bomba & Broggi, 2014; Brockmeyer et al., 2013; Castellon et al., 2020; Dalgleish et al., 2003; Huber et al., 2015; Keeler et al., 2022b; Laberg & Andersson, 2004; Nandrino et al., 2006; Rasmussen, Jørgensen, Connor, Bennedsen, et al., 2017; Ridout et al., 2015; Terhoeven, Nikendei, et al., 2023), an effect that is commonly associated with mood disorders such as depression and post-traumatic stress disorder (Williams et al., 2007). An overgeneralized memory is one that, instead of containing specific episodic details (e.g., “On my last birthday, John and I went for a walk in the park”), is relatively abstract, nonspecific, and often distended in time (e.g., “I usually go for a walk in the mornings”). This lack of specificity may reflect an implicit emotion regulation strategy, allowing individuals to avoid re-experiencing emotions from the past (Williams et al., 2007). However, it is also associated with poorer clinical outcomes among individuals with mood disorders, perhaps because it inhibits the very function of episodic memory—to allow details from the past to guide decision making, problem solving, and planning (Hallford et al., 2020). Indeed, recent studies suggest that overgeneralized memory among individuals with EDs may be associated with a reduced ability to think about the future and problem solve (Keeler et al., 2022a; Rasmussen, Jørgensen, Connor, Godt, & Berntsen, 2017; Ridout et al., 2015), potentially maintaining maladaptive ED behaviors.
1.2.2 ∣. Procedural memory (and learning) in EDs
There is a deep history of research relevant to eating behavior that falls within our broad definition of procedural memory. Indeed, seminal animal research on associative learning typically was (and still is) done using food stimuli, highlighting the power of food for shaping incrementally learned behavior (Anliker & Mayer, 1956; Pavlov, 2010; Pavlov, 1906; Skinner, 1971; Welzl et al., 2001). Recent work also suggests that procedural memory may contribute to a variety of eating behaviors and related experiences in humans, such as the craving and (over)consumption of palatable foods (Boswell & Kober, 2016; Jansen et al., 2016; Pearce et al., 2022). There has thus been consistent and growing interest in characterizing how aspects of procedural memory might contribute to EDs (Steinglass & Walsh, 2006; Steinglass & Walsh, 2016).
One line of research on procedural memory has focused on differences in learning associations between food and stimuli in the environment (i.e., Pavlovian/classical conditioning). For example, several theoretical models have proposed that learned associations between food and aversive stimuli (e.g., nausea) could lead to automatic fear or disgust responses to food, contributing to the onset of restrictive ED symptoms (Anderson et al., 2021; Murray et al., 2018; Strober, 2004). In addition, procedural memory for the associations between food and positive or neutral stimuli (e.g., popcorn and one's living room) could contribute to binge-type ED symptoms by increasing the number or strength of environmental cues that trigger food craving (Boswell & Kober, 2016; Pearce et al., 2022). Indeed, some evidence suggests that individuals with or at risk for AN may more easily learn to associate a novel stimulus with fear or disgust-inducing stimuli than healthy controls (Lambert & Mcgregor, 2021; Olatunji, 2020) (though see Fyer et al., 2020), and may have more difficulty learning new neutral or positive associations for a given stimulus once a negative association has already been formed (Hildebrandt et al., 2015; Lambert & Mcgregor, 2021). Furthermore, neural responses (i.e., prediction error measured via fMRI) to classically conditioned stimuli may be exaggerated in individuals with AN, while they may be reduced in individuals with BED, BN, and obesity (Deguzman et al., 2017; Frank et al., 2011; Frank et al., 2016; Frank et al., 2021). Thus, although existing findings are mixed and integrating results from distinct paradigms is difficult, there does appear to be evidence for abnormalities in the way that associations between stimuli are formed and updated among individuals with EDs.
Another line of research has focused on how individuals with EDs incrementally learn associations between stimuli (e.g., food), actions (e.g., dietary restriction), and outcomes (e.g., reduced anxiety) to maximize rewards or avoid punishments (i.e., instrumental/operant/reinforcement learning). For example, individuals with EDs may more readily learn to associate ED behaviors with rewarding outcomes, thus strengthening the propensity for engaging in those behaviors in the future (Schaefer, Forester, Burr, et al., 2023; Schaefer & Steinglass, 2021). Additionally, when contingencies between stimuli, actions, and outcomes change over time (e.g., if restriction in the presence of food no longer reduces anxiety or becomes associated with negative outcomes), individuals with EDs may fail to adjust their behavior, reflecting poor “reversal learning.” Lab-based assessment of basic deficits/enhancements of this type of procedural memory in EDs has been quite mixed, likely secondary to differences in study design (e.g., focus on initial learning vs. reversal learning) or population characteristics (diagnosis, age, illness stage). However, there does broadly appear to be evidence for altered outcome-guided procedural learning among individuals with EDs (Bernardoni et al., 2017; Celone et al., 2011; Foerde & Steinglass, 2017; Hagan & Forbush, 2021; Hildebrandt et al., 2018; Lao-Kaim et al., 2015; Ritschel et al., 2017; Shott et al., 2012; Wierenga et al., 2022), particularly with regard to reversal learning (Banca et al., 2016; Haynos et al., 2021; Kollei et al., 2018). Relatedly, there is also evidence that individuals with EDs may persist in learned behaviors (e.g., restriction) in response to a specific stimulus (e.g., food), even when the originally reinforcing/punishing outcomes have become irrelevant (e.g., if restriction is now punishing and no longer associated with social reinforcement; i.e., habit) (Davis et al., 2020; Foerde et al., 2021; Gillan et al., 2016; Seidel et al., 2022).
Although there has been more research on procedural than episodic and working memory in EDs, findings in this area are also conflicting, making it difficult to draw clear conclusions. There are likely several reasons for this beyond heterogeneity in populations and paradigms mentioned above. First, most existing research has explored procedural memory without consideration for how state or contextual factors may modulate performance. For instance, outside of EDs, consistent evidence indicates that emotions, stress, or other dynamic cognitive processes influence the encoding and retrieval of procedural memory (Packard et al., 2018; Packard et al., 2021) (as is also true for episodic and working memory) (Schweizer et al., 2019; Shields et al., 2017; Williams et al., 2022). These changing, state-based processes are particularly relevant given robust links between affective dysregulation and stress reactivity with EDs (Chami et al., 2019; Lavender et al., 2015; Wonderlich & Lavender, 2017). For instance, in a recent study in individuals with elevated depressive symptoms, researchers used a within-subjects design to explore the influence of state-based depressive rumination on reinforcement learning performance, finding that performance was impaired by engagement in rumination (Hitchcock, Forman, et al., 2022). Given the relevance of rumination and other forms of repetitive negative thinking in EDs (Palmieri et al., 2021), this type of manipulation may increase external validity of findings as well as inform more dynamic, context-dependent models of procedural memory in EDs (Hitchcock, Fried, & Frank, 2022). In addition to lack of attention toward state-based effects, the research designs utilized in ED research often do not allow for dissociating crucial subcomponents of procedural memory from each other, or from related neurocognitive functions such as attention, reward sensitivity, and executive function (see Figure 4) (Forester & Kamp, 2023; Gershman & Daw, 2017a; Huys et al., 2013; Ma et al., 2020; McGuire et al., 2014; Wildes et al., 2014). Furthermore, research on procedural memory has predominately focused on reward-based learning, while other forms of procedural memory (e.g., fear/disgust conditioning) have received relatively little attention (Anderson et al., 2021; Murray et al., 2018). Finally, as noted below, procedural memory has largely been studied in isolation by ED researchers, without accounting for the influence of episodic and working memory.
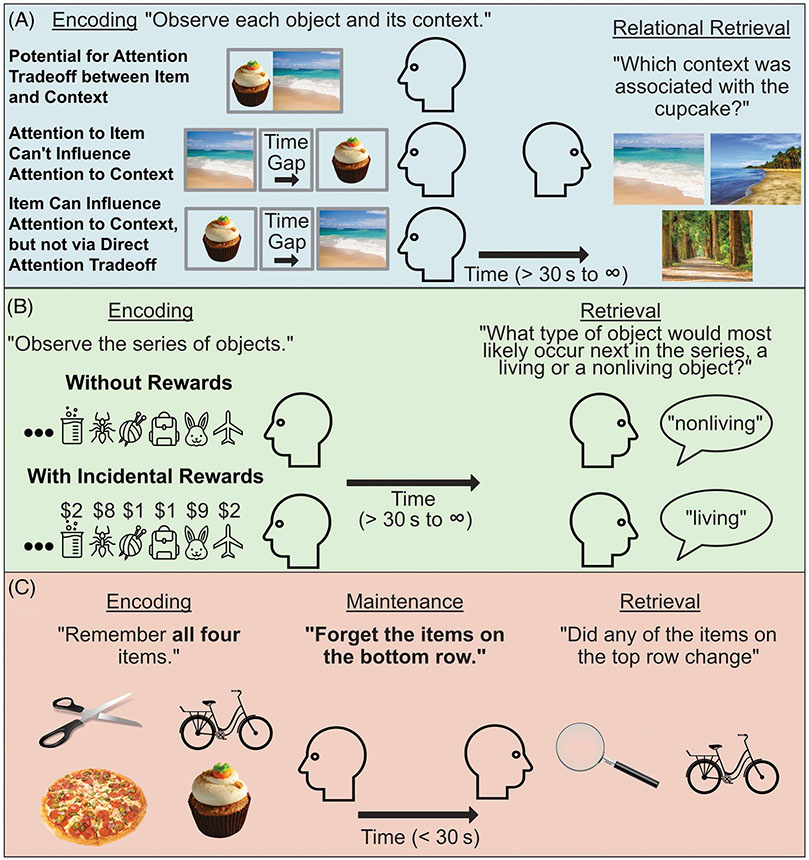
FIGURE 4.
Examples of dissociating memory subprocesses. (a) Multiple processes during encoding, storage, and/or retrieval could influence episodic relational memory. One simple mechanism could be imbalanced attention (i.e., to disorder-relevant cues) during encoding. This could be tested by varying the timing and order in which related information is encoded (Forester & Kamp, 2023). (b) Alterations in reward-based procedural memory could reflect differences in, for example, reward sensitivity or the ability to incrementally abstract statistical information (e.g., the relative probabilities of living and non-living objects). In this task, individuals especially sensitive to reward may be inappropriately biased by incidental associations with reward, while learning normally in a context without rewards (McGuire et al., 2014). (c) Working memory problems could occur, for example, due to impaired storage of task-relevant information or a failure to control access to working memory during either encoding or retrieval (see Figure 3c). But, it could also be due to an inability to remove no-longer-relevant information from working memory during storage (Lewis-Peacock et al., 2018).
1.2.3 ∣. Working memory in EDs
A growing number of studies suggest a relationship between working memory and food intake among non-ED samples (Dohle et al., 2018; Higgs & Spetter, 2018). For example, behavioral and electrophysiological (i.e., EEG) studies demonstrate that food is preferentially represented in working memory, which can have wide-ranging effects on cognition (Robinson et al., 2012; Rutters et al., 2014). Furthermore, higher (state and trait) working memory capacity has been associated with faster satiation and reduced food intake, which could reflect an enhanced ability to monitor bodily signals related to satiation (Nelson & Redden, 2017; Ward & Mann, 2000).
As with episodic memory, much of the research on working memory in EDs has focused on evaluating general impairments (or enhancements) of working memory (e.g., lower working memory capacity for words, digits, and other neutral stimuli, impaired ability to ignore food-related and neutral distractors when utilizing working memory, impaired working memory updating), and the results to date have been similarly mixed (Brooks, 2016; Voon, 2015). For example, some studies suggest that individuals with restricting-type EDs, such as AN, may have increased working memory capacity compared with individuals with binge-spectrum EDs or controls (Brooks et al., 2012; Dahlén et al., 2022; Israel et al., 2015), though other studies have found reduced capacity in AN or no difference between groups (Fowler et al., 2006; Weider et al., 2016). Research in BED and BN has produced similarly mixed evidence for general working memory dysfunction. For example, although two studies observed overall deficits in working memory among individuals with BED compared with overweight controls (Duchesne et al., 2010; Eneva et al., 2017), another study did not observe this difference (Manasse et al., 2015).
The causes of these discrepancies are likely diverse (e.g., heterogeneity in tasks, stimuli, and sample characteristics). However, one important factor may be an overreliance on working memory tasks that feature neutral (i.e., digits, neutral words, or images) rather than disorder-salient stimuli. The few studies utilizing disorder-relevant stimuli (e.g., food-related words) have revealed enhanced recall and working memory updating for food-related information among individuals with elevated levels of ED pathology (Fenton & Ecker, 2015) and difficulty keeping task-irrelevant food-related information out of working memory among individuals with BED (Svaldi et al., 2014). These findings may be particularly relevant given the large body of basic research showing that attention and cognition are biased by the contents of working memory (Soto et al., 2005). Thus, for example, difficulty in the ability to filter disorder-relevant information out of working memory may help explain well-known attentional and cognitive biases surrounding food or the body among individuals with EDs (Stott et al., 2021; Williamson et al., 1999). Although this idea has largely been untested in EDs, analogous work on anxiety has shown that anxious individuals involuntarily store threat-related cues in working memory, which may account for the persistence of anxious thoughts and actions in the absence of threat-related cues in the immediate environment (Stout et al., 2015) (see Figure 3c).
1.3 ∣. Moving forward: Integrating memory research in EDs
Although episodic, procedural, and working memory are subserved by (partially) distinct neural systems, it is becoming increasingly clear that these systems do not act in isolation (Collins & Frank, 2012; Duncan & Shohamy, 2020; Gershman & Daw, 2017a). Rather, most adaptive behavior is achieved through coordination and competition between memory systems. Thus, adopting a more integrative approach to studying memory (i.e., assessing the joint influence of different memory systems on behavior) will likely be important for gaining new insight into key facets of EDs. For example, the relationship between trauma and EDs likely depends in part on memory. However, there are several ways through which episodic, procedural, and working memory may connect past trauma to current ED behavior. Thus, a more holistic view of memory may help to understand and intervene in this relationship (Schwabe & Wolf, 2013).
To support a more integrative approach, memory researchers are developing novel paradigms to better assess the interaction between memory systems, and a key feature of many of these methods is a reliance on theory-driven computational modeling. In this context, computational modeling involves evaluating how well mathematical models that provide a formalized, mechanistic explanation of a given neurocognitive process (e.g., models of how individuals learn about cues and outcomes and incorporate this into future behavior) fit observed data. When coupled with strong experimental design, these models offer the opportunity to evaluate differing influences on a given behavioral outcome, and test competing explanations of observed behavior through model comparison. Indeed, computational modeling techniques have already begun to push ED memory research forward, providing a careful examination of the memory-related processes and subprocesses that may contribute to EDs (Bernardoni et al., 2017; Berner et al., 2023; Chan et al., 2014; Deguzman et al., 2017; Reiter et al., 2017; Smith et al., 2020; Wierenga et al., 2022). For example, one recent study using computational modeling found that what appears to be outcome-guided behavior in AN may actually reflect overreliance on learned stimulus–response associations (Foerde et al., 2021). However, computational modeling work in EDs has been conducted (or at least conceptualized) (Zhou et al., 2023; De Houwer, 2019; Gershman & Daw, 2017a) almost exclusively within the domain of procedural memory. Thus, future ED work would likely benefit from greater integration of episodic and working memory processes into these modeling approaches.
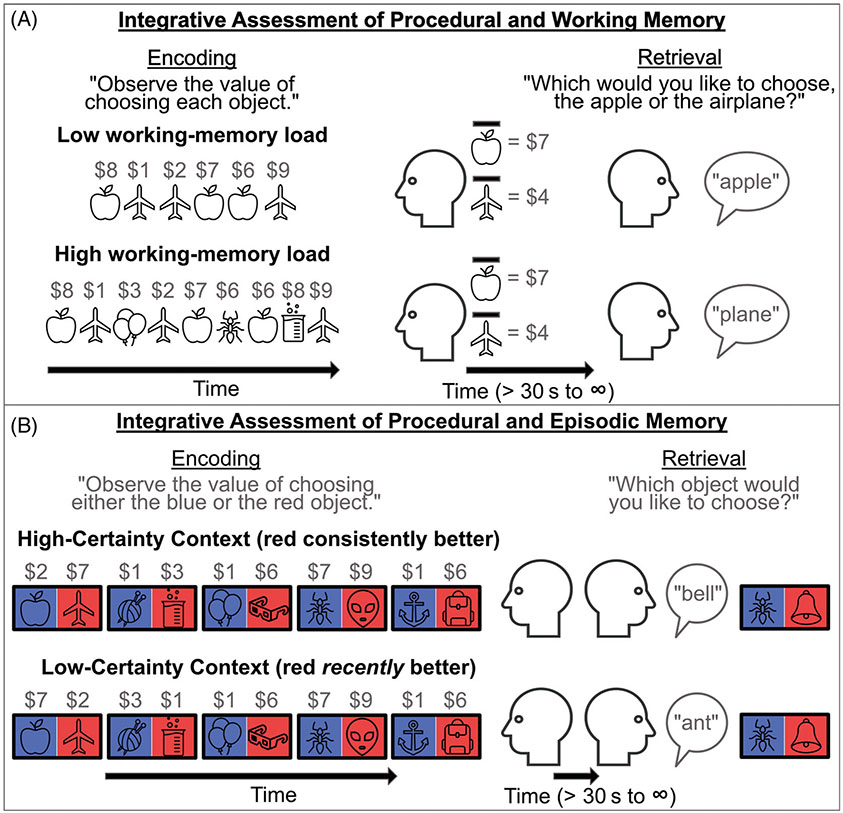
Such an approach has already led to novel insights in other areas of clinical research. For example, by assessing procedural and working memory jointly (see Figure 5a), recent work has found that apparent procedural memory deficits in schizophrenia may be fully explained by deficits in working memory (Collins et al., 2014; Collins et al., 2017). Thus, despite robust evidence for deficits in behavior guided by procedural memory, remediation approaches that target working (as opposed to procedural) memory may be more likely to benefit individuals with schizophrenia (Martin et al., 2020). Similar findings arise when examining the relationships between episodic and procedural memory. For example, recent work has shown that episodic memory can bias decision making, even maladaptively, when choice would otherwise be guided by procedural memory (Bornstein et al., 2017; Madan et al., 2014). Findings like these have led to innovative models of addiction, which account for otherwise inexplicable drug use behavior by incorporating episodic memory processes along-side procedural memory processes (Bornstein & Pickard, 2020). Finally, recent modeling work has shown that the relative influence of procedural and episodic memory on decision making (see Figure 5b) varies as a function of environmental uncertainty and that this balance may be related to (mal)adaptive choice (Nicholas et al., 2022). As EDs are characterized by greater intolerance of uncertainty (Kesby et al., 2017), similar research in this population may prove meaningful.
FIGURE 5.
Examples of integrative assessments of memory across memory domains. (a) Simplified task design to study the joint influence of procedural and working memory on decision making. The high working-memory load condition includes a greater number of unique stimuli, and more time in between repetitions, which taxes working memory. Based on procedural memory alone, however, the choice should be the same, because the average reward value for the apple/airplane is the same in both conditions. Differences in choice may therefore reflect the influence of working memory (Collins et al., 2014). (b) Simplified task design to study the joint influence of procedural and episodic memory on decision making. At the time of retrieval, procedural and episodic memory may have different preferences: procedural memory should favor the novel red object because red has recently been more rewarding (incremental generalization), while episodic memory might favor the ant because the ant was previously associated with a high reward (single instance detail). When certainty is low (due to volatility in whether red or blue is more rewarding), behavior may be guided more by episodic memory, even if this is maladaptive (Nicholas et al., 2022).
In addition to potentially clarifying some of the inconsistencies in the current ED memory literature, a more integrative approach may better support the translation of memory findings into clinical applications. For example, overgeneralized autobiographical memory, which is prospectively associated with poor clinical outcomes in other populations (Hallford et al., 2020) appears to be a robust memory deficit in EDs. The question that follows then, is: How do we target autobiographical memory in order to influence ED outcomes (Hitchcock et al., 2017)? Although autobiographical memory is best characterized as episodic, it also strongly depends on both procedural (especially semantic) and working memory, as well as other neurocognitive processes (Addis & Tanguay, 2022; Fan et al., 2023; Hill & Emery, 2013). Thus, an effective answer to the question will likely depend on a better understanding of the joint memory processes that contribute to the deficit (Moscovitch et al., 2023).
2 ∣. CONCLUSION
Memory is fundamental to almost all aspects of human behavior, leaving little doubt of its relevance to both the onset and maintenance of EDs. However, the term “memory” describes a complex collection of mechanisms. These memory mechanisms can be dissociated operationally into three primary domains: episodic, procedural, and working. To date, research on each facet of memory in EDs has typically been done in isolation, often with important methodological gaps, and has yielded mixed findings. We have attempted to identify key limitations within each area of ED memory research. In addition, we emphasize the highly interactive nature of these memory systems and the importance of studying memory in a more integrative fashion. Such efforts may support the translation of memory research into an increased understanding of how EDs emerge and are maintained over time, to inform critically needed novel treatment and prevention efforts.
FUNDING INFORMATION
This research was supported by funding from the National Institute of Mental Health (T32 MH082761, T32 MH096679, K23MH131871, and R15MH128896) and the National Institute of General Medical Sciences (P20 GM123969). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
Note that we include semantic memory (general knowledge that can be explicitly stated) as a type of procedural memory, though it is often paired more closely with episodic memory (Howard & Cohen, 2004). We do this because semantic memory reflects a generalized and relatively rigid form of memory, which is typically acquired gradually (Gershman & Daw, 2017a; Henke, 2010). For example, you likely know that coffee is associated with cream (a semantic memory) not because you can recall the first time you saw cream poured into coffee (an episodic memory), but because you have extracted the association from repeated experiences (a procedural memory).
Note that this type of memory is often referred “associative” rather than “relational” in episodic memory research. However, in this article, we save the term “associative” for describing “associative learning” in the context of procedural memory, to avoid confusion.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed for this manuscript.
REFERENCES
- Addis DR, & Tanguay A (2022). Prospective cognition and its links with memory. In Kahana M & Wagner AD (Eds.), Oxford handbook on human memory. Oxford University Press. 10.31234/OSF.IO/W69ND [DOI] [Google Scholar]
- Addis DR, Wong AT, & Schacter DL (2007). Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 45(7), 1377. 10.1016/J.NEUROPSYCHOLOGIA.2006.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Anderson LM, Berg H, Brown TA, Menzel J, & Reilly EE (2021). The role of disgust in eating disorders. Current Psychiatry Reports, 23(2), 1–12. 10.1007/S11920-020-01217-5/METRICS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anliker J, & Mayer J (1956). An operant conditioning technique for studying feeding-fasting patterns in normal and obese mice. Journal of Applied Physiology, 8(6), 667–670. 10.1152/JAPPL.1956.8.6.667 [DOI] [PubMed] [Google Scholar]
- Baddeley A. (1986). Working memory. Clarendon Press/Oxford University Press; Accessed March 13, 2023. https://psycnet.apa.org/record/1986-98526-000 [Google Scholar]
- Baddeley A. (2013). Working memory and emotion: Ruminations on a theory of depression. Review of General Psychology, 17(1), 20–27. 10.1037/a0030029 [DOI] [Google Scholar]
- Bakkour A, Palombo DJ, Zylberberg A, Kang YH, Reid A, Verfaellie M, Shadlen MN, & Shohamy D (2019). The hippocampus supports deliberation during value-based decisions. eLife, 8, e46080. 10.7554/eLife.46080.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banca P, Harrison NA, & Voon V (2016). Compulsivity across the pathological misuse of drug and non-drug rewards. Frontiers in Behavioral Neuroscience, 10, 154. 10.3389/FNBEH.2016.00154/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, & Haigh EAP (2014). Advances in cognitive theory and therapy: The generic cognitive model. Annual Review of Clinical Psychology, 10(1), 1–24. 10.1146/ANNUREV-CLINPSY-032813-153734 [DOI] [PubMed] [Google Scholar]
- Bernardoni F, Geisler D, King JA, Javadi A-H, Ritschel F, Murr J, Reiter AMF, Rössner V, Smolka MN, Kiebel S, & Ehrlich S (2017). Altered medial frontal feedback learning signals in anorexia nervosa. Biological Psychiatry, 83(3), 235–243. 10.1016/j.biopsych.2017.07.024 [DOI] [PubMed] [Google Scholar]
- Berner LA, Fiore VG, Chen JY, et al. (2023). Impaired belief updating and devaluation in adult women with bulimia nervosa. Translational Psychiatry, 13(1), 1–9. 10.1038/s41398-022-02257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biderman N, Bakkour A, & Shohamy D (2020). What are memories for? The hippocampus bridges past experience with future decisions trends in cognitive sciences. Trends in Cognitive Sciences, 24(7), 542–556. 10.1016/j.tics.2020.04.004 [DOI] [PubMed] [Google Scholar]
- Bomba M, & Broggi F (2014). Autobiographical memory in adolescent girls with anorexia nervosa. A clinical project for inpatients adolescents with a severe form of anorexia nervosa. European Eating Disorders Review, 22(6), 479–486. 10.1002/erv.2321 [DOI] [PubMed] [Google Scholar]
- Bornstein AM, Khaw MW, Shohamy D, & Daw ND (2017). Reminders of past choices bias decisions for reward in humans. Nature Communications 2, 8(1), 15958. 10.1038/ncomms15958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein AM, & Pickard H (2020). “Chasing the first high”: Memory sampling in drug choice. Neuropsychopharmacology, 45(6), 915. 10.1038/S41386-019-0594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell RG, & Kober H (2016). Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obesity Reviews, 17(2), 177. 10.1111/OBR.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR (2014). Episodic memory, perceptual memory, and their interaction: Foundations for a theory of posttraumatic stress disorder. Psychological Bulletin, 140(1), 69–97. 10.1037/A0033722 [DOI] [PubMed] [Google Scholar]
- Brewin CR, Gregory JD, Lipton M, & Burgess N (2010). Intrusive images in psychological disorders: Characteristics, neural mechanisms, and treatment implications. Psychological Review, 117(1), 210–232. 10.1037/A0018113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeyer T, Grosse Holtforth M, Bents H, Herzog W, & Friederich HC (2013). Lower body weight is associated with less negative emotions in sad autobiographical memories of patients with anorexia nervosa. Psychiatry Research, 210(2), 548–552. 10.1016/J.PSYCHRES.2013.06.024 [DOI] [PubMed] [Google Scholar]
- Brooks SJ (2016). A debate on working memory and cognitive control: Can we learn about the treatment of substance use disorders from the neural correlates of anorexia nervosa? BMC Psychiatry, 16(1), 1–16. 10.1186/S12888-016-0714-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Funk SG, Young SY, & Schiöth HB (2017). The role of working memory for cognitive control in anorexia nervosa versus substance use disorder. Frontiers in Psychology, 8, 1651. 10.3389/FPSYG.2017.01651/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, O'daly OG, Uher R, Schiöth HB, Treasure J, & Campbell IC (2012). Subliminal food images compromise superior working memory performance in women with restricting anorexia nervosa. Consciousness and Cognition, 21, 751–763. 10.1016/j.concog.2012.02.006 [DOI] [PubMed] [Google Scholar]
- Brunstrom JM, Burn JF, Sell NR, et al. (2012). Episodic memory and appetite regulation in humans. PLoS One, 7(12), e50707. 10.1371/JOURNAL.PONE.0050707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon P, Sudres JL, & Voltzenlogel V (2020). Self-defining memories in female patients with anorexia nervosa. European Eating Disorders Review, 28(5), 513–524. 10.1002/ERV.2739 [DOI] [PubMed] [Google Scholar]
- Celone KA, Thompson-Brenner H, Ross RS, Pratt EM, & Stern CE (2011). An fMRI investigation of the fronto-striatal learning system in women who exhibit eating disorder behaviors. NeuroImage, 56(3), 1749–1757. 10.1016/j.neuroimage.2011.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami R, Monteleone AM, Treasure J, & Monteleone P (2019). Stress hormones and eating disorders. Molecular and Cellular Endocrinology, 497, 110349. 10.1016/J.MCE.2018.12.009 [DOI] [PubMed] [Google Scholar]
- Chan TWS, Ahn WY, Bates JE, et al. (2014). Differential impairments underlying decision making in anorexia nervosa and bulimia nervosa: A cognitive modeling analysis. International Journal of Eating Disorders, 47(2), 157–167. 10.1002/EAT.22223 [DOI] [PubMed] [Google Scholar]
- Coffino JA, Udo T, & Grilo CM (2019). The significance of overvaluation of shape or weight in binge-eating disorder: Results from a National Sample of U.S. adults. Obesity (Silver Spring), 27(8), 1367–1371. 10.1002/OBY.22539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, & Poncelet M (2000). Working memory, long-term memory and language processing: Issues and future directions. Brain and Language, 71, 44–51. [DOI] [PubMed] [Google Scholar]
- Collins AGE, Albrecht MA, Waltz JA, Gold JM, & Frank MJ (2017). Interactions between working memory, reinforcement learning and effort in value-based choice: A new paradigm and selective deficits in schizophrenia. Biological Psychiatry, 82(6), 439. 10.1016/J.BIOPSYCH.2017.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AGE, Brown JK, Gold JM, Waltz JA, Michael X, & Frank J (2014). Behavioral/cognitive working memory Contributions to reinforcement learning impairments in schizophrenia. The Journal of Neuroscience, 34(41), 13747–13756. 10.1523/JNEUROSCI.0989-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AGE, & Frank MJ (2012). How much of reinforcement learning is working memory, not reinforcement learning? A behavioral, computational, and neurogenetic analysis. The European Journal of Neuroscience, 35(7), 1024. 10.1111/J.1460-9568.2011.07980.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R, & Stafford LD (2015). Feeling happy and thinking about food: Counteractive effects of mood and memory on food. Appetite, 84, 107–112. [DOI] [PubMed] [Google Scholar]
- Conway MA (2005). Memory and the self. Journal of Memory and Language, 53(4), 594–628. [Google Scholar]
- Cowan N. (2014). Working memory underpins cognitive development, learning, and education. Educational Psychology Review, 26(2), 223. 10.1007/S10648-013-9246-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CY, Talmi D, Daw N, & Mattar MG (2023). Episodic retrieval for model-based evaluation in sequential decision tasks. 14. 10.31234/OSF.IO/3SQJH [DOI] [Google Scholar]
- Dahlén AD, Gaudio S, Schiöth HB, & Brooks SJ (2022). Phonological working memory is adversely affected in adults with anorexia nervosa: A systematic literature review. Eating and Weight Disorders–Studies on Anorexia, Bulimia and Obesity, 27(6), 1931–1952. 10.1007/S40519-022-01370-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T, Tchanturia K, Serpell L, & Yiend J (2003). Self-reported abuse relates to autobiographical memory in patients with eating disorders an investigation of symptoms associated with autism Spectrum disorder in females with anorexia nervosa view project problematic eating on the autism Spectrum (PEAS) view project Tim Dalgleish Medical Research Council (UK). Emotion, 3(3), 211. 10.1037/1528-3542.3.3.211 [DOI] [PubMed] [Google Scholar]
- Davis L, Walsh BT, Schebendach J, Glasofer DR, & Steinglass JE (2020). Habits are stronger with longer duration of illness and greater severity in anorexia nervosa. The International Journal of Eating Disorders, 53(5), 419. 10.1002/EAT.23265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, & O'Doherty JP (2014). Multiple Systems for Value Learning. In Neuroeconomics (2nd ed., pp. 393–410). Academic Press. 10.1016/B978-0-12-416008-8.00021-8 [DOI] [Google Scholar]
- Dawe S, & Loxton NJ (2004). The role of impulsivity in the development of substance use and eating disorders. Neuroscience and Biobehavioral Reviews, 28(3), 343–351. 10.1016/j.neubiorev.2004.03.007 [DOI] [PubMed] [Google Scholar]
- De Houwer J. (2019). On how definitions of habits can complicate habit research. Frontiers in Psychology, 10, 2642. 10.3389/FPSYG.2019.02642/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguzman M, Shott ME, Yang TT, Riederer J, & Frank GKW (2017). Association of elevated reward prediction error response with weight gain in adolescent anorexia nervosa. The American Journal of Psychiatry, 174(6), 557–565. 10.1176/appi.ajp.2016.16060671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, & Duncan J (1995). Neural mechanisms of selective visual attention. Annual Review of Psychology, 18, 193–222. Accessed March 13, 2023. www.annualreviews.org/aronline [DOI] [PubMed] [Google Scholar]
- D'Esposito M, & Postle BR (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66, 115–142. 10.1146/ANNUREV-PSYCH-010814-015031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohle S, Diel K, & Hofmann W (2018). Executive functions and the self-regulation of eating behavior: A review. Appetite, 124, 4–9. 10.1016/J.APPET.2017.05.041 [DOI] [PubMed] [Google Scholar]
- Duchesne M, Mattos P, Appolinario JC, et al. (2010). Assessment of executive functions in obese individuals with binge eating disorder. Brazillian Journal of Psychiatry, 32, 381–388. [DOI] [PubMed] [Google Scholar]
- Duncan K, & Shohamy D (2020). In Poeppel D, Mangun GR, & Gazzaniga MS (Eds.), The cognitive neurosciences (6th ed.). The MIT Press. [Google Scholar]
- Eneva KT, Murray SM, & Chen EY (2017). Binge-eating disorder may be distinguished by visuospatial memory deficits. Eating Behaviors, 26, 159–162. 10.1016/J.EATBEH.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Fan C, Simpson S, Sokolowski HM, & Levine B (2023). In Kahana M & Wagner AD (Eds.), Autobiographical memory. Oxford Handbook of Human Memory. [Google Scholar]
- Fenton O, & Ecker UKH (2015). Memory updating in sub-clinical eating disorder: Differential effects with food and body shape words. Eating Behaviors, 17, 103–106. 10.1016/J.EATBEH.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Foerde K, Daw ND, Rufin T, Walsh BT, Shohamy D, & Steinglass JE (2021). Deficient goal-directed control in a population characterized by extreme goal pursuit. Journal of Cognitive Neuroscience, 33(3), 463. 10.1162/JOCN_A_01655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, & Steinglass JE (2017). Decreased feedback learning in anorexia nervosa persists after weight restoration. The International Journal of Eating Disorders, 50(4), 423. 10.1002/EAT.22709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester G, & Kamp SM (2023). Pre-associative item encoding influences associative memory: Behavioral and ERP evidence. Cognitive, Affective, & Behavioral Neuroscience, 11, 1–17. 10.3758/S13415-023-01102-7/METRICS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler L, Blackwell A, Jaffa A, et al. (2006). Profile of neurocognitive impairments associated with female in-patients with anorexia nervosa. Psychological Medicine, 36(4), 517–527. 10.1017/S0033291705006379 [DOI] [PubMed] [Google Scholar]
- Frank GKW, Collier S, Shott ME, & O'reilly RC (2016). Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. Journal of Psychiatry Neuroscience, 41(5), 304–311. 10.1503/jpn.150103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW, Reynolds JR, Shott ME, & O'Reilly RC (2011). Altered temporal difference learning in bulimia nervosa. Biological Psychiatry, 70(8), 735. 10.1016/J.BIOPSYCH.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW, Shott ME, Stoddard J, Swindle S, & Pryor TL (2021). Association of brain reward response with body mass index and ventral striatal-hypothalamic circuitry among young women with eating disorders. JAMA Psychiatry, 78(10), 1123–1133. 10.1001/JAMAPSYCHIATRY.2021.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyer AJ, Schneier FR, Simpson HB, et al. (2020). Heterogeneity in fear processing across and within anxiety, eating, and compulsive disorders. Journal of Affective Disorders, 275, 338. 10.1016/J.JAD.2020.03.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, & Daw ND (2017a). Reinforcement learning and episodic memory in humans and animals: An integrative. Framework, 68, 101–128. 10.1146/ANNUREV-PSYCH-122414-033625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, & Daw ND (2017b). Reinforcement learning and episodic memory in humans and animals: An integrative framework. Annual Review of Psychology, 68, 101–128. 10.1146/ANNUREV-PSYCH-122414-033625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Kosinski M, Whelan R, Phelps EA, & Daw ND (2016). Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. eLife, 5, e11305. 10.7554/ELIFE.11305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, & Robbins TW (2014). Goal-directed learning and obsessive–compulsive disorder. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1655), 20130475. 10.1098/RSTB.2013.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb EV, Fogelman N, & Sinha R (2020). Memory biases in alcohol use disorder: Enhanced memory for contexts associated with alcohol prospectively predicts alcohol use outcomes. Neuropsychopharmacology, 45(8), 1305. 10.1038/S41386-020-0650-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith E, Kuyken W, Watkins E, & Jones A (2015). Do females with bulimia nervosa and eating disorder not otherwise specified have selective memory biases? Behavioural and Cognitive Psychotherapy, 43(5), 602–613. 10.1017/S1352465814000058 [DOI] [PubMed] [Google Scholar]
- Hagan KE, & Forbush KT (2021). Reward learning in unmedicated women with bulimia nervosa: A pilot investigation. Journal of Psychiatric Research, 136, 70. 10.1016/J.JPSYCHIRES.2021.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan KE, & Walsh BT (2021). State of the art: The therapeutic approaches to bulimia nervosa. Clinical Therapeutics, 43(1), 40–49. 10.1016/J.CLINTHERA.2020.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallford DJ, Rusanov D, Yeow JJE, & Barry TJ (2020). Overgeneral and specific autobiographical memory predict the course of depression: An updated meta-analysis. Psychological Medicine, 51(6), 909–926. 10.1017/S0033291721001343 [DOI] [PubMed] [Google Scholar]
- Haynos AF, Berg KC, Cao L, et al. (2017). Trajectories of higher- and lower-order dimensions of negative and positive affect relative to restrictive eating in anorexia nervosa. Journal of Abnormal Psychology, 126(5), 495–505. 10.1037/ABN0000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynos AF, Camchong J, Pearson CM, et al. (2021). Resting state hypoconnectivity of reward networks in binge eating disorder. Cerebral Cortex, 31(5), 2504. 10.1093/CERCOR/BHAA369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TE, & Baumeister RE (1991). Binge eating as escape from self-awareness. Psychological Bulletin, 110(1), 86–108. [DOI] [PubMed] [Google Scholar]
- Heller AS, & Bagot RC (2020). Is hippocampal replay a mechanism for anxiety and depression? Neuroscience and Psychiatry, 77(4), 431–432. 10.1001/JAMAPSYCHIATRY.2019.4788 [DOI] [PubMed] [Google Scholar]
- Henke K. (2010). A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience, 11(7), 523–532. 10.1038/nrn2850 [DOI] [PubMed] [Google Scholar]
- Higgs S. (2002). Memory for recent eating and its influence on subsequent food intake. Appetite, 39(2), 159–166. 10.1006/APPE.2002.0500 [DOI] [PubMed] [Google Scholar]
- Higgs S, & Spetter MS (2018). Cognitive control of eating: The role of memory in appetite and weight gain. Current Obesity Reports, 7(1), 50–59. 10.1007/S13679-018-0296-9/METRICS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S, & Woodward M (2009). Television watching during lunch increases afternoon snack intake of young women. Appetite, 52(1), 39–43. 10.1016/J.APPET.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Grotzinger A, Reddan M, et al. (2015). Testing the disgust conditioning theory of food-avoidance in adolescents with recent onset anorexia nervosa. Behaviour Research and Therapy, 71, 131–138. 10.1016/j.brat.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Schulz K, Schiller D, Heywood A, Goodman W, & Sysko R (2018). Evidence of prefrontal hyperactivation to food-cue reversal learning in adolescents with anorexia nervosa. Behaviour Research and Therapy, 111, 36–43. 10.1016/j.brat.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Hill PF, & Emery LJ (2013). Episodic future thought: Contributions from working memory. Consciousness and Cognition, 22(3), 677–683. [DOI] [PubMed] [Google Scholar]
- Hitchcock C, Werner-Seidler A, Blackwell SE, & Dalgleish T (2017). Autobiographical episodic memory-based training for the treatment of mood, anxiety and stress-related disorders: A systematic review and meta-analysis. Clinical Psychology Review, 52, 92–107. 10.1016/J.CPR.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Forman E, Rothstein N, et al. (2022). Rumination derails reinforcement learning with possible implications for ineffective behavior. Clinical Psychological Science: A Journal of the Association for Psychological Science 10(4), 714. 10.1177/21677026211051324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock PF, Fried EI, & Frank MJ (2022). Computational Psychiatry Needs Time and Context. Annual Review of Psychology, 73, 243–270. 10.1146/ANNUREV-PSYCH-021621-124910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlstein LA, Smith GT, & Atlas JG (1998). An application of expectancy theory to eating disorders: Development and validation of measures of eating and dieting. Psychological Asessment, 10(1), 49–58. Accessed March 13, 2023. https://web.s.ebscohost.com/ehost/pdfviewer/pdfviewer?vid=0&sid=736506cc-7d3d-4a3f-85c0-af23353022ee%40redis [Google Scholar]
- Howard E, & Cohen NJ (2004). From conditioning to conscious recollection: Memory Systems of the Brain (Vol. 35, 1st ed.). Oxford University Press. [Google Scholar]
- Huber J, Salatsch C, Ingenerf K, et al. (2015). Characteristics of disorder-related autobiographical memory in acute anorexia nervosa patients. European Eating Disorders Review, 23(5), 379–389. 10.1002/ERV.2379 [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, & Kessler RC (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry, 61(3), 348–358. 10.1016/J.BIOPSYCH.2006.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, & Dayan P (2013). Mapping anhedonia onto reinforcement learning: A behavioural meta-analysis. Biology of Mood & Anxiety Disorders, 3(1), 1–16. 10.1186/2045-5380-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M, Klein M, Pruessner J, et al. (2015). n-Back task performance and corresponding brain-activation patterns in women with restrictive and bulimic eating-disorder variants: Preliminary findings. Psychiatry Research: Neuroimaging, 232(1), 84–91. 10.1016/j.pscychresns.2015.01.022 [DOI] [PubMed] [Google Scholar]
- Pavlov IP (2010). Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Annals of Neurosciences, 17(3), 141. 10.5214/ANS.0972-7531.1017309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Schyns G, Bongers P, & van den Akker K (2016). From lab to clinic: Extinction of cued cravings to reduce overeating. Physiology & Behavior, 162, 174–180. 10.1016/J.PHYSBEH.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Jung W, & Lee SH (2016). Memory deficit in patients with schizophrenia and posttraumatic stress disorder: Relational vs item-specific memory. Neuropsychiatric Disease and Treatment, 28, 1157–1166. 10.2147/NDT.S104384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler JL, Peters-Gill G, Treasure J, Himmerich H, Tchanturia K, & Cardi V (2022a). Difficulties in retrieving specific details of autobiographical memories and imagining positive future events in individuals with acute but not remitted anorexia nervosa. Journal of Eating Disorders, 10(1), 172. 10.1186/S40337-022-00684-W/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler JL, Peters-Gill G, Treasure J, Himmerich H, Tchanturia K, & Cardi V (2022b). Difficulties in retrieving specific details of autobiographical memories and imagining positive future events in individuals with acute but not remitted anorexia nervosa. Journal of Eating Disorders, 10(1), 1–15. 10.1186/S40337-022-00684-W/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby A, Maguire S, Brownlow R, & Grisham JR (2017). Intolerance of uncertainty in eating disorders: An update on the field. Clinical Psychology Review, 56, 94–105. 10.1016/J.CPR.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Kollei I, Rustemeier M, Schroeder S, Jongen S, Herpertz S, & Loeber S (2018). Cognitive control functions in individuals with obesity with and without binge-eating disorder. International Journal of Eating Disorders, 51(3), 233–240. 10.1002/EAT.22824 [DOI] [PubMed] [Google Scholar]
- Laberg S, & Andersson G (2004). Autobiographical memories in patients treated for bulimia nervosa. European Eating Disorders Review, 12, 34–41. 10.1002/erv.534 [DOI] [Google Scholar]
- Lally P, Van Jaarsveld CHM, Potts HWW, & Wardle J (2010). How are habits formed: Modelling habit formation in the real world. European Journal of Social Psychology, 40(6), 998–1009. 10.1002/EJSP.674 [DOI] [Google Scholar]
- Lambert E, & Mcgregor T (2021). Fear conditioning in women with anorexia nervosa and healthy controls: A preliminary study BEACON: Brain imaging of emotion and COgnition in anorexia nervosa view project Carers' assessment and skills intervention study for anorexia Neevosa view project. Article in Journal of Abnormal Psychology, 130(5), 490–497. 10.1037/abn0000549 [DOI] [PubMed] [Google Scholar]
- Lao-Kaim NP, Fonville L, Giampietro VP, Williams SCR, Simmons A, & Tchanturia K (2015). Aberrant function of learning and cognitive control networks underlie inefficient cognitive flexibility in anorexia nervosa: A cross-sectional fMRI study. PLoS One, 10(5), e0124027. 10.1371/JOURNAL.PONE.0124027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender JM, Wonderlich SA, Engel SG, Gordon KH, Kaye WH, & Mitchell JE (2015). Dimensions of emotion dysregulation in anorexia nervosa and bulimia nervosa: A conceptual review of the empirical literature. Clinical Psychology Review, 40, 111. 10.1016/J.CPR.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena SM, Fiocco AJ, & Leyenaar JAK (2004). The role of cognitive deficits in the development of eating disorders. Neuropsychology Review, 14(2), 99–113. 10.1023/B:NERV.0000028081.40907.DE/METRICS [DOI] [PubMed] [Google Scholar]
- Lewis-Peacock JA, Kessler Y, & Oberauer K (2018). The removal of information from working memory. Annals of the New York Academy of Sciences, 1424(1), 33–44. 10.1111/NYAS.13714 [DOI] [PubMed] [Google Scholar]
- Linardon J. (2018). Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: Meta-analysis. International Journal of Eating Disorders, 51(8), 785–797. 10.1002/EAT.22897 [DOI] [PubMed] [Google Scholar]
- Logie RH, Gilhooly KJ, & Wynn V (1994). Counting on working memory in arithmetic problem solving. Memory & Cognition, 22(4), 395–410. [DOI] [PubMed] [Google Scholar]
- Ma Y, Brooks J, Li H, & Principe JC (2020). Procedural memory augmented deep reinforcement learning. IEEE Transactions on Artificial Intelligence, 1(2), 105–120. 10.1109/TAI.2021.3054722 [DOI] [Google Scholar]
- Madan CR, Ludvig EA, & Spetch ML (2014). Remembering the best and worst of times: Memories for extreme outcomes bias risky decisions. Psychonomic Bulletin & Review, 21(3), 629–636. 10.3758/S13423-013-0542-9/FIGURES/4 [DOI] [PubMed] [Google Scholar]
- Manasse SM, Forman EM, Ruocco AC, Butryn ML, Juarascio AS, & Fitzpatrick KK (2015). Do executive functioning deficits underpin binge eating disorder? A comparison of overweight women with and without binge eating pathology. The International Journal of Eating Disorders, 48(6), 683. 10.1002/EAT.22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel A, & Wade TD (2013). Emotion regulation in broadly defined anorexia nervosa: Association with negative affective memory bias. Behaviour Research and Therapy, 51(8), 417–424. 10.1016/J.BRAT.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Martin RC, Shelton JR, & Yaffee LS (1994). Language processing and working memory: Neuropsychological evidence for separate phonological and semantic capacities. Journal of Memory and Language, 33(1), 83–111. 10.1006/JMLA.1994.1005 [DOI] [Google Scholar]
- Martin V, Brereton A, & Tang J (2020). Learning and motivation for rewards in schizophrenia: Implications for behavioral rehabilitation ∣ enhanced reader. Current Behavioral Neuroscience Reports, 7, 147–157. [Google Scholar]
- McGuire JT, Nassar MR, Gold JI, & Kable JW (2014). Functionally dissociable influences on learning rate in a dynamic environment. Neuron, 84(4), 870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele G, Alfano V, Cotugno A, & Longarzo M (2020). A broad-spectrum review on multimodal neuroimaging in bulimia nervosa and binge eating disorder. Appetite, 151, 104712. 10.1016/J.APPET.2020.104712 [DOI] [PubMed] [Google Scholar]
- Miller GA (1960). Plans and the structure of behavior. Holt. [Google Scholar]
- Moscovitch DA, Moscovitch M, & Sheldon S (2023). Neurocognitive model of schema-congruent and -incongruent learning in clinical disorders: Application to social anxiety and beyond ∣ enhanced reader. Perspectives on Psychological Science. 10.1177/17456916221141351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SB, Strober M, Craske MG, Griffiths S, Levinson CA, & Strigo IA (2018). Fear as a translational mechanism in the psychopathology of anorexia nervosa. Neuroscience and Biobehavioral Reviews, 95, 383–395. 10.1016/J.NEUBIOREV.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Nandrino JL, Doba K, Lesne A, Christophe V, & Pezard L (2006). Autobiographical memory deficit in anorexia nervosa: Emotion regulation and effect of duration of illness. Journal of Psychosomatic Research, 61(4), 537–543. 10.1016/J.JPSYCHORES.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Nelson NM, & Redden JP (2017). Remembering satiation: The role of working memory in satiation. Journal of Consumer Research, 44(3), 633–650. Accessed March 13, 2023. https://web.s.ebscohost.com/ehost/pdfviewer/pdfviewer?vid=0&sid=fab9ee24-8025-4dfb-9f6c-f0f7b11135ce%40redis [Google Scholar]
- Nicholas J, Daw ND, & Shohamy D (2022). Uncertainty alters the balance between incremental learning and episodic memory. Elife, 11, e81679. 10.7554/ELIFE.81679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikendei C, Weisbrod M, Schild S, et al. (2008). Anorexia nervosa: Selective processing of food-related word and pictorial stimuli in recognition and free recall tests. International Journal of Eating Disorders, 41(5), 439–447. 10.1002/EAT.20518 [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, & Dolan RJ (2002). Neural responses during anticipation of a primary taste reward. Neuron, 33(5), 815–826. 10.1016/S0896-6273(02)00603-7 [DOI] [PubMed] [Google Scholar]
- Olatunji BO (2020). Linking Pavlovian disgust conditioning and eating disorder symptoms: An analogue study. Behavior Therapy, 51(1), 178–189. 10.1016/J.BETH.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Old SR, & Naveh-Benjamin M (2008). Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging, 23(1), 104–108. [DOI] [PubMed] [Google Scholar]
- Orbell S, & Verplanken B (2010). The automatic component of habit in health behavior: Habit as cue-contingent automaticity. Health Psychology, 29(4), 374–383. 10.1037/A0019596 [DOI] [PubMed] [Google Scholar]
- Packard MG, Gadberry T, & Goodman J (2021). Neural systems and the emotion-memory link. Neurobiology of Learning and Memory, 185, 107503. 10.1016/J.NLM.2021.107503 [DOI] [PubMed] [Google Scholar]
- Packard MG, Goodman J, & Ressler RL (2018). Emotional modulation of habit memory: Neural mechanisms and implications for psychopathology. Current Opinion in Behavioral Sciences, 20, 25–32. 10.1016/J.COBEHA.2017.09.004 [DOI] [Google Scholar]
- Palmieri S, Mansueto G, Scaini S, et al. (2021). Repetitive negative thinking and eating disorders: A meta-analysis of the role of worry and rumination. Journal of Clinical Medicine, 10(11), 2448. 10.3390/JCM10112448/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MB, Higgs S, Cheke LG, & Kanoski SE (2022). Memory and eating: A bidirectional relationship implicated in obesity. Neuroscience and Biobehavioral Reviews, 132, 110–129. 10.1016/J.NEUBIOREV.2021.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP (1906). The scientific investigation of the psychical faculties or processes in the higher animals. Science, 24(620), 613–619. 10.1126/SCIENCE.24.620.613 [DOI] [PubMed] [Google Scholar]
- Pearce AL, Fuchs BA, & Keller KL (2022). The role of reinforcement learning and value-based decision-making frameworks in understanding food choice and eating behaviors. Frontiers in Nutrition, 9, 2867. 10.3389/FNUT.2022.1021868/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi JL, & Wade TD (2018). Imagery rescripting and cognitive dissonance: A randomized controlled trial of two brief online interventions for women at risk of developing an eating disorder. International Journal of Eating Disorders, 51(5), 439–448. 10.1002/EAT.22849 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, & Packard MG (2003). Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia, 41(3), 245–251. 10.1016/S0028-3932(02)00157-4 [DOI] [PubMed] [Google Scholar]
- Rasmussen AS, Jørgensen CR, Connor O, Bennedsen BE, Godt KD, Bøye R, & Berntsen D (2017). Eating disorder and obsessive-compulsive disorder. Journal: Psychology of Consciousness: Theory, 4(2), 190–210. 10.1037/cns0000109 [DOI] [Google Scholar]
- Rasmussen AS, Jørgensen CR, Connor O, Godt M, & Berntsen D (2017). Eating disorder and obsessive-compulsive disorder. Psychology of Consciousness: Theory, 4(2), 190–210. 10.1037/cns0000109 [DOI] [Google Scholar]
- Reiter AMF, Heinze HJ, Schlagenhauf F, & Deserno L (2017). Impaired flexible reward-based decision-making in binge eating disorder: Evidence from computational modeling and functional neuroimaging. Neuropsychopharmacology, 42(3), 628–637. 10.1038/npp.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout N, Matharu M, Sanders E, & Wallis DJ (2015). The influence of eating psychopathology on autobiographical memory specificity and social problem-solving. Psychiatry Research, 228(3), 295–303. [DOI] [PubMed] [Google Scholar]
- Ritschel F, Geisler D, King JA, et al. (2017). Neural correlates of altered feedback learning in women recovered from anorexia nervosa. Scientific Reports, 7(1), 1–10. 10.1038/s41598-017-04761-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E, Blissett J, & Higgs S (2012). Changing memory of food enjoyment to increase food liking, choice and intake. British Journal of Nutrition, 108, 1505–1510. 10.1017/S0007114511007021 [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, et al. (2012). Modelling neural correlates of working memory: A coordinate-based meta-analysis. NeuroImage, 60(1), 830–846. 10.1016/J.NEUROIMAGE.2011.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Dow S, Moscovitch M, & Suparna R (1998). What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychological Science, 9(5), 392–396. 10.1111/1467-9280.00073 [DOI] [Google Scholar]
- Rutters F, Kumar S, Higgs S, & Humphreys GW (2014). Electrophysiological evidence for enhanced representation of food stimuli in working memory short title: Enhanced food representation in working memory. Experimental Brain Research, 233(2), 519–528. [DOI] [PubMed] [Google Scholar]
- Schacter DL (2021). The seven sins of memory: An update. Memory, 30(1), 37–42. 10.1080/09658211.2021.1873391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, & Szpunar KK (2012). The future of memory: Remembering, imagining, and the brain. Neuron, 76(4), 677–694. 10.1016/J.NEURON.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer LM, Forester G, Burr EK, et al. (2023). Examining the role of craving in affect regulation models of binge eating. Evidence from an Ecological Momentary Assessment Study, 132(6), 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer LM, Forester G, Dvorak RD, Steinglass J, & Wonderlich SA (2023). Integrating aspects of affect, reward, and cognition to develop more comprehensive models of binge-eating pathology. International Journal of Eating Disorders, 1–9. 10.1002/EAT.23971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer LM, & Steinglass JE (2021). Reward learning through the lens of RDoC: A review of theory, assessment, and empirical findings in the eating disorders. Current Psychiatry Reports, 23, 1–11. 10.1007/S11920-020-01213-9 [DOI] [PubMed] [Google Scholar]
- Schwabe L, & Wolf OT (2013). Stress and multiple memory systems: From “thinking” to “doing”. Trends in Cognitive Sciences, 17(2), 60–68. 10.1016/j.tics.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Schweizer S, Satpute AB, Atzil S, et al. (2019). The impact of affective information on working memory: A pair of meta-analytic reviews of behavioral and neuroimaging evidence. Psychological Bulletin, 145(6), 566–609. Accessed April 17, 2023. https://psycnet.apa.org/fulltext/2019-22428-001.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel M, King JA, Fürtjes S, et al. (2022). Increased habit frequency in the daily lives of patients with acute anorexia nervosa. Nutrients, 14(19), 3905. 10.3390/NU14193905/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz BM, Tomiyama AJ, & Blaisdell AP (2021). Eating behavior as a new frontier in memory research. Neuroscience and Biobehavioral Reviews, 127, 795–807. 10.1016/J.NEUBIOREV.2021.05.024 [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, et al. (2004). Temporal difference models describe higher-order learning in humans. Nature, 429(6992), 664–667. 10.1038/nature02581 [DOI] [PubMed] [Google Scholar]
- Sherman B, & Turk-Browne N (2022). Oxford handbook of human memory. In Kahana MJ & Wagner AD (Eds.), Oxford Handbook of Memory. Oxford University Press. 10.31234/OSF.IO/XS6DB [DOI] [Google Scholar]
- Sherman BE, Turk-Browne NB, & Goldfarb EV (2023). Multiple Memory Subsystems: Reconsidering Memory in the Mind and Brain. Perspectives on Psychological Science, 30, 1–23. 10.1177/17456916231179146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, McCullough AM, & Yonelinas AP (2017). The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychological Bulletin, 143(6), 636–675. 10.1037/BUL0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shott ME, Filoteo JV, Jappe LM, Pryor T, Maddox WT, Rollin MDH, Hagman JO, & Frank GKW (2012). Altered implicit category learning in anorexia nervosa. Neuropsychology, 26(2), 191–201. 10.1037/A0026771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, De Wit S, Van Den Brink W, et al. (2013). Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Translational Psychiatry, 3(12), e337. 10.1038/tp.2013.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF (1971). Operant conditioning. The Encyclopedia of Education, 7, 29–33. [Google Scholar]
- Smink FRE, Van Hoeken D, & Hoek HW (2012). Epidemiology of eating disorders: Incidence, prevalence and mortality rates. Current Psychiatry Reports, 14(4), 406–414. 10.1007/s11920-012-0282-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Kuplicki R, Feinstein J, et al. (2020). A Bayesian computational model reveals a failure to adapt interoceptive precision estimates across depression, anxiety, eating, and substance use disorders. PLoS Computational Biology, 16(12), e1008484. 10.1371/JOURNAL.PCBI.1008484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Heinke D, Humphreys GW, & Blanco MJ (2005). Early, involuntary top-down guidance of attention from working memory. Journal of Experimental Psychology, 31(2), 248–261. 10.1037/0096-1523.31.2.248 [DOI] [PubMed] [Google Scholar]
- Stedal K, Broomfield C, Hay P, Touyz S, & Scherer R (2021). Neuropsychological functioning in adult anorexia nervosa: A meta-analysis. Neuroscience and Biobehavioral Reviews, 130, 214–226. 10.1016/J.NEUBIOREV.2021.08.021 [DOI] [PubMed] [Google Scholar]
- Steinglass J, & Walsh BT (2006). Habit learning and anorexia nervosa: A cognitive neuroscience hypothesis. The International Journal of Eating Disorders, 39(4), 267–275. 10.1002/EAT.20244 [DOI] [PubMed] [Google Scholar]
- Steinglass JE, & Walsh BT (2016). Neurobiological model of the persistence of anorexia nervosa. Journal of Eating Disorders, 4(1), 1–7. 10.1186/S40337-016-0106-2/METRICS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott N, Fox JRE, & Williams MO (2021). Attentional bias in eating disorders: A meta-review. International Journal of Eating Disorders, 54(8), 1377–1399. 10.1002/EAT.23560 [DOI] [PubMed] [Google Scholar]
- Stout DM, Shackman AJ, Johnson JS, & Larson CL (2015). Worry is associated with impaired gating of threat from working memory. Emotion, 15(1), 6. 10.1037/EMO0000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober M. (2004). Pathologic fear conditioning and anorexia nervosa: On the search for novel paradigms. International Journal of Eating Disorders, 35(4), 504–508. 10.1002/EAT.20029 [DOI] [PubMed] [Google Scholar]
- Svaldi J, Bender C, & Tuschen-Caffier B (2010). Explicit memory bias for positively valenced body-related cues in women with binge eating disorder. Journal of Behavior Therapy and Experimental Psychiatry, 41, 251–257. 10.1016/j.jbtep.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Svaldi J, Schmitz F, Trentowska M, Tuschen-Caffier B, Berking M, & Naumann E (2014). Cognitive interference and a food-related memory bias in binge eating disorder. Appetite, 72, 28–36. 10.1016/J.APPET.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Tekcan AI, Çağlar Taş A, Topçuoğlu V,& Yücel B (2008). Memory bias in anorexia nervosa: Evidence from directed forgetting. Journal of Behavior Therapy and Experimental Psychiatry, 39(3), 369–380. 10.1016/J.JBTEP.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Terhoeven V, Faschingbauer S, Huber J, et al. (2023). Verbal memory following weight gain in adult patients with anorexia nervosa: A longitudinal study. European Eating Disorders Review, 31(2), 271–284. 10.1002/ERV.2956 [DOI] [PubMed] [Google Scholar]
- Terhoeven V, Kallen U, Ingenerf K, et al. (2017). Meaningful memory in acute anorexia nervosa patients—Comparing recall, learning, and recognition of semantically related and semantically unrelated word stimuli. European Eating Disorders Review, 25(2), 89–97. 10.1002/ERV.2496 [DOI] [PubMed] [Google Scholar]
- Terhoeven V, Nikendei C, Faschingbauer S, et al. (2023). Neurophysiological correlates of disorder-related autobiographical memory in anorexia nervosa. Psychological Medicine, 53(3), 844–854. 10.1017/S003329172100221X [DOI] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, & O'Doherty JP (2009). A specific role for posterior dorsolateral striatum in human habit learning. The European Journal of Neuroscience, 29(11), 2232. 10.1111/J.1460-9568.2009.06796.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. (1983). Elements of episodic memory. Claredon Press. [Google Scholar]
- Tulving E. (1985). Memory and consciousness. Canadian Psychologist, 26(1), 1–12. 10.1037/H0080017 [DOI] [Google Scholar]
- Tulving E. (2007). Are there 256 different kinds of memory. In The foundations of remembering: Essays in honor of Henry L. Roediger (Vol. 3, pp. 39–52). [Google Scholar]
- Vartanian LR, Chen WH, Reily NM, & Castel AD (2016). The parallel impact of episodic memory and episodic future thinking on food intake. Appetite, 101, 31–36. [DOI] [PubMed] [Google Scholar]
- Voon V. (2015). Cognitive biases in binge eating disorder: The hijacking of decision making. CNS Spectrums, 20(6), 566–573. 10.1017/S1092852915000681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Rück C, et al. (2015). Disorders of compulsivity: a common bias towards learning habits. Molecular Psychiatry, 20(3), 345–352. 10.1038/mp.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BT (2013). The enigmatic persistence of anorexia nervosa. The American Journal of Psychiatry, 170(5), 477–484. 10.1176/APPI.AJP.2012.12081074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E, Bernardoni F, Batury VL, et al. (2022). Brain structure in acutely underweight and partially weight-restored individuals with anorexia nervosa: A coordinated analysis by the ENIGMA eating disorders working group. Biological Psychiatry, 92(9), 730–738. 10.1016/J.BIOPSYCH.2022.04.022 [DOI] [PubMed] [Google Scholar]
- Wang JX, Cohen NJ, & Voss JL (2015). Covert rapid action-memory simulation (CRAMS): A hypothesis of hippocampal-prefrontal interactions for adaptive behavior. Neurobiology of Learning and Memory, 117, 22–33. 10.1016/J.NLM.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, & Mann T (2000). Don't mind if I do: Disinhibited eating under cognitive load. Journal of Personality and Social Psychology, 78(4), 75–763. [DOI] [PubMed] [Google Scholar]
- Watson HJ, & Bulik CM (2013). Update on the treatment of anorexia nervosa: Review of clinical trials, practice guidelines and emerging interventions. Psychological Medicine, 43(12), 2477–2500. 10.1017/S0033291712002620 [DOI] [PubMed] [Google Scholar]
- Weider S, Indredavik MS, Lydersen S, & Hestad K (2016). Central coherence, Visuoconstruction and visual memory in patients with eating disorders as measured by different scoring methods of the Rey complex Figure test. European Eating Disorders Review, 24(2), 106–113. 10.1002/ERV.2385 [DOI] [PubMed] [Google Scholar]
- Welzl H, D'Adamo P, & Lipp HP (2001). Conditioned taste aversion as a learning and memory paradigm. Behavioural Brain Research, 125(1–2), 205–213. 10.1016/S0166-4328(01)00302-3 [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Ely A, Bischoff-Grethe A, Bailer UF, Simmons AN, & Kaye WH (2014). Are extremes of consumption in eating disorders related to an altered balance between reward and inhibition? Frontiers in Behavioral Neuroscience, 8, 410. 10.3389/FNBEH.2014.00410/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Reilly E, Bischoff-Grethe A, Kaye WH, & Brown GG (2022). Altered reinforcement learning from reward and punishment in anorexia nervosa: Evidence from computational modeling. Journal of the International Neuropsychological Society, 28(10), 1003–1015. 10.1017/S1355617721001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildes JE, Forbes EE, & Marcus MD (2014). Advancing research on cognitive flexibility in eating disorders: The importance of distinguishing attentional set-shifting and reversal learning. International Journal of Eating Disorders, 47(3), 227–230. 10.1002/EAT.22243 [DOI] [PubMed] [Google Scholar]
- Williams JMG, Barnhofer T, Crane C, et al. (2007). Autobiographical memory specificity and emotional disorder. Psychological Bulletin, 133(1), 122–148. Accessed March 13, 2023. https://psycnet.apa.org/fulltext/2006-23058-006.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Ford JH, & Kensinger EA (2022). The power of negative and positive episodic memories. Cognitive, Affective, & Behavioral Neuroscience, 22(5), 869–903. 10.3758/S13415-022-01013-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DA, Muller SL, Reas DL, & Thaw JM (1999). Cognitive bias in eating disorders: Implications for theory and treatment. Behavior Modification, 23(4), 556–577. 10.1177/0145445599234003 [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, & Lavender JM (2017). Eating disorders and obesity: A comprehensive handbook. In Brownell KD & Walsh BT (Eds.), Eating disorders and obesity (pp. 260–265). Guilford Publications; Accessed April 17, 2023. https://books.google.com/books?hl=en&lr=&id=e8fTDQAAQBAJ&oi=fnd&pg=PA260&dq=Wonderlich,+S.+A.,+%26+Lavender,+J.+M.+(2017).+Emotion+regulation+and+eating+disorders.+Eating+disorders+and+obesity:+a+comprehensive+handbook,+3,+260-264.&ots=ZhQaVRPHvN&sig=z7kEVog7eMLKf8j776pzCtmMJWI#v=onepage&q&f=false [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed for this manuscript.