Abstract
The chromogranin A-derived peptide catestatin (CST) exerts sympathoexcitatory and hypertensive effects when microinjected into the rostral ventrolateral medulla (RVLM: excitatory output); it exhibits sympathoinhibitory and antihypertensive effects when microinjected into the caudal ventrolateral medulla (CVLM: inhibitory output) of vagotomized normotensive rats. Here, continuous infusion of CST into the central amygdalar nucleus (CeA) of spontaneously hypertensive rats (SHRs) for 15 days resulted in a marked decrease of blood pressure (BP) in 6-month- (by 37 mmHg) and 9-month- (by 65 mmHg)old rats. Whole-cell patch-clamp recordings on pyramidal CeA neurons revealed that CST increased both spontaneous inhibitory postsynaptic current (sIPSC) amplitude plus frequency, along with reductions of sIPSC rise time and decay time. Inhibition of GABAA receptors (GABAARs) by bicuculline completely abolished CST-induced sIPSC, corroborating that CST signals occur through this major neuroreceptor complex. Hypertension is a major risk factor for cerebrovascular diseases, leading to vascular dementia and neurodegeneration. We found a marked neurodegeneration in the amygdala and brainstem of 9-month-old SHRs, while CST and the GABAAR agonist Muscimol provided significant neuroprotection. Enhanced phosphorylation of Akt and ERK accounted for these neuroprotective effects through anti-inflammatory and anti-apoptotic activities. Overall our results point to CST exerting potent antihypertensive and neuroprotective effects plausibly via a GABAergic output, which constitute a novel therapeutic measure to correct defects in blood flow control in disorders such as stroke and Alzheimer’s disease.
Keywords: hypertension, neurodegeneration, catestatin, GABAergic agonist, SHRs
INTRODUCTION
Hypertension (HTN) or high blood pressure (BP) is the most common lethal factor of cardiovascular disorders (D’Agostino et al., 2008), as its rising tendency accounts for increased risks of stroke and myocardial infarction (Chowdhury et al., 2014). These risks seem to be tightly correlated with the incidence of dementia (Qiu et al., 2005; Igase et al., 2012), Alzheimer’s disease (AD; Davies et al., 2011), and diminished cognitive abilities (Wysocki et al., 2012); yet, only the former two mental defects proved to be altered in middle-aged subjects (Whitmer et al., 2005). Interestingly, even though HTN treatment helped to prevent dementia (Peters et al., 2008), this treatment did not conclusively improve cognitive functions in longitudinal studies (Grassi et al., 2011; Guan et al., 2011). For HTN-related disorders, the spontaneously hypertensive rat (SHR), a rodent model developed by Okamoto (Okamoto and Aoki, 1963), has become a widely preferential experimental model (Pinto et al., 1998; Lerman et al., 2005). The usefulness of this rodent comes from the fact that like humans, SHRs develop BP gradually with age (Morris et al., 1983) and once HTN is established it is accompanied by neuronal degeneration in the hypothalamus (Eliash et al., 2005).
Chromogranin A (human CHGA/mouse Chga), a 48-kDa protein found in catecholamine secretory vesicles of chromaffin cells and postganglionic sympathetic axons, is the precursor of the catecholamine release inhibitory peptide catestatin (CST: CHGA352–372). In humans with essential (hereditary) HTN, lower levels of plasma CST play a pathogenic role in the development of HTN (O’Connor et al., 2002, 2008). CST was reported to exert antihypertensive effects on both monogenic (Chga knockout) (Mahapatra et al., 2005; Gayen et al., 2009; Biswas et al., 2012) and polygenic (high BP) HTN mice models (Biswas et al., 2012). When CST is microinjected into the rostral ventrolateral medulla (RVLM), it leads to an increase in resting arterial BP, sympathetic nerve activity and barosensitivity (Gaede and Pilowsky, 2010). On the other hand, the microinjection of CST into the caudal ventrolateral medulla (CVLM) tends to account for a decrease in resting arterial BP, sympathetic nerve activity and barosensitivity (Gaede and Pilowsky, 2012). Thus, the antihypertensive effects of CST led us to consider its potential protective role in SHRs against HTN-induced neurodegeneration.
It is well known that neuronal activities rely on constant blood supply, thus supporting the functional value of activated neurons coupled to microvessels and perivascular astrocytes (Hamel, 2006). Concomitantly, CgA-enriched brain areas surrounded by the major inhibitory GABAAR complex have pointed to this neuroreceptor as another main neuronal element responsible for stress-dependent hemodynamic dysfunctions (Zubcevic and Potts, 2010). Cerebral GABAAR is composed of different subunits, of which α-containing synaptic and extrasynaptic sites (Olsen and Sieghart, 2009) actively regulate psycho-physiological conditions. In the case of α1, which controls the assembly of the different receptor components (Olsen and Sieghart, 2009) together with other α subunits (α2, α4), seems to guarantee varying degrees of anti-sedative sensitivities during homeostatic events (Masneuf et al., 2012) and exerts protection against metabolic-/temperature-dependent sleeping and motor difficulties (Alò et al., 2010; Milić et al., 2012).
Based on these aspects, it was our intention to establish the effect exerted by intracranial infusions of the central nucleus of the amygdala (CeA) with CST and Muscimol (MUS; α1 GABAAR agonist) on HTN and neurodegeneration. CeA is one of the key limbic areas capable of linking psychologically induced stress with the BP-regulatory system (Saha, 2005). This amygdala (AMY) station sends projections to the nucleus tractus solitarius (NTS) and to RVLM and thus attributes to regulatory events of BP (Saha, 2005; Viltart et al., 2006). Additionally, earlier studies dealing with the electrical stimulation of AMY in rats and cats have shown that this limbic area is responsible for an increase in heart rate (HR), BP, and muscle blood flow (Galeno and Brody, 1983). Here, we sought to determine the effects of continuous infusion of CST and MUS in the CeA on the regulation of BP and neurodegeneration that may allow us to propose novel molecular mechanisms capable of rescuing neurogenic hypertensive disorders.
EXPERIMENTAL PROCEDURES
Animals
We used male SHRs aged 12–36 weeks (345.8 ± 50.2 g). They were housed two per cage, maintained under a 12:12-h light/dark cycle (lights on 07:00) at constant temperature (23 ± 1 °C) with free access to food and water. Rats adapted to their new laboratory conditions for at least 2 weeks before surgery. Animal maintenance and all experimental procedures were carried out in accordance with the Guide for Care and Use of Laboratory Animals issued by the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Telemetric continuous intra-arterial measurement of BP
SHRs of 3-, 6-, and 9-(overtly hypertensive) month-old (n = 108 animals, n = 36/age group) were anesthetized with 4% isoflurane (IsoSol; VedCo, St. Joseph, MO), maintained under 2% isoflurane, and placed in a Stoelting stereotaxic instrument. An Alzet osmotic minipump (Model 2004, Durect Corporation, Cupertino, CA) containing 200 μl of CST (1 mM), MUS (100 nM), CST + MUS (1 mM; 100 nM) or saline (0.9% NaCl) connected via polyethelene tubing to the intracranial cannulae (Alzet Brain Infusion Kits II) was stereotaxically implanted on the right CeA (coordinates relative to bregma: AP: −2.6 mm, ML: +4.3 mm, DV: −7.6 mm; Fig. 1) for delivery of drugs at a continuous rate for 15 days at 0.25 μl/h. The procedure for inserting the osmotic pump into the brain has required about 30–40 min for each animal. The coordinates used were taken from Paxinos and Watson stereotaxic atlas (Paxinos and Watson, 1982). The osmotic mini-pump was fixed to the skull with acrylic dental cement. Rats were implanted in the left carotid artery with a catheter coupled to a TA11PA-C40 (DSI, St. Paul, MN) transmitter for telemetric measurement of BP using the Data Sciences International (DSI; Transoma Medical) PhysioTel telemetry system. Telemetry signals were received by an antenna below the cage that relayed data to a signal processor (DataQuest A.R.T. Gold Version 2.3; DSI) connected to a Compaq desktop personal computer (Hewlett–Packard). BP was measured continuously over a 24-h period after 7 days of implantation surgery and normalization of the diurnal pattern of BP.
Fig. 1.
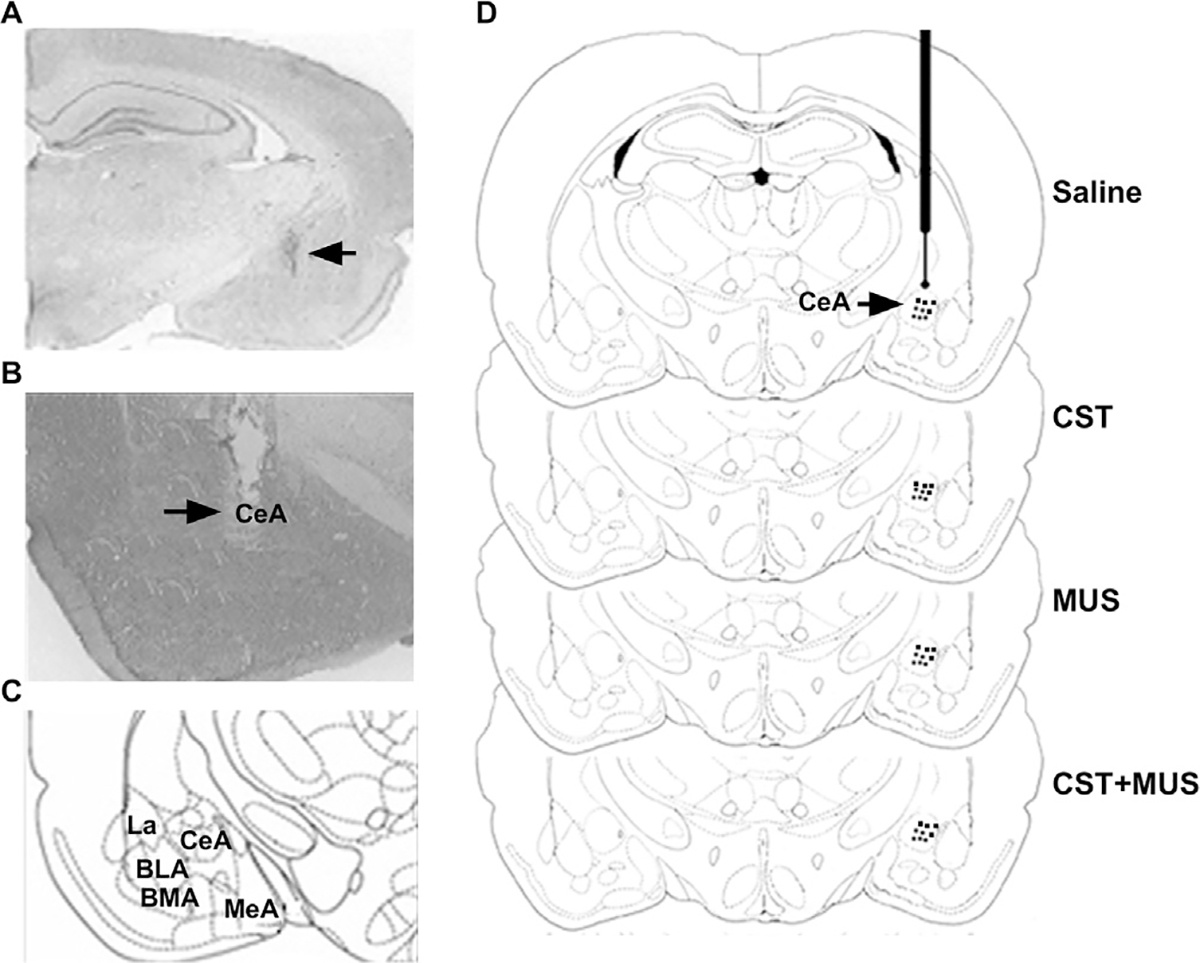
Histological analysis of intra-CeA microinfusion. (A) Representative injection location site within CeA. Arrow indicates the injection site. (B) Representative stimulation site within CeA. Arrow indicates electrode tip location. (C) Scheme showing the anatomical organization of AMY nuclei. (D) Schematic representation of intra-CeA injector locations for groups (saline; CST; MUS; CST + MUS). Abbreviations: BLA, basolateral amygdalar nucleus; BMA, basomedial amygdalar nucleus; CeA, central amygdalar nucleus; La, lateral amygdalar nucleus; MeA, medial amygdalar nucleus.
Whole-cell patch-clamp recordings
Electrophysiological whole-cell recordings in the presence of CST were carried out on pyramidal CeA neurons of overt SHRs (9 months old; n = 12) slices (thickness 300 μm) obtained with a Leica VT1200S vibratome (Leica, Buffalo Grove, IL) in ice-cold modified artificial cerebrospinal fluid (mACSF) as described (Sanna et al., 2004). Slices were immediately transferred to a nylon net submerged in standard ACSF; after a 30-min incubation at room temperature, hemi-slices were transferred to a recording chamber constantly perfused with ACSF (flow rate = ~2 ml/min). The resistance of the pipettes ranged from 2.5 to 4.5 MΩ when filled with an internal solution. Recordings with access resistance of <25 MΩ were only taken into consideration and cells were excluded from further analysis if the access resistance changed by >25%. Membrane currents were recorded, filtered at 2 kHz, and digitized at 5 kHz using an Axopatch 200-B amplifier (Axon Instruments). For all experiments, GABAergic-induced spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in ACSF containing 1 mM of the ionotropic glutamate receptor antagonist (kynurenic acid), used as control (CTRL) for this part. Next CST (1 μM) was applied for 5 min and washed out for at least 5 min; for some neurons sIPSCs was terminated by perfusion in GABAAR antagonist, bicuculline (20 μM). Mini-analysis 6.0 software was used in order to calculate the effects induced by CST with respect to the CTRL on the different kinetic parameters of GABAergic currents such as amplitude, rise time, decay time and frequency.
Stereotactic administration of CST and MUS for their effects on neuroprotection
The Alzet osmotic mini-pump, containing 200 μl of CST (1 mM), MUS (100 nM), CST + MUS (1 mM; 100 nM) or saline (0.9% saline) connected via polyethelene tubing to intracranial cannulae (Alzet Brain Infusion Kit II), was stereotaxically implanted on the right CeA (coordinates relative to bregma: AP: −2.6 mm, ML: +4.3 mm, DV: −7.6 mm; Fig. 1) for continuous delivery of drugs at a rate of 0.25 μl/h as described above. Nine groups of rats (3 months old, 3 groups; 6 months old, 3 groups; overt HTN 9 months old, 3 groups) received a microinjection of the following compounds: CST (n = 27) or the major GABAergic agonist MUS (n = 27) with respect to those treated with saline (n = 27). Another 3 rat groups (3 months old, 1 group; 6 months old, 1 group; 9 months old, 1 group) received the combined treatment of CST + MUS (n = 9 for each group) with respect to controls. First, the tubing that connected the mini-pump to the infusion cannula was exposed in euthanized rats and was cut approximately 2 cm from its point of entry into the dental cement. SHRs were injected (1 μl/rat) with 1% methylene blue solution in order to verify the injection site in CeA. At the end of 15 days, some SHRs were sacrificed to collect brain tissue for Western Blot (n = 3 for each treatment group of only 9-month-old rats) and neurodegenerative (n = 9 for each treatment group of 3- and 6-month-old rats; n = 3 for each treatment group of 9-month-old rats) studies while other rats (n = 3 for each treatment group of only 9-month-old rats) were anaesthetized with urethane (1.5 g/kg, i.p.) for terminal electrophysiological studies.
Drugs
The drugs used in the present study were CST (GenScript, Piscataway, NJ, USA), and the GABAergic agonist MUS (Sigma–Aldrich, Dorset, UK). CST was dissolved in PBS, while MUS in sterile 0.9% saline. Controls only received sterile 0.9% NaCl.
Analysis of neurodegeneration
The possibility that HTN may be linked to neurodegeneration was established using the amino cupric silver stain (ACS) method, which is applied for detecting both necrosis and apoptotic degeneration (Bueno et al., 2003). This method proved to be a valuable tool since it allowed a selective analysis of early and semiacute neurodegeneration events of not only advanced damaged cell bodies, dendrites, axons and terminals, but also the recruitment of new structures in progressive pathologic cases. For this part, a serial set of representative coronal sections (30 μm) was obtained at a cryostat (Microm-HM505E; Zeiss, Wallford, Germany) and selected at an interval of 240 lm for ACS procedures as described in previous studies (Avolio et al., 2011). Counterstained sections with 0.5% neutral red solution (Carlo Erba, Milan, Italy) were then mounted with DPX (Sigma, Milano, Italy) and observed using a bright-field Dialux EB 20 microscope (Leitz, Stuttgart, Germany). For the estimation of damaged fields, it was necessary to calculate the neuronal volumes (defined as Vref) in different brain sites using the following formula:
Nv = number of stained damaged neurons; N = number of damaged neurons/single section; Vsection = volume/single section; n = number of sections; Vref = total volume of brain regions.
Western Blot analysis
SHRs were sacrificed after completion of the study on day 15. The brains were removed and AMY and brainstem (BS) were dissected out and homogenized on ice in a lysis buffer [150 mM NaCl, 20 mM Tris (pH 7.5), 1 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, and 1% nonidet P-40] containing a cocktail of proteinase inhibitors (Roche) and a phosphatase inhibitor (Sigma). Samples were centrifuged at 12,500 rpm at 4 °C for 30 min and the supernatants were saved at −80 °C for immunoblotting. Protein concentrations were determined using the Bradford protein assay (Bio-Rad, Hercules, CA). Equal amounts of protein per sample (20 μg) were separated by electrophoresis on 8% and 10% Tris–glycine gels with 4% stacking gels then were transferred to PVDF membranes. The membranes were blocked with 5% non-fat dry milk or 5% serum albumin for 1 h. The primary antibodies were anti-pAkt/T-Akt and anti-pERK/T-ERK for both AMY and BS (1:1000; Cell Signaling Technology, Danvers, MA). The secondary antibodies (all 1:7000 diluted in blocking solution) were horseradish peroxidase conjugated anti-rabbit IgG (Chemicon International, Inc., Temecula). A horseradish peroxidase chemiluminescence kit with enhanced luminol and oxidizing reagents (Bio-Rad) was used to visualize the signal. The enhanced chemiluminescent signal of the blot was detected in a darkroom with a CCD camera (Molecular Imager Gel Doc XR System; Bio-Rad). The volume of the bands (i.e. area-intensity) was quantified using Quantity One Software (Bio-Rad).
Statistical analysis
Data are expressed as mean ± SEM. Multiple comparisons were made using a one-way ANOVA followed by the Bonferroni’s post hoc test (GraphPad InStat 3.0 for MacIntosh, La Jolla, CA). Statistical significance was concluded at p < 0.05.
RESULTS
Central effects of CST and MUS on BP and HR
Continuous infusion of 0.25 μl/h CST (1 mM) with an Alzet pump had no effect on BP and HR in 3-month-old SHR rats (Fig. 2A, D). CST, however, did evoke marked decreases of BP in 6-month (by ~21%; Fig. 2B) and 9-month-old SHRs (by ~32%; Fig. 2C) with respect to the saline-treated group. CST-induced decrease in BP was accompanied by a concomitant decrease in HR in 6-(by ~119 beats/min; Fig. 2E) and 9-month-(by ~85 beats/min, Fig. 2F) old SHRs. Even though the administration of 0.25 μl MUS (100 nM) per hour via an Alzet pump did not cause any changes in BP and HR in 3- and 6-month-old SHRs, a combination of MUS plus CST accounted for a marked reduction in BP (by ~32%) and HR (by 192 bpm) as compared to the saline-treated group in 6-month-old SHRs (Fig. 2B, E). Interestingly, MUS caused a reduction in BP (by ~23%) only in 9-month-old SHRs, while CST, in combination with the GABAergic agonist, caused a dramatic decrease in BP (by ~39%) and HR (by ~193 beats/min) in this same SHR with respect to the saline-treated group (Fig. 2C, F).
Fig. 2.
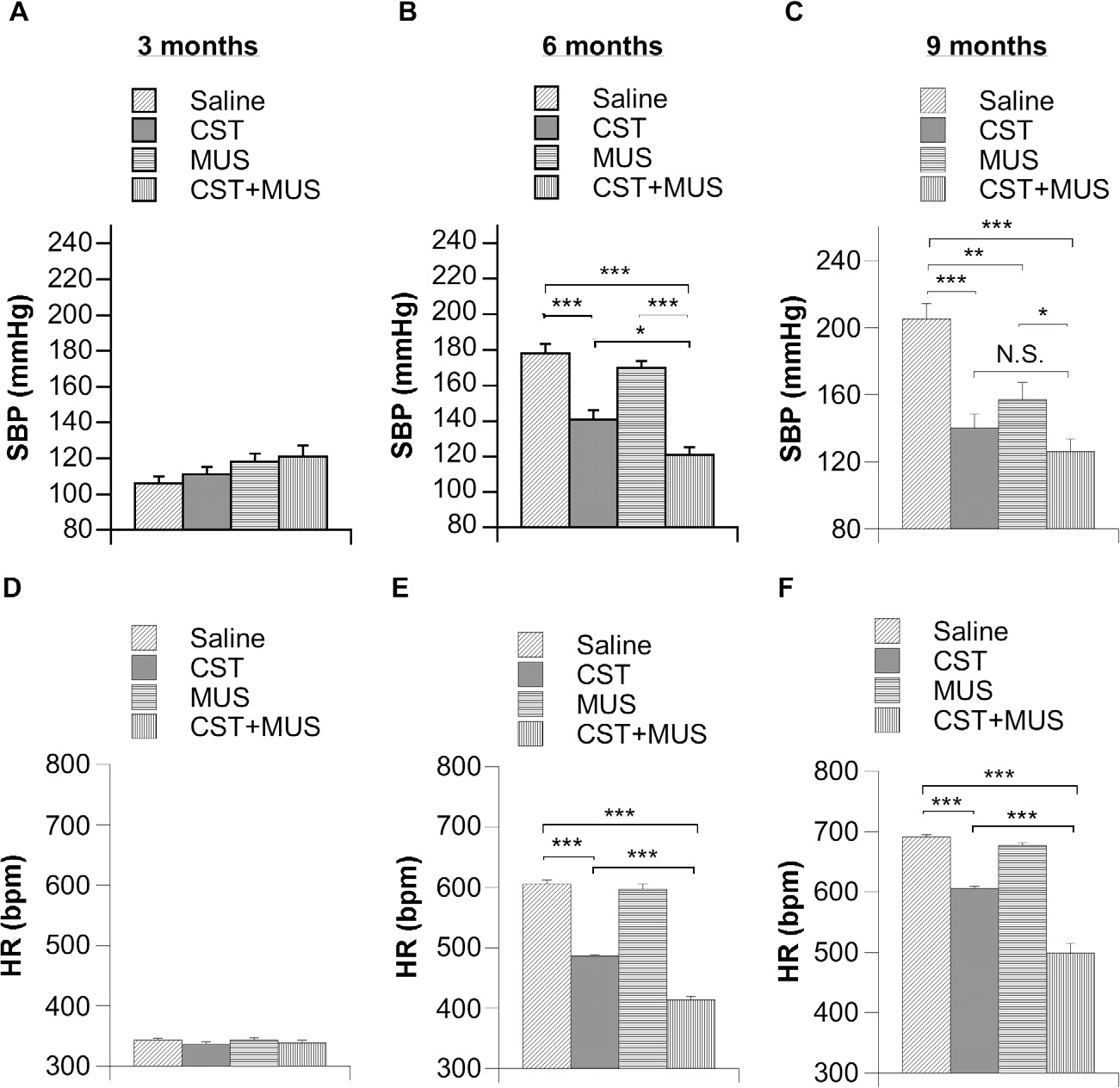
Effects of continuous infusion of CST, MUS and CST + MUS into CeA of SHRs (n = 6 per group) in 3-, 6- and 9-month-old SHRs for a radiotelemetric determination of BP (A–C) and HR (D–F). SHRs received chronic infusions of 0.25 μl/h containing one the following compounds: CST (1 mM); MUS (100 nM); CST + MUS and compared to controls (animals infused with only saline solution) as reported in Experimental procedures. *p < 0.05, **p < 0.01 and ***p < 0.001.
CST effects on GABAergic electrophysiological recordings
The effects of 1 μM CST on GABAergic transmission were determined by recording sIPSCs from single pyramidal neurons of 9-month-old SHR CeA slices under whole-cell voltage-clamp conditions (holding membrane voltage, −65 mV) in the presence of 1 mM of the ionotropic glutamate receptor antagonist (Fig. 3). Analysis of mean sIPSCs kinetic parameters in the presence of CST (check insert Fig. 3F) indicated that this CgA derivative was responsible for a significant increase in current amplitude (68% ± 5.5, p < 0.01 vs CTRL; Fig. 3A) as well as a parallel enhancement of current frequency (73% ± 12, p < 0.01 vs. CTRL; Fig. 3D). Surprisingly, perfusion with CST also led to a weak but significant alteration of both sIPSCs rise time and decay time (Fig. 3B, C). The presence of a major GABAAR antagonist bicuculline (20 μM) completely suppressed sIPSCs, indicating that the currents were mediated by GABAARs during hypertensive conditions (Fig. 3E). Such a feature tends to underlie a spontaneous burst of GABAergic-containing neurons operating under these hypertensive conditions, where CST displayed promising effects.
Fig. 3.
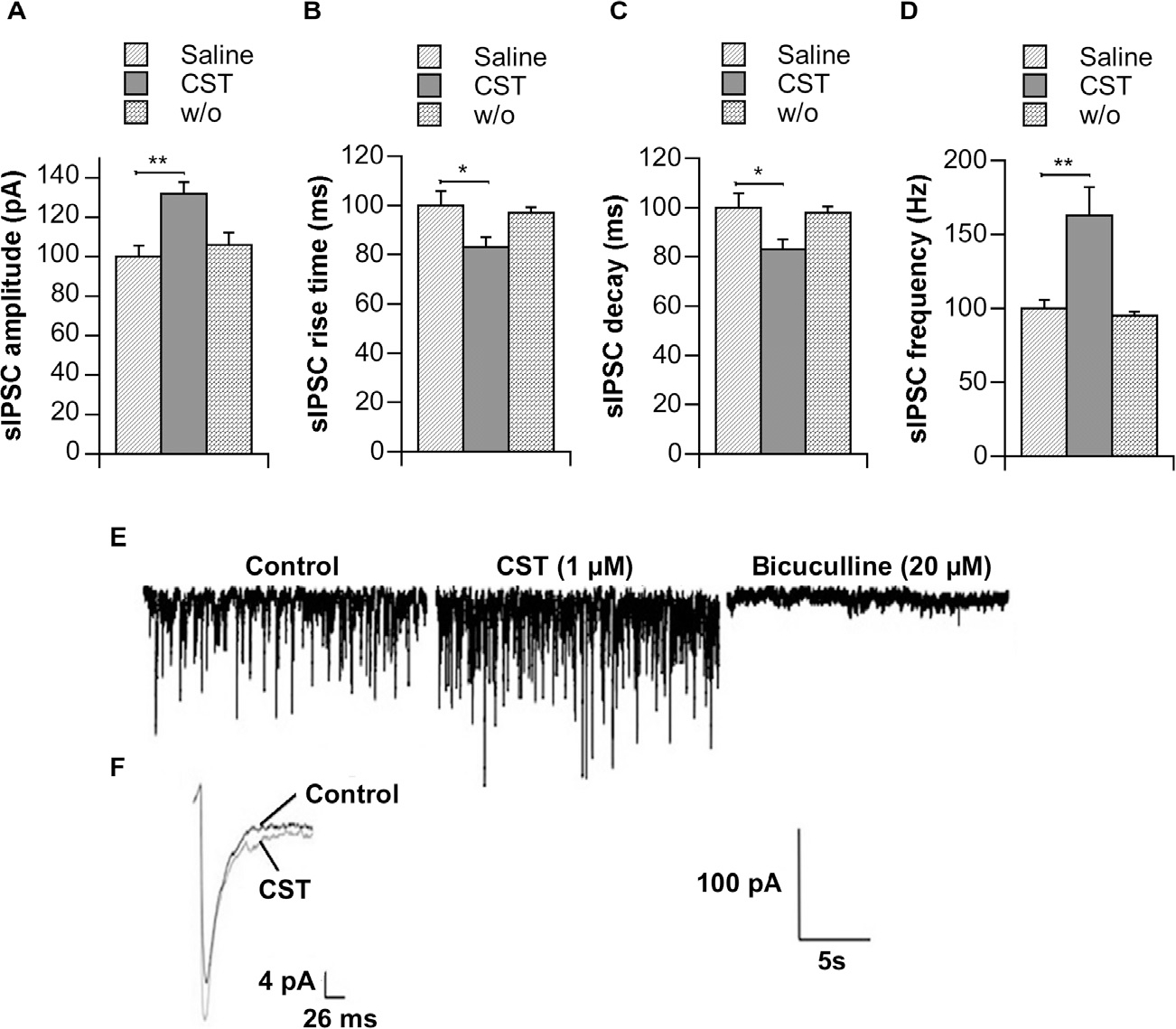
Effects of CST on GABAergic sIPSCs recorded for CeA pyramidal neurons. Absolute values (± s.e.m.) of different sIPSC kinetic parameters (A, amplitude; B, rise time; C, decay time; D, frequency) were collected from 5 different neurons of overt SHRs (9 months old) and recorded under control conditions (ACSF + 1 mM kynurenic acid), during CST (1 μM) perfusion (5 min) or 5 min after drug wash out (w/o). Representative traces of a single neuron under control conditions ± CST as well as during perfusion with the major GABAAR antagonist, i.e. bicuculline (20 μM), were illustrated (E). Additionally, mean average sIPSC traces under control conditions and during perfusion of CST were also added (F). *p < 0.05; **p < 0.01. Scale bar = 100 pA, 5 s.
Effects of CST and MUS on neuroprotection
Neurodegeneration, as judged by the presence of argyrophilic signals, was not seen in AMY and BS of 3-month-old SHRs (Fig. 4a–h). Neurodegenerating neurons (as shown by arrows) were detected in both AMY and BS of 6-month-old SHRs (Fig. 4i–p). Regarding AMY (Fig. 4i–l), these argyrophilic signals were reduced (~42%) after treatment with CST + MUS as compared to the saline-treated group (Fig. 4B). A significant neuroprotection (as judged by decreases in neurodegenerating neurons) was also seen in BS of 6-month-old rats (Fig. 4m–p) after treatments with CST (~60% reduction), MUS (~30% reduction) and especially after CST + MUS treatment (~90% reduction; Fig. 4B). Marked neurodegeneration (~3-fold increase over 6 month old) was detected in 9-month-old AMY and BS (Fig. 4q–x), where CST caused a ~61% reduction in neurodegenerating neurons as compared to saline treatment (Fig. 4C). These degenerating neurons exhibited increased argyrophilic reactions together with consistently dark and shrunken axonal and dendritic perikarya. Moreover, CST + MUS in AMY showed additive effects on neuroprotection with respect to single CST/MUS treatment (Fig. 4r–t) and these similar effects were even greater in BS (Fig. 4v–x).
Fig. 4.
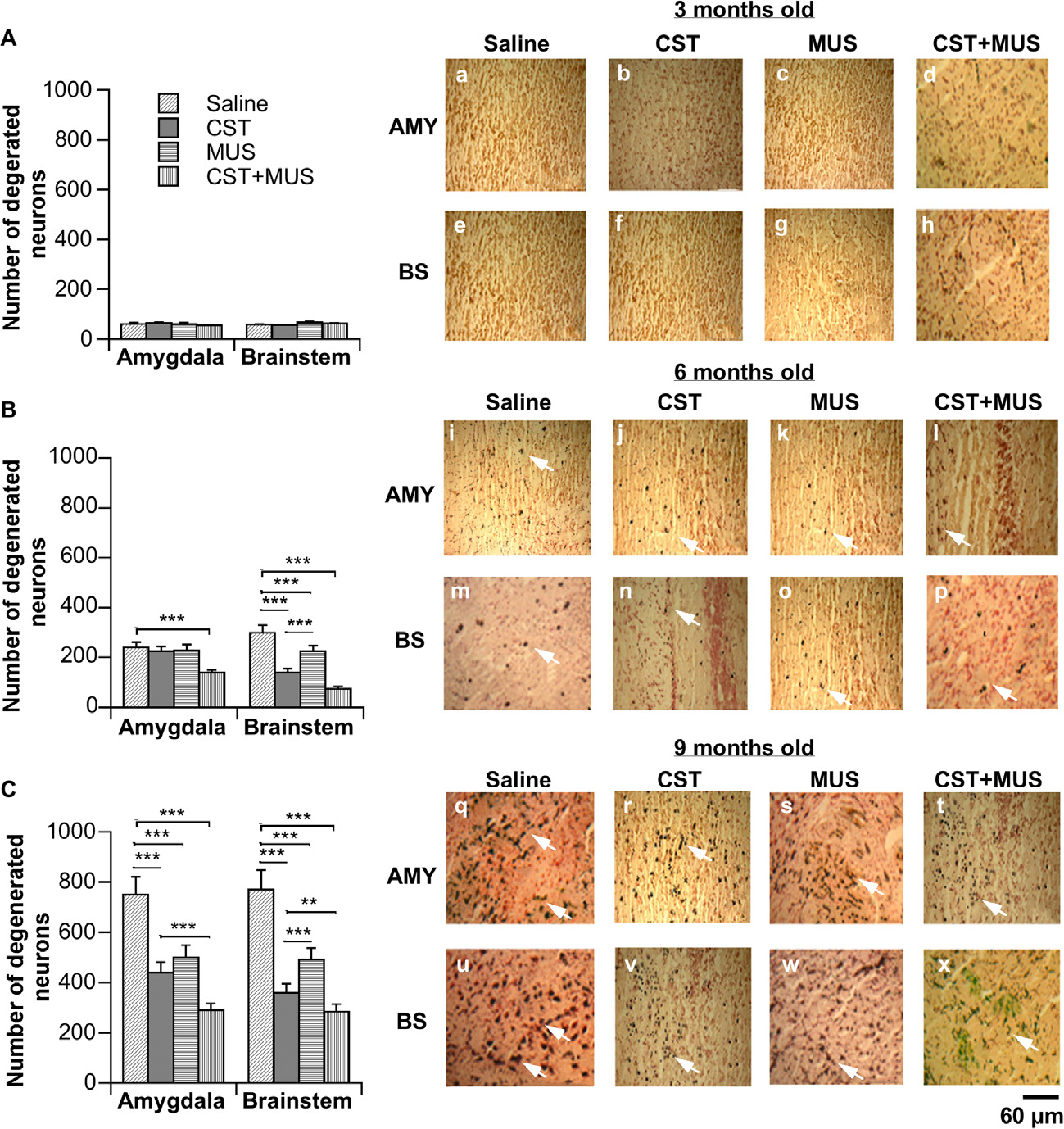
Neurodegeneration processes in AMY and BS of SHRs. Representative photograms of ACS (a–x) in which arrows point to dark neuronal perikarya that indicate damaged neurons showing the different levels of neurodegeneration/neuroprotection as reported for AMY and BS of SHRs that were 3 months old (Aa–h), SHRs that were 6 months old (Bi–p) and SHRs that were 9 months old (Cq–x) after brain injections of CST, MUS, CST + MUS in CeA with respect to controls that received only a saline solution. Scale bar = 60 μm. *p < 0.05, **p < 0.01 and ***p < 0.001.
CST and MUS signaling in AMY and BS
To further delineate the downstream signaling involved in the neuroprotective effects of CST, we looked at the major anti-apoptotic prosurvival mediators (Akt/ERK pathway) in only 9-month-old SHRs that showed overt HTN. Although CST and CST + MUS increased phosphorylation of Akt in the AMY, MUS alone had no effect on Akt phosphorylation as compared to the saline group (Fig. 5A). Moreover, ERK was phosphorylated by both CST and MUS, which in combination caused a partially additive effect (Fig. 5B). In the BS, CST increased phosphorylation of both Akt and ERK as compared to the saline-treated group (Fig. 5C, D). Even in this case CST and MUS injected together evoked additive effects on the phosphorylation of Akt and ERK (Fig. 5C, D).
Fig. 5.
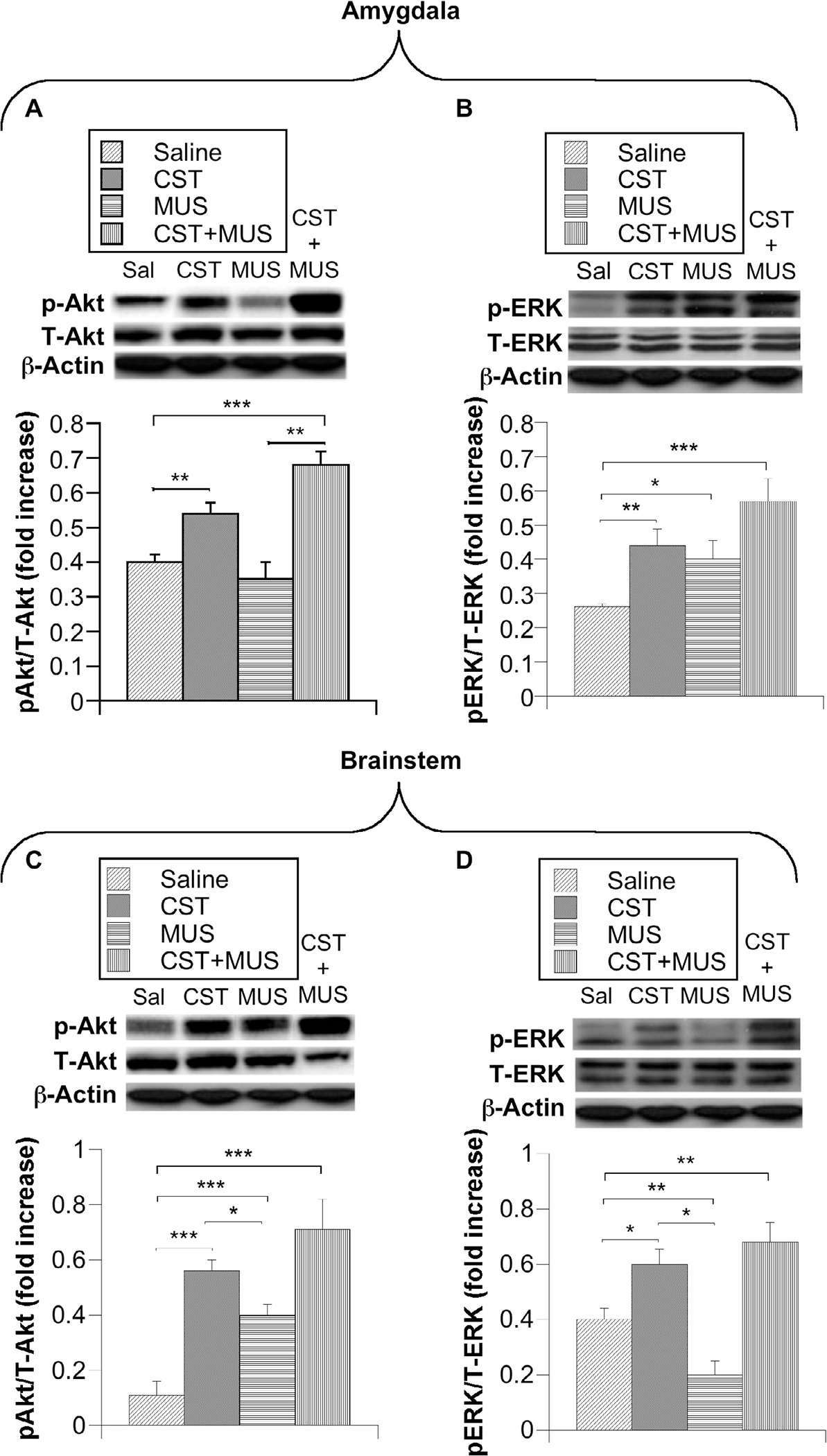
Immunoblotting was used to detect AMY (A, B) and BS (C, D) pAkt, pERK expression after brain injections of CST, MUS and CST + MUS in CeA of overt SHRs (9 months old). Quantification of immunoblotting results was expressed as either a ratio of pAkt/T-Akt or pERK/T-ERK. *p < 0.05, **p < 0.01 and ***p < 0.001.
DISCUSSION
In a similar manner to the antihypertensive effects on both monogenic (Chga knockout) (Mahapatra et al., 2005; Gayen et al., 2009; Biswas et al., 2012) and polygenic (high BP) HTN mice models (Biswas et al., 2012), continuous infusion of CST into CeA proved to be also responsible for a marked BP decrease (65 mmHg) in our HTN rodent model (SHR). Microinjections of CST into CVLM have recently been reported to cause a decrease in BP even in normotensive rats (Gaede and Pilowsky, 2012). However, peripheral administration of CST to normotensive mice caused a smaller decrease (5–15 mmHg) in BP (Mahapatra et al., 2005). How does the brain regulate BP? It may very well be that the crucial BS nuclei, which include CVLM, RVLM, and NTS constitute the central regulatory sites of BP. CVLM consists of small GABAergic interneurons that, by projecting in a rostral direction, inhibit the bulbospinal presympathetic neurons of RVLM (McAllen et al., 1994; Oshima et al., 2006), which have, instead, been shown to form synapses with the sympathetic preganglionic neurons (SPN). Moreover, studies established that increases in arterial BP activate baroreceptors in the periphery and consequently turn on excitatory baroreceptor-activated interneurons in NTS neurons, which then activate CVLM neurons eventuating in the reduction of sympathetic outflow (Lin et al., 2013). Curiously, while glutamate- and GABA-related mediations are primarily responsible for short-term regulation of BP, a long-term control is promoted, at least in part, by metabotropic neurotransmitters (Pilowsky et al., 2009), including peptides, such as neuropeptide Y (Kashihara et al., 2008), orexin (Machado et al., 2002) and substance P (Stornetta, 2009). In addition, the peripheral effects of CST have so far been reported to be mostly inhibitory in nature as demonstrated by the inhibition of catecholamine secretion (Mahata et al., 1997, 2000, 2010), accounting for inotropism and lusitropism (Angelone et al., 2008, 2012) together with a decrease in BP (Mahapatra et al., 2005; Biswas et al., 2012), whereas the central effects have instead been stimulatory (Gaede and Pilowsky, 2010, 2012). Consequently, microinjections of CST into RVLM evoked sympathoexcitatory activities, which resulted in a BP increase of the vagotomized normotensive rat (Gaede and Pilowsky, 2010). Likewise, microinjections of CST into CVLM caused sympathoinhibition, as it stimulated GABAergic interneurons that inhibited the excitatory outputs of RVLM leading to a decrease in BP (Gaede and Pilowsky, 2012).
It is becoming increasingly evident that BP can be regulated by the specific stimulation of neuronal cell bodies of CeA. This is line with the electrical stimulation of AMY in the rat and cat, accounting for an increase in HR, arterial BP and muscle blood flow (Stock et al., 1981; Galeno and Brody, 1983). However, electrical stimulation of CeA in the rabbit produced bradycardia, hypotension and hind limb vasodilation (Kapp et al., 1982; Dampney, 1994). Similar to previous reports, in the present study we found that continuous infusion of CeA neurons of SHRs with CST activated GABAergic interneurons in AMY (Gaede and Pilowsky, 2010, 2012), thus supplying diminished excitatory outputs from RVLM, which in turn accounted for decreased sympathetic outputs and consequently a decrease of BP (Fig. 6). Indeed, from whole-cell patch-clamp recordings of CeA pyramidal neurons in which CST increased both amplitude and frequency of sIPSC while bicuculline completely blocked sIPSC, it appears that the inhibitory effects were specifically GABAergic. This strengthens a novel antihypertensive action of CST through the stimulation of GABAARs belonging to such an AMY site.
Fig. 6.
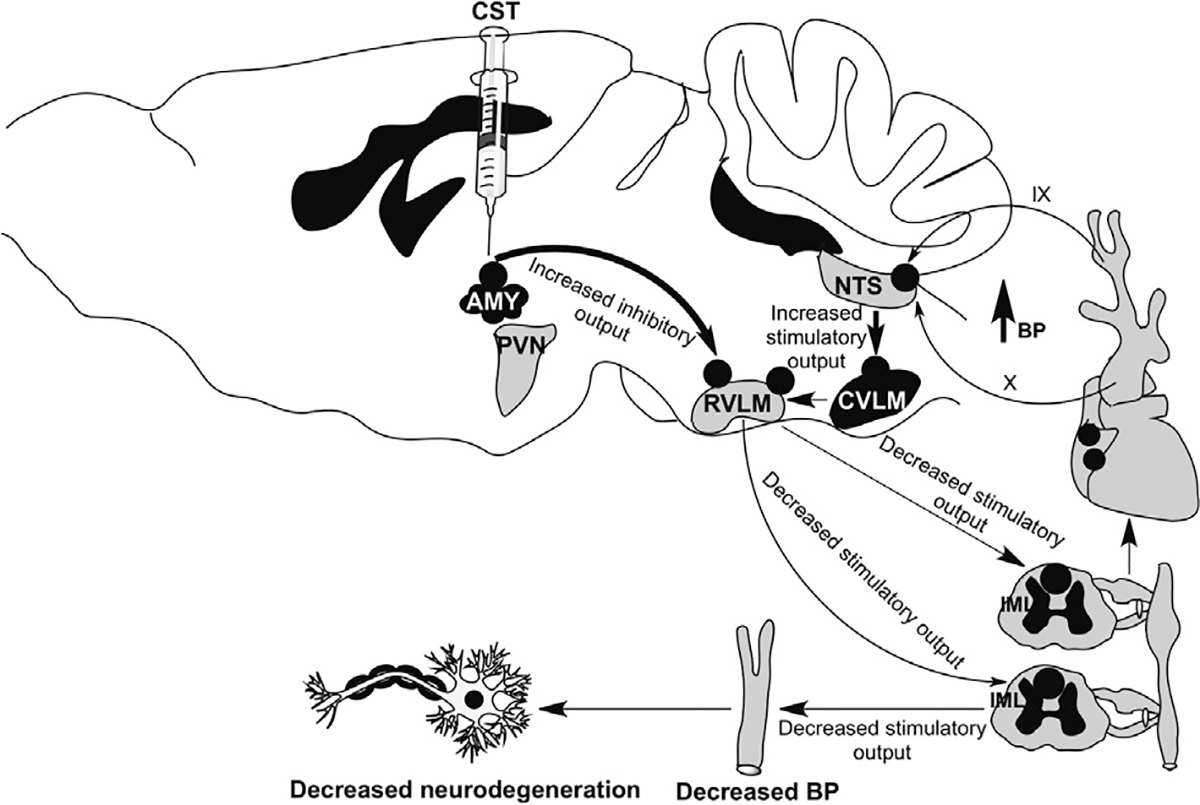
A schematic summary diagram showing the postulated pathways by which CeA regulates BP and neurodegeneration.
Another novel finding is the neuroprotective action of CST. Indeed, marked neurodegeneration signals that were detected in AMY and BS of 9-month-old SHRs were potently inhibited (>50%) by CST. In this case, the additive effects of CST and MUS being notably neurodegenerative in their nature tend to suggest that convergent signaling pathways of both compounds may be accounting for such an effect in a similar manner to the positive correlations between HTN and neurodegeneration detected in humans (Valerio Romanini et al., 2013). As far as HTN in older people is concerned, this disorder is associated with an increased risk of stroke and myocardial infarction (Chowdhury et al., 2014) together with an increased risk of cognitive decline (Wysocki et al., 2012), dementia (Qiu et al., 2005; Igase et al., 2012) and AD (Davies et al., 2011). On the other hand, high BP in middle-aged subjects is involved in the subsequent cognitive decline, mild cognitive impairment and dementia that occurs (Yamada et al., 2003; Whitmer et al., 2005). Moreover, it is believed that HTN potentiates aging-associated decreases in the number of cerebral capillaries coupled with thickening of the basement membrane (Farkas and Luiten, 2001), tortuous white matter vessels, and the consequent reduction in resting cerebral blood flow (Kalaria, 2009; Gasecki et al., 2013). At the same time, HTN is known to cause white matter hyper-intensities (Longstreth et al., 1996; Skoog, 1998), increased risk of incident infarcts (Knopman et al., 2011) and cerebral micro-bleeds (Loitfelder et al., 2012). These findings strongly point to chronic HTN interfering with the adaptive capacity of the brain to hypoperfusion conditions, thus accelerating the deteriorating effects of aging. Although anti-HTN treatments aimed to improve cognitive functions in longitudinal studies have not been shown to be conclusive (Grassi et al., 2011; Guan et al., 2011), such treatments did exert their greatest effects toward the prevention of dementia (Peters et al., 2008) despite other trials (MRC, SHEP, SCOPE, HYVET-COG) which found no significant effects (Duron and Hanon, 2010).
It was shown that Akt (Gomez del Pulgar et al., 2000) and ERK (Moranta et al., 2007) kinases participate in many pro-survival and anti-apoptotic processes inside and outside the brain (Dasari et al., 2008; Pignataro et al., 2008; Yune et al., 2008), including processes that rely on treatments with cannabinoids (Molina-Holgado et al., 2002; Ozaita et al., 2007). We found that continuous infusion of CST and especially CST + MUS caused significant neuroprotection by increased phosphorylation of Akt and ERK and consequent induction of their downstream anti-apoptotic pathways (Cohen-Yeshurun et al., 2011) in both AMY and BS. Furthermore, high levels of phosphorylated Akt/ERK in CeA could act as a “switching on” mechanism for sympathetic pathways as illustrated by increased sIPSC amplitudes and frequencies of pyramidal neurons in this same AMY site perfused with CST while they were suppressed by bicuculline, thus pointing to an inhibitory role played by this anti-hypertensive/cardioinhibitory CgA derivative.
CONCLUSION
The results of the present study corroborate for the first time that CST increases GABAergic effect and that CST + MUS injected in CeA preferentially exerts in vivo neuroprotective and anti-hypertensive effects in SHRs. Our results tend to suggest a cross-talking type of interaction between CST and GABAergic signals modulating brain vasoactive and anti-inflammatory pathways. Indeed, CST and MUS injections, by evoking decreased BP levels, seem to counteract high BP-dependent neuronal damages in hypertensive conditions. It appears that these effects are mediated at least in part by the induction of anti-apoptotic signals involving Akt/ERK phosphorylation. We are still at the beginning, but characterization of Akt/ERK signal cascades within AMY and BS sites via advanced pharmacological approaches may bring us closer to the unraveling of adaptive neurogenic mechanisms useful for understanding neurodegenerative disorders such as AD caused by cardiovascular impairment in HTN patients.
Acknowledgments—
We thank the Italian University Research Ministry (MIUR), Calabria Region (POR, FSE-2007/2013) for the financial support. S.K.M. is supported by the VA Research Career Scientist Award. We thank Sumana Mahata for editing the manuscript.
Abbreviations:
- ACS
amino cupric silver stain
- AMY
amygdala
- BP
blood pressure
- CeA
central amygdalar nucleus
- CST
catestatin
- CTRL
control
- HR
heart rate
- HTN
hypertension
- IML
intermediolateral cell coumn
- ix
glossopharyngeal nerve
- mACSF
modified artificial cerebrospinal fluid
- MUS
muscimol
- NTS
nucleus tractus solitarius
- PVN
paraventricular nucleus
- RVLM
rostral ventrolateral medulla
- SHR
spontaneously hypertensive rat
- sIPSC
spontaneous inhibitory postsynaptic current
- x
vagus nerve
REFERENCES
- Alò R, Avolio E, Di Vito A, Carelli A, Facciolo RM, Canonaco M (2010) Distinct α subunit variations of the hypothalamic GABAA receptor triplets (αβγ) are linked to hibernating state in hamsters. BMC Neurosci 11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC (2008) The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 149:4780–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelone T, Quintieri AM, Pasqua T, Gentile S, Tota B, Mahata SK, Cerra MC (2012) Phosphodiesterase type-2 and NO-dependent S-nitrosylation mediate the cardioinhibition of the antihypertensive catestatin. Am J Physiol Heart Circul Physiol 302:H431–H442. [DOI] [PubMed] [Google Scholar]
- Avolio E, Alo R, Carelli A, Canonaco M (2011) Amygdalar orexinergic–GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav Brain Res 218:288–295. [DOI] [PubMed] [Google Scholar]
- Biswas N, Gayen J, Mahata M, Su Y, Mahata SK, O’Connor DT (2012) Novel peptide isomer strategy for stable inhibition of catecholamine release: application to hypertension. Hypertension 60:1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno A, De Olmos S, Heimer L, De Olmos J (2003) NMDA-antagonist MK-801-induced neuronal degeneration in Wistar rat brain detected by the Amino-Cupric-Silver method. Exp Toxicol Pathol 54:319–334. [DOI] [PubMed] [Google Scholar]
- Cohen-Yeshurun A, Trembovler V, Alexandrovich A, Ryberg E, Greasley PJ, Mechoulam R, Shohami E, Leker RR (2011) Narachidonoyl-l-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Ceram Blood Flow Metab 31:1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury EK, Owen A, Krum H, Wing LM, Nelson MR, Reid CM (2014) Systolic blood pressure variability is an important predictor of cardiovascular outcomes in elderly hypertensive patients. J Hypertens 32:525–533. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB (2008) General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117:743–753. [DOI] [PubMed] [Google Scholar]
- Dampney RA (1994) Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74:323–364. [DOI] [PubMed] [Google Scholar]
- Dasari VR, Veeravalli KK, Saving KL, Gujrati M, Fassett D, Klopfenstein JD, Dinh DH, Rao JS (2008) Neuroprotection by cord blood stem cells against glutamate-induced apoptosis is mediated by Akt pathway. Neurobiol Dis 32:486–498. [DOI] [PubMed] [Google Scholar]
- Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM (2011) Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. JAD 26:699–708. [DOI] [PubMed] [Google Scholar]
- Duron E, Hanon O (2010) Antihypertensive treatments, cognitive decline, and dementia. JAD 20:903–914. [DOI] [PubMed] [Google Scholar]
- Eliash S, Shteter N, Eilam R (2005) Neuroprotective effect of rasagiline, a monamine oxidase-B inhibitor, on spontaneous cell degeneration in a rat model. J Neural Transm 112:991–1003. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG (2001) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64:575–611. [DOI] [PubMed] [Google Scholar]
- Gaede AH, Pilowsky PM (2010) Catestatin in rat RVLM is sympathoexcitatory, increases barosensitivity, and attenuates chemosensitivity and the somatosympathetic reflex. Am J Physiol Regul Integr Comp Physiol 299:R1538–R1545. [DOI] [PubMed] [Google Scholar]
- Gaede AH, Pilowsky PM (2012) Catestatin, a chromogranin A-derived peptide, is sympathoinhibitory and attenuates sympathetic barosensitivity and the chemoreflex in rat CVLM. Am J Physiol Regul Integr Comp Physiol 302:R365–R372. [DOI] [PubMed] [Google Scholar]
- Galeno TM, Brody MJ (1983) Hemodynamic responses to amygdaloid stimulation in spontaneously hypertensive rats. Am J Physiol 245:R281–R286. [DOI] [PubMed] [Google Scholar]
- Gasecki D, Kwarciany M, Nyka W, Narkiewicz K (2013) Hypertension, brain damage and cognitive decline. Curr Hypertens Rep 15:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen JR, Gu Y, O’Connor DT, Mahata SK (2009) Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin a null mouse. Endocrinology 150:5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez del Pulgar T, Velasco G, Guzman M (2000) The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J 347:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi D, Ferri L, Cheli P, Di Giosia P, Ferri C (2011) Cognitive decline as a consequence of essential hypertension. Curr Pharm Des 17:3032–3038. [DOI] [PubMed] [Google Scholar]
- Guan JW, Huang CQ, Li YH, Wan CM, You C, Wang ZR, Liu YY, Liu QX (2011) No association between hypertension and risk for Alzheimer’s disease: a meta-analysis of longitudinal studies. JAD 27:799–807. [DOI] [PubMed] [Google Scholar]
- Hamel E (2006) Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100:1059–1064. [DOI] [PubMed] [Google Scholar]
- Igase M, Kohara K, Miki T (2012) The association between hypertension and dementia in the elderly. Int J Hypertens 2012:320648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN (2009) Linking cerebrovascular defense mechanisms in brain ageing and Alzheimer’s disease. Neurobiol Aging 30:1512–1514. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Gallagher M, Underwood MD, McNall CL, Whitehorn D (1982) Cardiovascular responses elicited by electrical stimulation of the amygdala central nucleus in the rabbit. Brain Res 234:251–262. [DOI] [PubMed] [Google Scholar]
- Kashihara K, McMullan S, Lonergan T, Goodchild AK, Pilowsky PM (2008) Neuropeptide Y in the rostral ventrolateral medulla blocks somatosympathetic reflexes in anesthetized rats. Auton Neurosci 142:64–70. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, Mosley TH Jr (2011) Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology 76:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman LO, Chade AR, Sica V, Napoli C (2005) Animal models of hypertension: an overview. J Lab Clin Med 146:160–173. [DOI] [PubMed] [Google Scholar]
- Lin LH, Moore SA, Jones SY, McGlashon J, Talman WT (2013) Astrocytes in the rat nucleus tractus solitarii are critical for cardiovascular reflex control. J Neurosci 33:18608–18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loitfelder M, Seiler S, Schwingenschuh P, Schmidt R (2012) Cerebral microbleeds: a review. Panminerva Med 54:149–160. [PubMed] [Google Scholar]
- Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L (1996) Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 27:1274–1282. [DOI] [PubMed] [Google Scholar]
- Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ (2002) Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept 104:75–81. [DOI] [PubMed] [Google Scholar]
- Mahapatra NR, O’Connor DT, Vaingankar SM, Sinha Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J Jr, Mahata SK (2005) Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest 115:1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ (1997) Novel autocrine feedback control of catecholamine release. A discrete chromogranin A fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest 100:1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata SK, Mahata M, Wakade AR, O’Connor DT (2000) Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A344–364): identification of amino acid residues crucial for activity. Mol Endocrinol 14:1525–1535. [DOI] [PubMed] [Google Scholar]
- Mahata SK, Mahata M, Fung MM, O’Connor DT (2010) Catestatin: a multifunctional peptide from chromogranin A. Regul Pept 162:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masneuf S, Buetler J, Koester C, Crestani F (2012) Role of α1- and α2-GABA(A) receptors in mediating the respiratory changes associated with benzodiazepine sedation. Br J Pharmacol 166:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Habler HJ, Michaelis M, Peters O, Janig W (1994) Monosynaptic excitation of preganglionic vasomotor neurons by subretrofacial neurons of the rostral ventrolateral medulla. Brain Res 634:227–234. [DOI] [PubMed] [Google Scholar]
- Milić M, Divljaković J, Rallapalli S, Van Linn ML, Timić T, Cook JM, Savić MM (2012) The role of α1 and α5 subunit-containing GABAA receptors in motor impairment induced by benzodiazepines in rats. Behav Pharmacol 23:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C (2002) Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci 22:9742–9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranta D, Esteban S, Garcia-Sevilla JA (2007) Acute, chronic and withdrawal effects of the cannabinoid receptor agonist WIN55212–2 on the sequential activation of MAPK/Raf-MEK-ERK signaling in the rat cerebral frontal cortex: short-term regulation by intrinsic and extrinsic pathways. J Neurosci Res 85:656–667. [DOI] [PubMed] [Google Scholar]
- Morris M, Keller M, Sundberg DK (1983) Changes in paraventricular vasopressin and oxytocin during the development of spontaneous hypertension. Hypertension 5:476–481. [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ (2002) Early decline in the catecholamine releaseinhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens 20:1335–1345. [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, Mahata SK, Mahata M, Wang L, Zhang K, Greenwood TA, Shih PA, Cockburn MG, Ziegler MG, Stridsberg M, Martin NG, Whitfield JB (2008) Heritability and genome-wide linkage in US and Australian twins identify novel genomic regions controlling chromogranin A: implications for secretion and blood pressure. Circulation 118:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Aoki K (1963) Development of a strain of spontaneously hypertensive rats. Jap Circul J 27:282–293. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W (2009) GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima N, McMullan S, Goodchild AK, Pilowsky PM (2006) A monosynaptic connection between baroinhibited neurons in the RVLM and IML in Sprague-Dawley rats. Brain Res 1089:153–161. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R (2007) Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem 102:1105–1114. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C (2008) Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 7:683–689. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Gala R, Simon RP (2008) In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Ceram Blood Flow Metab 28:232–241. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Lung MS, Spirovski D, McMullan S (2009) Differential regulation of the central neural cardiorespiratory system by metabotropic neurotransmitters. Philos Trans R Soc Lond B Biol Sci 364:2537–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto YM, Paul M, Ganten D (1998) Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res 39:77–88. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L (2005) The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 4:487–499. [DOI] [PubMed] [Google Scholar]
- Saha S (2005) Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol 32:450–456. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G (2004) Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci 24:6521–6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I (1998) A review on blood pressure and ischaemic white matter lesions. Dement Geriatr Cogn Disord 9(Suppl. 1):13–19. [DOI] [PubMed] [Google Scholar]
- Stock G, Rupprecht U, Stumpf H, Schlor KH (1981) Cardiovascular changes during arousal elicited by stimulation of amygdala, hypothalamus and locus coeruleus. J Auton Nerv Syst 3:503–510. [DOI] [PubMed] [Google Scholar]
- Stornetta RL (2009) Neurochemistry of bulbospinal presympathetic neurons of the medulla oblongata. J Chem Neuroanat 38:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio Romanini C, Dias Fiuza Ferreira E, Correia Bacarin C, Verussa MH, Weffort de Oliveira RM, Milani H (2013) Neurohistological and behavioral changes following the fourvessel occlusion/internal carotid artery model of chronic cerebral hypoperfusion: comparison between normotensive and spontaneously hypertensive rats. Behav Brain Res 252:214–221. [DOI] [PubMed] [Google Scholar]
- Viltart O, Sartor DM, Verberne AJ (2006) Chemical stimulation of visceral afferents activate medullary neurones projecting to the central amygdala and periaqueductal grey. Brain Res Bull 71:51–59. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64:277–281. [DOI] [PubMed] [Google Scholar]
- Wysocki M, Luo X, Schmeidler J, Dahlman K, Lesser GT, Grossman H, Haroutunian V, Beeri MS (2012) Hypertension is associated with cognitive decline in elderly people at high risk for dementia. Am J Geriat Psychiatry 20:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G (2003) Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriat Soc 51:410–414. [DOI] [PubMed] [Google Scholar]
- Yune TY, Park HG, Lee JY, Oh TH (2008) Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/Akt-dependent CREB activation. J Neurotrauma 25:1121–1131. [DOI] [PubMed] [Google Scholar]
- Zubcevic J, Potts JT (2010) Role of GABAergic neurons in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp Physiol 95:909–918. [DOI] [PubMed] [Google Scholar]


