Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) prevalence is rapidly growing, and fatty liver has been found in a quarter of the US population. Increased liver lipids, particularly those derived from the pathway of de novo lipogenesis (DNL), have been identified as a hallmark feature in individuals with high liver fat. This has led to much activity in basic science and drug development in this area. No studies to date have investigated the contribution of DNL across a spectrum of disease, although it is clear that inhibition of DNL has been shown to reduce liver fat.
Objectives
The purpose of this study was to determine whether liver lipid synthesis increases across the continuum of liver injury.
Methods
Individuals (n = 49) consumed deuterated water for 10 d before their scheduled bariatric surgeries to label DNL; blood and liver tissue samples were obtained on the day of the surgery. Liver lipid concentrations were quantitated, and levels of protein and gene expression assessed.
Results
Increased liver DNL, measured isotopically, was significantly associated with liver fatty acid synthase protein content (R = 0.470, P = 0.003), total steatosis assessed by histology (R = 0.526, P = 0.0008), and the fraction of DNL fatty acids in plasma very low-density lipoprotein-triacylglycerol (R = 0.747, P < 0.001). Regression analysis revealed a parabolic relationship between fractional liver DNL (percent) and NAFLD activity score (R = 0.538, P = 0.0004).
Conclusion
These data demonstrate that higher DNL is associated with early to mid stages of liver disease, and this pathway may be an effective target for the treatment of NAFLD and nonalcoholic steatohepatitis.
This study was registered at clinicaltrials.gov as NCT03683589.
Keywords: de novo lipogenesis, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, triglycerides, cholesterol esters
Introduction
Nonalcoholic fatty liver disease (NAFLD) includes a spectrum of conditions ranging from the accumulation of lipids in the liver (steatosis) to inflammation and ballooning, which occurs in a condition called nonalcoholic steatohepatitis (NASH), which may advance to liver fibrosis and cirrhosis [1]. The epidemic of NAFLD and NASH continues to grow globally and is estimated to affect a quarter of the population of the US and worldwide [2]. Recently, NAFLD was predicted to replace viral hepatitis as a leading cause of end-stage liver disease, and NASH is now recognized as a major reason for hepatocellular carcinoma-related liver transplantation in the United States [1,3]. A wide range of metabolic abnormalities have been associated with NAFLD [4, 5] that are related to increased adipose fatty acid (FA) flux to the liver [6], decreased VLDL secretion [7], and within the liver, synthesis of newly-made FAs from the pathway of de novo lipogenesis (DNL) [[8], [9], [10]]. Data from an original publication suggested that the extent of liver lipogenesis can be assessed by measuring the amount of newly-made FAs found in plasma VLDL-triacylglycerol (VLDL-TAG), which are secreted from the liver [10]. Since then, in individuals with high levels of liver fat, approximately 26% to 50% of plasma VLDL-TAG palmitate has been shown to be derived from DNL [10,11]. Insulin resistance is an important feature of NAFLD development [[11], [12], [13]], and the sources of carbon used for FA synthesis primarily originate from carbohydrates including fructose and glucose [12,14,15]. In a detailed comparison between patients with NAFLD and equally insulin-resistant, weight-matched subjects with low liver fat, of the 20 metabolic variables tested (features of glucose and fat metabolism, hormones, age, body composition), hepatic DNL was the key distinguishing characteristic between those with and without fatty liver, i.e., DNL was significantly (3-fold) higher in the individuals with fatty liver compared with those without fatty liver [8].
Although the incidence of NAFLD is rising rapidly, no currently approved drug treatments exist, and numerous pharmaceutical strategies are under active investigation [[16], [17], [18], [19], [20], [21], [22]]. Within the stages of NAFLD development, steatosis appears to be an essential early step in the evolution of the disease [23]. We, and others, have shown that inhibiting DNL pharmacologically reduces liver fat and blood lipid levels, including blood cholesterol levels and liver enzymes, and some studies have demonstrated improvements in markers of insulin resistance [[17], [18], [19], [20], [21], [22]]. However, no study to date has compared DNL levels between groups with progressively worsening categories of liver health measured by liver biopsy. Since synthesis pathways for FAs, TAG, and cholesterol are coordinated in a molecular fashion [24,25], we developed 3 hypotheses for this study. First, elevated FA synthesis would increase the presence of newly-made FAs in liver TAG and cholesterol ester (CE) in concert. Second, lipogenesis levels would be higher in patients with greater levels of liver injury as assessed by histologic evaluation of liver tissue. Third, levels of lipogenesis measured directly in liver tissue would be accurately reflected in plasma VLDL-TAG. To study individuals whose liver health spanned the range from no indications of liver disease to those with potentially advanced levels of liver injury, patients scheduled for bariatric surgery were recruited for the present study. One benefit of this population is that individuals in this cohort have conditions that encompass a spectrum of liver health, varying from normal to cirrhosis [26]. These findings provide the first evidence that across a group of patients with worsening liver histology, DNL levels are increased. These results may aid in expanding our understanding of the role DNL plays in the different stages of NAFLD.
Material and Methods
All methods and procedures were approved by the University of Missouri Institutional Review Board (MU-IRB# 2012544), and the study was registered at clinicaltrials.gov (NCT03683589). This investigation does not meet the definition of a clinical trial. To describe the process of study activities, a study flow diagram is shown in Supplementary Figure S1. From a total of 142 patients undergoing bariatric surgery, 127 patients were identified to be introduced to the study during their presurgery orientation at the University of Missouri Bariatric Clinic. We excluded 71 patients for several reasons (outlined in Supplementary Figure S1). Therefore, we consented remaining 56 participants, and of them, 49 received the intervention and completed the study with data used for analyses.
Study design and visits
Before surgery, subjects were encouraged to consume a high-protein, liquid diet low in carbohydrates as per established presurgery guidelines [27]. This diet was prescribed to be consumed for 1 wk if the subject’s BMI was <50 kg/m2 (n = 34) and for 2 wk their BMI was >50 kg/m2 (n = 15). To maintain consistency among all groups, only participants who adhered to the diet (as indicated by modest levels of presurgery weight loss) were included in the study. As shown in Supplementary Figure S2, once the patient signed the consent form, they were provided with deuterated water (D2O) for 10 d prior to their surgery. The patient consumed 150 mL of D2O (50 mL doses consumed orally 3 times) on day 1 to increase the D2O enrichment in the plasma, then from days 2 to 10, 50 mL per day. On day 11, 2 h prior to the surgery, a fasting sample of the blood was obtained prior to anesthesia for all the measurements, and the FibroScan 530 Compact (Echosen North America) was performed to measure liver steatosis, assessed using the controlled attenuation parameter (CAP). A score of 238 to 260 dB/m represents 11% to 33% liver fat, 260 to 290 dB/m represents 34% to 66% liver fat, and >290 dB/m represents >67% liver fat. For liver stiffness, assessed using vibration-controlled transient elastography (VCTE), a score of 2 to 7 kPa represents F0 to F1, 7.5 to 10 kPa represents F2, 10 to 14 kPa represents F3, and >14 kPa represents F4 [28]. During surgery, a liver tissue sample (200–300 mg) was collected by the surgeon 30 min after induction of anesthesia using a standard wedge biopsy technique [29].
Blood sample processing and biochemical measurements
Blood samples were immediately processed. An aliquot was sent to an external laboratory for a complete metabolic panel, and the remaining plasma was stored in a −80oC freezer. Plasma concentrations of total cholesterol, TAG, LDL cholesterol, HDL cholesterol, hemoglobin A1c, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured by a CLIA-standardized laboratory (#26D0652092, Quest Diagnostics). The measurements of lipids were performed via autoanalyzer (Roche Cobas 8000 System, coefficient of variance [CV] 0.6–0.9%) using electrochemiluminescent immunoassay. Liver enzymes were measured using ultraviolet absorbance (Roche Cobas 8000 System, CV 0.5–3.2% for AST and 0.5–3.1% for ALT). Assay kits were used to measure the concentrations of plasma glucose (#439-90901, CV 6.6%, Wako), and plasma insulin was measured by enzyme-linked immunosorbent assay (#EZHI-14K, Human Insulin, CV 7.2%, EMD Millipore).
Liver histological scoring, western blotting, and qRT-PCR
Once liver tissue was obtained, the sample was immediately transferred to the research laboratory on ice and was weighed instantly in a 0.9% sterile sodium chloride solution (#306546, BD PosiFlush). Tissue (50 mg) was fixed to prepare for histology performed by an experienced hepatopathologist (AD-A) according to the Brunt scoring scale for NAFLD activity score (NAS) and fibrosis score [30,31]. For western blotting, liver tissue was washed with ice-cold phosphate-buffered saline (PBS) and lysed with a buffer solution, processed, and probed as described previously [32,33]. Blots were normalized to total protein staining. For RNA extraction, samples were washed with ice-cold PBS and lysed in the buffer, and RNA was isolated using the RNeasy mini kit (#74104, Qiagen GmbH) per the manufacturer’s instructions. A cDNA library was synthesized, and a Nanodrop spectrometer was used to measure cDNA and RNA purity and assess quality. A list of primers is presented in Supplementary Table S1. Data are presented relative to GAPDH using the 2−ΔΔCT method [32]. All analyses were performed without the knowledge of the histologic score.
Liver-TAG and -CE content and FA composition
For liver-TAG, approximately 30 mg of tissue was extracted using the Folch method [34] and processed for biochemical analysis, mass spectrometry, and chromatography. Lipogenesis was measured as described previously [10] with minor modifications detailed in the Supplementary Methods. The fractional DNL (expressed as a percentage) reflects intrahepatic assembly of lipid and is a read-out of whether one lipid source is preferred over another (nonDNL derived from the diet or adipose free FA) for intrahepatic TAG (IHTAG) synthesis. By contrast, the total liver FA synthesis is presented in units of mg/g liver and referred to as absolute DNL (absDNL). AbsDNL is calculated by multiplying the percent DNL (14:0, 16:0, and 18:0) by total lipid concentration (e.g., percent DNL in 16:0 FA from liver-TAG is multiplied by total liver-TAG 16:0 concentration) [35]. In VLDL, the absDNL has units of mg/dL and represents the total quantity of 14:0, 16:0, and 18:0 FAs made de novo that are carried in the VLDL particles in plasma. Additional details pertaining to liver tissue processing, histological scoring, western blotting, and lipid and DNL quantification are presented in Supplementary Methods (Appendices A1.1–A1.4).
Data and statistical analysis and calculations
Once the liver histologic scoring was obtained from the histopathologist, patients were separated into 4 groups, as described previously [36], based on the NAS [31]. Using the NAS includes a recording of the absence of characteristics; for example, histologic steatosis of <5% of the liver surface area (under low or medium power) gives a steatosis score of 0. Here, the No-NAFLD category shown in Table 1 is defined as containing structural zeros for steatosis, inflammation, and cellular ballooning, as described previously [37]. Data are presented as mean ± SD. HOMA-IR was calculated using the formula [Ins(U/L)×glu(mg/dL)]/405. One-factor analysis of variance (ANOVA) was performed using the IBM Statistical Package for the Social Sciences (SPSS, v26, 2019) to test differences between groups for anthropometric and biochemical variables where NAS group classification was used as a between-subject variable. For anthropometric and biochemical measurements, Bonferroni post hoc analysis was performed if P < 0.10 to identify differences between individual groups. However, for ANOVA, only P ≤ 0.05 were considered significant. For all other variables, Kruskal–Wallis ANOVA was performed using GraphPad Prism (v9.3.1 GraphPad Software) to test for the overall difference. If significant, Dunn’s multiple comparisons test was performed. Regression analysis was performed using SPSS to test the relationship between 2 variables. A polynomial regression analysis was performed to test the relationship between liver-TAG fxn DNL and NAS (Figure 1C). Pearson correlation analysis was used to test relationships between variables as presented in Figure 2A, B, F, G and Figure 4C, D.
TABLE 1.
Characteristics of subjects by histologic grouping
| Subject characteristics | No-NAFLD (n = 8) | NAFL (n = 11) | Borderline NASH (n = 14) | NASH (n = 16) | ANOVA P |
|---|---|---|---|---|---|
| NAS | 0.0 ± 0.0 | 1.6 ± 0.8 a | 2.9 ± 0.8 a | 4.8 ± 0.8 b | <.00011 |
| Steatosis (0–3) | 0.0 ± 0.0 | 1.6 ± 0.8 a | 1.9 ± 0.7 a | 2.4 ± 0.5 b | .00981 |
| Inflammation (0–3) | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.1 ± 0.5 a | 1.3 ± 0.5 a | <.00012 |
| Ballooning (0–2) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 a | 1.1 ± 0.3 b | <.00012 |
| Fibrosis | 0.0 ± 0.0 a,b | 0.0 ± 0.0 a | 0.6 ± 1.3 a,b | 0.8 ± 1.0 b | .01111 |
| Female/male (n/n) | 8/0 | 9/2 | 11/3 | 11/5 | |
| Ethnicity (%) | |||||
| White | 100 | 82 | 86 | 88 | |
| African American | 0 | 18 | 7 | 6 | |
| Others | 0 | 0 | 7 | 6 | |
| Hispanic/Latino (%) | 0 | 0 | 0 | 0 | |
| Age (y) | 46.9 ± 12.4 | 47.1 ± 7.7 | 46.0 ± 12.8 | 54.8 ± 10.5 | .122 |
| BMI (kg/m2) | 43.5 ± 10.8 | 48.0 ± 8.6 | 45.0 ± 7.1 | 46.5 ± 8.5 | .500 |
| Body weight (kg) | 121.3 ± 23.2 | 137.2 ± 34.1 | 128.6 ± 25.9 | 136.0 ± 20.9 | .503 |
| FibroScan | |||||
| CAP (dB/m) | 323 ± 51 | 325 ± 64 | 313 ± 32 | 350 ± 54 | .475 |
| VCTE (kPa) | 5.2 ± 1.6 | 9.7 ± 6.2 | 7.2 ± 3.7 | 12.0 ± 9.0 | .181 |
| Plasma glucose (mg/dL) | 109 ± 43 | 123 ± 60 | 106 ± 49 | 120 ± 39 | .774 |
| Hemoglobin A1c (%) | 6.2 ± 1.3 | 6.6 ± 1.6 | 6.1 ± 1.4 | 6.8 ± 1.9 | .602 |
| Insulin (U/L) | 6.2 ± 2.6 a | 6.6 ± 3.3 a | 9.9 ± 3.8 a,b | 15.6 ± 14.5 b | .037 |
| HOMA-IR {[Ins(U/L)×glu(mg/dL)]/405} | 1.82 ± 1.37 a | 1.81 ± 0.80 a | 2.68 ± 1.76 a,b | 4.51 ± 4.31 b | .047 |
| AST (U/L) | 20.1 ± 5.8 a | 29.8 ± 20.3 a | 22.7 ± 9.8 a | 37.3 ± 20.1 b | .053 |
| ALT (U/L) | 23.6 ± 8.8 a | 33.2 ± 25.9 a | 29.4 ± 20.6 a | 45.9 ± 26.0 b | .099 |
| Triacylglycerols (mg/dL) | 124 ± 35 | 145 ± 79 | 129 ± 58 | 161 ± 61 | .434 |
| Total cholesterol (mg/dL) | 150 ± 19 | 163 ± 42 | 172 ± 28 | 155 ± 37 | .406 |
| LDLc (mg/dL) | 86 ± 19 | 98 ± 35 | 110 ± 23 | 95 ± 32 | .266 |
| HDLc (mg/dL) | 41 ± 15 | 40 ± 9 | 39 ± 10 | 37 ± 9 | .760 |
Data are mean ± SD. From the subject liver histological analysis, a NAFLD activity score (NAS) was calculated, and the components of NAS were used a priori to categorize individuals into 4 groups as follows. No-NAFLD: healthy liver, NAS=0. Nonalcoholic fatty liver (NAFL): All subjects with steatosis only (steatosis grades of 1, 2, or 3; inflammation and ballooning scores = 0). Borderline NASH: The presence of steatosis at any level and either inflammation or ballooning scores greater than 0. NASH: The presence of steatosis, inflammation, and ballooning scores, each greater than 0.
For anthropometric and biochemical measurements, 1-way ANOVA was performed, and if significance was achieved (P < 0.10), Bonferroni post hoc tests were performed.
Within a row, superscript lowercase letters that are not shared represent values that are significantly different from one another.
FibroScan CAP score represents liver fat (n = 32), and the FibroScan VCTE score represents a measure of stiffness (n = 35).
Abbreviations: ANOVA, analysis of variance; AST, aspartate transaminase; ALT, alanine aminotransferases; BMI, body mass index; CAP, controlled attenuation parameter from the FibroScan; LDLc, low-density lipoprotein cholesterol; HDLc, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; NAFL, nonalcoholic fatty live; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; VCTE, vibration-controlled transient elastography.
Kruskal–Wallis ANOVA was used to determine whether any of the 4 groups’ means for a given outcome were different from another. If significant, Dunn’s multiple comparisons test was performed.
Student t-test was used to test for significance between Borderline NASH and NASH groups only.
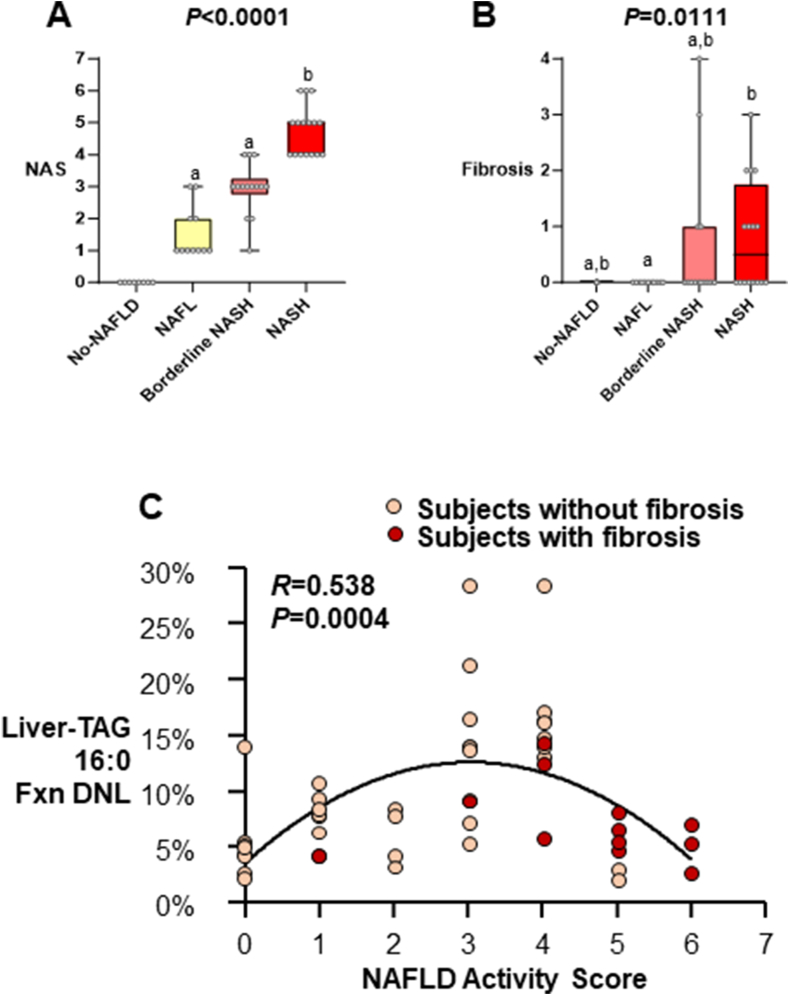
FIGURE 1.
NAS and fibrosis score by subject group. Data are mean ± SD (total n = 49, No-NAFLD n = 8, NAFL n = 11, Borderline NASH n = 14, NASH n = 16). Subject liver histologies were used a priori to categorize individuals into 4 groups based on NAS. (A) Kruskal–Wallis ANOVA was performed for the NAS between the 3 groups that had scores greater than zero. Dunn’s multiple comparisons test was performed to reveal differences between groups. Lowercase letters that are not shared represent values that are significantly different from one another. (B) Although fibrosis scores were not used to categorize subjects into groups, Kruskal–Wallis ANOVA revealed a significant difference between groups, and Dunn’s multiple comparisons test revealed no significant difference in fibrosis between Borderline NASH and NASH. (C) Relationship between liver-TAG Fxn DNL and NAS. Polynomial regression analysis was performed, and a significant relationship was found between liver-TAG Fxn DNL and NAS (total n = 49, No-NAFLD n =8, NAFL n = 11, Borderline NASH n = 14, NASH n = 16, R = 0.538, P = 0.0004). Abbreviations: ANOVA, analysis of variance; DNL, de novo lipogenesis; Fxn, fractional; NAFL, nonalcoholic fatty liver; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis; SD, standard deviation; TAG, triacylglycerol.
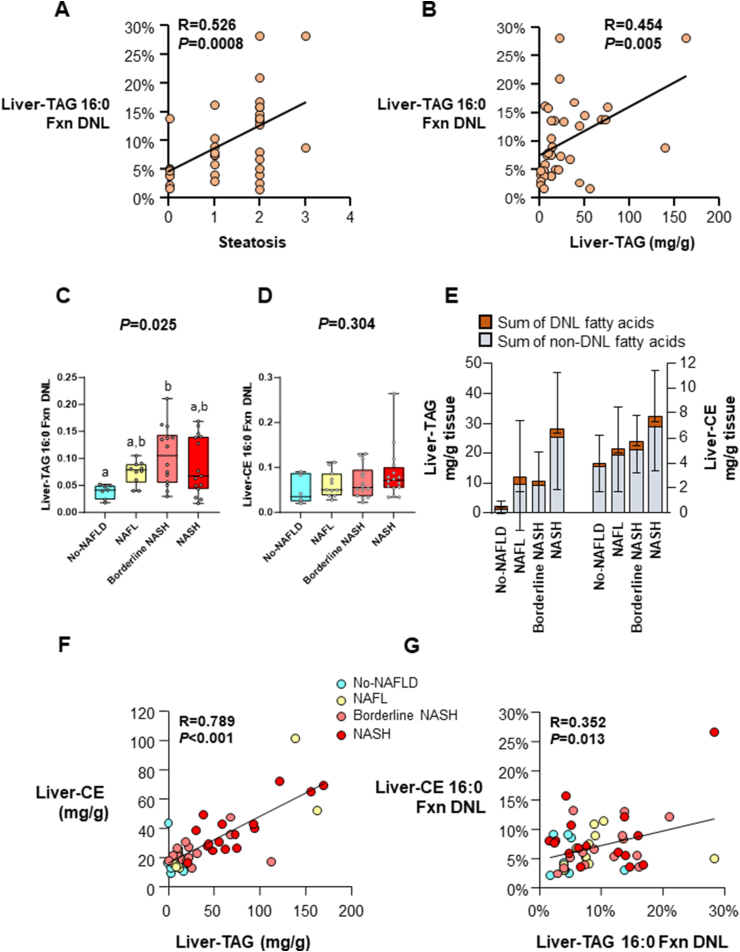
FIGURE 2.
Liver fractional lipogenesis, lipid contents, and interrelationships. Total n = 49, No-NAFLD n = 8, NAFL n = 11, Borderline NASH n = 14, NASH n = 16, unless otherwise noted.
All liver lipid was presented in units of mg lipid per g liver tissue wet weight. (A, B) Pearson correlation analysis was performed in patients without fibrosis (total n = 37, No-NAFLD n = 8, NAFL n = 11, Borderline NASH n = 10, NASH n = 8), and a significant relation was observed between liver-TAG Fxn DNL and (A) steatosis grade by histology and (B) liver-TAG (mg/g).
(C, D) Data are reported as median with confidence interval. Kruskal–Wallis ANOVA was performed between the groups, and the P value is presented above each bar graph. If significant, Dunn’s multiple comparisons test was performed to test the significance of each group. For comparisons that were significant, lowercase letters that are not shared represent values that are significantly different from one another. (C) Fxn DNL in liver-TAG palmitate (16:0). Total n = 46, No-NAFLD n = 7, NAFL n = 10, Borderline NASH n = 14, NASH n = 15; and (D) Fxn DNL in liver-CE 16:0. (E) DNL and non-DNL sources of liver-TAG and liver-CE. Data are reported in mean ± SD. On the left axis, the top of the columns represents the sum of DNL-fatty acids found in liver-TAG myristate (14:0), palmitate (16:0), and stearate (18:0), and the bottom of these columns represent the content of these fatty acids from other unlabeled sources such as the diet, plasma NEFA pool, or stored TAG that did not turn over during the 2 wk of labeling. Similarly, for liver-CE (right axis) the sum of 14:0, 16:0, and 18:0 made from DNL is at the top of the column, and non-DNL sources of these fatty acids are shown at the bottom of the columns. Absolute DNL was calculated by multiplying the percent DNL of each fatty acid (14:0, 16:0, and 18:0) with the concentration of the specific fatty acids in these lipids (14:0, 16:0, and 18:0). No statistical analysis was performed for this figure. (F, G) Bivariate Pearson correlation analysis with 2-tailed significance was performed to test the correlation between (F) liver-CE and liver-TAG content and (G) the fractional DNL of the 2 lipids. Abbreviations: CE, cholesterol ester; DNL, de novo lipogenesis; Fxn, fractional; NAFL, nonalcoholic fatty liver; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis; NEFA, nonesterified fatty acid; TAG, triacylglycerol.
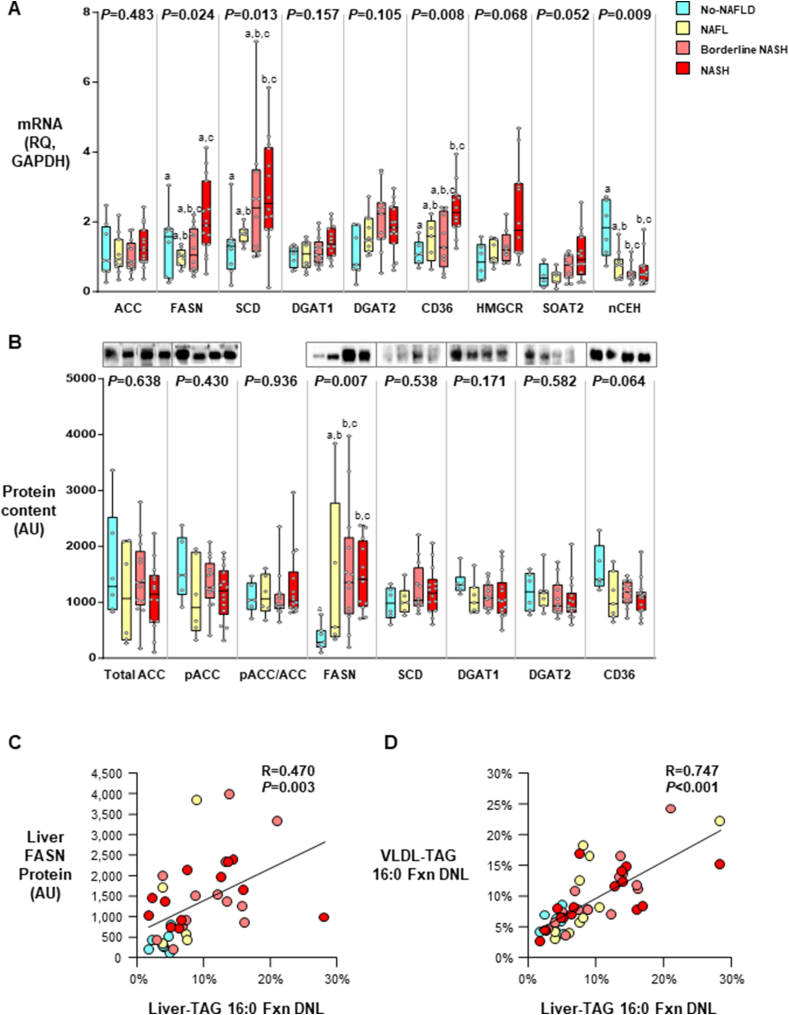
FIGURE 4.
Liver mRNA expression and protein content and relationships to liver-TAG DNL and VLDL-TAG DNL. Data are reported as median with confidence interval, with sample sizes below. Kruskal–Wallis ANOVA was performed between the groups, and the P value is presented above each bar graph. If significant, Dunn’s multiple comparisons test was performed to test the significance of each group. (A) Liver mRNA expression of key enzymes involved in the DNL and TAG synthesis pathways (n = 32–44 per gene). In the NAFL, Borderline NASH, and NASH groups, the data suggested that excess liver-CE (Figure 2) was due to decreased hydrolase activity, whereas in the NASH group, the data suggest an additional effect of increased esterification by SOAT2 and decreased nCEH activity. (B) Liver protein contents of enzymes for TAG and fatty acid synthesis and fatty acid transport (n = 37–40 per protein).
Pearson correlation analysis was performed, and a significant relation was observed between (C) liver FASN protein level and fractional DNL in liver-TAG (total n = 37, No-NAFLD n = 7, NAFL n = 5, Borderline NASH n = 12, NASH n = 12, R = 0.470, P = 0.003), and (D) fractional DNL in VLDL-TAG and fractional DNL in liver-TAG (total n = 49, No-NAFLD n = 8, NAFL n = 11, Borderline NASH n = 14, NASH n = 16; R = 0.747, P < 0.001). Abbreviations: ANOVA, analysis of variance; AU, arbitrary unit; CE, cholesterol ester; DNL, de novo lipogenesis; Fxn, fractional; NAFL, noalcoholic fatty liver; NAFLD, nonalcholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RQ, fold change relative to GAPDH; TAG, triacylglycerol; VLDL, very low-density lipoprotein.
Results
As described in the Supplementary Materials, 49 subjects scheduled for bariatric surgery consumed D2O to label DNL, their anthropometrics and blood biochemistries were assessed, and liver samples were taken during surgery and graded by a single pathologist using the NAS system [31]. Based on histologic assessment, liver histology was used a priori to group individuals into 4 categories [37]. Categories included No-NAFLD (healthy liver); those with nonalcoholic fatty liver (NAFL), which included all subjects with steatosis only (grades of 1, 2, or 3); Borderline NASH, which was indicated by the presence of steatosis and either inflammation or ballooning; and the category of NASH, which was indicated by the presence of steatosis, inflammation, and ballooning. Considering the way total NAS is calculated, it was anticipated, but not guaranteed, that the NAS would be different between the groups. This was found to be the case, as shown in Table 1 and Figure 1. For the subscores, steatosis increased progressively with the severity of the disease, as expected (Table 1). Inflammation was similar between Borderline NASH and NASH; however, ballooning was greater in NASH compared with Borderline NASH. The mean fibrosis score (a characteristic not used in the NAS calculation) was similar between Borderline NASH and NASH. With regard to the other characteristics of these groups, the proportion of White ethnicity was higher in all groups (Table 1), as was the proportion of females in the No-NAFLD group. The groups were matched for age, BMI, and body weight. Results from the FibroScan revealed no differences between the groups in the CAP and VCTE scores (Table 1) or differences in glucose concentrations or HbA1c. Insulin concentrations were significantly higher in the group with the most severe disease (NASH, P = 0.035), as was HOMA-IR (P = 0.047). AST and ALT tended to rise progressively across the groups, but this did not reach significance, suggesting that these blood markers are poor indicators of liver fat content/function. Plasma lipids (Table 1) were not different between the groups; although, as a cohort, TAGs tended to be higher and HDLc concentrations lower than levels observed in non-NAFLD populations.
Comparison of liver lipids across groups with worsening NAS
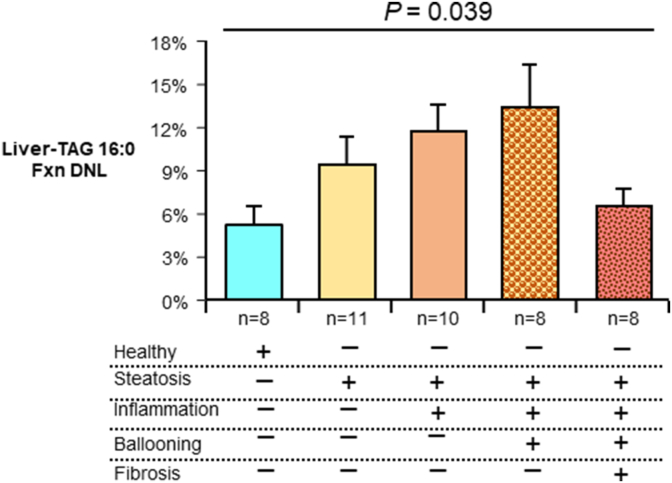
As shown in Figure 1C, the proportion of TAG palmitate (16:0) derived from DNL (referred to as fractional [fxn] DNL) increased as NAS increased until a NAS of 4 and as the disease progressed to severe form (fibrosis, fxnDNL then started to decline (R = 0.538, P = 0.004). Liver-TAG fxnDNL was significantly associated with steatosis by histology (Figure 2A, R = 0.526, P = 0.008), and it significantly correlated with total liver-TAG concentrations measured biochemically (Figure 2B, P = 0.005). As shown in Figure 2C, fxnDNL was significantly different between the groups even when controlling for differences in liver fat in these groups (P = 0.009, data not shown); Borderline NASH subjects demonstrated higher fxnDNL compared with subjects with no NAFLD. Concentrations of total liver-TAG (P < 0.0001, Supplementary Figure S3A) and total liver-CE (P = 0.0006, Supplementary Figure S3B) increased across the groups. The product of fxnDNL and the content of liver-TAG 16:0 is a quantitative measurement of the absolute liver-TAG 16:0 derived from the DNL pathway (Supplementary Figure S3C). This was significantly different by ANOVA, with the NASH subjects demonstrating greater levels of absDNL compared with all other groups. This was due to both a greater proportion of newly-made FAs in the liver (Supplementary Figure S2C) and greater amounts of total lipid present (Supplementary Figure S3A). Thus, both excess stimulation of FA synthase (FASN) and greater esterification and storage result in expanded liver-TAG pools. Further, when stimulated, lipogenesis could also lead to greater quantities of CE [38]. However, the fxnDNL in CE 16:0 was not greater with more advanced liver injury (P = 0.304, Figure 2D). By contrast, for absDNL in CE, NASH subjects demonstrated greater levels compared with all other groups (P = 0.0005, Supplementary Figure S3D). This greater absDNL in CE should not be simply interpreted as a mathematical result of great liver lipid present. Rather, the combined fxn and abs data suggest that stimulation of DNL is contributing to the excess total CE in a similar manner as other nonDNL sources are. As described previously [10] and shown in Figure 2E, the DNL contribution to hepatic lipid is less than that from nonDNL sources (adipose free FA and diet). Moreover, the content of the 2 liver lipids, TAG and CE, correlated tightly with each other (R = 0.789, P < 0.001, Figure 2F), and fxnDNL palmitate present in each of the lipid species was weakly related (R = 0.352, P = 0.013, Figure 2G). Since directly measured lipogenesis has not been previously compared to liver histology, for the reader’s consideration, the level of DNL is presented in one other way—by grouping subjects demonstrating successively greater numbers of components of liver disease, including fibrosis. This analysis, shown in Figure 3, demonstrated a higher level of DNL across progressive indications of liver pathology (ANOVA P = 0.039), which occurred until fibrosis was found, and lipogenesis was then lower (discussed below) indicating that the DNL contributes not only during steatosis but perhaps during inflammation and ballooning, a finding consistent with data presented in Figure 1C.
FIGURE 3.
Liver DNL in relation to NAS components, including fibrosis, as determined by histology, an alternate approach to compare DNL based on the number of NAFLD characteristics present. For example, 11 subjects were found to have steatosis only but no inflammation, ballooning, or fibrosis, whereas 10 subjects were found to have steatosis and inflammation but no ballooning or fibrosis. One-way ANOVA was performed for Liver-TAG amount, as progressive histologic factors were detected in subjects. Four participants that did not fall into any of these categories (had mixed characteristics) were excluded from this analysis. The Liver-TAG 16:0 Fxn DNL for the 4 was 8 ± 4%. Abbreviations: ANOVA, analysis of variance; DNL, de novo lipogenesis; Fxn, fractional; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; TAG, triacylglycerol.
Lipogenic gene expression, liver protein content, and relationships with isotopic DNL
Shown in Figure 4A are the mRNA expression of genes associated with lipid synthesis. While no changes were observed for acetyl-CoA carboxylase (ACC) mRNA expression, FASN and stearoyl-CoA desaturase (SCD) mRNA increased with the severity of the disease—with the expression of FASN in the NASH group being higher than in those with NAFL (Figure 4A). HOMA-IR was significantly associated with liver-TAG absDNL (n = 44, R = 0.341, P = 0.023). Liver sterol regulatory binding protein-1c (SREBP1c) protein content was weakly associated with liver-TAG absDNL (n = 38, R = 0.296, P = 0.071). Diacylglycerol O-acyltransferases 1 and 2 (DGAT1 and DGAT2) tended to increase across the groups, while significance was achieved for CD36 and sterol O-acyltransferase 2 (SOAT2, a cholesterol esterification enzyme) by ANOVA. In the No-NAFLD group, neutral cholesterol ester hydrolase (NCEH) expression was significantly higher compared with that of all other groups (P = 0.009) indicating CE hydrolase activity was reduced as the disease progressed, contributing to increased liver-CE content.
Figure 4B displays the levels of protein, and no significant differences were found for total ACC, phospho-ACC (pACC), or the pACC/ACC ratio, an indicator of ACC activation. FASN protein was significantly different between the groups with the highest expression in the Borderline NASH and NASH groups compared with the No-NAFLD group (P ≤ 0.05 posthoc comparison). No changes were observed for SCD1, DGAT1, and DGAT2; whereas CD36 protein tended to be lower in all groups compared with that of No-NAFLD. For the group as a whole, protein levels of SREBP1c 120 kDa were significantly and positively associated with pACC (R = 0.522, P = 0.001), SCD (R = 0.454, P = 0.005), and FASN (R = 0.493, P = 0.003, data not shown for all 3), but SREBP1c was not related to the total NAS (data not shown). Figure 4C demonstrates a significant positive relationship between FASN protein expression and direct isotopic evidence of newly-made FAs in human liver-TAG. Further, the liver-TAG DNL pool size was significantly and strongly related to the level of DNL found in plasma VLDL-TAG (Figure 4D). This has only been shown once before, by us, in a smaller study [39].
Discussion
NAFLD is a spectrum of diseases that initiates with the accumulation of lipids, primarily TAG, in hepatocytes. Past human studies have used isotopic labeling to measure liver lipid derived from various DNL and nonDNL sources of FAs derived from the diet, the adipose, and stored lipid. This labeling method was first developed in healthy subjects [9,35,40] to assess the origin of FAs in VLDL-TAG, and we demonstrated a natural circadian rhythm of DNL starting from a nadir in the early morning. Following this work, labeling was combined with direct analysis of TAG from liver biopsies from a small group of NAFLD patients [10] showing significantly higher levels of DNL in liver-TAG that correlated with the levels found in plasma VLDL-TAG. Since that time, DNL has been estimated using FA biomarkers [41], and isotopic labeling in the plasma VLDL-TAG pool demonstrated that, compared with lean healthy subjects, it is elevated in obesity, insulin resistance [14], NAFLD [11, 42] and genetic conditions associated with elevated liver fat [43, 44]. In the present study, DNL was quantified, only for the second time, directly in the liver-TAG and CE pools along with concurrent assessment of liver histology. Compared to individuals with histologically-identified normal liver tissue, DNL was found to be elevated in individuals whose liver histology classified them as having Borderline NASH and NASH. These data provide direct evidence that liver-TAG from DNL is higher in patients with more liver injury. These findings serve as the basis for consideration of the development of therapies that may inhibit FA and TAG synthesis.
Lipogenic gene expression and protein contents
Consistent with a previous lipidomic analysis by Puri et al. [45], the content of liver-TAG was increased in subjects with greater disease severity, with liver-TAG being significantly higher in patients with NAFLD and NASH compared to healthy individuals. In the present study, this observation has been extended by analysis of expression of genes associated with lipid synthesis and their proteins, which echoed the biochemical findings. SCD1 and CD36 mRNA increased significantly with the severity of the disease, suggesting both increased liver FA uptake and desaturation in NAFLD. Elevated liver-CE was observed in NASH compared with the other groups, which was consistent with increased activity of SOAT2 and decreased activity of NCEH (Figure 4A). Puri et al. [45] and Min et al. [46] did not find increases in liver-CE content across disease groups, which may have been because of the lower BMI of their subjects (35 kg/m2 compared with 46 kg/m2 in the present cohort). Puri et al. [45] did find increased liver free cholesterol, which was not assessed here due to a limitation in sample. We observed a decreased NCEH activity with increased severity whereas Min et al. [46] reported the opposite (i.e., higher in NASH patients). Both FASN mRNA and protein were elevated as disease severity increased, similar to previous findings in human liver biopsy sample analysis by Mitsuyoshi et al. [47]. These findings were strengthened by a significant association between protein levels of SREBP1c and FASN (P = 0.003), which is in line with the known regulatory mechanism [48].
Elevated lipogenesis in greater levels of disease
Although excess liver lipid may be an important early feature of NAFLD, some believe a threshold may exist for steatosis to exert its negative effects and thus, this feature is sometimes referred to as ‘benign steatosis.’ The absDNL measure is a way to consider the total quantity of these FAs that are present in the liver. By contrast, the observed fxnDNL is independent of the total quantity of lipids and reflects the extent to which this biochemical pathway, stimulated within the liver, results in lipids that are stored. The significant relationship between fxnDNL and increasing clusters of liver component scores (Figure 3) supports the concept that lipogenesis is present during inflammation and ballooning and may not be just a source of inert lipids—a key concept in need of confirmation. The present results suggested that once fibrosis occurred, the FA synthetic machinery may become impaired (Figure 1C). Recently, in a comprehensive study by Lawitz et al. [49], no difference in hepatic fxnDNL was observed between NAFLD patients with and without cirrhosis. Those patients had BMIs 30 to 36 kg/m2, whereas in our study, liver tissue from bariatric patients was studied, and no patients with cirrhosis were included in the present cohort.
With regard to the role of DNL in adding FAs to the total liver lipid pool, higher absDNL levels correlated with a greater steatosis grade by histology (Figure 2B) and worsening of liver disease (Figure 2C). This occurs even though DNL levels were quantitatively small compared with that of nonDNL sources (Figure 3E). The mechanism for this effect is supported by numerous studies that have shown activation of the DNL pathway stimulates the esterification of FAs coming into the liver from both the DNL and nonDNL sources [10,50,51]. In the present study, stimulation of DNL was also associated with cholesterol (Supplementary Figure S3D). Elevated cholesterol (free and esterified) has been postulated to increase liver injury [52]. Stimulation of lipogenesis also leads to several other biochemical pathways implicated in oxidative damage and injury. The primary product of this pathway is the saturated FA palmitate, and human hepatic lipidomic studies have found increased liver saturated fat content in patients with NAFLD/NASH compared to patients without the disease [53]. The present data suggest these associations are related to the production of saturated FAs. Palmitic acid added to cells in culture substantially contributes to oxidative stress and inflammation [54], and rodent data show that upregulation of DNL through dietary supplementation of sucrose and fructose exacerbates the hepatotoxic effects of excess dietary FAs [55, 56]. Further, a byproduct of DNL, malonyl-CoA, reduces the activity of the carnitine-palmitoyl transferase-1 [57,58], which would reduce mitochondrial uptake of long-chain FAs and increase oxidative stress [59]. We have shown that, compared to subjects without liver injury, liver mitochondria from subjects with Borderline NASH and NASH exhibited mitochondrial toxicity evidenced by reductions in complete FA oxidation to CO2 and increased ROS production [37]. Thus, even though DNL FAs may be in small number, when production is up, this can increase the lipid burden and inflammatory milleu of the liver.
The biochemical analysis of liver tissue provides direct measurement of DNL in liver TAG. Two recent technical developments suggest that such measurements may be able to be made noninvasively in the future. First, Roumans et al. [60] have developed an elegant method to measure SFAs, PUFAs, and MUFAs using a 1H-magnetic resonance spectroscopy (MRS) postprocessing tool. They combined these measurements with MRS measures of total IHTAG along with D2O administration to measure VLDL-TAG DNL in healthy, overweight, obese and type 2 diabetic (T2D) subjects. The percentage of SFA in liver measured by MRS was positively correlated with VLDL-TAG DNL (r = 0.52, P = 0.047), which is very similar to the current findings using isolated lipid from liver tissue. Liver SFA percentage was significantly higher in patients with fatty liver and T2D compared with healthy controls. Second, a new method developed by Burgess and colleagues [61] uses high-resolution Orbitrap GC-MS analysis of blood TAG after D2O administration, which has the advantage of greater sensitivity and lack of convolution with 13C-labeled molecules. With the availability of these new tools, noninvasive methods can be used to facilitate testing in the future in larger numbers of patients without and with the use of heavy isotopes. Importantly, these methods will allow for repeated assessments of DNL during intervention studies.
Limitations of the study
The strengths of this study included isotopic labeling and mass spectrometry to directly assess DNL in concert with measurement of phenotypic, biochemical, and molecular parameters in humans. The key limitation relates to the nature of samples obtained from subjects preparing for bariatric surgery [19, 49] in which body weight would be higher than may be found in a NAFLD clinic, and our cohort contained more females. Further, patients preparing for surgery are typically encouraged to lose weight before the procedure [27]. No differences between the 4 groups were found in presurgery weight loss (3 ± 4% of body weight, P = 0.815 by ANOVA). We have found no evidence that this effect would be different across the groups studied; however, the levels of DNL would be lower than other reports of patients with NAFLD. When compared with data from a review by Machado et al. [26] of 12 studies that assessed liver histology in patients undergoing bariatric surgeries, the present cohort had a similar prevalence of steatosis (84% in the present study compared with 91% on average across the 12 studies) and similar inflammation (59% compared with 50%). Second, although our sample size was sufficient to detect significant differences in DNL measurements and mRNA and protein of several enzymes, other transcripts and proteins may not have had adequate numbers to detect differences by ANOVA. Last, the liver tissue sample was obtained at a single time point, and repeated liver sampling over time is not medically warranted, yet the identification of the contribution of DNL to NAFLD progression, within a single individual, is of interest. The data presented here strongly support the use of DNL measured in VLDL-TAG as an efficient marker of liver steatosis and thus, future studies can monitor liver fat over time using MRS while simultaneously assessing lipogenesis in plasma lipids.
Conclusions
Using isotopic labeling, we found that DNL increases across groups of individuals with progressive indications of NAFLD/NASH. Beyond the direct production of FAs, activation of lipogenesis is part of a coordinated stimulation of biochemical pathways leading to increased lipid storage and inflammation. Therapies designed to lower lipogenesis may reduce inflammation and cell death. Since the level of newly-made FAs in plasma VLDL particles showed strong agreement with that found in the liver-TAG, future studies may continue to assess lipogenesis in VLDL-TAG palmitate as an objective biomarker for liver FA synthesis and one that reflects liver mRNA and protein levels of FASN. Such studies will support investigations of the role of DNL in liver toxicity and injury as treatments for this devastating disease emerge.
Author contributions
The authors’ responsibilities were as follows—MMS-A, EJP: contributed to the study concept and design; MMS-A, MPM, AAW, RRG, AD-A, RSR, JAI, EJP: aided in data acquisition; MMS-A, EJP analyzed data and interpreted results; MMS-A, GFP, EJP: provided statistical analysis of the data; MMS-A: drafted the manuscript; EJP, RSR, JAI: provided valuable edits, additions, and analysis; and all authors: conceived the article and read and approved the final manuscript.
Funding
This study was supported by a Predoctoral Fellowship from the American Society for Nutrition (ASN# 0058251 to MMS-A). Additional support was derived from the National Institutes of Health (R01 DK113701 to RSR, JAI, EJP), a VA-Merit Grant (I01BX003271 to RSR), and some use of resources and facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
Data availability
Data described in the manuscript, code book, and analytic code will be made available from the corresponding author upon reasonable request.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Majid Syed-Abdul reports financial support was provided by American Society for Nutrition. Elizabeth Parks reports financial support was provided by National Institutes of Health. R. Scott Rector reports financial support was provided by National Institutes of Health. Jamal Ibdah reports financial support was provided by National Institutes of Health. R. Scott Rector reports financial support was provided by US Department of Veterans Affairs. Elizabeth Parks reports a relationship with Pfizer Inc that includes: consulting or advisory.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgments
We acknowledge Drs. Pamela Bruzina, Jill Kanaley, Brian Bostick, and Stephen D. Ball for enlightening discussions on this project [62] and the University of Missouri’s Bariatric Clinic, Department of Surgery, and Pre-operations unit staff for their support. We appreciate Nhan Le, Jennifer Anderson, and Megan Searles, who provided technical support, and we are indebted to the study volunteers for their participation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.09.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kanwal F., Shubrook J.H., Younossi Z., Natarajan Y., Bugianesi E., Rinella M.E., et al. Preparing for the NASH epidemic: a call to action. Gastroenterology. 2021;161(3):1030–1042.e8. doi: 10.1053/j.gastro.2021.04.074. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M., Blissett D., Blissett R., Henry L., Stepanova M., Younossi Y., et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z., Stepanova M., Ong J.P., Jacobson I.M., Bugianesi E., Duseja A., et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 2019;17(4):748–755.e3. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 4.Chakravarthy M.V., Neuschwander-Tetri B.A. The metabolic basis of nonalcoholic steatohepatitis. Endocrinol. Diabetes Metab. 2020;3(4) doi: 10.1002/edm2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bence K.K., Birnbaum M.J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 2021;50 doi: 10.1016/j.molmet.2020.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bril F., Barb D., Lomonaco R., Lai J., Cusi K. Change in hepatic fat content measured by MRI does not predict treatment-induced histological improvement of steatohepatitis. J. Hepatol. 2020;72(3):401–410. doi: 10.1016/j.jhep.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Al-Mrabeh A., Zhyzhneuskaya S.V., Peters C., Barnes A.C., Melhem S., Jesuthasan A., et al. Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss. Cell Metab. 2020;31(2):233–249.e4. doi: 10.1016/j.cmet.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timlin M.T., Parks E.J. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am. J. Clin. Nutr. 2005;81(1):35–42. doi: 10.1093/ajcn/81.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W., et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 2020;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarz J.M., Clearfield M., Mulligan K. Conversion of sugar to fat: is hepatic de novo lipogenesis leading to metabolic syndrome and associated chronic diseases? J. Am. Osteopath. Assoc. 2017;117(8):520–527. doi: 10.7556/jaoa.2017.102. [DOI] [PubMed] [Google Scholar]
- 13.Jelenik T., Kaul K., Séquaris G., Flögel U., Phielix E., Kotzka J., et al. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes. 2017;66(8):2241–2253. doi: 10.2337/db16-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz J.M., Linfoot P., Dare D., Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 2003;77(1):43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Silva J.C.P., Marques C., Martins F.O., Viegas I., Tavares L., Macedo M.P., et al. Determining contributions of exogenous glucose and fructose to de novo fatty acid and glycerol synthesis in liver and adipose tissue. Metab. Eng. 2019;56:69–76. doi: 10.1016/j.ymben.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson D., Finck B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021;17(8):484–495. doi: 10.1038/s41574-021-00507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syed-Abdul M.M., Parks E.J., Gaballah A.H., Bingham K., Hammoud G.M., Kemble G., et al. Fatty acid synthase inhibitor TVB-2640 reduces hepatic de novo lipogenesis in males with metabolic abnormalities. Hepatology. 2020;72(1):103–118. doi: 10.1002/hep.31000. [DOI] [PubMed] [Google Scholar]
- 18.Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X., et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26(2):394–406.e6. doi: 10.1016/j.cmet.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawitz E.J., Coste A., Poordad F., Alkhouri N., Loo N., McColgan B.J., et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2018;16(12):1983–1991.e3. doi: 10.1016/j.cgh.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Loomba R., Kayali Z., Noureddin M., Ruane P., Lawitz E.J., Bennett M., et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(5):1463–1473.e6. doi: 10.1053/j.gastro.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stiede K., Miao W., Blanchette H.S., Beysen C., Harriman G., Harwood H.J., Jr., et al. Acetyl-coenzyme A carboxylase inhibition reduces de novo lipogenesis in overweight male subjects: a randomized, double-blind, crossover study. Hepatology. 2017;66(2):324–334. doi: 10.1002/hep.29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergman A., Carvajal-Gonzalez S., Tarabar S., Saxena A.R., Esler W.P., Amin N.B. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a liver-targeting acetyl-CoA carboxylase inhibitor (PF-05221304): a three-part randomized phase 1 study. Clin. Pharmacol. Drug Dev. 2020;9(4):514–526. doi: 10.1002/cpdd.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parks E.J. On the front line: obesity and NAFLD. Cell Metab. 2020;31(4):655–657. doi: 10.1016/j.cmet.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Liang G., Yang J., Horton J.D., Hammer R.E., Goldstein J.L., Brown M.S. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 2002;277(11):9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaka T., Shimano H. Insulin-dependent and -independent regulation of sterol regulatory element-binding protein-1c. J. Diabetes Investig. 2013;4(5):411–412. doi: 10.1111/jdi.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado M., Marques-Vidal P., Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J. Hepatol. 2006;45(4):600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Mechanick J.I., Apovian C., Brethauer S., Timothy Garvey W., Joffe A.M., Kim J., et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery. Obesity Medicine Association, and American Society of Anesthesiologists, Obesity (Silver Spring) 2020;28(4):O1–O58. doi: 10.1002/oby.22719. [DOI] [PubMed] [Google Scholar]
- 28.Bonder A., Afdhal N. Utilization of FibroScan in clinical practice. Curr. Gastroenterol. Rep. 2014;16(2):372. doi: 10.1007/s11894-014-0372-6. [DOI] [PubMed] [Google Scholar]
- 29.Udelsman B.V., Corey K.E., Lindvall C., Gee D.W., Meireles O.R., Hutter M.M., et al. Risk factors and prevalence of liver disease in review of 2557 routine liver biopsies performed during bariatric surgery. Surg. Obes. Relat. Dis. 2019;15(6):843–849. doi: 10.1016/j.soard.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 30.Brunt E.M., Kleiner D.E., Wilson L.A., Belt P., Neuschwander B.A. -Tetri, NASH Clinical Research Network (CRN), Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai R.K., Jairath V., Hogan M., Zou G., Adeyi O.A., Anstee Q.M., et al. Reliability of histologic assessment for NAFLD and development of an expanded NAFLD activity score. Hepatology. 2022;76(4):1150–1163. doi: 10.1002/hep.32475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore M.P., Cunningham R.P., Kelty T.J., Boccardi L.R., Nguyen N.Y., Booth F.W., et al. Ketogenic diet in combination with voluntary exercise impacts markers of hepatic metabolism and oxidative stress in male and female Wistar rats. Appl. Physiol. Nutr. Metab. 2020;45(1):35–44. doi: 10.1139/apnm-2019-0042. [DOI] [PubMed] [Google Scholar]
- 33.Rector R.S., Thyfault J.P., Morris R.T., Laye M.J., Borengasser S.J., Booth F.W., et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima fatty rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294(3):G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 34.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226(1):497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 35.Parks E.J., Skokan L.E., Timlin M.T., Dingfelder C.S. Dietary sugars stimulate fatty acid synthesis in adults. J. Nutr. 2008;138(6):1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caussy C., Ajmera V.H., Puri P., Hsu C.L., Bassirian S., Mgdsyan M., et al. Serum metabolites detect the presence of advanced fibrosis in derivation and validation cohorts of patients with non-alcoholic fatty liver disease. Gut. 2019;68(10):1884–1892. doi: 10.1136/gutjnl-2018-317584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore M.P., Cunningham R.P., Meers G.M., Johnson S.A., Wheeler A.A., Ganga R.R., et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology. 2022;76(5):1452–1465. doi: 10.1002/hep.32324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahik V.D., Frisdal E., Le Goff W. Rewiring of lipid metabolism in adipose tissue macrophages in obesity: impact on insulin resistance and type 2 diabetes. Int. J. Mol. Sci. 2020;21(15):5505. doi: 10.3390/ijms21155505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnelly K.L., Margosian M.R., Sheth S.S., Lusis A.J., Parks E.J. Increased lipogenesis and fatty acid reesterification contribute to hepatic triacylglycerol stores in hyperlipidemic Txnip-/- mice. J. Nutr. 2004;134(6):1475–1480. doi: 10.1093/jn/134.6.1475. [DOI] [PubMed] [Google Scholar]
- 40.Barrows B.R., Parks E.J. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J. Clin. Endocrinol. Metab. 2006;91(4):1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 41.Sevastianova K., Santos A., Kotronen A., Hakkarainen A., Makkonen J., Silander K., et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am. J. Clin. Nutr. 2012;96(4):727–734. doi: 10.3945/ajcn.112.038695. [DOI] [PubMed] [Google Scholar]
- 42.Diraison F., Moulin P., Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29(5):478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 43.Baykal A.P., Parks E.J., Shamburek R., Syed-Abdul M.M., Chacko S., Cochran E., et al. Leptin decreases de novo lipogenesis in patients with lipodystrophy. JCI Insight. 2020;5(14) doi: 10.1172/jci.insight.137180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santoro N., Caprio S., Pierpont B., Van Name M., Savoye M., Parks E.J. Hepatic de novo lipogenesis in obese youth is modulated by a common variant in the GCKR gene. J. Clin. Endocrinol. Metab. 2015;100(8):E1125–E1132. doi: 10.1210/jc.2015-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puri P., Baillie R.A., Wiest M.M., Mirshahi F., Choudhury J., Cheung O., et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 46.Min H.K., Kapoor A., Fuchs M., Mirshahi F., Zhou H., Maher J., et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15(5):665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsuyoshi H., Yasui K., Harano Y., Endo M., Tsuji K., Minami M., et al. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol. Res. 2009;39(4):366–373. doi: 10.1111/j.1872-034X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 48.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawitz E.J., Li K.W., Nyangau E., Field T.J., Chuang J.C., Billin A., et al. Elevated de novo lipogenesis, slow liver triglyceride turnover, and clinical correlations in nonalcoholic steatohepatitis patients. J. Lipid Res. 2022;63(9) doi: 10.1016/j.jlr.2022.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts R., Bickerton A.S., Fielding B.A., Blaak E.E., Wagenmakers A.J., Chong M.F., et al. Reduced oxidation of dietary fat after a short term high-carbohydrate diet. Am. J. Clin. Nutr. 2008;87(4):824–831. doi: 10.1093/ajcn/87.4.824. [DOI] [PubMed] [Google Scholar]
- 51.Chong M.F., Fielding B.A., Frayn K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007;85(6):1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 52.Ioannou G.N. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol. Metab. 2016;27(2):84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Gorden D.L., Myers D.S., Ivanova P.T., Fahy E., Maurya M.R., Gupta S., et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res. 2015;56(3):722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das S.K., Mondal A.K., Elbein S.C. Distinct gene expression profiles characterize cellular responses to palmitate and oleate. J. Lipid Res. 2010;51(8):2121–2131. doi: 10.1194/jlr.M004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Softic S., Meyer J.G., Wang G.X., Gupta M.K., Batista T.M., Lauritzen H.P.M.M., et al. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab. 2019;30(4):735–753.e4. doi: 10.1016/j.cmet.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Softic S., Cohen D.E., Kahn C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016;61(5):1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith B.K., Perry C.G., Koves T.R., Wright D.C., Smith J.C., Neufer P.D., et al. Identification of a novel malonyl-CoA IC(50) for CPT-I: implications for predicting in vivo fatty acid oxidation rates. Biochem. J. 2012;448(1):13–20. doi: 10.1042/BJ20121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster D.W. Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. J. Clin. Invest. 2012;122(6):1958–1959. doi: 10.1172/jci63967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGarry J.D. Travels with carnitine palmitoyltransferase I: from liver to germ cell with stops in between. Biochem. Soc. Trans. 2001;29(2):241–245. doi: 10.1042/0300-5127:0290241. [DOI] [PubMed] [Google Scholar]
- 60.Roumans K.H.M., Lindeboom L., Veeraiah P., Remie C.M.E., Phielix E., Havekes B., et al. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat. Commun. 2020;11(1):1891. doi: 10.1038/s41467-020-15684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu X., Deja S., Fletcher J.A., Anderson N.N., Mizerska M., Vale G., et al. Measurement of lipogenic flux by deuterium resolved mass spectrometry. Nat. Commun. 2021;12(1):3756. doi: 10.1038/s41467-021-23958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Syed Abdul M.M. University of Missouri--Columbia; 2020. Contribution of de novo lipogenesis to the progression of nonalcoholic fatty liver disease. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available from the corresponding author upon reasonable request.