Abstract
Background
The acquisition of single-lead electrocardiogram (ECG) from mobile devices offers a more practical approach to arrhythmia detection. Using artificial intelligence for atrial fibrillation (AF) identification enhances screening efficiency. However, the potential of single-lead ECG for AF identification during normal sinus rhythm (NSR) remains under-explored. This study introduces a method to identify AF using single-lead mobile ECG during NSR.
Methods
We employed three deep learning models: recurrent neural network (RNN), long short-term memory (LSTM), and residual neural networks (ResNet50). From a dataset comprising 13,509 ECGs from 6,719 patients, 10,287 NSR ECGs from 5,170 patients were selected. Single-lead mobile ECGs underwent noise filtering and segmentation into 10-second intervals. A random under-sampling was applied to reduce bias from data imbalance. The final analysis involved 31,767 ECG segments, including 15,157 labeled as masked AF and 16,610 as Healthy.
Results
ResNet50 outperformed the other models, achieving a recall of 79.3%, precision of 65.8%, F1-score of 71.9%, accuracy of 70.5%, and an area under the receiver operating characteristic curve (AUC) of 0.79 in identifying AF from NSR ECGs. Comparative performance scores for RNN and LSTM were 0.75 and 0.74, respectively. In an external validation set, ResNet50 attained an F1-score of 64.1%, recall of 68.9%, precision of 60.0%, accuracy of 63.4%, and AUC of 0.68.
Conclusion
The deep learning model using single-lead mobile ECG during NSR effectively identified AF at risk in future. However, further research is needed to enhance the performance of deep learning models for clinical application.
Keywords: Artificial Intelligence, Atrial Fibrillation, Electrocardiography, Mobile Applications, Probability Learning
Graphical Abstract

INTRODUCTION
Atrial fibrillation (AF), the primary cause of embolic stroke, presents a significant healthcare challenge.1,2 Early detection of AF is crucial for preventing severe complications such as heart failure and stroke, as well as averting cardiac structural abnormalities. Moreover, early identification of AF encourages lifestyle changes that can reduce healthcare costs and associated problems.3,4 However, detecting AF before clinical symptoms appear is complex due to many individuals exhibiting paroxysmal and asymptomatic characteristics.4,5 Furthermore, the limited effectiveness of a 12-lead electrocardiogram (ECG) in identifying transient arrhythmias and the impracticality of prolonged monitoring complicate the identification of undetected or potential AF.1,5,6
The application of artificial intelligence through deep learning algorithms can classify 12-lead ECGs by arrhythmia types and even detect undiagnosed AF.7,8,9,10 This research hypothesizes the presence of subtle features of AF indicators in 12-lead ECGs during normal sinus rhythm (NSR).
Recent advances have led to the development of more practical and convenient ECG recording devices.11,12,13 Technological progress now allows cardiac rhythm monitoring using single-lead ECG equipment, which is effective for AF diagnosis and screening.12,14,15 It has been established that using a single-lead ECG, acquired by a mobile device, is a good alternative to Holter monitoring for diagnosing AF.16,17,18 Unlike 12-lead ECGs, single-lead ECG recordings are more user-friendly for capturing cardiac rhythm and offer advantages in continuous, long-term ECG acquisition compared to Holter monitoring. Although deep learning algorithms could detect AF in NSR using 12-lead ECG, these ECGs are less convenient than single-lead mobile ECG devices and offer limited continuous monitoring capabilities, thus restricting ongoing AF monitoring and individual data collection. Holter monitors can be beneficial in certain cases, but they are cumbersome to wear and can disrupt daily life.19
With the advent of single-lead mobile ECG devices, both the quality and quantity of data have significantly improved. Recent studies have explored the classification of AF using deep learning techniques with single-lead ECGs.20,21 However, the identification of AF remains a challenge with single-lead mobile ECG devices, primarily due to the subtle and often undetectable features of AF. Furthermore, the focus on screening rather than determining the prevalence of AF presents additional complexities.4,5,22 Therefore, there has been limited understanding in identifying AF using single-lead mobile ECG devices.
In this context, similar to previous studies conducted with 12-lead ECGs,9,10 we propose the hypothesis that deep neural networks can identify subtle features of AF characteristics in single-lead mobile ECG data during NSR, which are typically imperceptible to the human eye.
METHODS
Our methodology comprises five essential components: mobile ECG datasets, preprocessing procedures, segmentation, deep learning models, and outputs (Fig. 1). We ensured the reliability of our analysis by having all mobile ECGs meticulously diagnosed and labeled by clinical experts.
Fig. 1. Proposed method for AF prediction using single-lead mobile ECG in normal sinus rhythm.
(A) Input stage with raw ECG signal. (B) Stage of data processing. (C) Segments of filtered ECG. (D) Application of ResNet50. (E) Final output stage.
ECG = electrocardiogram, AF = atrial fibrillation, ResNet = residual neural network, Conv = convolutional layer, ReLU = rectified linear unit.
Data collection
We collected mobile ECGs prospectively and consecutively from the patients who consented to the data collection and those who visited or were admitted at the Departments of Cardiology and Neurology, Chonnam National University Hospital. From July 2021 to December 2022, we acquired 13,059 mobile ECGs from 6,720 patients using the mobiCARE-MC100 device (Seers Technology, Seongnam, Korea). These ECGs had a sampling rate of 256 Hz and comprised single-lead recordings of approximately 60 seconds each.
To ensure data suitability for analysis, we applied specific exclusion criteria: ECGs with AF or non-sinus rhythms, those lacking a confirmed patient identification number (PID), or containing incomplete digital information. Additionally, ECGs from patients who underwent radiofrequency catheter ablation (RFCA) for AF were excluded changes in ECG waveforms.23,24,25,26 As a result, 3,222 ECGs from 1,549 patients were excluded: 1,096 ECGs from 495 patients due to missing digital information or unverified PID, 2,108 ECGs from 1,045 patients with confirmed AF or non-sinus rhythm, and 18 ECGs from 9 patients with a history of AF RFCA (Fig. 2). We utilized 10,287 NSR ECGs from 5,170 patients for analysis. Within this subset, 2,178 NSR ECGs from 1,089 patients with an AF history were labeled “AF” and 8,109 ECG recordings from 4,081 patients were labeled “Healthy.” AF history was determined by at least one documented 12-lead ECG with AF. Notably, a history of AF without a documented 12-lead ECG was not considered as AF history. The external validation dataset comprised 160 72-hour-Holter ECGs from 160 distinct patients, using the same device.
Fig. 2. Flow diagram of patient data.
PID = patient identification number, AF = atrial fibrillation, ECG = electrocardiogram, NSR = normal sinus rhythm.
Data preprocessing
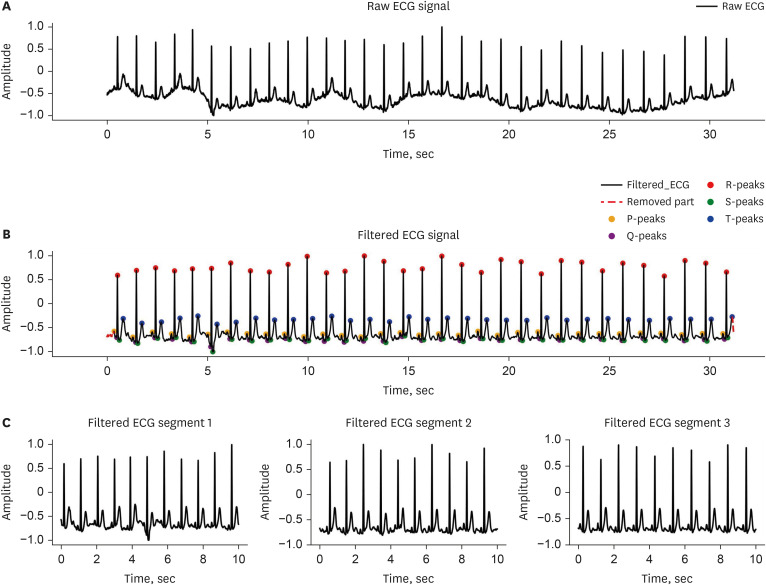
To eliminate noise, the PQRST peaks of each ECG were labeled, and segments not containing these waveforms were removed. The initial segment preceding the first P-Peak and the trailing segment following the last T-Peak were also removed. Subsequently, the ECG baseline was adjusted, and a 0.5 Hz high-pass Butterworth filter applied to further denoise the ECG waveform. ECGs were then segmented into 10-second increments to standardize ECG length across individuals (Fig. 3).
Fig. 3. Process of data preprocessing for atrial fibrillation detection in mobile ECG under normal sinus rhythm.
(A) Raw ECG signal. (B) Filtered ECG signal. (C) Filtered ECG segments.
ECG = electrocardiogram.
To counteract data imbalance and enhance the generalizability of our models while reducing bias, a random under-sampling technique27,28 was employed. This involved the random exclusion of 38,760 10-second segments labeled as Healthy. The dataset was divided into training, internal validation, and testing sets, adhering to a distribution ratio of 6:2:2, respectively.
Deep learning model
Residual neural networks (ResNet) were utilized as deep learning models for identifying potential AF in NSR mobile ECGs.
ResNet, a type of convolutional neural network, demonstrates high accuracy even with deeper layers, effectively addressing the gradient vanishing and exploding problems. Additionally, it offers excellent generalization capabilities, facilitating rapid learning and convergence.29,30 For comparative purposes, recurrent neural network (RNN) and long short-term memory (LSTM) models were also evaluated. RNNs are primarily employed in time series analysis,31,32 whereas LSTM models address long-term dependencies inherent in RNNs.33,34,35 Supplementary Data 1 provides detailed descriptions of RNN and LSTM models.
Model optimization and implementation
Training was conducted using the AdamW36,37 optimizer, with a learning rate of 0.0001, a batch size of 32, and over 50 epochs. However, early stopping was implemented at the 12th epoch to prevent overfitting. Cross-entropy loss served as the loss function.
All mobile ECGs underwent preprocessing using Python 3.9. Pytorch 2.0.1 was utilized for model implementation, with training conducted on a computer equipped with an NVIDIA GeForce RTX3090 TI graphics processing unit (Nvidia, Santa Clara, CA, USA).
Data analysis
The performance of the model was evaluated and compared using accuracy, recall, and precision. These evaluation measures are defined as:
| Accuracy = (TP+TN)/(TP+TN+FP+FN) |
| Recall = TP/(TP+FN) |
| Precision = TN/(TN+FP) |
, where TP represents true positive, TN signifies true negative, FP is false positive, and FN stands for false negative. To provide a comprehensive assessment of model performance, the F1-score and the area under the receiver operating characteristic (ROC) curve (AUC) were calculated. The F1-score, representing the harmonic average of precision and recall, is calculated as follows:
The ROC curve plots the true positive rate (recall) against the false positive rate (1 − Specificity), varying the classification threshold. The AUC value was determined by integrating the ROC curve.
Ethics statement
The study protocol received approval from the Chonnam National University Hospital Institutional Review Board (IRB) in South Korea (IRB No. CNUH-2021-176). Written informed consent was obtained from all patients.
RESULTS
Data characteristics
The dataset includes a total of 31,767 segments, each 10 seconds long. Of these segments, 15,157 (47.7%) were categorized as AF and 16,610 (52.3%) as Healthy. The training set comprised 19,060 of these 10-second segments, with 9,094 labeled as AF and 9,966 as Healthy. The test set contained 6,354 segments, of which 3,031 were identified as AF and 3,322 as Healthy. Additionally, the internal validation set consisted of 6,353 segments, with 3,032 labeled as AF and 3,321 as Healthy.
Performance comparison of different models
Three deep learning models—RNN, LSTM, and one-dimensional convolutional ResNet50—were employed to analyze the dataset refined through specific exclusion criteria. The one-dimensional convolutional ResNet50 model exhibited F1-score, recall, precision, and accuracy values of 71.9%, 79.3%, 65.8%, and 70.5%, respectively, for identifying AF in the test set. In contrast, the RNN model achieved an F1-score of 59.5%, recall of 50.1%, precision of 73.1%, and accuracy of 67.4%, while the LSTM model recorded an F1-score of 62.2%, recall of 53.7%, precision of 73.8%, and accuracy of 68.8%. In the training set, ResNet50 demonstrated F1-score, recall, precision, and accuracy values of 79.3%, 77.8%, 80.8%, and 80.6%, respectively. The RNN in the training set yielded an F1-score of 66.0%, recall of 64.1%, precision of 68.0%, and accuracy of 68.5%. Furthermore, the LSTM’s performance in the training set included an F1-score of 65.5%, recall of 63.0%, precision of 68.2%, and accuracy of 68.3%. For the internal validation set, ResNet50 showed F1-score, recall, precision, and accuracy values of 69.1%, 76.2%, 63.2%, and 67.4%, respectively. The RNN in the internal validation set exhibited an F1-score of 59.6%, recall of 51.3%, precision of 71.1%, and accuracy of 66.8%. Similarly, the LSTM model in the internal validation set displayed an F1-score of 61.1%, recall of 53.2%, precision of 71.5%, and accuracy of 67.6% (Table 1).
Table 1. Performance of proposed models in predicting atrial fibrillation in mobile electrocardiogram during normal sinus rhythm.
| Dataset | Performance | Deep learning model | ||
|---|---|---|---|---|
| RNN | LSTM | ResNet50 | ||
| Training set | F1-score, % | 66.0 | 65.5 | 79.3 |
| Recall, % | 64.1 | 63.0 | 77.8 | |
| Precision, % | 68.0 | 68.2 | 80.8 | |
| Accuracy, % | 68.5 | 68.3 | 80.6 | |
| AUC | 0.75 | 0.81 | 0.88 | |
| Internal validation set | F1-score, % | 59.6 | 61.1 | 69.1 |
| Recall, % | 51.3 | 53.2 | 76.2 | |
| Precision, % | 71.1 | 71.5 | 63.2 | |
| Accuracy, % | 66.8 | 67.6 | 67.4 | |
| AUC | 0.72 | 0.74 | 0.75 | |
| Test set | F1-score, % | 59.5 | 62.2 | 71.9 |
| Recall, % | 50.1 | 53.7 | 79.3 | |
| Precision, % | 73.1 | 73.8 | 65.8 | |
| Accuracy, % | 67.4 | 68.8 | 70.5 | |
| AUC | 0.74 | 0.75 | 0.79 | |
RNN = recurrent neural network, LSTM = long short-term memory, ResNet = residual neural network, AUC = area under the receiver operating characteristic curve.
Confusion matrices were employed to visually assess and compare the classification performance of each model. Fig. 4 depict the confusion matrices for the classification results of ResNet50, RNN, and LSTM, respectively. In the context of classifying AF from NSR, ResNet50 demonstrated the highest AUC of 0.79, followed by RNN at 0.75, and LSTM at 0.74 for the test set. For the training set, the AUCs of ResNet50, RNN, and LSTM were 0.88, 0.75, and 0.81, respectively. Additionally, ResNet50, RNN, and LSTM exhibited AUCs of 0.75, 0.72, and 0.74, respectively, for the internal validation set. The ROC curves and AUC values for identifying potential AF in the training set, internal validation set, and test set are presented in Fig. 5.
Fig. 4. Performance evaluation of proposed models using confusion matrices on the test set.
(A) Confusion matrix of ResNet50, (B) Confusion matrix of RNN, (C) Confusion matrix of LSTM.
ResNet = residual neural network, RNN = recurrent neural network, LSTM = long short-term memory.
Fig. 5. ROC curves demonstrating the performance of proposed models for atrial fibrillation prediction in mobile electrocardiogram during normal sinus rhythm.
(A) ROC curve for the training set. (B) ROC curve for the internal validation set. (C) ROC curve for the test set.
ROC = receiver operating characteristic, AUC = area under the receiver operating characteristic curve, ResNet = residual neural network, LSTM = long short-term memory, RNN = recurrent neural network.
Performance on external validation set
In the external validation, we employed the same filtering approach as in the pre-existing dataset of labeled 72-hour Holter ECGs, ensuring consistency in our analysis. This process involved dividing the ECGs into 10-second segments. We then utilized random sampling without replacement to select these segments. Consequently, we extracted 3,555 (54.21%) segments labeled as Healthy and 3,003 (45.79%) segments labeled as AF. In this external set, ResNet50 demonstrated an F1-score of 64.1%, a recall of 68.9%, a precision of 60.0%, an accuracy of 63.4%, and an AUC of 0.68.
DISCUSSION
In this study, we developed and evaluated a deep learning-based algorithm for detecting potential AF in NSR using single-lead mobile ECG. The algorithm achieved an AUC of 0.79, with external validation yielding 0.68. An AUC value of 0.7 or higher from the test set is generally regarded as indicative of good performance.38 Our results were also compared with previous studies that detected AF using 12-lead ECG,9,10 revealing that single-lead ECG is adequate for obtaining reliable results. These outcomes indicate that potential AF can be identified in NSR ECG using only single-lead data, rather than relying on all 12-lead data.
Few studies have employed a single-lead ECG, collected using single-lead mobile ECG devices, to classify potential AF from ECG during NSR. Most prior research has focused on identifying AF using 12-lead ECG.7,8,9,10 The single-lead mobile ECG device utilized in this study offers convenient body attachment and high versatility, enabling extended ECG monitoring and real-time data collection. Considering the paroxysmal nature of AF, long-term ECG monitoring is often essential for its detection.39 This study’s results validate the reliability of single-lead ECG collected with a single-lead mobile ECG device and demonstrate its potential as a convenient, highly useful method for ECG recording. Such recordings can be analyzed to identify arrhythmia occurrences, thereby allowing healthcare providers to mitigate the risk of arrhythmias and other diseases and to develop innovative patient management strategies, such as timely medication.
The study also implemented crucial steps to acquire the necessary single-lead ECG for identifying potential AF. Our data collection involved 6,720 patients, facilitating the acquisition and analysis of 13,059 mobile ECGs. A common issue in many medical datasets is the data imbalance problem, where specific health conditions are underrepresented in classification algorithms. This problem was evident in our study, particularly with the scarcity of ECGs labeled as AF. Predominantly, the data were marked as Healthy, which could hinder the detection of AF. To mitigate this data imbalance and enhance the generalization capabilities of our models while minimizing bias, a random under-sampling technique was employed. This involved randomly discarding 38,760 10-second Healthy-labeled segments, effectively addressing the data imbalance and leading to more accurate classification outcomes. For the external validation dataset, the same methodology was applied as with our training, testing, and internal validation sets. The 160 72-hour Holter ECGs from 160 distinct patients were divided into 10-second segments, and random sampling without replacement was conducted to form a representative subset for validation. This method of random sampling without replacement ensures a more unbiased analysis by avoiding potential bias from the researchers’ preferences or assumptions. Furthermore, three deep learning models—RNN, LSTM, and ResNet50—were utilized to detect potential AF in mobile ECGs during NSR, and their performances were compared. The ResNet50 model exhibited superior performance, with an F1-score, recall, precision, accuracy, and AUC of 71.9%, 79.3%, 65.8%, 70.5%, and 0.79, respectively. These results suggest that ResNet50 is more accurate in identifying the subtle patterns indicative of potential AF. The methodologies of data collection, preprocessing, and model selection are critical in determining the efficacy of a detection model. Through this approach, we demonstrated the viability of using single-lead mobile ECG and highlighted the potential of deep learning algorithms to discern subtle signals in ECGs for the detection and identification of cardiovascular events such as AF.
Further research is necessary to enhance the performance and generalizability of deep learning models for use in clinical settings. Although our model, which uses a single-lead ECG, demonstrated promising results in identifying AF with an AUC above 0.7 in the test set, its performance was less impressive in the external validation set. Previous studies have indicated that the RR interval, P-wave morphology, and QT interval are significant factors affecting AF risk.40,41 Our study relied solely on ECGs to train the deep learning algorithm, but future research could improve model accuracy by incorporating additional relevant features as AF indicators.
About 30% of patients with cardiogenic stroke, specifically embolic stroke of unknown source (ESUS), exhibit AF during long-term ECG monitoring.42,43 In instances of cryptogenic strokes where the cause is unclear, detecting AF is crucial; the presence of AF necessitates a shift in treatment from standard antiplatelet therapy to anticoagulation to lower the recurrence risk of strokes. Therefore, more studies focused on detecting AF in patients with ESUS are imperative. Employing our developed deep learning model in specific cohorts, such as those with ESUS, will aid in verifying its clinical utility. The data processing methods and deep learning models developed in this study are foundational for broader clinical investigations. As we continue this research and enhance our models, their significant impact on patient healthcare and cost-effectiveness will become increasingly evident in the future.
This study presents several limitations. Firstly, the constraints of a single-lead mobile ECG device compromise data quality compared to 12-lead ECGs. As a result, rhythms that were unidentified or vague were systematically excluded from the analysis, leading to a higher prevalence of exclusions and a more restrictive analysis. Secondly, the study lacks prospective validation. However, to address this limitation, ongoing studies are currently applying and validating the algorithm in acute ischemic stroke patients. These patients are undergoing serial follow-up with 72-hour Holter ECGs at three-month intervals. The primary objective of this validation is to identify AF and evaluate the algorithm’s performance.
Finally, we developed an AI model in this study for detecting potential AF using short-read ECG data from a single-lead mobile ECG device, and we validated its performance to ensure reliable results. The data and AI techniques utilized in this study have the potential to significantly enhance early identification and patient management of potential heart diseases.
ACKNOWLEDGMENTS
Special thanks to Dr. Seong Won Jeon, Dr. Changhyun Kim, and Dr. Dong Kyun Kim for the diagnosis and labeling mobile electrocardiograms.
Footnotes
Funding: This work was supported by a grant (2021R1F1A1048115) of the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) and a grant (BCRI23088) from Chonnam National University Hospital Biomedical Research Institute.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim J, Ko B, Lee KH.
- Data curation: Kim J, Lee KH.
- Formal analysis: Kim J.
- Funding acquisition: Lee KH.
- Investigation: Kim J, Ko B, Lee YS.
- Methodology: Kim J, Ko B, Lee YS.
- Software: Kim J, Lee KH, Lee YS.
- Validation: Ko B, Lee M, Lee KH.
- Visualization: Kim J.
- Writing - original draft: Kim J, Lee SJ.
- Writing - review & editing: Lee SJ, Lee M, Lee YS, Lee KH.
SUPPLEMENTARY MATERIALS
Descriptions of recurrent neural network (RNN) and long short-term memory (LSTM)
References
- 1.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 2.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 3.Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet. 2016;388(10046):806–817. doi: 10.1016/S0140-6736(16)31257-0. [DOI] [PubMed] [Google Scholar]
- 4.Barbarossa A, Guerra F, Capucci A. Silent atrial fibrillation: a critical review. J Atr Fibrillation. 2014;7(3):1138. doi: 10.4022/jafib.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation. 2011;124(4):477–486. doi: 10.1161/CIRCULATIONAHA.111.029801. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler PD, Glotzer TV, Daoud EG, Singer DE, Ezekowitz MD, Hoyt RH, et al. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am J Cardiol. 2012;110(9):1309–1314. doi: 10.1016/j.amjcard.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Somani S, Russak AJ, Richter F, Zhao S, Vaid A, Chaudhry F, et al. Deep learning and the electrocardiogram: review of the current state-of-the-art. Europace. 2021;23(8):1179–1191. doi: 10.1093/europace/euaa377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro AH, Ribeiro MH, Paixão GM, Oliveira DM, Gomes PR, Canazart JA, et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat Commun. 2020;11(1):1760. doi: 10.1038/s41467-020-15432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baek YS, Lee SC, Choi W, Kim DH. A new deep learning algorithm of 12-lead electrocardiogram for identifying atrial fibrillation during sinus rhythm. Sci Rep. 2021;11(1):12818. doi: 10.1038/s41598-021-92172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394(10201):861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 11.Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B, Armoundas AA. Wearable devices for ambulatory cardiac monitoring: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(13):1582–1592. doi: 10.1016/j.jacc.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang J, Kim J, Choi KJ, Cho MS, Nam GB, Kim YH. Assessing accuracy of wrist-worn wearable devices in measurement of paroxysmal supraventricular tachycardia heart rate. Korean Circ J. 2019;49(5):437–445. doi: 10.4070/kcj.2018.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramkumar S, Nerlekar N, D’Souza D, Pol DJ, Kalman JM, Marwick TH. Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis. BMJ Open. 2018;8(9):e024178. doi: 10.1136/bmjopen-2018-024178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giebel GD, Gissel C. Accuracy of mHealth devices for atrial fibrillation screening: systematic review. JMIR Mhealth Uhealth. 2019;7(6):e13641. doi: 10.2196/13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon S, Lee SR, Choi EK, Ahn HJ, Song HS, Lee YS, et al. Validation of adhesive single-lead ECG device compared with holter monitoring among non-atrial fibrillation patients. Sensors (Basel) 2021;21(9):3122. doi: 10.3390/s21093122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nault I, André P, Plourde B, Leclerc F, Sarrazin JF, Philippon F, et al. Validation of a novel single lead ambulatory ECG monitor - Cardiostat™ - Compared to a standard ECG Holter monitoring. J Electrocardiol. 2019;53:57–63. doi: 10.1016/j.jelectrocard.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Karaoğuz MR, Yurtseven E, Aslan G, Deliormanlı BG, Adıgüzel Ö, Gönen M, et al. The quality of ECG data acquisition, and diagnostic performance of a novel adhesive patch for ambulatory cardiac rhythm monitoring in arrhythmia detection. J Electrocardiol. 2019;54:28–35. doi: 10.1016/j.jelectrocard.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Cheung CC, Kerr CR, Krahn AD. Comparing 14-day adhesive patch with 24-h Holter monitoring. Future Cardiol. 2014;10(3):319–322. doi: 10.2217/fca.14.24. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh J, Solatidehkordi Z, Aburukba R, Sagahyroon A. Atrial fibrillation classification with smart wearables using short-term heart rate variability and deep convolutional neural networks. Sensors (Basel) 2021;21(21):7233. doi: 10.3390/s21217233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tutuko B, Rachmatullah MN, Darmawahyuni A, Nurmaini S, Tondas AE, Passarella R, et al. Short single-lead ECG signal delineation-based deep learning: implementation in automatic atrial fibrillation identification. Sensors (Basel) 2022;22(6):2329. doi: 10.3390/s22062329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khairuddin A, Azir KF, Kan PE. Limitations and future of electrocardiography devices: a review and the perspective from the Internet of Things; Proceedings of 2017 International Conference on Research and Innovation in Information Systems (ICRIIS); 2017 July 16–17; Langkawi, Malaysia. New York, NY, USA: IEEE; 2017. pp. 1–7. [Google Scholar]
- 23.Maan A, Mansour M, Ruskin JN, Heist EK. Impact of catheter ablation on P-wave parameters on 12-lead electrocardiogram in patients with atrial fibrillation. J Electrocardiol. 2014;47(5):725–733. doi: 10.1016/j.jelectrocard.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Hu X, Jiang J, Ma Y, Tang A. Novel P wave indices to predict atrial fibrillation recurrence after radiofrequency ablation for paroxysmal atrial fibrillation. Med Sci Monit. 2016;22:2616–2623. doi: 10.12659/MSM.896675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao Y, Xu M, Yang L, Zhang C, Liu H, Shao X. Investigating the association between P wave duration and atrial fibrillation recurrence after radiofrequency ablation in early persistent atrial fibrillation patients. Int J Cardiol. 2022;351:48–54. doi: 10.1016/j.ijcard.2021.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Letsas KP, Pappas LK, Gavrielatos G, Efremidis M, Sideris A, Kardaras F. ST-segment elevation induced during the transseptal procedure for radiofrequency catheter ablation of atrial fibrillation. Int J Cardiol. 2007;114(1):e12–e14. doi: 10.1016/j.ijcard.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 27.Lee W, Seo K. Downsampling for binary classification with a highly imbalanced dataset using active learning. Big Data Research. 2022;28:100314 [Google Scholar]
- 28.Qazi N, Raza K. Effect of feature selection, SMOTE and under sampling on class imbalance classification; Proceedings of 2012 UKSim 14th International Conference on Computer Modelling and Simulation; 2012 March 28–30; Cambridge, UK. New York, NY, USA: IEEE; 2012. pp. 145–150. [Google Scholar]
- 29.He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition; Proceedings of 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 2016 June 27–30; Las Vegas, NV, USA. New York, NY, USA: IEEE; 2016. pp. 770–778. [Google Scholar]
- 30.He F, Liu T, Tao D. Why ResNet works? Residuals generalize. IEEE Trans Neural Netw Learn Syst. 2020;31(12):5349–5362. doi: 10.1109/TNNLS.2020.2966319. [DOI] [PubMed] [Google Scholar]
- 31.Hüsken M, Stagge P. Recurrent neural networks for time series classification. Neurocomputing. 2003;50:223–235. [Google Scholar]
- 32.Singh S, Pandey SK, Pawar U, Janghel RR. Classification of ECG arrhythmia using recurrent neural networks. Procedia Comput Sci. 2018;132:1290–1297. [Google Scholar]
- 33.Hochreiter S, Schmidhuber J. Long short-term memory. Neural Comput. 1997;9(8):1735–1780. doi: 10.1162/neco.1997.9.8.1735. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Si X, Hu C, Zhang J. A review of recurrent neural networks: LSTM cells and network architectures. Neural Comput. 2019;31(7):1235–1270. doi: 10.1162/neco_a_01199. [DOI] [PubMed] [Google Scholar]
- 35.Karim F, Majumdar S, Darabi H, Chen S. LSTM fully convolutional networks for time series classification. IEEE Access. 2018;6:1662–1669. [Google Scholar]
- 36.Reyad M, Sarhan AM, Arafa M. A modified Adam algorithm for deep neural network optimization. Neural Comput Appl. 2023;35(23):17095–17112. [Google Scholar]
- 37.Jia X, Feng X, Yong H, Meng D. Weight decay with tailored Adam on scale-invariant weights for better generalization. IEEE Trans Neural Netw Learn Syst. 2022 doi: 10.1109/TNNLS.2022.3213536. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 38.Simundic AM. Diagnostic accuracy—part 1: basic concepts: sensitivity and specificity, ROC analysis, STARD Statement. Point Care. 2012;11(1):6–8. [Google Scholar]
- 39.Nigusse AB, Mengistie DA, Malengier B, Tseghai GB, Langenhove LV. Wearable smart textiles for long-term electrocardiography monitoring—a review. Sensors (Basel) 2021;21(12):4174. doi: 10.3390/s21124174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppola EE, Gyawali PK, Vanjara N, Giaime D, Wang L. Atrial fibrillation classification from a short single lead ECG recording using hierarchical classifier; Proceedings of 2017 Computing in Cardiology (CinC); 2017 September 24–27; Rennes, France. New York, NY, USA: IEEE; 2017. [Google Scholar]
- 41.Yazdani S, Laub P, Luca A, Vesin JM. Heart rhythm classification using short-term ECG atrial and ventricular activity analysis; Proceedings of 2017 Computing in Cardiology (CinC); 2017 September 24–27; Rennes, France. New York, NY, USA: IEEE; 2017. [Google Scholar]
- 42.Bahit MC, Sacco RL, Easton JD, Meyerhoff J, Cronin L, Kleine E, et al. Predictors of atrial fibrillation development in patients with embolic stroke of undetermined source: an analysis of the RE-SPECT ESUS trial. Circulation. 2021;144(22):1738–1746. doi: 10.1161/CIRCULATIONAHA.121.055176. [DOI] [PubMed] [Google Scholar]
- 43.Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015;46(4):936–941. doi: 10.1161/STROKEAHA.115.008714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptions of recurrent neural network (RNN) and long short-term memory (LSTM)