To the Editor
The development of Venetoclax (Ven) and Azacytidine (Aza) as treatment option in Acute Myeloid Leukemia (AML) has been a breakthrough especially for patients unfit for intensive chemotherapy (IC). Ven/Aza induced complete remission (CR) in 36.7% of newly diagnosed elderly AML patients [1]. However, long term response rates remain poor with a median event-free survival of 9.8 months and a median overall survival of 14.1 months [1], due to persistence of measurable residual disease (MRD) that drives relapse. Novel therapeutic strategies are therefore urgently needed. Data from B-cell malignancies have demonstrated that T-cell based immunotherapy platforms, e.g. T-cell bispecific antibodies (TCBs) or chimeric antigen receptor (CAR) engineered T cells, are able to induce long-term remission and even cure in this patient cohort. Treatment with Blinatumomab in B-cell precursor acute lymphoblastic leukemia resulted in significant elimination of MRD in low-burden disease [2]. Therefore, utility of immunotherapies such as TCBs post Ven/Aza could be envisaged to eradicate the MRD pool in AML and to improve survival rates in this patient population. This concept is further supported by preclinical data that demonstrated a Venetoclax induced increase in reactive oxygen-species (ROS) production in T cells, which resulted in enhanced T-cell effector function [3]. In addition, Azacytidine increased the susceptibility of AML cells for T-cell mediated lysis [3]. Multiple early phase clinical trials with TCBs targeting CD123 (Flotetuzumab and Vibecotamab) or the intracellular tumor antigen Wilms tumor 1 (WT1) presented on HLA-A*02 are currently underway in AML [4–6]. In the current report, we investigate the immune modulating impact of Ven/Aza on the efficacy of a WT1-targeted TCB in AML in vitro and in vivo.
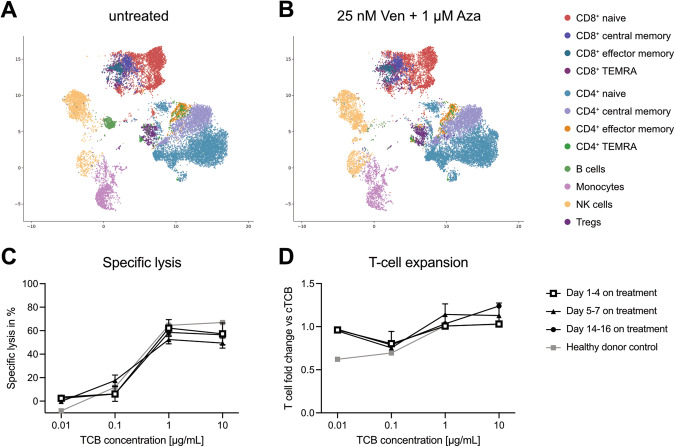
To this end, we analyzed the PBMC composition of three healthy donors (HD) in vitro by flow cytometry after three days of treatment with 25 nM Ven and 1 µM Aza, reflecting patient serum concentrations [7, 8], and compared to an untreated control. High dimensional clustering showed no changes in clustering of NK cells and monocytes but revealed a depletion of B cells (Fig. 1A, B). Interestingly, it has recently been shown that bone marrow inflammation in AML is associated with an enrichment of atypical B cells likely conveying suppressive function [9]. Importantly, the clustering of the T-cell compartment remained largely unaffected in our analysis with the exception of CD8+ naïve T cells that were partially depleted in response to Ven/Aza (Fig. 1A, B). Ten-fold higher concentrations of Ven further enhanced the reduction of CD8+ naïve T cells and led to an overall reduction in viable T cells. A more detailed analysis revealed that the cytoreductive effect in this experiment was only mediated by Ven but not Aza (Supplementary Fig. 1). Taken together, these results confirm previous observations on distinct Ven-sensitivity of lymphocytes [10–12].
Fig. 1. Ven/Aza has limited impact on the T-cell compartment.
A High dimensional clustering by Cytolution of HD PBMCs (A) left untreated or (B) treated with 25 nM Ven and 1 µM Aza for three days in vitro (n = 3). C Specific lysis of OCI-AML3 cells and (D) T-cell expansion in cytotoxicity assays with WT1-TCB and T cells from Ven/Aza-treated patients during their first cycle of treatment (n = 3 per time point). HD T cells were included as control (n = 3). Dots represent mean ± SEM.
Next, we studied primary patient samples derived from Ven/Aza- treated patients. T cells were extracted from peripheral blood on days 1–4, 5–7 and 14–16 post Ven/Aza treatment. We first evaluated the WT1-TCB mediated cytotoxicity against OCI-AML3 cells in a co-culture with the extracted T cells and observed that WT1-TCB consistently and dose-dependently induced target cell lysis (mean specific lysis with 1 µg/mL WT1-TCB: d1-4: 62.2 ± 7.3%; d5-7: 52.5 ± 3.7%; d14-16: 58.5 ± 5.6%; ±SEM; all n = 3; Fig. 1C). Importantly, results showed low inter-patient variability and reflected data obtained from HD T cells. In line with these findings, a dose-dependent T-cell expansion was observed throughout the longitudinal monitoring (Fig. 1D). Taken together, the data indicate that Ven/Aza does not change T-cell function in AML patients during the first two weeks of treatment.
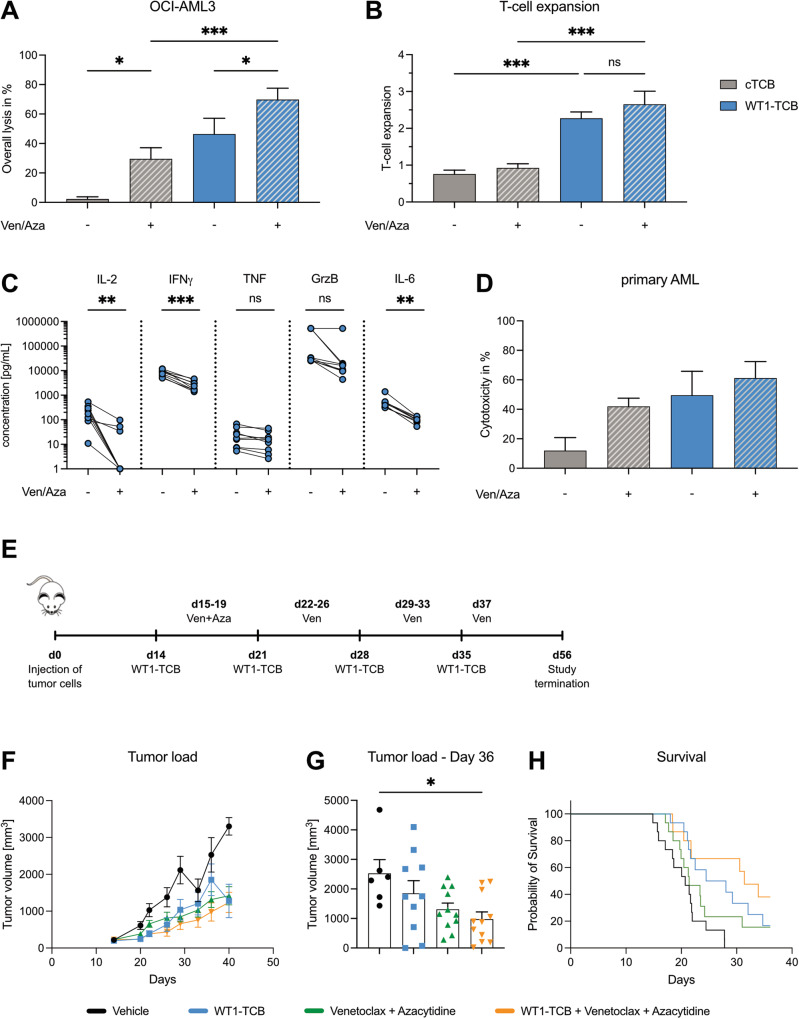
Next, we investigated whether Ven/Aza sensitized AML cells to TCB-mediated lysis. First, we tested the combination of Ven/Aza and WT1-TCB in vitro in cocultures with T cells from nine healthy donors and OCI-AML3 (Fig. 2) or SKM1 cells (Supplementary Fig. 2A, B) for six days in the presence of 1 µg/mL WT1-TCB, 25 nM Ven and 1 µM Aza mimicking concentrations reported for AML patients. A control TCB (cTCB) recognizing a nontumor target antigen was included as control. Ven/Aza mediated cytotoxicity of OCI-AML3 cells was significantly increased in combination with the WT1-TCB from 29.5 ± 7.6% to 69.8 ± 7.8% (p = 0.0004; ±SEM; n = 9) (Fig. 2A). Moreover, WT1-TCB led to a significant increase in T-cell proliferation in the presence of Ven/Aza (mean fold expansion compared to day 0: 2.7 ± 0.4) (Fig. 2B). Importantly, this was comparable to monotherapy with WT1-TCB (2.3 ± 0.2), whereas Ven/Aza and cTCB treatments did not lead to a T-cell expansion (0.9 ± 0.1 and 0.8 ± 0.1, respectively) (Fig. 2B). Furthermore, we observed lower levels of secreted proinflammatory cytokines IFNγ (p < 0.0001), IL-6 (p = 0.004), and TNF (p = 0.056), as well as secretion of Granzyme B (p = 0.125) and IL-2 (p = 0.004) in the Ven/Aza and WT1-TCB combination treatment compared to WT1-TCB monotherapy (Fig. 2C).
Fig. 2. Ven/Aza augments WT1-TCB mediated lysis in vitro and in vivo.
A Lysis of OCI-AML3 cells and (B) T-cell expansion compared to day 0 in cytotoxicity assays with HD T cells mediated by 1 µg/mL cTCB and WT1-TCB after six days in the presence or absence of 25 nM Ven and 1 µM Aza. Bars represent the mean ± SEM (n = 9). Statistical analysis: One-way ANOVA with Tukey post hoc test, *p < 0.05, ***p < 0.001; (C) cytokine secretion after six days in cytotoxicity assays with OCI-AML3 cells and HD T cells. Statistical analysis: paired t test, **p < 0.01, ***p < 0.001; ns, not significant. D Lysis of primary AML cells in cytotoxicity assays with HD T cells mediated by cTCB and WT1-TCB after six days in the presence or absence of Ven/Aza. Bars represent the mean ± SEM (n = 9). E Experimental set-up for in vivo testing of WT1-TCB in combination with Ven/Aza in humanized NSG mice bearing SKM1 tumor. F, G Tumor load of SKM1 cells over time and on day 36, and (H) overall survival of humanized mice treated with WT1-TCB and Ven/Aza (n = 15 per treatment group). Bars represent mean ± SEM. Statistical analysis: Dunn’s test, *p < 0.01.
We have furthermore validated synergism between Ven/Aza and WT1-TCB in cytotoxicity experiments with OCI-AML3 cells and T cells isolated from three AML patients obtained after their first cycle of Ven/Aza. Again, the combinatorial approach increased the lysis compared to monotherapy with Ven/Aza or WT1-TCB (Supplementary Fig. 2C).
Analysis of four primary AML samples further validated our findings. The combination of Ven/Aza and WT1-TCB led to the highest cytotoxicity in cocultures with healthy donor T cells over seven days. While Ven/Aza and WT1-TCB already achieved 40 – 50% lysis, the combinatorial approach enhanced cytotoxicity up to 61.2 ± 11.2% (n = 4; Fig. 2D). Similar observations were made using a CD33 targeting TCB with AML cell lines and primary AML cells showing that enhanced tumor cell killing by the combination of Ven/Aza and TCBs is tumor target independent (Supplementary Fig. 2D–F).
Lastly, we applied a humanized HLA-A*02+ NSG mouse model bearing SKM1 tumors with 15 animals per treatment group to evaluate the combinatorial effect of Ven/Aza and TCB in vivo. Mice received daily treatments with 1 mg/kg Aza (days 15–19) and 50 mg/kg Ven (days 15–37, except for weekends) and weekly injections with the WT1-TCB (0.05 mg/kg) upon reaching an average tumor size of 198 mm3 on day 14 (Fig. 2E). Monotherapies with either WT1-TCB or Ven/Aza inhibited tumor growth (day 36: 1850 ± 432 mm3 and 1316 ± 213 mm3, respectively), compared to the corresponding vehicle control (2528 ± 468 mm3). However, the combination of WT1-TCB and Ven/Aza resulted in significant anti-tumor activity in terms of reduced tumor growth (978 ± 247 mm3; p = 0.0023) but also prolongation of the median survival (p = 0.0034) compared to the vehicle control on day 36 (Fig. 2F–H). Furthermore, treatment with Ven/Aza but not WT1-TCB resulted in a decreased body weight compared to the vehicle control, which was not further increased by the combinatorial treatment (Supplementary Fig. 3). Other apparent signs for clinical toxicities were not observed, indicating no synergistic side effects of Ven/Aza and WT1-TCB.
Taken together, our data provide a first rationale for combining Ven/Aza and TCBs as demonstrated by the significantly increased anti-tumor activity observed in in vitro and in vivo assays. Moreover, we also observed that synergies between Ven/Aza and TCBs are likely independent of the specific tumor target, based on our observations with a CD33-TCB. A challenge in the application of TCBs is the risk for higher-grade cytokine release syndrome which can be mitigated by step-up dosing as shown in trials [13]. Notably, through combination of Ven/Aza and WT1-TCB we observed a reduced secretion of the proinflammatory cytokines IFNγ and IL-6 compared to TCB alone. Therefore, the combination of these drugs might even increase the TCB tolerability, albeit this needs further pre-clinical investigation. Furthermore, the optimal scheduling of Ven/Aza and TCBs whether in parallel or sequential, to increase patients’ benefit/risk ratio requires further evaluation. Overall, our data support the combination of TCBs with Ven/Aza, the current gold standard for unfit and high-risk AML patients, as potentially more efficacious and less toxic anti-leukemic therapy. Clearly, further research is needed to dissect the impact of Ven/Aza on the immune contexture of AML and to identify the most effective treatment strategy.
Supplementary information
Acknowledgements
We thank Sabine Sandner-Thiede and Simone Pentz (LMU University Hospital, LMU Munich) for their excellent technical support. We acknowledge the iFlow Core Facility of the LMU University Hospital, LMU Munich (INST 409/225-1 FUGG) for assistance with the generation of flow cytometry data. This work was supported by a Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) research grant provided within the Sonderforschungsbereich SFB-TRR 388/1 2021—452881907, and DFG research grant 451580403 (to MSu). The work was further supported by the Bavarian Elite Graduate Training Network, the Wilhelm–Sander Stiftung (project no. 2018.087.1), the Else–Kröner–Fresenius Stiftung, the Bavarian Center for Cancer Research (BZKF), and research funding from Roche (all to MSu).
Author contributions
CK, and MSu designed the study and supervised the project; GH, KK, VK, CK, and MSu, wrote the manuscript; GH and AS performed the experiments and analyzed and/or interpreted the data; DN, NP; ASN; MSp; AL; VB; JE; DD; VK; KK; SC and PU were involved in research design and data interpretation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
AS, VK, KK, SC, PU and CK are employed at the Roche Innovation Center Zurich, Schlieren, Switzerland and JE is employed at the Roche Innovation Center Munich, Penzberg, Germany. These authors also declare patents and ownership of Roche stock. DD is employed at Genentech Inc., South San Francisco, CA. MSu has received industry research support from Amgen, Gilead, Miltenyi Biotec, Morphosys, Roche, and Seattle Genetics, and has served as a consultant/advisor to Amgen, BMS, Celgene, Gilead, Pfizer, Novartis, and Roche. She sits on the advisory boards of Amgen, Celgene, Gilead, Janssen, Novartis, Pfizer, and Seattle Genetics, and serves on the speakers’ bureau at Amgen, Celgene, Gilead, Janssen, and Pfizer. GH, ASN and MSu declare patents together with Roche. DN, NP, MSp, AL and VB declare no relevant competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was obtained by the Institutional Review Board of the LMU Munich.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christian Klein, Email: christian.klein.ck1@roche.com.
Marion Subklewe, Email: marion.subklewe@med.uni-muenchen.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-023-02127-0.
References
- 1.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–29. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 2.Subklewe M. BiTEs better than CAR T cells. Blood Adv. 2021;5:607–12. doi: 10.1182/bloodadvances.2020001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JB, Khan DH, Hurren R, Xu M, Na Y, Kang H, et al. Venetoclax enhances T cell–mediated antileukemic activity by increasing ROS production. Blood. 2021;138:234–45. doi: 10.1182/blood.2020009081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uy GL, Aldoss I, Foster MC, Sayre PH, Wieduwilt MJ, Advani AS, et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood. 2021;137:751–62. doi: 10.1182/blood.2020007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravandi F, Bashey A, Stock W, Foran JM, Mawad R, Egan D, et al. Complete responses in relapsed/refractory acute myeloid leukemia (AML) patients on a weekly dosing schedule of vibecotamab (XmAb14045), a CD123 x CD3 T cell-engaging bispecific antibody; initial results of a phase 1 study. Blood. 2020;136:4–5. doi: 10.1182/blood-2020-134746. [DOI] [Google Scholar]
- 6.Augsberger C, Hänel G, Xu W, Pulko V, Hanisch LJ, Augustin A, et al. Targeting intracellular WT1 in AML with a novel RMF-peptide-MHC specific T-cell bispecific antibody. Blood. 2021;135:1–15. doi: 10.1182/blood.2020010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AbbVie Ltd. Venclyxto [Assessment report]. European Medicines Agency. EMA/725631/2016. https://www.ema.europa.eu/en/documents/assessment-report/venclyxto-epar-public-assessment-report_en.pdf (accessed 31 Jul2023).
- 8.Bristol-Myers Squibb Pharma EEIG. Vidaza [Assessment report for paediatric studies submitted according to Article 46 of the Regulation (EC) No 1901/2006]. European Medicines Agency. EMA/295254/2020. https://www.ema.europa.eu/en/documents/variation-report/voxzogo-h-c-005475-p46-007-epar-assessment-report_en.pdf (accessed 31 Jul2023).
- 9.Lasry A, Nadorp B, Fornerod M, Nicolet D, Wu H, Walker CJ et al. An inflammatory state remodels the immune microenvironment and improves risk stratification in acute myeloid leukemia. Springer US, 2023 10.1038/s43018-022-00480-0. [DOI] [PMC free article] [PubMed]
- 10.Ludwig LM, Hawley KM, Banks DB, Thomas-Toth AT, Blazar BR, McNerney ME et al. Venetoclax imparts distinct cell death sensitivity and adaptivity patterns in T cells. Cell Death Dis. 2021; 12 10.1038/s41419-021-04285-4. [DOI] [PMC free article] [PubMed]
- 11.Kohlhapp FJ, Haribhai D, Mathew R, Duggan R, Ellis PA, Wang R, et al. Venetoclax increases intratumoral effector T cells and antitumor efficacy in combination with immune checkpoint blockade. Cancer Discov. 2021;11:68–79. doi: 10.1158/2159-8290.CD-19-0759. [DOI] [PubMed] [Google Scholar]
- 12.Teh CE, Peng H, Luo M, Tan T, Trussart M, Howson LJ et al. Venetoclax treatment in cancer patients has limited impact on circulating T and NK cells. Blood Adv. 2023;7:2733–2745. [DOI] [PMC free article] [PubMed]
- 13.Hosseini I, Gadkar K, Stefanich E, Li CC, Sun LL, Chu YW, et al. Mitigating the risk of cytokine release syndrome in a Phase I trial of CD20/CD3 bispecific antibody mosunetuzumab in NHL: impact of translational system modeling. NPJ Syst Biol Appl. 2020;6:28. doi: 10.1038/s41540-020-00145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during the current study are available from the corresponding author on reasonable request.