Abstract
Background
Gestational weight gain (GWG) below or above the Institute of Medicine (IOM) recommendations has been associated with adverse perinatal outcomes. Few studies have examined the effect of prenatal nutrient supplementations on GWG in low- and middle-income countries (LMICs).
Objectives
We aimed to investigate the effects of multiple micronutrient supplements (MMSs) and small-quantity lipid-based nutrient supplements (LNSs) on GWG in LMICs.
Methods
A 2-stage meta-analysis of individual participant data was conducted to examine the effects of MMSs (45,507 women from 14 trials) and small-quantity LNSs (6237 women from 4 trials) on GWG compared with iron and folic acid supplements only. Percentage adequacy of GWG and total weight gain at delivery were calculated according to the IOM 2009 guidelines. Binary outcomes included severely inadequate (percentage adequacy <70%), inadequate (<90%), and excessive (>125%) GWG. Results from individual trials were pooled using fixed-effects inverse-variance models. Heterogeneity was examined using I2, stratified analysis, and meta-regression.
Results
MMSs resulted in a greater percentage adequacy of GWG [weighted mean difference (WMD): 0.86%; 95% CI: 0.28%, 1.44%; P < 0.01] and higher GWG at delivery (WMD: 209 g; 95% CI: 139, 280 g; P < 0.01) than among those in the control arm. Women who received MMSs had a 2.9% reduced risk of severely inadequate GWG (RR: 0.971; 95% CI: 0.956, 0.987; P < 0.01). No association was found between small-quantity LNSs and GWG percentage adequacy (WMD: 1.51%; 95% CI: −0.38%, 3.40%; P = 0.21). Neither MMSs nor small-quantity LNSs were associated with excessive GWG.
Conclusions
Maternal MMSs were associated with greater GWG percentage adequacy and total GWG at delivery than was iron and folic acid only. This finding is consistent with previous results on birth outcomes and will inform policy development and local recommendations of switching routine prenatal iron and folic acid supplements to MMSs.
Keywords: multiple micronutrient supplements, small-quantity lipid-based nutrient supplements, gestational weight gain, randomized controlled trials, meta-analysis, low- and middle-income countries
Introduction
Pregnancy is characterized by multiple metabolic changes with additional requirements for nutrients and energy intake. As pregnancy progresses, the maternal basal metabolic rate continues to increase, reaching 10%–20% more than nonpregnancy rates (1). Maternal weight gain is small and primarily due to fat deposition and placental development during the first trimester. The fastest weight gain occurs in the second trimester, with a slightly decreasing rate during the third trimester. Weight gain in the later trimesters is more related to fetal growth as well as maternal fat stores and total body water accretion (2). Overall ∼50% of total gestational weight gain (GWG) during pregnancy is attributed to the fetoplacental unit (fetus, placenta, amniotic fluid, and gravid uterus); another 25% is attributed to increases in blood volume, extravascular fluid, and breast tissue; and the remaining 25% to maternal fat stores (1, 2).
Undernutrition is common among women in low- and middle-income countries (LMICs) (3). Pregnant women in these settings are often at higher risk of multiple micronutrient deficiencies owing to food insecurity, low dietary diversity, and the increased demands of the developing fetus (4, 5). Currently, the most widely available prenatal multiple micronutrient supplement (MMS) product is the UN International Multiple Micronutrient Antenatal Preparation (UNIMMAP) tablet, which contains 15 micronutrients including 30 mg Fe and 0.4 mg folic acid. Data from previous meta-analyses have shown that, compared with iron and folic acid supplements, prenatal MMSs decrease the risk of low birth weight and small-for-gestational-age birth (6, 7), and particularly benefit infants born to underweight or anemic women (6).
Prenatal small-quantity lipid-based nutrient supplements (LNSs), providing ∼120 kcal/d, offer another strategy for delivering not only vitamins and minerals, but also essential fatty acids and macronutrients not incorporated in MMS tablets. Two meta-analyses reported that prenatal LNSs, including those providing much more than 120 kcal/d, significantly increased birth weight and length and reduced the risk of small-for-gestational-age birth (8, 9). However, meta-analysis focused on the effect of small-quantity LNSs is lacking.
GWG is widely used as an indicator of the adequacy of nutrition during pregnancy. Inadequate GWG has been consistently associated with adverse birth outcomes such as prematurity (10, 11, 12), small-for-gestational-age birth (12, 13, 14), low birth weight (10, 12, 13, 14, 15), and infant mortality (16). On the other hand, excessive GWG has been associated with increased risks of large-for-gestational-age birth, macrosomia, cesarean delivery, gestational diabetes, and subsequent maternal obesity (17, 18). Demographic surveillance data from sub-Saharan Africa and India suggest that average weight gain among pregnant women is only ∼60% of the recommended amount for normal-weight women (19). A more recent modeling analysis using Demographic and Health Surveys data revealed inadequate GWG in most LMICs and regions (20).
Because weight gain during pregnancy is often monitored in prenatal clinical care, it is a modifiable risk factor for adverse birth and maternal outcomes. However, few existing randomized controlled trials have been designed to examine the effect of prenatal nutritional supplements on GWG (21, 22, 23), and direct evidence of the effect on GWG is limited. We conducted a systematic review and meta-analysis using individual participant data from randomized controlled trials to examine the effects of MMSs and small-quantity LNSs on GWG among pregnant women in LMICs. We further aimed to identify potential modifiers of the effect of these nutritional supplements on GWG.
Methods
Identification of eligible trials and individual participants
We conducted a systematic search using PubMed, Embase, and Web of Science to identify randomized controlled trials among pregnant women published after January 2000 up to December 2021 (Supplemental Material: search strategy). Study-level inclusion criteria included 1) randomized controlled trials of prenatal nutrient supplements from LMICs, including trials of MMSs or small-quantity LNSs; and 2) studies that had measured maternal weight during pregnancy. Trials conducted exclusively among pregnant women with a health condition, such as anemia, HIV, or diabetes, were excluded. We also reviewed the references of the included trials and previous systematic reviews to identify additional relevant studies. The study protocol was developed with predefined outcome metrics and a predefined analysis plan while we were conducting the literature search and screening.
We contacted the principal investigators of all identified trials to seek collaboration and data sharing. For those who agreed to participate in these individual participant data meta-analyses, the Knowledge Integration (Ki) team at the Bill & Melinda Gates Foundation and study principal investigators executed data contributor agreements with the corresponding institutions. Once data were obtained from each trial, we checked data completeness and mapped all the variables we had requested. All data queries were resolved with individual principal investigators, and there was no critical issue regarding data integrity. In order to facilitate pooling of data across trials, data items were recoded into a common format, classifications of participant characteristics and their disease/condition status were standardized, and variables were named consistently across studies. We further applied individual-level criteria to identify eligible individual participants, including 1) singleton pregnancies, 2) ≥1 weight measurement in the second or third trimesters, 3) known gestational ages at the time of weight measurements, and 4) availability of a maternal height measure. Data from pregnancies that resulted in stillbirths or neonatal deaths were included. The balance across intervention and control arms with respect to baseline subject characteristics was checked for each trial separately.
Estimation of prepregnancy weight and BMI
An accurate assessment of GWG during pregnancy requires a prepregnancy weight measure, which is often unavailable in epidemiologic studies. In this analysis, we used first-trimester weight as a proxy for maternal prepregnancy weight. Overall, 60% of pregnant women included in the analysis had prepregnancy weight or weight measured in the first trimester. We developed an imputation model for women who did not have an observed prepregnancy or first-trimester weight measure to impute their first-trimester weight using weights measured later during pregnancy. The details of the model development, selection, and validation have been published elsewhere (24). Briefly, mixed-effects models and restricted cubic splines were used to impute weight at 9 weeks of gestation. We chose to impute weight at 9 weeks of gestation because it is consistent with the first available weight measure during pregnancy used in the INTERGROWTH-21st Study, an international research project that developed GWG standards among prepregnancy normal-weight women (25). Supplemental Table 1 presents the availability of an observed prepregnancy or first-trimester weight measure and the average total number of weight measures during pregnancy by trial. BMI (in kg/m2) was calculated by dividing prepregnancy (observed) or first-trimester weight (observed or imputed) in kilograms by the square of height in meters. For women aged ≥20 y, we used the WHO BMI cutoffs to define underweight (BMI <18.5), normal weight (18.5 ≤ BMI < 25.0), overweight (25.0 ≤ BMI < 30.0), and obesity (BMI ≥30.0) (26). For adolescent women (<20 y old), we used the WHO adolescent growth reference to define underweight (BMI-for-age z score: < −2), normal weight (BMI-for-age z score: −2 to <1), overweight (BMI-for-age z score: 1 to <2), and obesity (BMI-for-age z score: ≥2) (27).
Outcome metrics
Percentage adequacy of GWG
First, GWG at the time of last weight measure during pregnancy was calculated for each woman by subtracting prepregnancy or first-trimester weight from the last available weight measurement during pregnancy. Second, following the Institute of Medicine (IOM) 2009 recommendation (2), we estimated the expected weight gain for each woman at the time of their last observed weight measure using the following formula:
recommended GWG = (expected first-trimester weight gain/13.86) × (13.86 − gestational age at first observed or imputed weight measurement) + (gestational age at the last weight measurement − 13.86) × recommended rate of GWG for the second and third trimesters by BMI category based on IOM guidelines (1)
We assumed that the expected first-trimester weight gain was 2 kg for underweight and normal-weight women, 1 kg for overweight women, and 0.5 kg for women with obesity (22). The recommended rates of GWG for the second and third trimesters were 0.51, 0.42, 0.28, and 0.22 kg/wk for women with underweight, normal weight, overweight, and obesity, respectively (2).
Finally, the percentage adequacy of GWG was calculated by dividing the observed GWG at the time of the last weight measurement by the expected GWG for that week of gestation based on the IOM recommendations, multiplied by 100. This continuous outcome is independent of gestational age at the time of weight measurement and has been used previously (22).
Severely inadequate, inadequate, and excessive GWG
The percentage adequacy of GWG defined as aforementioned was considered adequate between 90% and 125%. The cutoffs 90% and 125% correspond to the lower and upper limits of the recommended total weight gain during pregnancy by the IOM guideline (2). Severely inadequate GWG was defined as percentage adequacy of GWG <70%, inadequate GWG as percentage adequacy of GWG <90%, and excessive GWG as percentage adequacy of GWG >125% (22).
Estimated total GWG at delivery
The median time interval between last weight measurement and delivery was 6.0 wk (IQR: 3.2–8.4 wk). The total GWG at delivery was estimated by multiplying the percentage adequacy of GWG (estimated as aforementioned) by the IOM-recommended GWG at delivery, which was calculated based on the gestational age at delivery and BMI category for each individual woman.
Statistical analysis
Within each trial, we used multiple linear regression models to examine the association between MMSs or small-quantity LNSs and continuous outcomes, including percentage adequacy of GWG and estimated total GWG at delivery. Mean differences in percentage adequacy and estimated total GWG and their 95% CIs were reported for continuous outcomes. We used modified Poisson regression with robust variance estimation to estimate the association between MMSs or small-quantity LNSs compared with iron and folic acid only and binary outcomes, including severely inadequate, inadequate, and excessive GWG. Risk ratios (RRs) and 95% CIs were reported for binary outcomes. For cluster-randomized controlled trials, compound symmetry correlation structure was used to account for the fact that clusters were randomly assigned instead of individual participants. For factorial design trials with MMSs and another intervention, the interaction test between the 2 interventions was examined within each trial first, and if no interaction was found, all intervention arms were collapsed based on whether MMSs were received.
To identify potential subgroups of women who might experience a greater effect from MMSs or small-quantity LNSs, we conducted stratified analyses by categories of the following factors for each trial: 1) prepregnancy BMI (underweight, normal-weight, overweight, or obese); 2) adherence to the assigned regimen (<90% or ≥90%); 3) maternal age (<20 y, 20–29 y, and ≥30 y); 4) gestational age at randomization (<20 wk or ≥20 wk); 5) parity (0 or ≥1); 6) maternal education level (<8 y or ≥8 y); 7) maternal anemia status (hemoglobin <11.0 g/dL or ≥11.0 g/dL); 8) maternal height (<150 cm or ≥150 cm); and 9) infant sex (male or female). These factors and their cutoffs were selected based on their inclusion in existing literature, data availability, and distribution in the current analysis. Individual data on pill count or intervention uptake of the assigned regimen from each trial were collected, and adherence was assessed by dividing the amount of regimen consumed by the amount distributed to each woman during the overall study period. Mean differences for continuous outcomes and their corresponding 95% CIs were estimated by subgroups within each trial.
After analyses were completed for each trial, fixed-effect inverse-variance meta-analyses were conducted to pool study-specific overall and subgroup effects. Heterogeneity across trials was assessed using the I2 statistic, with thresholds of <30%, 30–60%, and >60% considered low, moderate, and high heterogeneity, respectively. Meta-regression analysis was used to examine the statistical difference in the effect of MMSs or small-quantity LNSs on GWG across categories of potential effect modifiers with P < 0.05 considered as indicative of effect modification.
As a secondary analysis, we calculated GWG z score using the INTERGROWTH-21st maternal weight gain standards (25) and further examined the association of this z score with MMSs and small-quantity LNSs among normal-weight women.
Random-effect meta-analyses were conducted as a sensitivity analysis for continuous outcomes. To evaluate whether our results were driven by the JiVitA-3 trial (28) owing to its large sample size, or the Women First trial (29) owing to the provision of extra calories by its study design, we conducted a sensitivity analysis for GWG percentage adequacy excluding these 2 trials. In another sensitivity analysis, we excluded pregnant women who had the last weight measure in the second trimester and restricted our analysis to those who had the final weight measure in the third trimester. In a similar analysis, we restricted our analysis to women who had imputed first-trimester weight to evaluate the potential bias by use of the imputation.
All individual trials were approved by their respective ethics committees. Two-tailed P values < 0.05 were considered significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute) and Stata version 16 (StataCorp).
Results
General characteristics of the included studies
Table 1 shows a summary of the characteristics of each trial included in the analysis. We identified 17 randomized controlled trials that met our eligibility criteria, and 16 of them with a combined sample size of 50,927 pregnant women were included in this analysis (22, 23, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41) (Supplemental Material: PRISMA IPD flow diagram). For interventions, 12 of 16 trials included had an MMS arm, 2 trials had a small-quantity LNS arm, and another 2 trials had both MMS and small-quantity LNS arms. The only eligible trial not included in the analysis owing to nonresponse to invitation had an MMS arm (42). In all trials, women in the control arm were provided daily supplementation of iron with (n = 15) or without folic acid (n = 1) by the study team or had access to prenatal supplementation from local health services. Of the included trials, 6 were cluster-randomized (23, 28, 30, 37, 38, 40) and the remainder were individually randomized. Pregnant women were enrolled before or at 20 weeks of gestation in 14 of 16 trials. The participants’ characteristics and cumulative incidence of binary outcomes by trial are presented in Supplemental Table 2 for the analysis of MMSs and Supplemental Table 3 for the analysis of small-quantity LNSs. We updated our search in August 2022 and did not find any new eligible trial published.
TABLE 1.
Characteristics of 16 trials of MMSs or small-quantity LNSs included in the meta-analysis of the effect of prenatal nutritional supplements on gestational weight gain1
| Authors (ref.) | Country | Years of study | Control arm | Intervention arms | Participants included in analysis, n | Median weeks of gestation at enrollment | Median BMI,2 kg/m2 | Median adherence, % |
|---|---|---|---|---|---|---|---|---|
| Christian et al. (30)3 | Nepal | 1998–2001 | 60 mg Fe/d + 400 μg folic acid/d + 1000 μg vitamin A | MMSs | 1193 | 9.6 | 18.9 | 93.7 |
| Friis et al. (31) | Zimbabwe | 1996–1997 | 60 mg Fe/d + 400 μg folic acid/d | MMSs | 415 | 26.0 | 22.0 | 80.0 |
| Osrin et al. (32) | Nepal | 2002–2004 | 60 mg Fe/d + 400 μg folic acid/d | MMSs | 1108 | 15.9 | 19.2 | 98.1 |
| Ramakrishnan et al. (41) | Mexico | 1997–2000 | 60 mg Fe/d | MMSs | 353 | 9.0 | 23.3 | 95.0 |
| Fawzi et al. (33) | Tanzania | 2001–2004 | 60 mg Fe/d + 250 μg folic acid/d | MMSs | 7421 | 21.6 | 22.5 | 96.4 |
| Zeng et al. (37)3 | China | 2002–2006 | 60 mg Fe/d + 400 μg folic acid/d | MMSs | 2653 | 13.6 | 20.1 | 98.7 |
| Roberfroid et al. (34) | Burkina Faso | 2004–2006 | 60 mg Fe/d + 400 μg folic acid/d | MMSs | 1091 | 15.7 | 20.0 | 84.3 |
| Bhutta et al. (38)3 | Pakistan | 2002–2004 | 60 mg Fe/d + 400 μg folic acid/d | MMSs | 1507 | 12.9 | 20.6 | 81.4 |
| Persson et al. (39)4 | Bangladesh | 2001–2003 | 60 mg Fe/d + 400 μg folic acid/d | MMSs | 2329 | 9.0 | 19.7 | 70.0 |
| Moore et al. (35)4 | The Gambia | 2010–2012 | 60 mg Fe/d + 400 μg folic acid/d | MMSs | 803 | 13.4 | 20.4 | 87.9 |
| West et al. (28)3 | Bangladesh | 2008–2012 | 27 mg Fe/d + 600 μg folic acid/d | MMSs | 23,577 | 9.7 | 18.8 | 95.0 |
| Ashorn et al. (36) | Malawi | 2011–2013 | 60 mg Fe/d + 400 μg folic acid/d | MMSs; small-quantity LNSs | 1321 | 17.1 | 20.8 | 92.1 |
| Matias et al. (23)3 | Bangladesh | 2011–2012 | 60 mg Fe/d + 400 μg folic acid/d | Small-quantity LNSs | 3343 | 13.4 | 19.5 | 80.0 |
| Adu-Afarwuah et al. (22) | Ghana | 2009–2011 | 60 mg Fe + 400 μg folic acid/d | MMSs; small-quantity LNSs | 1114 | 15.9 | 23.1 | 80.9 |
| Hambidge et al. (29)5 | Guatemala, India, and Pakistan | 2013–2014 | Iron + folic acid | Small-quantity LNSs | 1277 | 12.0 | 21.0 | 88.0 |
| Isanaka et al. (40)3 | Niger | 2014–2019 | 60 mg Fe/d + 400 μg folic acid/d | MMSs | 1422 | 11.0 | 21.1 | 85.4 |
LNS, lipid-based nutrient supplement; MMS, multiple micronutrient supplement.
BMI observed during the first trimester or imputed for 9 weeks of gestation.
Cluster-randomized trial.
Randomized controlled trial with factorial design, and intervention arms were collapsed based on MMSs received or not.
Data from the Democratic Republic of the Congo were excluded owing to missing gestational age data. The preconceptional supplementation arm was excluded because the analysis focused on prenatal supplementation during pregnancy.
Continuous outcomes: percentage adequacy and total GWG
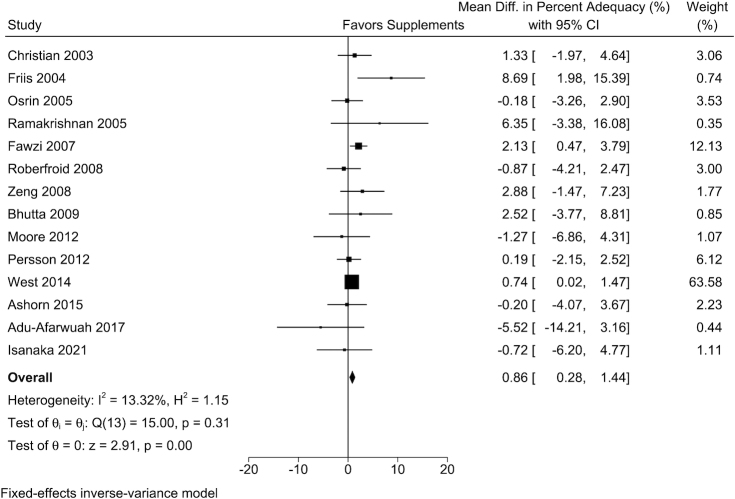
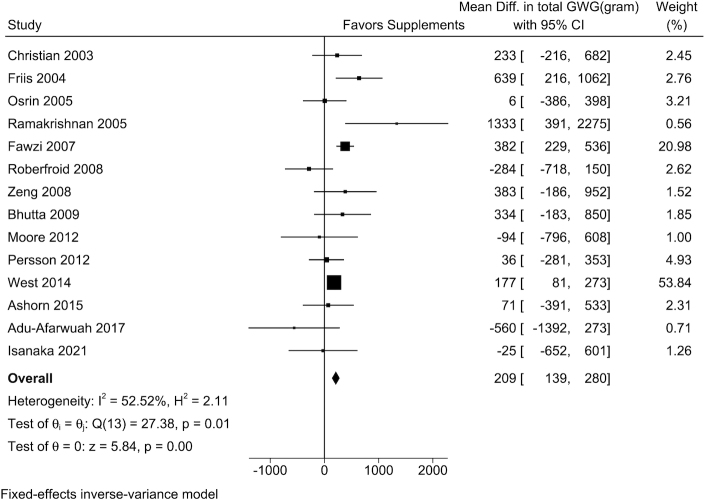
The mean GWG percentage adequacy was 77%, ranging from 60% to 107% across the 16 trials included in the analysis. Pregnant women who received maternal MMSs had greater percentage adequacy of GWG and estimated total GWG at delivery than those in the control arm (Table 2). Study-specific results demonstrated that in 9 of the 14 trials, MMSs had positive effects on percentage adequacy of GWG. The weighted mean difference (WMD) from fixed-effects meta-analyses was 0.86% (95% CI: 0.28%, 1.44%; I2 = 13.3%) (Figure 1) and the WMD in estimated total GWG at delivery was 209 g (95% CI: 139, 280 g; I2 = 52.5%) (Figure 2).
TABLE 2.
The effect of prenatal nutritional supplements on percentage adequacy of GWG and estimated total GWG at delivery1
| Intervention | ||
|---|---|---|
| Outcome | MMSs | Small-quantity LNSs |
| Percentage adequacy,2 % | ||
| Studies, n | 14 | 4 |
| Participants, total n | 45,507 | 6237 |
| Participants, n by intervention/control arms | 22,940/22,567 | 2335/3902 |
| WMD (95% CI), fixed-effects | 0.86 (0.28, 1.44) | 1.51 (−0.38, 3.40) |
| WMD (95% CI), random-effects | 0.90 (0.08, 1.71) | 2.55 (−1.42, 6.52) |
| I2,3 % | 13.3 | 69.5 |
| P-heterogeneity | 0.31 | 0.02 |
| Total GWG, g | ||
| Studies, n | 14 | 4 |
| Participants, total n | 45,455 | 6026 |
| Participants, n by intervention/control arms | 22,914/22,541 | 2287/3739 |
| WMD (95% CI), fixed-effects | 209 (139, 280) | 152 (−71, 376) |
| WMD (95% CI), random-effects | 186 (43, 329) | 203 (−123, 529) |
| I2,3 % | 52.5 | 42.2 |
| P-heterogeneity | 0.01 | 0.16 |
GWG, gestational weight gain; LNS, lipid-based nutrient supplement; MMS, multiple micronutrient supplement; WMD, weighted mean difference.
The percentage adequacy of GWG was calculated by dividing the actual GWG at the last weight measure during pregnancy by the recommended GWG according to the Institute of Medicine (IOM) 2009 guideline, multiplied by 100.
I2 is a statistic index used to assess heterogeneity across trials, with thresholds of <30%, 30–60%, and >60% considered low, moderate, and high heterogeneity, respectively.
FIGURE 1.
The effect of MMSs on the percentage adequacy of GWG. Percentage adequacy of GWG was calculated by dividing the actual GWG at the last weight measure during pregnancy by the recommended GWG according to the Institute of Medicine (IOM) 2009 guideline, multiplied by 100. The sample size by MMS/control arms for each trial was 648/545, 210/205, 559/549, 176/177, 3701/3704, 535/556, 1323/1330, 713/794, 409/394, 1156/1173, 11,994/11,583, 443/447, 375/370, and 682/740, respectively. GWG, gestational weight gain; MMS, multiple micronutrient supplement.
FIGURE 2.
The effect of MMSs on estimated total GWG at delivery. The total GWG at delivery was estimated by multiplying the percentage adequacy of GWG by the IOM-recommended GWG at delivery, which was calculated based on the gestational age at delivery and BMI category for each individual woman. The sample size by MMS/control arms for each trial was 648/545, 210/205, 559/549, 176/177, 3701/3704, 526/549, 1323/1330, 696/775, 409/394, 1156/1173, 11,994/11,583, 443/447, 375/370, and 682/740, respectively. GWG, gestational weight gain; MMS, multiple micronutrient supplement.
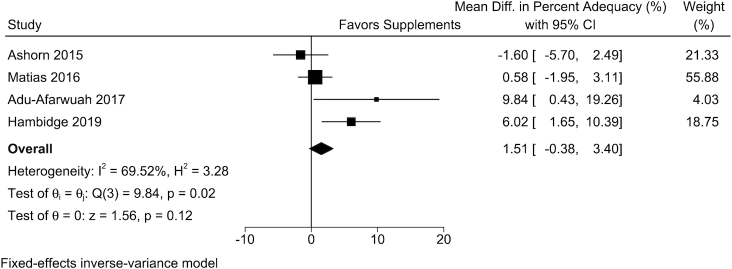
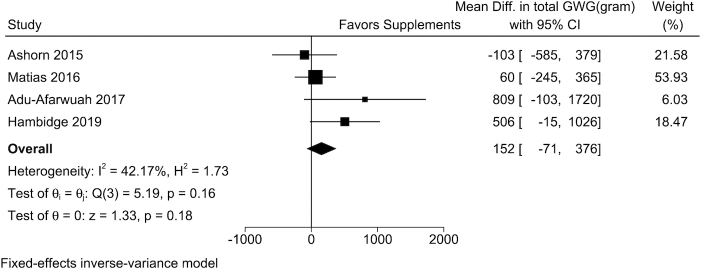
Individual data from 4 trials of small-quantity LNSs were included in the analysis. In the study-specific analysis, maternal small-quantity LNSs were positively associated with GWG percentage adequacy in 3 of the 4 trials, and 2 of these associations were statistically significant. The overall WMD from the fixed-effects meta-analysis comparing women who received small-quantity LNSs with those in the control arm was 1.51% (95% CI: −0.38%, 3.40%; I2 = 69.5%) (Table 2, Figure 3). For estimated absolute GWG at delivery, the WMD from the fixed-effect meta-analysis between women who received small-quantity LNSs and those in the control arm was 152 g (95% CI: −71, 376 g; I2 = 42.2%) (Table 2, Figure 4). Random-effects meta-analysis produced similar results on the effect of MMSs or small-quantity LNSs on continuous outcomes (Table 2).
FIGURE 3.
The effect of small-quantity LNSs on the percentage adequacy of GWG. Percentage adequacy of GWG was calculated by dividing the actual GWG at the last weight measure during pregnancy by the recommended GWG according to the Institute of Medicine (IOM) 2009 guideline, multiplied by 100. The sample size by small-quantity LNS/control arms for each trial was 431/447, 865/2478, 369/370, and 670/607, respectively. GWG, gestational weight gain; LNS, lipid-based nutrient supplement.
FIGURE 4.
The effect of small-quantity LNSs on estimated total GWG at delivery. The total GWG at delivery was estimated by multiplying the percentage adequacy of GWG by the IOM-recommended GWG at delivery, which was calculated based on the gestational age at delivery and BMI category for each individual woman. The sample size by small-quantity LNS/control arms for each trial was 431/447, 817/2315, 369/370, and 670/607, respectively. GWG, gestational weight gain; LNS, lipid-based nutrient supplement.
Binary outcomes: severely inadequate, inadequate, and excessive GWG
On average, 70% of women in the analysis had inadequate GWG and 45% had severely inadequate GWG. Compared with women in the control arm, women in the MMS arm had a 2.9% reduced risk of severely inadequate GWG (fixed-effect RR: 0.971; 95% CI: 0.956, 0.987; I2 = 57.6%) and a 1.4% reduced risk of inadequate GWG (RR: 0.986; 95% CI: 0.978, 0.995; I2 = 48.4%). No significant association was found between MMSs and risk of excessive (RR: 1.042; 95% CI: 0.975, 1.113) GWG (Table 3, Supplemental Figures 1–3). There were no significant associations of maternal small-quantity LNSs with the risks of severely inadequate (fixed-effect RR: 0.952; 95% CI: 0.903, 1.005), inadequate (RR: 0.992; 95% CI: 0.962, 1.022), or excessive (RR: 1.131; 95% CI: 0.970, 1.318) GWG (Table 3, Supplemental Figures 4–6). Results from random-effects meta-analysis were similar to those from fixed-effects meta-analysis (Table 3).
TABLE 3.
The effect of prenatal nutritional supplements on the risk of severely inadequate, inadequate, and excessive GWG1
| Intervention | ||
|---|---|---|
| Outcome | MMSs | Small-quantity LNSs |
| Severely inadequate | ||
| Studies, n | 14 | 4 |
| Participants, total n | 45,507 | 6237 |
| Participants, n by intervention/control arms | 22,940/22,567 | 2335/3902 |
| RR (95% CI), fixed-effects | 0.971 (0.956, 0.987) | 0.952 (0.903, 1.005) |
| RR (95% CI), random-effects | 0.959 (0.922, 0.997) | 0.935 (0.829, 1.055) |
| I2,2 % | 57.6 | 71.1 |
| P-heterogeneity | <0.01 | 0.02 |
| Inadequate | ||
| Studies, n | 14 | 4 |
| Participants, total n | 45,507 | 6237 |
| Participants, n by intervention/control arms | 22,940/22,567 | 2335/3902 |
| RR (95% CI), fixed-effects | 0.986 (0.978, 0.995) | 0.992 (0.962, 1.022) |
| RR (95% CI), random-effects | 0.982 (0.963, 1.002) | 0.976 (0.927, 1.028) |
| I2,2 % | 48.4 | 46.8 |
| P-heterogeneity | 0.02 | 0.13 |
| Excessive | ||
| Studies, n | 14 | 4 |
| Participants, total n | 45,507 | 6237 |
| Participants, n by intervention/control arms | 22,940/22,567 | 2335/3902 |
| RR (95% CI), fixed-effects | 1.042 (0.975, 1.113) | 1.131 (0.970, 1.318) |
| RR (95% CI), random-effects | 1.032 (0.944, 1.127) | 1.127 (0.946, 1.342) |
| I2,2 % | 18.3 | 15.6 |
| P-heterogeneity | 0.25 | 0.31 |
The percentage adequacy of GWG was calculated by dividing the actual GWG at the last weight measure during pregnancy by the recommended GWG according to the Institute of Medicine (IOM) 2009 guideline, multiplied by 100. Severely inadequate GWG was defined as percentage adequacy <70%, inadequate GWG as percentage adequacy <90%, and excessive GWG as percentage adequacy >125%. GWG, gestational weight gain; LNS, lipid-based nutrient supplement; MMS, multiple micronutrient supplement; RR, risk ratio.
I2 is a statistic index used to assess heterogeneity across trials, with thresholds of <30%, 30–60%, and >60% considered low, moderate, and high heterogeneity, respectively.
Potential effect modifiers
Adherence to the assigned regimen modified the effect of MMSs on percentage adequacy of GWG. Maternal MMSs were associated with greater percentage adequacy of GWG among women with adherence of ≥90% (WMD: 1.4%; 95% CI: 0.6%, 2.1%), but not among women with adherence <90% (WMD: 0.1%; 95% CI: −0.9%, 1.1%; P-interaction = 0.04) (Table 4). Maternal MMSs had a greater effect on percentage adequacy of GWG among women enrolled at 20 weeks of gestation or later (WMD: 2.3%; 95% CI: 0.8%, 3.7%) than among those enrolled earlier (WMD: 0.7%; 95% CI: 0.1%, 1.3%; P-interaction = 0.054).
TABLE 4.
The effect of prenatal nutrient supplements on percentage adequacy of GWG, by potential modifiers1
| MMSs (14 trials) | Small-quantity LNSs (4 trials) | |||||
|---|---|---|---|---|---|---|
| Subgroup | n | WMD (95% CI) | P-interaction2 | n | WMD (95% CI) | P-interaction2 |
| Estimated prepregnancy BMI,3 kg/m2 | 0.48 | 0.04 | ||||
| <18.5 | 10,330 | 1.6 (0.7, 2.5) | 1116 | −0.2 (−3.4, 2.9) | ||
| 18.5 to <25.0 | 31,671 | 0.8 (0.1, 1.4) | 4423 | 0.6 (−1.4, 2.6) | ||
| ≥25.0 | 3506 | 2.8 (−0.1, 5.7) | 698 | 15.5 (7.0, 23.9) | ||
| Maternal adherence to regimen, % | 0.04 | 0.98 | ||||
| <90 | 16,208 | 0.1 (−0.9, 1.1) | 1265 | −2.0 (−7.2, 3.2) | ||
| ≥90 | 25,421 | 1.4 (0.6, 2.1) | 999 | −2.1 (−6.3, 2.1) | ||
| Maternal age, y | 0.79 | 0.44 | ||||
| <20 | 10,659 | 0.8 (−0.3, 1.8) | 1657 | 9.1 (−3.7, 21.9) | ||
| 20–29 | 26,970 | 0.9 (0.1, 1.6) | 3289 | 2.7 (−1.9, 7.2) | ||
| ≥30 | 7812 | 1.0 (−0.5, 2.4) | 783 | 8.1 (2.9, 13.3) | ||
| Gestational age at enrolment, wk | 0.054 | 0.15 | ||||
| <20 | 37,922 | 0.7 (0.1, 1.3) | 5779 | 1.7 (−0.3, 3.6) | ||
| ≥20 | 7252 | 2.3 (0.8, 3.7) | 240 | −4.7 (−13.2, 3.8) | ||
| Parity | 0.99 | 0.13 | ||||
| 0 | 16,983 | 1.1 (−0.3, 2.5) | 870 | −3.0 (−9.3, 3.4) | ||
| ≥1 | 27,025 | 1.0 (0.2, 1.7) | 3671 | 2.3 (−1.4, 6.1) | ||
| Maternal education, y | 0.51 | 0.89 | ||||
| <8 | 29,703 | 0.9 (0.2, 1.6) | 4027 | 1.8 (−0.9, 4.5) | ||
| ≥8 | 14,109 | 10.7 (−26.3, 47.8) | 2206 | 1.9 (−3.8, 7.7) | ||
| Maternal hemoglobin at enrolment, g/dL | 0.79 | 0.52 | ||||
| <11.0 | 7402 | 0.4 (−1.7, 2.5) | 1506 | 2.4 (−1.1, 6.0) | ||
| ≥11.0 | 7001 | 0.3 (−1.3, 1.9) | 1947 | 5.0 (−0.5, 10.5) | ||
| Maternal height, cm | 0.26 | <0.001 | ||||
| <150 | 16,754 | 0.5 (−0.3, 1.3) | 2348 | 5.3 (2.7, 7.9) | ||
| ≥150 | 28,753 | 1.2 (0.4, 1.9) | 3889 | −1.2 (−3.6, 1.3) | ||
| Infant sex | 0.65 | |||||
| Female | 21,881 | 1.0 (−0.3, 2.4) | 0.66 | 2947 | 1.2 (−1.8, 4.3) | |
| Male | 23,154 | 0.7 (−0.1, 1.5) | 2960 | 3.2 (−1.7, 8.1) | ||
The percentage adequacy of GWG was calculated by dividing the actual GWG at the last weight measure during pregnancy by the recommended GWG according to the Institute of Medicine (IOM) 2009 guideline, multiplied by 100. GWG, gestational weight gain; LNS, lipid-based nutrient supplement; MMS, multiple micronutrient supplement; WMD, weighted mean difference.
P value for interaction was obtained from meta-regression analysis.
BMI observed during the first trimester or imputed for 9 weeks of gestation.
We found that small-quantity LNSs increased percentage adequacy of GWG among women with overweight and obesity (WMD: 15.5%; 95% CI: 7.0%, 23.9%), but not among underweight (−0.2%; 95% CI: −3.4%, 2.9%) and normal-weight women (0.6%; 95% CI: −1.4%, 2.6%; P-interaction = 0.04) (Table 4). Also, small-quantity LNSs increased percentage adequacy of GWG among women with height shorter than 150 cm (WMD: 5.3%; 95% CI: 2.7%, 7.9%), but not among taller women (WMD: −1.2%; 95% CI: −3.6%, 1.3%; P-interaction < 0.001) (Table 4). We did not find any other factors that modified the effect of MMSs or small-quantity LNSs on GWG.
Results from secondary and sensitivity analyses
No association was found between the INTERGROWTH-21st GWG z score and MMSs or small-quantity LNSs among normal-weight women (Supplemental Figures 7 and 8). With >23,000 study subjects, the JiVtA-3 trial from Bangladesh had a much larger sample size than other trials and was weighted heavily in the meta-analysis. In a sensitivity analysis excluding this trial, we found that MMSs were still associated with GWG percentage adequacy with a WMD of 1.06% (95% CI: 0.10%, 2.02%) (Supplemental Figure 9). Similarly, we conducted a sensitivity analysis excluding the Women First trial from the meta-analysis because it was not a typical small-quantity LNS trial because individuals in the intervention arm who were underweight or had weight gain that did not meet expectation received additional daily lipid-based protein-energy supplements. The result in GWG percentage adequacy remained nonsignificant with a WMD of 0.47% (95% CI: −1.63%, 2.56%) (Supplemental Figure 10).
There were 3148 (6.2%) women for whom the last weight measure was in the second trimester. In a sensitivity analysis, we removed these women and restricted our analysis to those who had weight measures in the third trimester. The significant association between MMSs and GWG percentage adequacy persisted (Supplemental Figure 11), as did the lack of association between small-quantity LNSs and GWG percentage adequacy (Supplemental Figure 12). When we restricted our analysis to women with imputed first-trimester weight, point effect estimates for MMSs (Supplemental Figure 13) and small-quantity LNSs (Supplemental Figure 14) were not materially different from their original estimates and not significant.
Discussion
In these meta-analyses using individual participant data, mean GWG percentage adequacy according to the IOM recommendation was 77%; 45% of pregnant women had severely inadequate GWG and 70% had inadequate GWG. MMSs increased GWG percentage adequacy and total weight gain at delivery and reduced the risks of severely inadequate and inadequate GWG. The beneficial effect of maternal MMSs was only observed among those with ≥90% adherence to their assigned regimen. Only 4 eligible trials were identified to examine the effect of small-quantity LNSs on GWG. No association was found in the overall analysis, but small-quantity LNSs were associated with greater GWG adequacy in the subgroups of women with overweight or obesity and those with height <150 cm. Neither MMSs nor small-quantity LNSs were associated with excessive GWG.
Our estimate that 70% of pregnant women had inadequate GWG in the current analysis is consistent with the previous findings from similar settings. In a recently published meta-analysis of studies conducted in pregnant women in sub-Saharan Africa, the percentage of inadequate GWG was >50% in 9 of 16 studies (43). Using data from Demographic and Health Surveys, Wang et al. (20) reported that the mean estimated GWG did not meet the minimum recommendation by the IOM in most developing regions and countries. Data from individual studies indicated inadequate GWG among 74% of pregnant women in Bangladesh (14) and 52% in Tanzania (44). In the current meta-analysis, we found that prenatal MMSs were associated with a 209-g increase in total GWG at delivery and a 1.4% reduced risk of inadequate GWG. Although the effect size seems small, given the high proportion (∼70%) of inadequate GWG in LMICs, the small reduction in risk would correspond to shifting 1% of the total number of pregnant women in these settings from inadequate GWG to adequate GWG. With the expectation that the fetus constitutes 27% of GWG (45), we estimate that 55 g of the 209-g increase in GWG would be fetal growth and manifest as higher birth weight, a number consistent with previously reported effect sizes of MMSs on birth weight from individual trials (28, 30, 32, 33, 46).
Previous randomized controlled trials in pregnant women have focused on the effect of MMSs on birth outcomes, rather than GWG. Several meta-analyses have been conducted to assess the effect of prenatal MMSs on birth outcomes (6, 47, 48, 49), and it has consistently been shown that the provision of MMSs reduced the risk of low birth weight and small-for-gestational-age birth (8, 9). In response to new evidence from randomized controlled trials, in 2020, the WHO updated their guidelines on prenatal nutritional interventions and recommended the use of MMSs in the context of rigorous implementation research to establish the impact of switching from iron and folic acid supplements to MMSs containing iron and folic acid (50). Our findings that MMSs increase GWG and reduce the risks of severely inadequate GWG compared with iron and folic acid provide further evidence supporting the WHO’s updated recommendation. This position is further reinforced by the results of a systematic review of >1.3 million pregnancies reporting that inadequate weight gain was associated with an increased risk of small-for-gestational-age births and preterm birth (18). Because birth outcomes have long been prioritized over maternal outcomes, more efforts should be made in future research to study the determinants and consequences of maternal outcomes of pregnancy.
There are several plausible mechanisms through which prenatal micronutrient supplements can affect GWG. First, nutritional supplements may reduce the risk of infections and morbidities during pregnancy (51, 52). Micronutrients included in the prenatal supplements might help improve immune function, increase iron absorption, and reduce the risks of anemia, pre-eclampsia, and eclampsia during pregnancy (53, 54). Second, supplementation with micronutrients may improve appetite, leading to increases in food intake by influencing the gut microbiome as well as peptide hormone concentrations and neurotransmitters that affect satiety and appetite (55, 56). Third, micronutrients included in the supplements directly improve fetal development and growth, thereby leading to greater GWG (57, 58). For example, iron, zinc, vitamin C, and B-vitamins are involved in protein and energy metabolism, DNA and RNA synthesis, and cell division (59, 60, 61, 62); further, antioxidants, including vitamins C and E, protect against free radical generation and damage caused by increased oxidative stress during pregnancy (63, 64), which has been associated with adverse pregnancy outcomes, including low birth weight and preterm birth (65, 66).
Small-quantity LNS was provided as the intervention supplement in 4 trials included in the analysis. However, it should be noted that women enrolled in the intervention arm of the Women First trial received an extra daily lipid-based protein-energy supplement, which provided 300 kcal/d and 11 g protein/d, if they had a BMI <20 at any time during the study period or had weight gain in the second or third trimester less than the IOM guidelines (29). To avoid the possibility that our pooled results were driven by the Women First trial, in sensitivity analyses, we excluded this trial from the meta-analysis and found that the results attenuated toward the null and remained statistically nonsignificant. Consistent with the previous meta-analysis published in 2018 (8), we did not find an association between small-quantity LNSs and GWG with participant data from 2 more trials included (29, 36). However, we found that small-quantity LNSs were associated with a greater adequacy of GWG in the subgroup of women with overweight or obesity and the subgroup with short height (<150 cm). Women with overweight or obesity have a lower GWG recommendation than women with underweight or normal weight according to the IOM guideline; this may at least partially explain why the effect of small-quantity LNSs on GWG percentage adequacy, which was assessed based on the IOM recommendation, was greater in this subgroup. Women with short height might tend to have low socioeconomic status and suffer from long-term undernutrition and concurrent nutritional deprivation (67, 68), and thereby potentially benefit more in GWG from the prenatal small-quantity LNSs. As a highly nutrient-dense supplement, LNSs could be a good source of macronutrients and micronutrients for malnourished pregnant women in LMICs. The effect of medium-quantity LNSs and other balanced energy-protein interventions among pregnant women in food insecurity contexts warrants further research (69).
Our study has several strengths. It is the first individual participant data meta-analysis to synthesize the effect of nutrient supplementation on GWG. Although weight gain during pregnancy is widely used in prenatal clinics as an indicator of the adequacy of maternal nutrition, it is often not reported as one of the primary outcomes in randomized controlled trials among pregnant women. By contacting each principal investigator for their originally collected data, we were able to include trials from 14 LMICs. Furthermore, our analysis is the first meta-analysis to examine the effect of small-quantity LNS, which provides <120 kcal/d, on GWG among pregnant women. Although most of the energy is supplied from fat, the energy contents of different types of LNS vary widely. Previous meta-analyses usually included LNS trials in which larger quantities of energy were provided (8, 9). By confining our analysis to trials of small-quantity LNSs, our pooled results were more likely to reflect the effect of the multiple micronutrients plus essential fatty acids included in the LNSs.
Limitations of these analyses should be noted. First, a direct measure of GWG during the entire pregnancy period was not always available because of the lack of prepregnancy weight and large variance in gestational age at enrollment and last weight measure before delivery. To overcome this limitation, we estimated early pregnancy weight at 9 weeks of gestation for women without a weight measure in the first trimester by applying a validated statistical modeling approach to their individual weight measures during pregnancy. We then developed several GWG outcome metrics including GWG percentage adequacy and estimated total GWG at delivery according to the IOM GWG guideline. We further calculated GWG z score by applying the INTERGROWTH-21st GWG standards in normal-weight women (25), and obtained similar results when we examined the association of GWG z score with MMSs and small-quantity LNSs among normal-weight women, indicating that our findings are robust. However, random or systematic measurement errors in weight and gestational age during pregnancy and their influence on the results could not be ruled out. Second, we were not able to examine whether food insecurity was an effect modifier of the associations between prenatal nutrient supplements and GWG given limited data on food insecurity. We did perform stratified analysis by baseline BMI categories and found that baseline BMI modified the effect of small-quantity LNSs, but not MMSs, on GWG adequacy. Third, even with 2 more trials included than in the previous meta-analysis on LNSs, our sample size was still relatively small, and this may have limited our power to detect the effect of small-quantity LNSs on GWG among underweight and normal-weight women.
In conclusion, by using individual participant data we conducted a 2-stage meta-analysis and found that the provision of prenatal MMSs increases GWG compared with iron and folic acid supplements only in LMICs. Given that previous trials of maternal MMS have been mainly focused on birth outcomes, our result on GWG might help to explain and further understand its beneficial effect on birth outcomes observed previously. This finding provides additional evidence to support the recently updated WHO guidelines on prenatal MMSs and lends support to switching prenatal supplements to MMSs instead of iron and folic acid alone. The contribution of LNSs of different quantities and balanced energy-protein supplements to GWG and birth outcomes warrants further study.
Acknowledgments
Members of the GWG Pooling Project Consortium: Seth Adu-Afarwuah and Anna Lartey (Department of Nutrition and Food Science, University of Ghana, Legon), Abu Ahmed Shamim and Malay Kanti Mridha (Center for Non-communicable Diseases and Nutrition, BRAC James P Grant School of Public Health, BRAC University), Shams Arifeen (International Center for Diarrheal Disease Research, Bangladesh), Per Ashorn and Ulla Ashorn (Center for Child, Adolescent and Maternal Health Research, Faculty of Medicine and Health Technology, Tampere University and Tampere University Hospital), Zulfiqar A Bhutta (Centre for Global Child Health, Hospital for Sick Children and Institute for Global Health & Development, The Aga Khan University), Yue Cheng (Department of Nutrition and Food Safety Research, School of Public Health, Xi’an Jiaotong University Health Science Center), Anthony M Costello (Global Health and Sustainable Development, UCL Institute for Global Health), Henrik Friis (Department of Nutrition, Exercise and Sports, University of Copenhagen), Exnevia Gomo (Faculty of Medicine and Health Sciences, University of Zimbabwe), Rebecca Grais (Epicentre, Paris), Ousmane Guindo (Epicentre Niger), K Michael Hambidge and Nancy F Krebs (University of Colorado School of Medicine), Lieven Huybregts (Department of Food Technology, Safety and Health, Ghent University and Poverty, Health and Nutrition Division, International Food Policy Research Institute), Sheila Isanaka (Epicentre, Paris and Departments of Nutrition and Global Health and Population, Harvard TH Chan School of Public Health), Patrick Kolsteren and Carl Lachat (Department of Food Technology, Safety and Health, Ghent University), Steven C LeClerq, Kerry Schulze, Keith P West Jr, and Lee Wu (Center for Human Nutrition, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University), Kenneth Maleta (School of Public Health and Family Medicine, College of Medicine, University of Malawi), Dharma S Manandhar [Mother and Infant Research Activities (MIRA)], Reynaldo Martorell and Usha Ramakrishnan (Hubert Department of Global Health, Rollins School of Public Health, Emory University), Susana L Matias (Department of Nutritional Sciences and Toxicology, University of California, Berkeley), Elizabeth M McClure (RTI International), Sophie E Moore (Department of Women and Children’s Health, King’s College London; St Thomas’ Hospital, London; and MRC Unit The Gambia at London School of Hygiene & Tropical Medicine, Banjul), David Osrin (UCL Institute for Global Health), Andrea B Pembe (Department of Obstetrics and Gynaecology, Muhimbili University of Health and Allied Sciences), Andrew M Prentice (MRC Unit The Gambia at London School of Hygiene & Tropical Medicine, Banjul), Juan Rivera (National Institute of Public Health, Mexico), Sajid Soofi (Centre of Excellence in Women and Child Health, The Aga Khan University), Willy Urassa (Department of Microbiology and Immunology, Muhimbili University of Health and Allied Sciences), and Lingxia Zeng and Zhonghai Zhu (Department of Epidemiology and Biostatistics, School of Public Health, Xi’an Jiaotong University Health Science Center). We thank Sun-Eun Lee, Jian Yan, and Karen T Cuenco at the Bill and Melinda Gates Foundation for their support of the Gestational Weight Gain Pooling Project. We thank Nita Bhandari at the Society for Applied Studies for her support as a member of the technical advisory group.
The authors’ responsibilities were as follows—EL, DW, AMD, NP, MW, and WWF: designed the study (project conception, development of the overall research plan, and study oversight) with substantial input from technical advisory group members (PC, KGD, GK, SK, and TA); all members of the GWG Pooling Project Consortium: contributed data, provided feedback on the study methods and interpretation of the findings, and critically reviewed the manuscript for important intellectual content; VS, BB, and DW: provided technical support and coordination for data collection, data management, and harmonization; DW, EL, AMD, and NP: had access to the pooled data; EL: led the statistical analysis and drafted and revised the manuscript; EL and WWF: have primary responsibility for the final content; and all authors: contributed to, read, and approved the final manuscript.
Data Availability
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Footnotes
Supported by Bill and Melinda Gates Foundation grant OPP1204850 (to WWF). The funder did not play a role in the study design or implementation or result interpretation.
Author disclosures: The authors report no conflicts of interest.
Supplemental Material, Supplemental Tables 1–3, and Supplemental Figures 1–14 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Contributor Information
Enju Liu, Email: enju.liu@childrens.harvard.edu.
Wafaie W Fawzi, Email: mina@hsph.harvard.edu.
SUPPORTING INFORMATION
nqac259_sup1
References
- 1.Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, et al., editors. Williams obstetrics. 24th ed. McGraw-Hill Medical; New York: 2018. [Google Scholar]
- 2.Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. National Academies Press (US); Washington (DC): 2009. [PubMed] [Google Scholar]
- 3.Bailey RL, West KP, Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl. 2):22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 4.Darnton-Hill I, Mkparu UC. Micronutrients in pregnancy in low- and middle-income countries. Nutrients. 2015;7(3):1744–1768. doi: 10.3390/nu7031744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SE, Talegawkar SA, Merialdi M, Caulfield LE. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013;16(8):1340–1353. doi: 10.1017/S1368980012004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith ER, Shankar AH, Wu LS-F, Aboud S, Adu-Afarwuah S, Ali H, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health. 2017;5(11):e1090–e1100. doi: 10.1016/S2214-109X(17)30371-6. [DOI] [PubMed] [Google Scholar]
- 7.Keats EC, Neufeld LM, Garrett GS, Mbuya MNN, Bhutta ZA. Improved micronutrient status and health outcomes in low- and middle-income countries following large-scale fortification: evidence from a systematic review and meta-analysis. Am J Clin Nutr. 2019;109(6):1696–1708. doi: 10.1093/ajcn/nqz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela-Rubio NG, Weise Prinzo Z, et al. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database Syst Rev. 2018;8(8):CD012610. doi: 10.1002/14651858.CD012610.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto E. Effectiveness of prenatal lipid-based nutrient supplementation to improve birth outcomes: a meta-analysis. Am J Trop Med Hyg. 2019;101(5):994–999. doi: 10.4269/ajtmh.19-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Z, Lutsiv O, Mulla S, Rosen A, Beyene J, McDonald SD, et al. Low gestational weight gain and the risk of preterm birth and low birthweight: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2011;90(9):935–954. doi: 10.1111/j.1600-0412.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 11.Durie DE, Thornburg LL, Glantz JC. Effect of second-trimester and third-trimester rate of gestational weight gain on maternal and neonatal outcomes. Obstet Gynecol. 2011;118(3):569–575. doi: 10.1097/AOG.0b013e3182289f42. [DOI] [PubMed] [Google Scholar]
- 12.Soltani H, Lipoeto NI, Fair FJ, Kilner K, Yusrawati Y. Pre-pregnancy body mass index and gestational weight gain and their effects on pregnancy and birth outcomes: a cohort study in West Sumatra, Indonesia. BMC Womens Health. 2017;17(1):102. doi: 10.1186/s12905-017-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009;201(4):339. doi: 10.1016/j.ajog.2009.07.002. e1–14. [DOI] [PubMed] [Google Scholar]
- 14.Kac G, Arnold CD, Matias SL, Mridha MK, Dewey KG. Gestational weight gain and newborn anthropometric outcomes in rural Bangladesh. Matern Child Nutr. 2019;15(4):e12816. doi: 10.1111/mcn.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauserman MS, Bann CM, Hambidge KM, Garces AL, Figueroa L, Westcott JL, et al. Gestational weight gain in 4 low- and middle-income countries and associations with birth outcomes: a secondary analysis of the Women First Trial. Am J Clin Nutr. 2021;114(2):804–812. doi: 10.1093/ajcn/nqab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis RR, Hofferth SL. The association between inadequate gestational weight gain and infant mortality among U.S. infants born in 2002. Matern Child Health J. 2012;16(1):119–124. doi: 10.1007/s10995-010-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group. Voerman E, Santos S, Inskip H, Amiano P, Barros H, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321(17):1702–1715. doi: 10.1001/jama.2019.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffey D. Prepregnancy body mass and weight gain during pregnancy in India and sub-Saharan Africa. Proc Natl Acad Sci U S A. 2015;112(11):3302–3307. doi: 10.1073/pnas.1416964112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Wang M, Darling AM, Perumal N, Liu E, Danaei G, et al. Gestational weight gain in low-income and middle-income countries: a modelling analysis using nationally representative data. BMJ Glob Health. 2020;5(11):e003423. doi: 10.1136/bmjgh-2020-003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Changamire FT, Mwiru RS, Peterson KE, Msamanga GI, Spiegelman D, Petraro P, et al. Effect of multivitamin supplements on weight gain during pregnancy among HIV-negative women in Tanzania. Matern Child Nutr. 2015;11(3):297–304. doi: 10.1111/mcn.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Ashorn U, Zeilani M, et al. Maternal supplementation with small-quantity lipid-based nutrient supplements compared with multiple micronutrients, but not with iron and folic acid, reduces the prevalence of low gestational weight gain in semi-urban Ghana: a randomized controlled trial. J Nutr. 2017;147(4):697–705. doi: 10.3945/jn.116.242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matias SL, Mridha MK, Paul RR, Hussain S, Vosti SA, Arnold CD, et al. Prenatal lipid-based nutrient supplements affect maternal anthropometric indicators only in certain subgroups of rural Bangladeshi women. J Nutr. 2016;146(9):1775–1782. doi: 10.3945/jn.116.232181. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Wang D, Darling AM, Liu E, Perumal N, Fawzi WW, et al. Methodological approaches to imputing early-pregnancy weight based on weight measures collected during pregnancy. BMC Med Res Method. 2021;21(1):24. doi: 10.1186/s12874-021-01210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheikh Ismail L, Bishop DC, Pang R, Ohuma EO, Kac G, Abrams B, et al. Gestational weight gain standards based on women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: a prospective longitudinal cohort study. BMJ. 2016;352:i555. doi: 10.1136/bmj.i555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO . World Health Organization; London (United Kingdom): 2000. Obesity: preventing and managing the global epidemic. Report on a WHO consultation. [PubMed] [Google Scholar]
- 27.WHO . World Health Organization; Geneva (Switzerland): 2021. Growth reference data for 5–19 years: indicators: BMI-for-age (5–19 years) [Internet]. [Cited 2021 Jul 4]. Available from: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age. [Google Scholar]
- 28.West KP, Jr, Shamim AA, Mehra S, Labrique AB, Ali H, Shaikh S, et al. Effect of maternal multiple micronutrient vs iron–folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial. JAMA. 2014;312(24):2649–2658. doi: 10.1001/jama.2014.16819. [DOI] [PubMed] [Google Scholar]
- 29.Hambidge KM, Westcott JE, Garces A, Figueroa L, Goudar SS, Dhaded SM, et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial. Am J Clin Nutr. 2019;109(2):457–469. doi: 10.1093/ajcn/nqy228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, Jr, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003;326(7389):571. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friis H, Gomo E, Nyazema N, Ndhlovu P, Krarup H, Kaestel P, et al. Effect of multimicronutrient supplementation on gestational length and birth size: a randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am J Clin Nutr. 2004;80(1):178–184. doi: 10.1093/ajcn/80.1.178. [DOI] [PubMed] [Google Scholar]
- 32.Osrin D, Vaidya A, Shrestha Y, Baniya RB, Manandhar DS, Adhikari RK, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365(9463):955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 33.Fawzi WW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356(14):1423–1431. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 34.Roberfroid D, Huybregts L, Lanou H, Henry MC, Meda N, Menten J, et al. Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88(5):1330–1340. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 35.Moore SE, Fulford AJ, Darboe MK, Jobarteh ML, Jarjou LM, Prentice AM. A randomized trial to investigate the effects of pre-natal and infant nutritional supplementation on infant immune development in rural Gambia: the ENID trial: Early Nutrition and Immune Development. BMC Pregnancy Childbirth. 2012;12(1):107. doi: 10.1186/1471-2393-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Harjunmaa U, et al. The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. Am J Clin Nutr. 2015;101(2):387–397. doi: 10.3945/ajcn.114.088617. [DOI] [PubMed] [Google Scholar]
- 37.Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:a2001. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhutta ZA, Rizvi A, Raza F, Hotwani S, Zaidi S, Moazzam Hossain S, et al. A comparative evaluation of multiple micronutrient and iron–folic acid supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food Nutr Bull. 2009;30(4_suppl4):S496–S505. doi: 10.1177/15648265090304S404. [DOI] [PubMed] [Google Scholar]
- 39.Persson LÅ, Arifeen S, Ekström E-C, Rasmussen KM, Frongillo EA, Yunus M, et al. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: the MINIMat randomized trial. JAMA. 2012;307(19):2050–2059. doi: 10.1001/jama.2012.4061. [DOI] [PubMed] [Google Scholar]
- 40.Isanaka S, Garba S, Plikaytis B, Malone McNeal M, Guindo O, Langendorf C, et al. Immunogenicity of an oral rotavirus vaccine administered with prenatal nutritional support in Niger: a cluster randomized clinical trial. PLoS Med. 2021;18(8):e1003720. doi: 10.1371/journal.pmed.1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramakrishnan U, Gonzalez-Cossio T, Neufeld LM, Rivera J, Martorell R. Effect of prenatal multiple micronutrient supplements on maternal weight and skinfold changes: a randomized double-blind clinical trial in Mexico. Food Nutr Bull. 2005;26(3):273–280. doi: 10.1177/156482650502600304. [DOI] [PubMed] [Google Scholar]
- 42.Shankar AH, Jahari AB, Sebayang SK, Aditiawarman AM, Harefa B, Muadz H, et al. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet. 2008;371(9608):215–227. doi: 10.1016/S0140-6736(08)60133-6. [DOI] [PubMed] [Google Scholar]
- 43.Asefa F, Cummins A, Dessie Y, Hayen A, Foureur M. Gestational weight gain and its effect on birth outcomes in sub-Saharan Africa: systematic review and meta-analysis. PLoS One. 2020;15(4):e0231889. doi: 10.1371/journal.pone.0231889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu E, Wang D, Darling AM, Perumal N, Wang M, Urassa W, et al. Multivitamin supplementation is associated with greater adequacy of gestational weight gain among pregnant women in Tanzania. J Nutr. 2022;152(4):1091–1098. doi: 10.1093/jn/nxab448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Champion ML, Harper LM. Gestational weight gain: update on outcomes and interventions. Curr Diab Rep. 2020;20(3):11. doi: 10.1007/s11892-020-1296-1. [DOI] [PubMed] [Google Scholar]
- 46.Huybregts L, Roberfroid D, Lanou H, Menten J, Meda N, Van Camp J, et al. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2009;90(6):1593–1600. doi: 10.3945/ajcn.2009.28253. [DOI] [PubMed] [Google Scholar]
- 47.Fall CH, Fisher DJ, Osmond C, Margetts BM, The Maternal Micronutrient Supplementation Study Group Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull. 2009;30(4_suppl4):S533–S546. doi: 10.1177/15648265090304S408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2017;4(4):CD004905. doi: 10.1002/14651858.CD004905.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;3:CD004905. doi: 10.1002/14651858.CD004905.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO . World Health Organization; Geneva (Switzerland): 2020. WHO antenatal care recommendations for a positive pregnancy experience: nutritional interventions update: multiple micronutrient supplements during pregnancy. [PubMed] [Google Scholar]
- 51.Bendich A. Micronutrients in women’s health and immune function. Nutrition. 2001;17(10):858–867. doi: 10.1016/s0899-9007(01)00649-9. [DOI] [PubMed] [Google Scholar]
- 52.Christian P, Mullany LC, Hurley KM, Katz J, Black RE. Nutrition and maternal, neonatal, and child health. Semin Perinatol. 2015;39(5):361–372. doi: 10.1053/j.semperi.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Gomes F, Agustina R, Black RE, Christian P, Dewey KG, Kraemer K, et al. Multiple micronutrient supplements versus iron-folic acid supplements and maternal anemia outcomes: an iron dose analysis. Ann N Y Acad Sci. 2022;1512(1):114–125. doi: 10.1111/nyas.14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh C, Keats EC, Bhutta ZA. Vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low- and middle-income countries: a systematic review and meta-analysis. Nutrients. 2020;12(2):491. doi: 10.3390/nu12020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Major GC, Doucet E, Jacqmain M, St-Onge M, Bouchard C, Tremblay A. Multivitamin and dietary supplements, body weight and appetite: results from a cross-sectional and a randomised double-blind placebo-controlled study. Br J Nutr. 2008;99(5):1157–1167. doi: 10.1017/S0007114507853335. [DOI] [PubMed] [Google Scholar]
- 56.Kyei-Arthur F, Situma R, Aballo J, Mahama AB, Selenje L, Amoaful E, et al. Lessons learned from implementing the pilot Micronutrient Powder Initiative in four districts in Ghana. BMC Nutr. 2020;6:50. doi: 10.1186/s40795-020-00382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terrin G, Berni Canani R, Di Chiara M, Pietravalle A, Aleandri V, Conte F, et al. Zinc in early life: a key element in the fetus and preterm neonate. Nutrients. 2015;7(12):10427–10446. doi: 10.3390/nu7125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H-K, Han SN. Vitamin E: regulatory role on gene and protein expression and metabolomics profiles. IUBMB Life. 2019;71(4):442–455. doi: 10.1002/iub.2003. [DOI] [PubMed] [Google Scholar]
- 59.Kontic-Vucinic O, Sulovic N, Radunovic N. Micronutrients in women’s reproductive health: II. Minerals and trace elements. Int J Fertil Womens Med. 2006;51(3):116–124. [PubMed] [Google Scholar]
- 60.Kontic-Vucinic O, Sulovic N, Radunovic N. Micronutrients in women’s reproductive health: I. Vitamins. Int J Fertil Womens Med. 2006;51(3):106–115. [PubMed] [Google Scholar]
- 61.Froese DS, Fowler B, Baumgartner MR. Vitamin B12, folate, and the methionine remethylation cycle—biochemistry, pathways, and regulation. J Inherit Metab Dis. 2019;42(4):673–685. doi: 10.1002/jimd.12009. [DOI] [PubMed] [Google Scholar]
- 62.Ramakrishnan U, Gonzalez-Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: a randomized controlled trial in a semirural community in Mexico. Am J Clin Nutr. 2003;77(3):720–725. doi: 10.1093/ajcn/77.3.720. [DOI] [PubMed] [Google Scholar]
- 63.Duhig K, Chappell LC, Shennan AH. Oxidative stress in pregnancy and reproduction. Obstet Med. 2016;9(3):113–116. doi: 10.1177/1753495X16648495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiarello DI, Abad C, Rojas D, Toledo F, Vázquez CM, Mate A, et al. Oxidative stress: normal pregnancy versus preeclampsia. Biochim Biophys Acta Mol Basis Dis. 2020;1866(2):165354. doi: 10.1016/j.bbadis.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Toboła-Wróbel K, Pietryga M, Dydowicz P, Napierała M, Brązert J, Florek E. Association of oxidative stress on pregnancy. Oxid Med Cell Longev. 2020:6398520. doi: 10.1155/2020/6398520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol. 2017;77(5):e12653. doi: 10.1111/aji.12653. [DOI] [PubMed] [Google Scholar]
- 67.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7(Suppl 3):5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith Fawzi MC, Andrews KG, Fink G, Danaei G, McCoy DC, Sudfeld CR, et al. Lifetime economic impact of the burden of childhood stunting attributable to maternal psychosocial risk factors in 137 low/middle-income countries. BMJ Glob Health. 2019;4(1):e001144. doi: 10.1136/bmjgh-2018-001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christian P, Smith ER, Zaidi A. Addressing inequities in the global burden of maternal undernutrition: the role of targeting. BMJ Glob Health. 2020;5(3):e002186. doi: 10.1136/bmjgh-2019-002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
nqac259_sup1
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.