Abstract
Background
Platinum-sensitivity is a phenotypic biomarker of Poly (ADP-ribose) polymerase inhibitors (PARPi) sensitivity in histotypes where PARPi are approved. Approximately one-third of non-small cell lung cancers (NSCLC) are platinum-sensitive. The double-blind, randomized phase II PIPSeN (NCT02679963) study evaluated olaparib, a PARPi, as maintenance therapy for patients with platinum-sensitive advanced NSCLC.
Methods
Chemonaïve patients with ECOG performance status of 0–1, platinum-sensitive, EGFR- and ALK-wild-type, stage IIIB-IV NSCLC were randomized (R) to receive either olaparib (O) maintenance or a placebo (P). The primary objective was progression-free survival (PFS) from R. Secondary objectives included overall survival (OS) and safety. With an anticipated hazard ratio of 0.65, 144 patients were required to be randomized, and approximately 500 patients enrolled.
Results
The trial was prematurely terminated because anti-PD(L)1 therapy was approved during the trial recruitment. A total of 182 patients were enrolled, with 60 patients randomized: 33 and 27 in the O and P arms, respectively. Patient and tumor characteristics were well-balanced between arms, except for alcohol intake (33% vs 11% in the O and P arms, respectively, p = 0.043). The median PFS was 2.9 and 2.0 months in the O and P arms, respectively (logrank p = 0.99). The median OS was 9.4 and 9.5 months in the O and P arms, respectively (p = 0.28). Grade ≥3 toxicities occurred in 15 and 8 patients in O and P arms, with no new safety concerns.
Conclusion
PIPSeN was terminated early after enrollment of only 50% of the pre-planned population, thus being statistically underpowered. Olaparib maintenance did neither improve median PFS nor OS in this patient population.
Subject terms: Non-small-cell lung cancer, Targeted therapies
Introduction
Platinum-based doublet chemotherapy regimens have been the cornerstone of first-line treatment in advanced non-small cell lung cancer (NSCLC) until the advent of immune checkpoint blockers, with a median overall survival (OS) of 8 months and objective response rates (ORR) of 15–27% [1]. This modest benefit prompted a search for additional treatment strategies, in combination or in the maintenance setting, including targeted therapies, antiangiogenics, immune checkpoint blockers, or more recently, Poly (ADP-ribose) polymerase (PARP) inhibitors. A first strategy consisted of continuation maintenance, as exemplified by the continuation of pemetrexed after 4 cycles of platinum doublet, which increased OS compared to best supportive care and became standard of care in non-squamous NSCLC [2, 3]. Another strategy consisted of switch maintenance, with the use of a drug not previously employed in the platinum-based induction setting, such as PARP inhibitors (PARPi) [4].
PARPi act by two main mechanisms: (i) inhibiting the catalytic activity of the PARP enzymes (PARYlation), which play a key role in the repair of DNA single-strand breaks through base excision repair; and (ii) trapping PARP1 on the DNA, thereby stalling the replication forks during S-phase [5, 6]. Upon cell replication, unprocessed stalled replication forks eventually lead to DNA double-strand breaks (DSB), which are the most toxic cellular insult. In cells that are deficient in DSB repair (notably following mutations or loss-of-function of the homologous recombination repair (HRR) BRCA1/2 enzymes), the accumulation of DSB eventually causes cell death through synthetic lethality. Because platinum salts generate platinum adducts, which are handled by the same DNA repair pathways as DNA lesions caused by trapped PARP1, platinum-sensitivity is traditionally considered as a phenotypic biomarker for PARPi sensitivity. This suggests that PARPi may be beneficial to patients with platinum-sensitive diseases. Since concomitant administration of PARPi and platinum agents is not tolerable (with the exception of veliparib that has no trapping capabilities) [5, 7], post-platinum switch maintenance represents an attractive setting for these agents. Notably, PARPi have first shown efficacy as a maintenance therapy in advanced ovarian cancer, where they remarkably improved progression-free survival (PFS) in patients with platinum-sensitive disease [8–10]. Similarly, maintenance olaparib recently brought significant improvement in PFS (7.4 months vs. 3.8 months; hazard ratio 0.53) and ORR (23% vs. 12%) in patients with germline BRCA-mutated metastatic pancreatic cancer that did not progress on platinum therapy [11].
We previously found that NSCLC preclinical models that are deficient in the Excision repair cross-complementation group 1 (ERCC1) DNA repair enzyme (defective in 20% of NSCLC) were exquisitely selectively sensitive to platinum salts and PARPi [12]. Based on this data, we hypothesized that maintenance PARPi may be beneficial for patients with chemo-naïve platinum-sensitive NSCLC. We therefore conducted an academy-sponsored randomized double-blind phase II study to evaluate the efficacy of the PARPi olaparib as a maintenance therapy in patients with platinum-sensitive advanced NSCLC (PIPSeN, NCT02679963). This trial started recruiting patients in 2016 and was prematurely stopped once immune-checkpoint inhibitors became standard of care in the first line setting of advanced NSCLC.
Patients and methods
Study design and treatment
PIPSeN was a multicenter randomized double-blind phase II trial, which aimed at assessing the efficacy of olaparib versus placebo as a maintenance therapy after platinum-doublet therapy. The study was sponsored by Gustave Roussy; it involved one French center and the Spanish Lung Cancer Group, including 14 centers.
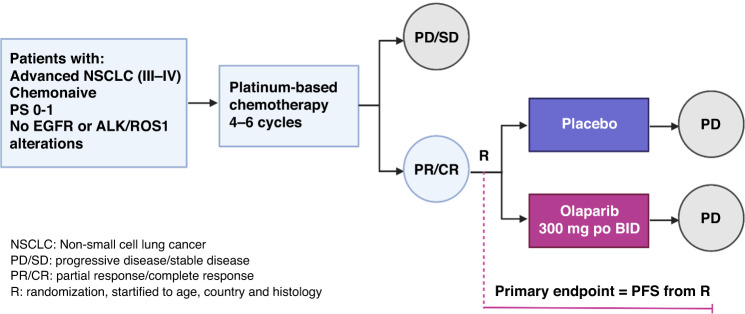
Eligible patients were required to be at least 18 years old, with an ECOG performance status of 0–1, and have a histologically confirmed diagnosis of advanced NSCLC (stage IIIB or IV according to AJCC 7th edition) without EGFR or ALK/ROS1 alterations. Other eligibility criteria included being chemonaïve for NSCLC and having adequate organ and bone marrow function. Full eligibility criteria are available in the trial protocol (Supplementary File 1). Patients initially received the standard-of-care: four to six 21-day cycles of any platinum-doublet therapy, excluding taxane-based doublets [13–15]. Patients displaying progressive disease or stable disease after induction chemotherapy were excluded from the trial and further optimally managed according to local practice. Patients with partial or complete response (based on RECIST v1.1) after 4–6 cycles of platinum-based chemotherapy were randomly assigned, in a one-to-one ratio, to receive maintenance olaparib or placebo. Random assignment was stratified by age, country, and histology. Olaparib or placebo was administered orally, at a dose of 300 mg twice a day in 28-day cycles, and started no later than 6 weeks after the last administration of chemotherapy. Treatment was administered until disease progression or unacceptable toxicity (Fig. 1). Crossover to olaparib was not allowed. After discontinuation of the trial intervention, patients received treatment according to local guidelines, at the investigators’ discretion.
Fig. 1. Study design.
Chemonaive patients with advanced non small cell lung cancer (NSCLC) who presented with partial or complete response after platinum-based chemotherapy were randomized between placebo and olaparib. CR complete response, PD progessive disease, PR partial response, SD stable disease.
The study was approved by the institutional review board at all participating sites, by the French and Spanish regulatory authorities, and was conducted in accordance with the Declaration of Helsinki. This study was registered at ClinicalTrials.gov with study reference number NCT02679963. All patients provided written informed consent that included provision of an archival tumor sample and collection of blood samples for future biomarker analyses.
End points and assessments
The primary objective was PFS, which was assessed from randomization until disease progression or death from any cause, whichever occurred first. Secondary objectives included OS and safety. OS was measured from patient randomization until death, regardless of the cause, or to last follow-up. Treatment efficacy was assessed by a chest- abdomen-pelvic CT-scan, every 2 cycles after randomization, according to RECIST v1.1. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for AEs Version 4.0 (NCI CTCAE v 4.0).
Statistical analysis
The calculation for the sample size aimed to achieve a true PFS hazard ratio of 0.65 (primary objective).Based on literature data available at the time of the trial design [2, 16–18], the median PFS from randomization for patients receiving olaparib or placebo were anticipated to be 4.6 and 3 months, respectively [19]. With a two-sided log-rank test at α = 0.20 level (type I error) and 80% power, 114 subjects (97 events) were required to show a statistically significant difference for an anticipated true hazard ratio (HR) of 0.65. The median PFS was calculated for each arm of the trial, and the Logrank test was used to compare PFS between the two arms; Kaplan–Meier curves were used to describe PFS by trial arms. A Logrank p-value below 0.20, associated with a HR < 1, would have signaled a benefit from olaparib as compared to placebo.
Secondary analysis included safety, ORR, and OS, was assessed using Kaplan–Meier curves and compared using Logrank tests with a two-sided alpha of 0.20. The study of interaction between treatment effect on the main endpoints and the following patient characteristics was performed: (i) histology (squamous vs non-squamous), and (ii) smoking status, defined as non-smoker (less than 100 cigarettes in the whole life), current smoker, and ex-smoker (patient having stopped smoking >15 years ago). Two Cox models were computed to detect an interaction between treatment effect and histology, or treatment effect and smoking status, respectively.
Results
Patients
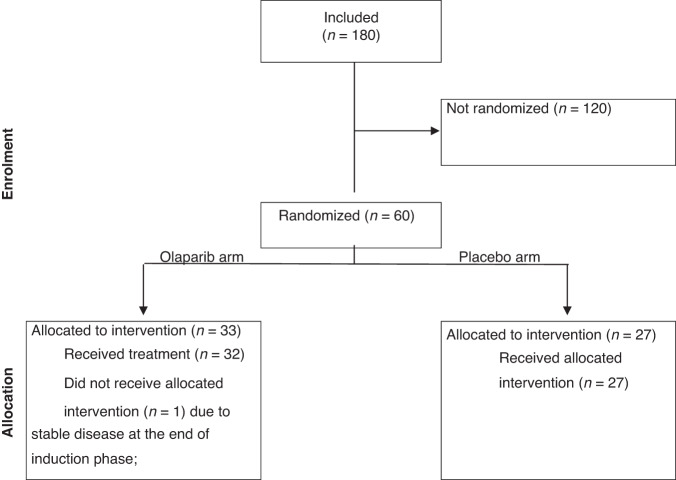
Five hundred patients were initially required for enrollment and 144 randomized patients were needed to assess the trial’s primary endpoint based on statistical hypotheses (HR PFS = 0.65). However, due to changes in the standard of care for NSCLC treatment and the introduction of anti-PD-(L)1 therapy in the first-line setting, the trial was prematurely closed in August 2019 after enrolling 180 patients. Among them, 60 were randomized: 33 patients were allocated to the olaparib arm and 27 to the placebo arm (Fig. 2). At the time of trial data cut-off, two patients were still receiving placebo, and none were on olaparib.
Fig. 2. CONSORT diagram of PIPSeN.
Among 180 enrolled patients, 60 presented with partial response after platinum-based induction chemotherapy and were randomized between olaparib (n = 33) and placebo (n = 27).
Baseline patient characteristics (Table 1) showed no significant differences between arms for age, sex, smoking status, histology, number of platinum cycles or the presence of brain metastases. Most patients were male, with a smoking history, and with stage IV NSCLC. Over 60% of the patients had adenocarcinoma. Significantly more patients in the olaparib group had a history of alcohol intake (11 vs 3, p = 0.043).
Table 1.
Patients’ characteristics
| arm: Placebo N = 27 | arm: Olaparib N = 33 | Total N = 60 | p-value (test of chi²) | |
|---|---|---|---|---|
| Age | ||||
| Median | 65 | 62 | 63 | |
| Range | 47–82 | 53–86 | 47–86 | |
| Gender | 0.73 | |||
| Male | 23 (85%) | 27 (82%) | 50 (83%) | |
| Female | 4 (15%) | 6 (18%) | 10 (17%) | |
| Previous cancer | 0.26 | |||
| No | 26 (96%) | 33 (100%) | 59 (98%) | |
| Yes | 1 (4%) | 0 | 1 (2%) | |
| Alcohol abuse | 0.043 | |||
| No | 24 (89%) | 22 (67%) | 46 (77%) | |
| Yes | 3 (11%) | 11 (33%) | 14 (23%) | |
| Smoking history | 0.28 | |||
| Never been a smoker | 2 (7.4%) | 0 | 2 (3.3%) | |
| Ex-smoker | 16 (59.3%) | 21 (63.6%) | 37 (61.7%) | |
| Current smoker | 9 (33.3%) | 12 (36.4%) | 21 (35%) | |
| Median number of pack years | ||||
| Median | 20 | 20 | 20 | |
| Range | 8–80 | 2–60 | 2–80 | |
| Stage at diagnosis | 0.84 | |||
| Stage III-B | 2 (7%) | 2 (6%) | 4 (7%) | |
| Stage IV | 25 (93%) | 31 (94%) | 56 (93%) | |
| Histology | 0.95 | |||
| Adenocarcinoma | 18 (67%) | 20 (61%) | 38 (63%) | |
| Squamous cell | 7 (26%) | 10 (30%) | 17 (28%) | |
| Large cell | 1 (4%) | 1 (3%) | 2 (3%) | |
| Other | 1 (4%) | 2 (6%) | 3 (5%) | |
| Number of metastasis | ||||
| Median | 2 | 3 | 2 | |
| Range | 1–7 | 0–5 | 0–7 | |
| Brain | 0.70 | |||
| No | 21 (78%) | 27 (82%) | 48 (80%) | |
| Yes | 6 (22%) | 6 (18%) | 12 (20%) | |
| Bone | 0.31 | |||
| No | 19 (70%) | 19 (58%) | 38 (63%) | |
| Yes | 8 (30%) | 14 (42%) | 22 (37%) | |
| Peripheral adenopathy | 0.099 | |||
| No | 23 (85%) | 22 (67%) | 45 (75%) | |
| Yes | 4 (15%) | 11 (33%) | 15 (25%) | |
| Liver | 0.17 | |||
| No | 20 (74%) | 29 (88%) | 49 (82%) | |
| Yes | 7 (26%) | 4 (12%) | 11 (18%) | |
| Median/mean number of induction cycles number | 0.75 | |||
| 4 | 7 (26%) | 6 (18%) | 13 (22%) | |
| 5 | 1 (4%) | 1 (3%) | 2 (3%) | |
| 6 | 19 (70%) | 26 (79%) | 45 (75%) | |
Treatment outcomes
Survival outcomes
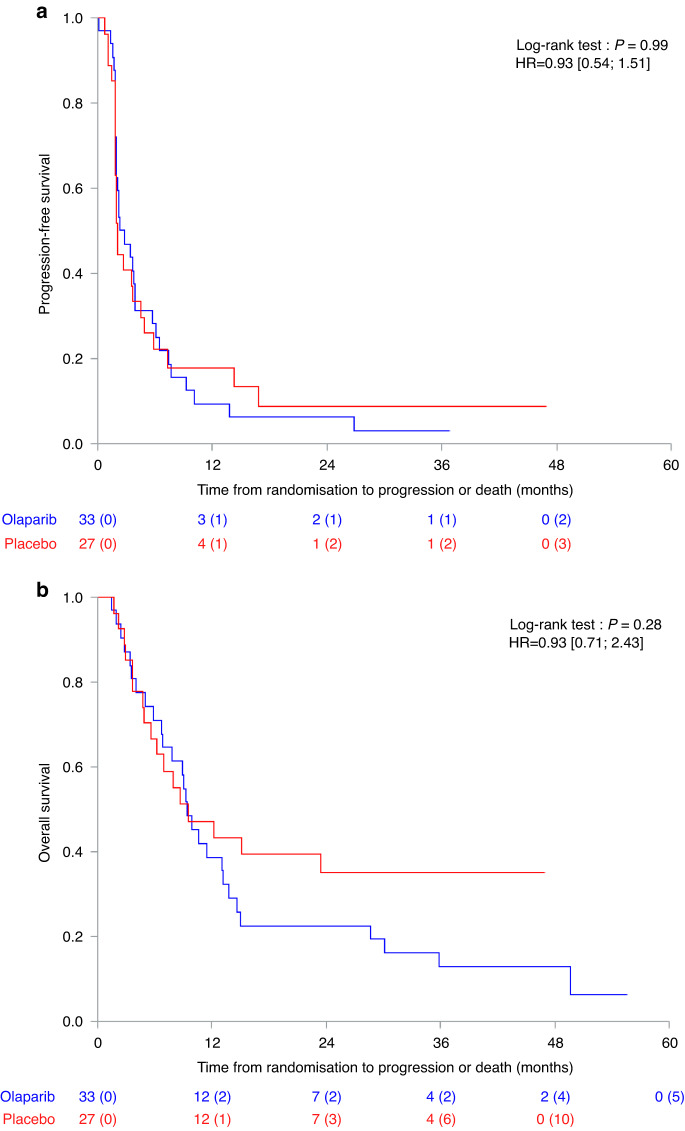
All randomized patients were included in the intent-to-treat efficacy analysis. At the time of analysis, the median duration of follow-up was 39.3 months [CI 95%: 27.3–46.0]. Median PFS was 2.9 and 2.0 months in the olaparib and placebo arms, respectively (p = 0.99) (Fig. 3a). PFS rates at 6, 12 and 24 months were 28%, 9% and 6% in the olaparib arm; and 22%, 18% and 9% in the placebo arm (Supplementary Table 1). Median OS was 9.4 and 9.5 months in the olaparib and placebo arms, respectively (p = 0.28) (Fig. 3b).
Fig. 3. Treatment outcomes.
a Progression-Free Survival; b Overall Survival.
Response rate
Among the 60 patients evaluable for efficacy, six patients did not have any radiological evaluation: one was lost to follow-up, and the other five patients did not have radiological evaluation due to the worsening of their clinical condition; the latter were therefore considered as Progressive Disease. Response rates are depicted in Table 2. Two (6%) and three (11%) patients presented an objective response in the olaparib arm and placebo arm, respectively. Thirteen (41%) patients on olaparib and 7 (36%) patients on placebo presented stable disease at the first radiological assessment. Overall, the disease control rate at the first assessment was 47% (15 patients) and 37% (10 patients) in the olaparib and placebo arms, respectively.
Table 2.
Response evaluation at first radiological assessment (n = 59)
| Olaparib arm (n = 32) | Placebo arm (n = 27) | |||||
|---|---|---|---|---|---|---|
| N | % | IC 95% | N | % | IC 95% | |
| Objective response | 2 | 6.3 | [0.8–20.8] | 3 | 11.1 | [2.4–29.2] |
| Disease control | 15 | 46.9 | [29.1–65.3] | 10 | 37 | [19.4–57.6] |
| Complete response | 0 | 0 | - | 0 | 0 | - |
| Partial response | 2 | 6.3 | [0.8–20.8] | 3 | 11.1 | [2.4–29.2] |
| Stable disease | 13 | 40.6 | [23.7–59.4] | 7 | 25.9 | [11.1–46.3] |
| Progression | 17 | 53.1 | [34.7–70.9] | 17 | 63 | [42.4–80.6] |
Subgroup analysis
No statistically significant interaction was identified between the treatment effect (as measured by the PFS) and histology or smoking status. However, there was a trend suggesting that current smokers might benefit more from olaparib (HR = 0.51; 95% CI 0.20–1.30; p = 0.11) (Supplementary Table 2).
Safety profile
All patients who received at least one treatment dose were evaluated for safety. One patient randomized in the olaparib arm, who did not start investigational treatment, was excluded from the safety analysis. Treatment/placebo exposure was similar between arms. The median number of treatment cycles received by patients was two for both olaparib and placebo. A total of 20 (63%) patients in the olaparib arm and 8 (30%) in the placebo arm experienced a grade ≥3 AE. AEs led to drug discontinuation in four patients: two in the olaparib arm (one patient had grade 3 anemia and diarrhea, and one patient had grade 3 vomiting) and two in the placebo arm (one patient had grade 3 migraine and one had grade 3 pulmonary infection). One death, deemed unrelated to the study drug, occurred in the olaparib arm (sepsis caused by lung infection). Table 3 lists serious AEs in both groups and Supplementary Table 3 shows all grade side effects. AEs were in line with the known safety profile of PARPi, with fatigue, anemia and nausea being the most common toxicities.
Table 3.
Serious Adverse events (classified by SOC) - maximum grade per cycle
| Olaparib (n = 32) | Placebo (n = 27) | |||||
|---|---|---|---|---|---|---|
| Grade | Grade | |||||
| AE soc | ae_term | 3 | 4 | 5 | 2 | 3 |
| Blood and lymphatic system disorders | Anemia | 1(3%) | 1(3%) | |||
| Gastrointestinal disorders | Diarrhea | 1(3%) | ||||
| Rectal fistula | 1(4%) | |||||
| Vomiting | 1(3%) | |||||
| Intestinal stoma obstruction | 1(3%) | |||||
| Infections and infestations | Lung infection | 2(6%) | 1(3%) | |||
| Sepsis | 1(3%) | 1(3%) | ||||
| Upper respiratory infection | 1(3%) | |||||
| Urinary tract infection | 1(3%) | |||||
| Vascular disorders - Other, specify | 1(4%) | |||||
| Investigations | Creatinine increased | 1(4%) | ||||
| Musculoskeletal and connective tissue disorders | Pain | 1(4%) | ||||
| Nervous system disorders | Edema cerebral | 1(3%) | ||||
| Headache | 1(4%) | |||||
| Stroke | 1(4%) | |||||
| Nervous system disorders - Other, specify | 1(3%) | |||||
| Renal and urinary disorders | Acute kidney injury | 1(3%) | ||||
| Respiratory, thoracic and mediastinal disorders | Infections and infestations - Other, specify | 1(4%) | ||||
| Dyspnea | 1(3%) | |||||
| Vascular disorders | Thromboembolic event | 1(4%) | ||||
| Vascular disorders - Other, specify | 1(3%) | |||||
Discussion
The PIPSeN trial investigated the activity of olaparib as a switch maintenance therapy in chemotherapy-naïve patients with advanced NSCLC, who achieved an objective response after 4–6 cycles of platinum-based chemotherapy. The shift in therapeutic standard in the first line setting of advanced NSCLC following the advent of immune-checkpoint inhibitors led to the premature discontinuation of the trial. The final analysis included only half of the pre-planned patient population, thus being statistically underpowered. No signal of efficacy was detected when using olaparib as a maintenance therapy, with no improvement in PFS or OS in this patient population. The safety profile of olaparib was similar to that observed in other patient populations, with predominantly gastrointestinal and hematological toxicity.
To date, all trials investigating PARPi in NSCLC have failed to show a significant benefit, despite various trial designs. Veliparib, a PARPi with no PARP trapping capabilities, has been compared to placebo in a phase III randomized trial, where it was associated with platinum doublet in treatment-naïve patients with advanced squamous NSCLC [20, 21]. This trial failed to show any difference in OS or PFS between arms [20]. However, the LP52 signature, which distinguishes adenocarcinoma vs non-adenocarcinoma tumors based on a 52-gene signature, identified a subgroup of patients who may derive benefit from veliparib with chemotherapy: mOS was more favorable with veliparib in the LP52-positive population (i.e., tumors with non-adenocarcinoma characteristics; median 14.0 v 9.6 months; HR, 0.66; 95% CI, 0.49–0.89). The phase II PIN trial (NCT01788332) randomized maintenance olaparib 300 mg bd versus placebo in patients with chemosensitive advanced NSCLC after 3–4 cycles of platinum-based chemotherapy. Like PIPSeN, PIN failed to demonstrate a statistically significant benefit of olaparib (PFS HR of 0.83 with a one-sided 80% CI upper limit of 1.03; p = 0.23). However, after adjustment on histology and smoking status (never vs ever smoker), ITT Cox-adjusted model showed a HR of 0.73 (one-sided 80% CI upper limit 0.91, one sided p-value = 0.11, which was consideredstatistically significant at the 0.2 level [22]), suggesting that PARPi may bring some benefit in platinum-sensitive NSCLC. In PIPSeN, age was initially chosen as a stratification factor, presuming that elderly patients may have a worse outcome, either due to comorbidities or poorer treatment tolerability which could limit the number of administered cycles of induction platinum-based chemotherapy or full dosing of PARPi. Subgroup analysis identified a trend for current smokers to derive more benefit from olaparib. Whether this is due to increased genomic instability or secondary mutations caused by tobacco exposure This calls for a meta-analysis on individual data of both PIN and PIPSeN, which may allow to better define the role of PARPi, and potentially smoking status, in this setting. It is also likely that not all platinum-sensitive patients equally benefit from PARPi and that a more stringent molecular selection is needed. Notably, PARPi were evaluated in the non-randomized phase II Lung-MAP Substudy S1400G, where patients with advanced platinum-sensistive and DDR-deficient squamous NSCLC received talazoparib as a monotherapy. The primary endpoint was objective response in patients harboring BRCA1, BRCA2, ATM, ATR, and PALB2 alterations. Patients had an ORR of only 4%, a mPFS of 2.4 months and mOS of 5.2 months, suggesting that these selection criteria were still insufficient to predict benefit from PARPi [23], or that tissue-specific characteristics (e.g., genetic or epigenetic background, limited drug penetration, etc.) may hamper PARPi activity in NSCLC. Currently, PARPi are being evaluated in NSCLC in combination with anti-PD-(L)1 immunotherapy in multiple trials [24], based on the observation that PARPi elicit a cell-autonomous type I interferon cGAS-STING response in contexts where they also elicit synthetic lethality [25]. In the maintenance setting, these include the phase II ORION trial (NCT03775486), and the phase III KEYLYNK-006 (NCT03976323) and KEYLYNK-008 (NCT03976362) studies.
To date, PARPi have demonstrated their effectiveness as a monotherapy mostly in selected tumor types that harbor germline alterations in the HRR pathway and are platinum-sensitive [26, 27]. Since response to platinum (as opposed to stable disease only) is traditionally used as a surrogate marker of PARPi sensitivity and it has been employed to select patients with ovarian cancer for maintenance therapy in trials that led to PARPi approval [10, 28, 29], we decided to exclude patients with disease stabilization from randomization, to better select the patient population, even if maintenance studies in NSCLC classically include all the non-progressing patients. To this point, the most significant benefit of PARPi has been noted in BRCA-altered ovarian, breast, prostate and pancreatic cancers [6, 11, 27, 30]. In other DDR defects, such as ATM alterations, PARPi benefit is less clear [30]. In the aforementioned histotypes, as opposed to lung cancer, HRR defects are bi-allelic and directly involved in their pathogenesis [30–32], a feature that is required for causing genomic instability [33]. Also, in these cancer types, the most frequently altered DDR gene is BRCA [31, 32], while in NSCLC, ERCC1 defects predominate (20% of cases), followed by ATM (3.5%) and BRCA2 alterations (2% of cases) [32]. ERCC1 mutations predict sensitivity to PARPi in vitro [12], but result in predominant nucleotide excision repair and interstrand crosslink repair defects, thereby leading to a less profound synthetic lethality than canonical HRR defects. Also, BRCA alterations may simply be incidental events unrelated to the pathogenesis of lung cancer, being more prevalent amongpatients with a heavy smoking history [34]. As NSCLC frequently displays high TMB and a smoking genomic signature (rather than BRCAness signature), DDR alterations may occur as a consequence of genomic instability, as passenger or subclonal events, rather than driver events. A more stringent molecular selection is therefore likely required to identify NSCLC patients who may benefit from PARPi. Further, other molecular biomarkers, such as PARP1 or SLFN11 expression [35, 36], would also need to be assessed to better select patients. Although some translational studies were initially planned in this pragmatic academic clinical trial, the very limited number of collected tumor samples unfortunately precluded from any statistically relevant biomarker investigation.
The limitations of the PIPSeN trial include its underpowers and absence of molecular selection. Indeed, its rationale was mainly based on targeting ERCC1 defects with PARPi, for which no reliable biomarker exists. This is due to the presence of four closely-related isoforms, of which only one is functional [37]; these isoforms cannot be distinguished by available antibodies [38] and molecular screening by RNA-Seq was not routinely done at the time of trial design. Future translational studies on biological samples collected from patients treated with PARPi should interrogate biomarkers of efficacy, including biallelic BRCA alterations, BRCAness signatures, HRD loss, ERCC1 or composite scores. Also, patients with ATM alterations might more likely benefit from ATR inhibitors [39]. Notably, in the phase II HUDSON trial (NCT03334617), the ATR inhibitor ceralasertib combined with durvalumab showed promising results in 66 ATM-mutant or -WT patients with advanced NSCLC who previously received chemotherapy and immunotherapy, with an ORR of 16.7%, a median PFS of 6 months and median OS of 15.9 months [40]. A phase III trial is currently ongoing (NCT05450692).
Conclusions
The PIPSeN trial prematurely terminated and was statistically underpowered. Olaparib maintenance did not improve median PFS nor median OS in this subset of patients with platinum-sensitive NSCLC. Further translational studies are warranted to identify which molecular subset of NSCLC patients might truly benefit from PARPi.
Supplementary information
Acknowledgements
Figure 1 has been created by Mihaela Aldea with Biorender.com. English editing was performed with ChatGPT v4.
Author contributions
Study design, SPV, JCS and RR; Methodology & statistics, SPV, JP, MT, JCS and RR; Investigation, patient screening and enrollment: SPV, JC, AG, MD, DP, RDLP, MASG, SV, JP, ANO, TM, CC, ALM, MP, JCS, BB, BM, RR; Writing – Original Draft: MA and SPV; Writing – Review & Editing; all co-authors; Funding Acquisition, SPV and JCS.
Data availability
Original data could be made available upon request for research purposes on a case-by-case basis, in a pseudonymized way and under current GDPR policies, so that patient protection is fully ensured.
Competing interests
SPV and AG: Principal/sub-Investigator of Clinical Trials for Abbvie, Adaptimmune, Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Arno Therapeutics, Astex Pharmaceuticals, Astra Zeneca Ab, Aveo, Basilea Pharmaceutica International Ltd, Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Ca, Celgene Corporation, Chugai Pharmaceutical Co, Cullinan-Apollo, Daiichi Sankyo, Debiopharm, Eisai, Eisai Limited, Eli Lilly, Exelixis, Forma Tharapeutics, Gamamabs, Genentech, GlaxoSmithKline, H3 Biomedicine, Hoffmann La Roche Ag, Imcheck Therapeutics, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Janssen Cilag, Janssen Research Foundation, Kyowa Kirin Pharm. Dev, Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncopeptides, Orion Pharma, Oxford Therapeutics, Ose Pharma, Pfizer, Pharma Mar, PEP-therapy, Pierre Fabre, Medicament, Roche, Sanofi Aventis, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Xencor. SPV: Preclinical research funding: IMCore Hoffman La-Roche; AstraZeneca for work unrelated to this manuscript; Advisory board: Daiichi-Sankyo, Amgen. MA Travel: Sandoz ; Recherche grant : Sandoz ; Advisory : Viatris. DP: Consulting, advisory role or lectures: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche, Samsung; Honoraria: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche, Samsung; Clinical trials research as principal or co-investigator (Institutional financial interests): AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, Medimmun, Sanofi-Aventis, Taiho Pharma, Novocure, Daiichi Sankyo; Travel, Accommodation, Expenses: AstraZeneca, Roche, Novartis, prIME Oncology, Pfizer. MD: MD: Consultant or Advisory Role: Astra-Zeneca, Boehringer Ingelheim, Janssen Cilag, MSD, Pfizer, Roche, Sanofi, Takeda. Speaking: Astra-Zeneca, BMS, MSD, Pfizer, Roche, Takeda. MP: MP: Consulting, advisory role or lectures: AstraZeneca, Bristol-Myers Squibb (BMS), Merck, Novartis, Pfizer, Travel, Accommodation, Expenses: AstraZeneca, Roche, Novartis,BMS. ALM: No conflicts of interest related to this study. Other conflicts of interest: - Consultant or advisory board: Amgen, Boehringer. Speaking: BMS, MSD, Pierre Fabre, Astra Zeneca. TM: Dr. Moran declares consulting/advisory role fees from Roche, Bristol Myers, Boeringher, Astra Zeneca, Lilly, and Research funding from Kyowa Kirin and Janssen, all of them unrelated with the present project. JCS is a full time-employee at Amgen since August 2021. He owns stock at Gritstone Bio, Relay Therapeutics and Amgen. BB: Contracted / supported research grants: Abbvie, Amgen, AstraZeneca, BeiGene, Blueprint Medicines, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Ignyta, IPSEN, Inivata, Janssen, Merck KGaA, MSD, Nektar, Onxeo, OSE immunotherapeutics, Pfizer, Pharma Mar, Roche-Genentech, Sanofi, Servier, Spectrum Pharmaceuticals, Takeda, Tiziana Pharma, Tolero Pharmaceuticals JCS: Personal fees outside of this work: Astex, AstraZeneca, Bayer, Blend Therapeutics, Boehringer-Ingelheim, Clovis, Eli Lilly, Gammamabs, Merus, Mission Therapeutics, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Symphogen, Tarveda; Gritstone; AstraZeneca All other authors declare no conflicts of interest.
Ethics approval and consent to participate
PIPSeN was granted central approval by the French Regulatory Authority ANSM under the reference 150127A12, on 10 April 2015, and Ethics Committee CPP Ile de France 8 on 10 Feb 2015. In Spain, PIPSeN was approved by the Agencia Española del Medicamento y Productos Sanitarios under the reference MUH/AEC, and the EC of Hospital Germans Trias i Pujol. Each enrolled patient provided informed consent as described in the manuscript and Supplementary Materials.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02514-5.
References
- 1.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 3.Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 4.Blais N, Kassouf E. Maintenance therapies for non-small cell lung cancer. Front Oncol. 2014;4:213. doi: 10.3389/fonc.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–8. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mateo J, Lord CJ, Serra V, Tutt A, Balmana J, Castroviejo-Bermejo M, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30:1437–47. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govindan R, Lind M, Insa A, Khan SA, Uskov D, Tafreshi A, et al. Veliparib plus carboplatin and paclitaxel versus investigator’s choice of standard chemotherapy in patients with advanced non-squamous non-small cell lung cancer. Clin Lung Cancer. 2022;23:214–25. doi: 10.1016/j.cllc.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Mirza MR, Coleman RL, Gonzalez-Martin A, Moore KN, Colombo N, Ray-Coquard I, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31:1148–59. doi: 10.1016/j.annonc.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, et al. Olaparib plus Bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 11.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance Olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–27. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postel-Vinay S, Bajrami I, Friboulet L, Elliott R, Fontebasso Y, Dorvault N, et al. A high-throughput screen identifies PARP1/2 inhibitors as a potential therapy for ERCC1-deficient non-small cell lung cancer. Oncogene. 2013;32:5377–87. doi: 10.1038/onc.2013.311. [DOI] [PubMed] [Google Scholar]
- 13.Postel-Vinay S, Vanhecke E, Olaussen KA, Lord CJ, Ashworth A, Soria JC. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat Rev Clin Oncol. 2012;9:144–55. doi: 10.1038/nrclinonc.2012.3. [DOI] [PubMed] [Google Scholar]
- 14.Sung M, Giannakakou P. BRCA1 regulates microtubule dynamics and taxane-induced apoptotic cell signaling. Oncogene. 2014;33:1418–28. doi: 10.1038/onc.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boukovinas I, Papadaki C, Mendez P, Taron M, Mavroudis D, Koutsopoulos A, et al. Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PloS one. 2008;3:e3695. doi: 10.1371/journal.pone.0003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelman MJ, Le Chevalier T, Soria JC. Maintenance therapy and advanced non-small-cell lung cancer: a skeptic’s view. J Thorac Oncol. 2012;7:1331–6. doi: 10.1097/JTO.0b013e3182629e37. [DOI] [PubMed] [Google Scholar]
- 17.Azzoli CG, Temin S, Aliff T, Baker S, Jr., Brahmer J, Johnson DH, et al. 2011 Focused update of 2009 American Society of clinical oncology clinical practice guideline update on chemotherapy for Stage IV non-small-cell lung cancer. J Clin Oncol. 2011;29:3825–31. doi: 10.1200/JCO.2010.34.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, et al. Non-small cell lung cancer. J Natl Compr Cancer Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 19.Edelman MJ, Harb WA, Pal SE, Boccia RV, Kraut MJ, Bonomi P, et al. Multicenter trial of EC145 in advanced, folate-receptor positive adenocarcinoma of the lung. J Thorac Oncol. 2012;7:1618–21. doi: 10.1097/JTO.0b013e318267d051. [DOI] [PubMed] [Google Scholar]
- 20.Ramalingam SS, Novello S, Guclu SZ, Bentsion D, Zvirbule Z, Szilasi M, et al. Veliparib in combination with platinum-based chemotherapy for first-line treatment of advanced squamous cell lung cancer: a randomized, multicenter Phase III study. J Clin Oncol. 2021;39:3633–44. doi: 10.1200/JCO.20.03318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reck M, Blais N, Juhasz E, Gorbunova V, Jones CM, Urban L, et al. Smoking history predicts sensitivity to PARP inhibitor veliparib in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:1098–108. doi: 10.1016/j.jtho.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Fennell DA, Porter C, Lester J, Danson S, Blackhall F, Nicolson M, et al. Olaparib maintenance versus placebo monotherapy in patients with advanced non-small cell lung cancer (PIN): a multicentre, randomised, controlled, phase 2 trial. EClinicalMed. 2022;52:101595. doi: 10.1016/j.eclinm.2022.101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owonikoko TK, Redman MW, Byers LA, Hirsch FR, Mack PC, Schwartz LH, et al. Phase 2 study of Talazoparib in patients with homologous recombination repair-deficient squamous cell lung cancer: lung-MAP substudy S1400G. Clin Lung Cancer. 2021;22:187–94.e1. doi: 10.1016/j.cllc.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chabanon RM, Rouanne M, Lord CJ, Soria JC, Pasero P, Postel-Vinay S. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer. 2021;21:701–17. doi: 10.1038/s41568-021-00386-6. [DOI] [PubMed] [Google Scholar]
- 25.Chabanon RM, Muirhead G, Krastev DB, Adam J, Morel D, Garrido M, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Investig. 2019;129:1211–28. doi: 10.1172/JCI123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenblick A, de Azambuja E, Azim HA, Jr., Piccart M. An update on PARP inhibitors–moving to the adjuvant setting. Nat Rev Clin Oncol. 2015;12:27–41. doi: 10.1038/nrclinonc.2014.163. [DOI] [PubMed] [Google Scholar]
- 27.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–54. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 29.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 30.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 31.Sokol ES, Pavlick D, Khiabanian H, Frampton GM, Ross JS, Gregg JP, et al. Pan-cancer analysis of BRCA1 and BRCA2 genomic alterations and their association with genomic instability as measured by genome-wide loss of heterozygosity. JCO Precis Oncol. 2020;4:442–65. [DOI] [PMC free article] [PubMed]
- 32.Heeke AL, Pishvaian MJ, Lynce F, Xiu J, Brody JR, Chen WJ, et al. Prevalence of homologous recombination-related gene mutations across multiple cancer types. JCO Precis Oncol. 2018;2. [DOI] [PMC free article] [PubMed]
- 33.Westphalen CB, Fine AD, Andre F, Ganesan S, Heinemann V, Rouleau E, et al. Pan-cancer analysis of homologous recombination repair-associated gene alterations and genome-wide loss-of-heterozygosity score. Clin Cancer Res. 2022;28:1412–21. doi: 10.1158/1078-0432.CCR-21-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remon J, Besse B, Leary A, Bieche I, Job B, Lacroix L, et al. Somatic and Germline BRCA 1 and 2 mutations in advanced NSCLC from the SAFIR02-lung trial. JTO Clin Res Rep. 2020;1:100068. doi: 10.1016/j.jtocrr.2020.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lok BH, Gardner EE, Schneeberger VE, Ni A, Desmeules P, Rekhtman N, et al. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res. 2017;23:523–35. doi: 10.1158/1078-0432.CCR-16-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler C, Armenia J, Jones GN, Tobalina L, Sale MJ, Petreus T, et al. SLFN11 informs on standard of care and novel treatments in a wide range of cancer models. Br J cancer. 2021;124:951–62. doi: 10.1038/s41416-020-01199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101–10. doi: 10.1056/NEJMoa1214271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma D, Baruch D, Shu Y, Yuan K, Sun Z, Ma K, et al. Using protein microarray technology to screen anti-ERCC1 monoclonal antibodies for specificity and applications in pathology. BMC Biotechnol. 2012;12:88. doi: 10.1186/1472-6750-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafiei S, Fitzpatrick K, Liu D, Cai MY, Elmarakeby HA, Park J, et al. ATM loss confers greater sensitivity to ATR inhibition than PARP inhibition in prostate cancer. Cancer Res. 2020;80:2094–100. doi: 10.1158/0008-5472.CAN-19-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besse B, Awad MM, Forde PM, Thomas M, Goss G, Aronson B, et al. OA15.05 HUDSON: an open-label, multi-drug, biomarker-directed Phase 2 study in NSCLC patients who progressed on Anti-PD-(L)1 therapy. J Thorac Oncol. 2022;17:S41–S2. doi: 10.1016/j.jtho.2022.07.074. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data could be made available upon request for research purposes on a case-by-case basis, in a pseudonymized way and under current GDPR policies, so that patient protection is fully ensured.