Abstract
Pbs21 is a surface protein of the ookinete of Plasmodium berghei, which can induce a potent transmission-blocking immune response. Pbs21 is normally expressed only by parasite stages in the mosquito, i.e., female gametes/zygotes, ookinetes, and oocysts. However, the Pbs21 gene is transcribed in female gametocytes which circulate in the bloodstream of the host, where translation of the resulting mRNA is totally repressed. Episomal transfection has been used to investigate whether expression of Pbs21 protein could be achieved in blood stages of the parasite. By using plasmid pMD221, the complete mRNA-encoding region of Pbs21, flanked only by 218 nucleotides (nt) of its promoter region and 438 nt of its 3′ region downstream from the polyadenylation site, was introduced into the blood stages of gametocyte-producing and non-gametocyte-producing clones of P. berghei. In both of these transformed parasite lines, Pbs21 protein was expressed in asexual trophozoites, schizonts, and, when present, in both male and female gametocytes. Hence, the flanking regions present are sufficient to allow transcription but lack the elements that exert natural control of sex- and stage-specific transcription. The mRNA and the protein expressed by transformed blood stages were indistinguishable from the wild-type forms by the criteria tested, and the protein was recognized by both conformation-dependent and conformation-independent monoclonal antibodies raised against native Pbs21. In mice infected with transformed non-gametocyte-producing parasites, a Pbs21-specific immune response was induced and characterized with respect to isotype (IgG2a/IgG2b) and quantity (11.5 ± 10 μg/ml) of antibody produced. However, as found in previous studies, these antibody levels were insufficient to inhibit development of the parasites in the mosquito. The ability to express mosquito midgut-stage antigens in blood-stage parasites will facilitate further investigations of molecular and immunological properties of these proteins.
Pbs21 is a stage-specific surface protein of the rodent malaria parasite Plasmodium berghei, which is expressed in mosquito midgut stages (23, 24, 33). This protein has been used extensively as a model to study transmission-blocking immunity. Immunization with native or recombinant Pbs21 can elicit antibody responses in mice that completely block the development of the parasite in the mosquito midgut (14, 16, 17, 27). Pbs21 and Pfs28, its homolog from the human malaria parasite P. falciparum, are members of a family of glycosylphosphatidylinositol (GPI)-anchored proteins typified by the presence of three or four epidermal growth factor-like domains. The studies mentioned above and others on homologous proteins (5) have suggested that the conformation of these molecules is crucial for the induction of transmission-blocking immune responses. Both Pfs28 and Pfs25 are candidates for the development of a transmission-blocking vaccine; Pfs25 is currently undergoing human vaccine trials (6, 10).
Pbs21 not only is of interest to investigate the immunological features of proteins which elicit transmission-blocking immune responses but also is of great importance in the study of the control of stage- and sex-specific protein expression in Plasmodium. Expression of Pbs21 is regulated both at the level of gene transcription and that of mRNA translation. Transcription of the gene is confined to female gametocytes and begins 16 to 19 h into their development in the blood of the vertebrate host, resulting in the localized accumulation of mRNA in mature female gametocytes (18, 22, 26, 31). In contrast, translation of the Pbs21 mRNA normally occurs only after gametocyte activation in the mosquito midgut in macrogametes, zygotes, ookinetes, and young oocysts (23, 24) and is triggered at the same time as gametogenesis (1).
We report here the transgenic production of Pbs21 protein in its native conformation in blood stages of P. berghei parasites in the vertebrate host. These transgenic parasites were generated by the introduction of episomally maintained plasmids containing the Pbs21 gene under the control of short but homologous 5′ and 3′ untranslated regions of the Pbs21 gene. Truncation of the flanking regions removed the normal sex- and stage-specific expression. Comparison of the antibody responses to infection by transformed and nontransformed parasites showed an additional and specific response to Pbs21 in the former.
The technology of production of mosquito midgut-stage antigens by blood stages provides an additional tool for the analysis of regulation of protein expression and of immunological features of transmission-blocking vaccine proteins.
MATERIALS AND METHODS
Nucleic acids.
The oligonucleotides used in this study and their sequences were (with appropriate melting temperatures) as follows: L15T, TATCGTATTGCAAGTTGG (50°C); L24D, TAAGATTGAAATGGAAAAGAG (54°C); L56O, CAATTGATATTTATTTTAATTTGCTCAC (68°C); L59O, GTCATGGATCCCAATAAGGAGAACAAG (78°C); L71O, TGTTAAACTTAAAAAAATGTATAAAATGG (68°C); L113O, TAGCTTTTTAGCTACCTAAAATCGA (68°C); L134O, GTATAAAATGGAATAAAAATAAAATATATA (64°C); 645D, GGGCCGGATCCTTAAGCTGCCATATCCATATT; 5′ RACE anchor, CACGAATTCACTATCGATTCTGGAACCTTCAGAGG; anchor PCR primer, CTGGTTCGGCCCACCTCTGAAGGTTCCAGAATCGATAG; and oligo(dT) for 3′ RACE, CGCAAGCTTTTTTTTTTTTTTTTT. Oligonucleotides were end labelled with T4 polynucleotide kinase by using [γ-32P]ATP in reaction mixtures containing 50 pmol of oligonucleotide. Labelled oligonucleotides were purified from unincorporated label by chromatography on Sephadex G-100.
RNA analysis.
RNA prepared from purified parasites was subjected to Northern analysis by using previously described methods (18). Final washes for complex probes are described in the figure legends. All hybridization mixtures used with oligonucleotides were given a final wash in 3× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.5% sodium dodecyl sulfate (SDS) for 5 min at the melting temperature minus 5°C.
Primer extension analysis.
Primer extensions were performed by standard methods (21). Five micrograms of total RNA isolated from purified gametocytes was annealed to 5 pmol of end-labelled oligonucleotide. The resulting hybrid was specifically elongated in a reaction mixture containing 5 U of reverse transcriptase and 1 mmol of deoxynucleoside triphosphates. Reactions were terminated by the addition of sequencing stop buffer containing formamide to the mixtures, which were then heat denatured and loaded on a 5% polyacrylamide sequencing gel. Radiolabelled molecular weight standards and a standard sequencing reaction mix primed with the same oligonucleotide were run alongside the primer extension reaction.
5′ and 3′ RACE technologies.
For determination of the polyadenylation site of Pbs21 mRNA, total cDNA of P. berghei was made with oligo(dT) plus a HindIII linker (see above) from 5 μg of DNase I-treated total RNA obtained from gametocytes. Reverse PCR was performed with the same oligo(dT) primer and L59O and the following PCR cycles: 94°C for 45 s, 44°C for 1 min, and 72°C for 1 min 30 s (for 35 cycles). Products were digested with appropriate restriction enzymes, gel purified, and cloned into PUC19, and plasmids were isolated, purified, and sequenced.
The 5′ end of the Pbs21 mRNA was characterized by two different reactions by using the cDNA synthesized in the 3′ RACE analysis ligated to the anchor primer supplied (Ambion). This was then amplified in two separate nested reactions, each with an anchor-derived oligonucleotide and either L15T or L71O (PCR conditions: 94°C for 45 s, 44°C for 1 min, 72°C for 1 min 30 s [for 35 cycles]). cDNA products were gel purified, cloned, and sequenced as described above.
Parasites.
Two clones of the ANKA strain of P. berghei were used: clone 2.33, which does not produce mature gametocytes or Pbs21 mRNA (18, 31), and clone 15cy1 (termed clone HP here), which is a high gametocyte producer (29) producing abundant Pbs21 mRNA in macrogametocytes.
Transfection.
Introduction of plasmids into the blood stages of clones 2.33 and HP was performed as described previously (29, 30). The plasmid used, pMD221, contains a modified form of the homologous dihydrofolate reductase-thymidylate synthase (DHFR-TS) gene (28) as a selectable marker, which confers pyrimethamine resistance to transformants. In addition, it contains a 1.9-kb DNA fragment of the Pbs21 gene (18) consisting of the Pbs21 open reading frame (ORF) flanked by 0.4 and 0.9 kb of the 5′ and 3′ genomic regions, respectively (29).
Maintenance of transformed parasites.
Transformed parasite clones of clones 2.33 and HP, containing the pMD221 plasmid, are called Pb233/221 and PbHP/221, respectively. To obtain parasites with a high plasmid copy number, Theiler’s Original (TO) mice infected with 107 to 108 transformed parasites were treated with pyrimethamine (10 mg/kg of body weight) for 2 to 3 consecutive days at 3- to 5-day intervals starting 24-h post infection (29).
Detection and quantification of plasmids in transformed parasites.
For Southern blot analyses and plasmid rescue experiments, DNA was extracted from parasites by phenol-chloroform. Total parasite DNA was digested with ClaI, fractionated by agarose gel electrophoresis, and transferred to a Hybond nylon membrane (Amersham, Amersham, United Kingdom) by capillary transfer. A 32P-labelled DHFR-specific 1.5-kb probe was prepared by PCR with primers L24D and 645D (29). This probe hybridizes with a 3.4-kb DNA fragment in ClaI-digested genomic P. berghei DNA (29).
For plasmid rescue experiments, 40 μl of Escherichia coli cells (Sure cells; Stratagene, Cambridge, United Kingdom) were transformed by electroporation (with a Gene Pulser set at 25 μF and 2.5 kV and a Pulse Controller set at 200 Ω) with 1 μl of total parasite DNA or, as a control, with plasmid pMD221. Plasmid DNA from randomly picked colonies was purified by using standard methods. Five microliters of each miniprep was cut with PvuII and visualized on ethidium bromide-stained agarose gels (1%). Restriction enzyme PvuII cuts plasmid pMD221 twice, resulting in three fragments of 2.4, 3.1, and 5.0 kb (29).
Detection of Pbs21 by Western blot analyses.
Infected mouse blood was collected into syringes and transferred immediately into a surplus of 37°C RPMI medium (Sigma, Poole, United Kingdom) (pH 7.3) without sodium bicarbonate (2). Parasites were separated from the bulk of the erythrocytes by ammonium chloride lysis (33); for clones HP and PbHP/221, this procedure was carried out at 37°C for 1 to 3 min. Samples of approximately 2 × 107 to 5 × 107 parasites, diluted with nonreducing SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer, were loaded per well. As a positive control for Pbs21, either (i) enriched ookinete cultures, consisting of asexual parasite stages, macrogametes/zygotes, and ookinetes (33) (ca. 5 × 104 ookinetes per well), or (ii) affinity-purified recombinant Pbs21 (amino acids [aa] 1 to 188, lacking the GPI anchor) expressed in a baculovirus expression system (15), diluted in nonreducing buffer, was used. Phase separation of proteins in samples of transformed parasites was accomplished by the method of Bordier (3). Proteins were separated on SDS–12 or 14% PAGE gels by using a Mini Protean II cell system (Bio-Rad), transferred to nitrocellulose (Schleicher & Schuell, Dassel, Germany), and probed with either anti-Pbs21 monoclonal antibodies (MAbs; final concentration, 50 to 500 ng/ml) (MAb 13.1 recognizes a conformation-independent epitope; MAbs 12.1 and 17.9 react with conformation-dependent epitopes [33]), with polyvalent immune sera (diluted 1:500 with phosphate-buffered saline [PBS]-Tween 20-skim milk) obtained from mice infected with transfected or nontransfected parasites, or with normal mouse serum (NMS; dilution 1:500) (negative control). Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG; Southern Biotechnologies, Birmingham, Ala.) was used as a secondary antibody. Blots were developed by using nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate as the substrate.
Detection of Pbs21 by IFAT and enzyme-linked immunosorbent assay (ELISA).
Indirect immunofluorescence antibody tests (IFATs) were carried out as described previously (14). Blood smears of transfected and nontransfected parasites were made from blood passed over CF11 columns to remove leukocytes and washed once in PBS. In experiments where biotinylated secondary antibodies were used, an additional blocking step with 0.1 M ammonium chloride was introduced. An anti-Pbs21-specific MAb (13.1) and NMS (negative control), diluted 1:500 with normal goat serum-PBS, were used as primary antibodies. Either fluorescein isothiocyanate-labelled goat anti-mouse antibody (Sigma) or biotinylated goat anti-mouse antibody (Vector Laboratories, Peterborough, United Kingdom) was used as a secondary antibody. Texas red-labelled streptavidin was used to reveal biotinylated secondary antibodies. A biotinylated goat anti-streptavidin antibody (Vector Laboratories) was added; this was followed by a second incubation with Texas red-labelled streptavidin. Parasite nuclei were stained with either propidium iodide (Sigma) (11) or Sytox Green (Molecular Probes, Leiden, The Netherlands) as described in the manufacturer’s recommendations. Slides were read in an MRC 600 argon-krypton laser confocal microscope (Bio-Rad).
ELISA was performed as described previously (27). Briefly, plates (Immunoadsorb; Life Technologies, Paisley, United Kingdom) were coated with 50 μl of affinity-purified recombinant Pbs21 (aa 1 to 188) at a concentration of 2 μg/ml. After blocking, dilutions of immune sera (1:125 with PBS-Tween 20-normal goat serum) in triplicate were incubated for 1 h at room temperature. An alkaline phosphatase-labelled goat anti-mouse antibody (Southern Biotechnologies) was used as a secondary antibody, and p-nitrophenyl phosphate was used as the substrate. Plates were read at an optical density (OD) of 405 nm. Known concentrations of purified MAb 13.1 were used as standards to convert OD readings into approximate immunoglobulin concentrations.
Immunization of mice with transformed parasites.
To maintain a prolonged blood infection at sublethal parasitemias, TO mice infected with transformed parasites (Pb233/221) and nontransformed control parasites were fed dried milk pellets. The resulting slow growth permits the mouse to develop an immune response against the normally lethal P. berghei infection (8). Recognizing that the lower growth rate was induced by a para-aminobenzoic acid (pABA) deficiency (9, 13), we were able to restore normal growth within a few days by providing normal food pellets. The mice infected with transformed parasites were treated daily with 12 to 15 mg of pyrimethamine per kg of body mass to prevent plasmid loss. Sera were collected on day 26 (clone 2.33) or day 33 (Pb233/221) postinfection to determine the specificity and intensity of the immune response by Western blot analyses and ELISA. To control for the possibility that the pABA deficiency in immune sera, caused by the milk diet, had a negative effect on mosquito infection (19), all mice were fed normal food pellets for at least 3 days before sera were collected for feeding to mosquitoes. The mosquitoes were subsequently maintained on fructose plus pABA.
Effect of immune sera on mosquito infection.
Immune sera of mice infected with transformed and nontransformed parasites were tested via membrane feeds of the sera to starved Anopheles stephensi mosquitoes. Membrane feeds were carried out either with 24-h ookinete cultures as described before (20) or with gametocytes. For membrane feeds with gametocytes, 250 μl of blood from mice infected with normal parasites collected 3 days postinfection was mixed with 250 μl of immune serum or NMS and added to membrane feeders at 37°C on top of a Parafilm membrane. An additional blood meal of noninfected blood was given 4 to 5 days later. Oocysts on mosquito midguts were counted on days 9 to 11 after the infectious membrane feed. Data were statistically analyzed by Student’s t test.
RESULTS
Transcription of the Pbs21 gene initiates at different sites upstream of the ORF.
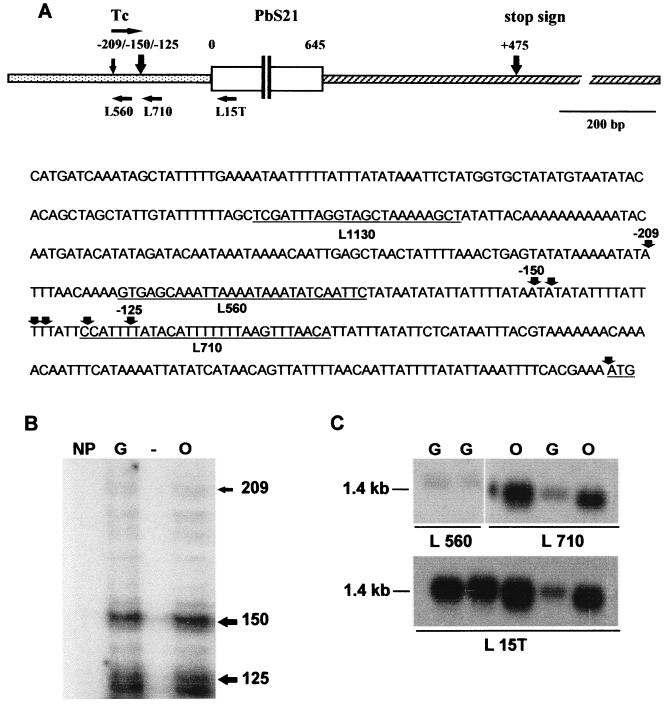
The normal transcription initiation site of the chromosomal Pbs21 gene was located by using primer extension analysis, 5′ RACE methodologies, and oligonucleotide hybridization (Fig. 1). The results indicate that transcription initiated at two loci: a discrete site 209 nucleotides (nt) upstream of the ORF and a clustered region (125 to 150 nt upstream of the ORF) giving rise to an mRNA population that exhibits slight length heterogeneity (Fig. 1B). Parallel control reactions performed on the A-type precursor rRNA from the same RNA preparation indicated the anticipated discrete initiation site for transcription, thus confirming that the RNA was of sufficient quality to conclude that the observed heterogeneity of the Pbs21 transcripts was not an artefact (data not shown). Oligonucleotide hybridization to two regions adjacent to the initiation sites demonstrated the larger transcript that results from the use of the more-upstream site (Fig. 1C). 3′ RACE experiments indicated the preferred site of polyadenylation 475 nt (10 of 16 clones sequenced) downstream of the ORF. The data are summarized in Fig. 1A. The 1,985-bp fragment of the Pbs21 locus cloned into plasmid pMD221 contained the region encoding the mRNA transcripts of discrete sizes between 1,267 and 1,329 nt, 218 nt of 5′ flanking region upstream of the transcription start site at position −209 that might contain elements that act as promoters of transcription, and 438 of the 913 nt of the 3′ flanking region containing elements that direct termination of transcription and polyadenylation. The promoter region included in pMD221 is 85% AT with short CG-rich motifs, none of which corresponds to the known binding sites of general and conserved transcription factors such as Oct-1.
FIG. 1.
Transcription of the Pbs21 gene of P. berghei. (A) Positions of the transcription start sites and oligonucleotides used in the analysis of the Pbs21 gene of P. berghei. A diagram of the Pbs21 gene locus included in plasmid pMD221 indicating the relative positions of the oligonucleotides and the positions of the transcription start sites and site of polyadenylation is shown at the top. The nucleotide sequence of the 5′ region of the Pbs21 gene up to the BclI site used to clone the gene into the expression plasmid pMD221 is shown below the diagram. Precise positions and sequences of the oligonucleotides are indicated by underlining. The cDNA ends isolated through the 5′ RACE procedure are indicated and numbered with respect to the ORF. The start codon is underlined. (B) Primer extension analysis of the Pbs21 gene. The products of a cDNA synthesis primed by oligonucleotide L15T were resolved on a 5% polyacrylamide sequencing gel and run next to size markers and a sequencing reaction. Corresponding nucleotide positions are indicated to the right of the gel. Reactions were performed with RNA isolated from a non-gametocyte producer clone (NP clone 2.33), pure gametocytes (G; clone HP), and ookinetes (O; clone HP). (C) Northern analysis of the transcripts of the Pbs21 gene. RNA was isolated from gametocytes and ookinetes and fractionated and prepared for Northern analysis as described in Materials and Methods. The blot was first probed with oligonucleotide L15T, which was used for the primer extension reactions. The blot was then stripped and cut into two pieces as illustrated and probed with either oligonucleotide L56O or L71O.
Transformation of parasites with plasmid pMD221 and transcription of the Pbs21 gene.
The previously described plasmid pMD221 (29) contains the pyrimethamine-resistant form of the DHFR-TS gene of P. berghei as a selectable marker and the Pbs21 gene detailed above (Fig. 1A). Promoters of gene transcription generally consist of a core promoter region located adjacent to the transcription initiation site whose activity is modulated by additional enhancer activities located further upstream. Having established that the plasmid pMD221 contained sufficient DNA upstream of the transcription initiation site that might act as a core promoter, we introduced this plasmid into blood stages of two P. berghei clones, clone HP (a gametocyte producer) and clone 2.33 (a non-gametocyte producer) as described previously (29). We then selected for pyrimethamine-resistant parasites. From both populations of transformed parasites, we obtained clones that were resistant to pyrimethamine and designated them PbHP/221 and Pb233/221, respectively. The transformed parasites were maintained in mice in the presence of pyrimethamine to prevent the loss of plasmids, which inevitably occurs in the absence of the drug (29). Restriction fragments of total DNA digested with ClaI were hybridized to a probe recognizing the coding region of the DHFR-TS gene. This probe recognized the genomic copy of the DHFR-TS gene and, in transformed parasites, an additional fragment of 10.5 kb which was the plasmid copy of this gene (Fig. 2A). The plasmid copy number in the two transformed parasite populations was estimated by quantitative Southern hybridization, demonstrating that in the presence of pyrimethamine, both transformed lines maintained between 2 and 6 copies of the plasmid per parasite nucleus (data not shown).
FIG. 2.
Characterization of the recombinant P. berghei clones expressing transgenic Pbs21. (A) The non-gametocyte-producing transgenic clone Pb233/221 and the gametocyte-producing clone PbHP/221 contain the same copy numbers of pMD221. DNA was isolated from the recombinant clones digested with ClaI and prepared for Southern analysis. Hybridization with the DHFR-TS-specific probe reveals the genomic single copy of the DHFR-TS gene and the copy carried on the plasmid in the transfected clones. The copy number was estimated to be 2 to 6 per parasite nucleus. (B) Stage-specific expression of the transgenes in PbHP/221. Aliquots of synchronous and parallel cultures of PbHP/221 (B1) or wild-type HP (B2) were removed at the stages shown, RNA was prepared, and equal amounts were subjected to Northern analysis. The blots were simultaneously hybridized with complex probes representing the ORFs of both the DHFR and Pbs21 genes as indicated. The Pbs21 signal in HP wild-type parasites results from gametocytes present in the preparations. R, ring form; T, trophozoite; S, schizont. (C) Relative expression of the Pbs21 and DHFR genes in the different transgenic parasites. RNAs isolated from the same samples as those in panel A were subjected to Northern analysis. Hybridization was performed sequentially with probes for Pbs21 (top), DHFR-TS (middle), and rRNA (bottom). 221, pMD221 isolated from bacteria; HP, untransfected P. berghei ANKA; HP/221, clone PbHP/221; 233/221, clone Pb233/221; 233, untransfected clone 2.33; gam., gametocytes.
Plasmid pMD221 was rescued from the transformed parasites by transformation of E. coli with total parasite DNA and showed the same restriction pattern as the original pMD221 plasmid when digested with PvuII (data not shown), indicating that the plasmid DNA was maintained in an unrearranged form in the parasites (see also reference 29).
Plasmid pMD221 carried only 218 bp of the region that would normally be expected to contain the Pbs21 promoter of transcription (see above). When we examined the transcription of the Pbs21 gene in both PbHP/221 and Pb233/221, significant transcription of the Pbs21 gene was observed. Throughout the 24-h cycle of synchronized transformed PbHP/221 parasites, the levels of both Pbs21 and DHFR-TS mRNA were elevated compared with their respective normal levels of stage-specific expression that were observed in nontransformed parasites (Fig. 2B) (see also references 18 and 30). The Pbs21 gene is not transcribed in clone 2.33 (18), but Pbs21 mRNA was found in transformed Pb233/221 blood-stage parasites (Fig. 2C). The two transformed clones produced significant steady-state levels of transgene Pbs21 mRNA, indicating that expression results from the plasmid-borne copy of Pbs21 in both transgenic backgrounds (Fig. 2C). This result suggests that the small 5′ region of the Pbs21 gene that flanks the plasmid copy of the Pbs21 gene is able to drive transcription in blood stages but lacks the elements that normally control the stage specificity which would result in macrogametocyte-specific transcription. The size of the Pbs21 mRNA produced under the control of the truncated promoter contained on pMD221 was indistinguishable from that of the wild-type mRNA (Fig. 2B and C).
Translation of Pbs21 mRNA occurs in asexual and sexual blood stages of transformed parasites.
It has been shown before that in nontransformed parasites, Pbs21 protein is not expressed in asexual blood stages, in male gametocytes, nor in female gametocytes circulating in the peripheral blood of the vertebrate host despite the presence of abundant mRNA in the latter (26, 31). The protein first appears after activation in female gametes and is continuously produced in zygotes and ookinetes (24, 33). Recognizing that transformed parasites failed to demonstrate normal stage-specific transcription of the Pbs21 gene, we investigated whether the translation of the Pbs21 mRNA in these parasites was similarly deregulated.
In Western blot analysis, nontransformed asexual blood stages of clone 2.33 and blood stages (asexual and sexual stages) of clone HP probed with the Pbs21-specific transmission-blocking MAb 13.1 did not show any signal whereas in the transformed blood stages (Pb233/221 and PbHP/221) Pbs21 was detected with this and other transmission-blocking MAbs (12.1 and 17.9) (Fig. 3A, lanes 2 to 8). The molecular mass of the plasmid-derived Pbs21 in the blood stages of transformed Pb233/221 was, under nonreducing conditions, indistinguishable from that of native Pbs21 of ookinetes (20 to 21 kDa).
FIG. 3.
Western blot analyses of P. berghei antigen and immune sera raised against nontransformed (2.33) and transformed (Pb233/221) P. berghei clones. (A and B) Antigen preparations were obtained from ookinete cultures, nontransformed parental P. berghei blood-stage parasites (non-gametocyte-producing clone 2.33 and gametocyte-producing clone HP), or transformed Pb233/221 and PbHP/221 blood-stage parasites (expressing transgenic Pbs21) and separated on an SDS–14% PAGE gel (C) recombinant Pbs21 was separated on an SDS–12% PAGE gel under nonreducing conditions and transferred to a nitrocellulose membrane. Pbs21-specific MAbs recognizing conformation-independent (MAb 13.1) or conformation-dependent (MAbs 17.9 and 12.1) epitopes were used. (A) Lane 1, whole ookinete culture homogenate (ca. 5 × 104 ookinetes) containing asexual, sexual, and ookinete-stage proteins probed with MAb 13.1; lane 2, clone 2.33 homogenate (ca. 5 × 107 parasites) probed with MAb 13.1; lanes 3 to 6, Pb233/221 homogenate (ca. 5 × 107 parasites) probed with MAb 13.1, MAb 17.9, MAb 12.1, and NMS, respectively; lane 7, HP homogenate (ca. 5 × 107 parasites) probed with MAb 13.1; lane 8, PbHP/221 homogenate (ca. 2 × 107 parasites) probed with MAb 13.1. (B) Lanes 9 to 12, whole ookinete homogenate probed with MAb 13.1, NMS, anti-2.33 immune serum, and anti-Pb233/221 immune serum, respectively. (C) Lanes 13 to 16, affinity-purified recombinant Pbs21 (aa 1 to 188) probed with MAb 13.1, NMS, anti-2.33 immune serum, and anti-Pb233/221 immune serum, respectively.
Following phase separation of a Triton X-114 extract, ookinete-derived Pbs21 was found exclusively in the detergent phase (data not shown and reference 1). The demonstrated hydrophobicity of Pbs21 has been shown to be due solely to the presence of a GPI anchor (1). Pbs21 expressed by transgenic parasites partitioned exclusively in the detergent phase following phase separation (data not shown), indicating the presence of a hydrophobic domain in transgenically expressed Pbs21, which is most likely to be a GPI membrane anchor.
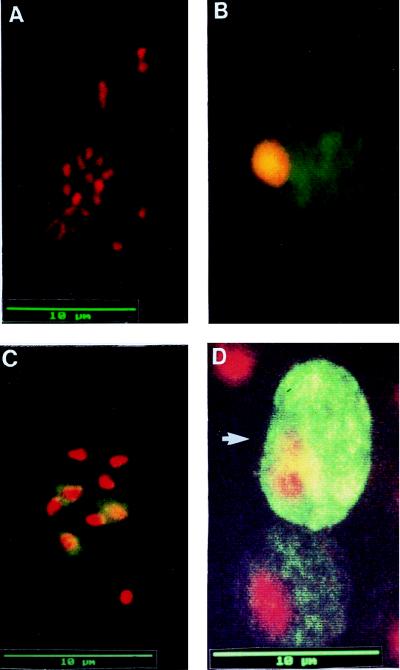
In IFAT analysis of nontransfected parasites of both P. berghei clones, 2.33 and HP, in samples collected 5 to 10 days postinfection, no signal was detected with MAb 13.1 (Fig. 4A). Pbs21-specific fluorescence was observed in asexual blood stages (trophozoites and schizonts) on smears of transfected parasites of both lines, Pb233/221 and PbHP/221 (Fig. 4B and C). Interestingly, in the gametocyte-producing strain PbHP/221, Pbs21 expression was found in both male (Fig. 4D, lower image) and female (Fig. 4D, upper image) gametocytes, with a stronger intensity of MAb 13.1 staining in female than in male gametocytes. These results show that plasmid-derived Pbs21 expression occurs in blood stages of transformed parasites and that not only transcriptional control but also translational control was altered after transformation with plasmid pMD221. Within individual segmenting schizonts, fluorescence was not invariably detected in all the merozoites. While this is consistent with the observation of van Dijk et al. (30), i.e., that in episome-based transfection segregation of plasmid DNA during schizogony is not uniform, the mechanism by which the protein could be unequally distributed at schizogony is not understood.
FIG. 4.
IFAT on acetone-fixed blood smears of nontransformed P. berghei parasites and P. berghei parasites transformed with plasmid pMD221 probed with anti-Pbs21-specific MAb 13.1 as the primary antibody. For consistency of appearance, antigen-specific red fluorescence in samples probed with streptavidin-Texas red conjugate was artificially changed to green; therefore, for all panels, stained nuclei appear red and stained Pbs21-specific signal appears green. (A) Nontransformed parasites of the gametocyte-producing P. berghei clone HP. No Pbs21-specific signal was detected with MAb 13.1. (B) A late trophozoite of the transformed non-gametocyte-producing clone Pb233/221 showing Pbs21-specific fluorescence distributed throughout the cell. (C) A segmenter of the transformed gametocyte-producing clone PbHP/221. In three of the eight merozoites, antigen-specific fluorescence was detected. (D) A male and a female gametocyte (arrow) of transformed clone PbHP/221. In both cells, the Pbs21-specific signal is distributed throughout the cells, with the intensity of fluorescence being stronger in the female than in the male gametocyte.
Induction of an immune response by transformed parasites expressing Pbs21 in blood stages.
Pbs21 expression is usually found only after gametocyte activation (see above). We noted in the present study that due to unintentional activation of macrogametocytes during handling, a weak primary antibody response to Pbs21 was induced following mechanical transfer of mouse blood infected with nontransformed HP parasites. Consequently, the specific response to plasmid-encoded Pbs21 in PbHP/221 could not be accurately estimated. In further experiments, we therefore focused on the plasmid-derived Pbs21 expression in the non-gametocyte-producing clone 2.33. Mice were infected with transformed or nontransformed clone 2.33 parasites, and the usual lethal course of the infection was suppressed through dietary modulation (see Materials and Methods). Sera were collected on day 26 or 33 postinfection. Mice infected with P. berghei clone 2.33 or Pb233/221 developed a complex antibody response to asexual-stage P. berghei proteins (Fig. 3B); antibodies were predominantly of IgG2a/IgG2b isotypes. Sera of mice infected with nontransformed parasites of clone 2.33 did not react with Pbs21 (Fig. 3B and C). In contrast, mice infected with transformed Pb233/221 parasites additionally developed anti-Pbs21 antibodies as revealed by Western blot analysis using a homogenate of ookinete cultures (consisting of mixed asexual and sexual blood stages and early mosquito stages) and purified recombinant Pbs21 as antigens (Fig. 3B and C). By using ELISA, Pbs21-specific antibody titers of 5, 6, 9, and 26 μg/ml (mean ± standard deviation, 11.5 ± 10 μg/ml) were determined for sera from mice infected with Pb233/221 parasites. Anti-Pbs21 antibody subclasses were again of IgG2a/IgG2b isotypes.
In previous immunization studies, it was shown that immune sera containing antibodies against Pbs21 can inhibit the development of P. berghei in the mosquito (14, 27). The impact of Pbs21-specific immune sera induced by transformed blood stages of Pb233/221 upon parasite transmission to mosquitoes was tested.
Gametocytes and cultured ookinetes were fed to mosquitoes by membrane feeding in the presence of the immune sera. These sera did not show a significant inhibition of parasite transmission when compared to that of control immune sera from mice infected with clone 2.33 or NMS (Table 1). Pbs21-specific MAb 13.1 significantly blocked parasite development when a concentration of 150 μg/ml (Table 1) was used, confirming earlier data (20).
TABLE 1.
Mosquito oocyst infection following gametocyte and ookinete membrane feeds with pooled immune sera (n = 4) from mice infected with P. berghei clone 2.33 or transformed clone Pb233/221 parasites or with Pbs21-specific transmission-blocking MAb 13.1
| Membrane feed | Serum or antibody (concn) | No. of mosquitoes infected/total no. dissected (%) | Geometric mean no. of oocysts (±SE) | % of controla |
|---|---|---|---|---|
| Gametocyte (n = 3) | NMS | 92/118 (78) | 11.34 (±8) | 100 |
| Clone 2.33-immune serum | 96/120 (80) | 9.16 (±5) | 81 | |
| Clone Pb233/221-immune serum | 91/117 (78) | 10.01 (±5) | 88 | |
| Gametocyte plus MAb 3.1 (n = 3) | NMS | 63/87 (72) | 6.20 (±0.8) | 100 |
| MAb 13.1 (150 μg/ml) | 44/107 (41) | 1.42 (±0.9) | 23b | |
| MAb 13.1 (10 μg/ml) | 78/114 (68) | 3.90 (±1.4) | 63 | |
| Ookinete (n = 1) | NMS | 30/30 (100) | 7.83 (±1.2) | 100 |
| Clone Pb233-immune serum | 30/30 (100) | 7.52 (±1.0) | 96 | |
| Clone Pb233/221-immune serum | 27/30 (90) | 5.72 (±1.0) | 73 | |
| MAb 13.1 (150 μg/ml) | 19/30 (63) | 0.79 (±0.2) | 10b |
The value for the NMS control was defined as 100%.
Significantly different (P < 0.001) from value for NMS control as analyzed by Student’s t test.
DISCUSSION
Using genetic transformation of blood stages of P. berghei, we were able to express artificially a protein in the blood stages of the parasite that is normally present only during development of mosquito midgut stages of the parasite. In the transformed parasites, the gene coding for this protein, Pbs21, is additionally present on an episomally maintained plasmid. For a stage-specific protein to be correctly expressed, the cell has to accurately perform transcription, translation, and subcellular protein trafficking.
Transcription.
In nontransformed parasites, expression of Pbs21 is regulated by stage-specific transcriptional and translational control mechanisms. Transcription of the Pbs21 gene is confined to the female gametocytes and starts during development of the immature macrogametocyte in the blood of the vertebrate host (18, 22, 26, 31). In transgenic parasites, this stage-specific transcription was lost, resulting in transcription of the plasmid copy of the gene in all blood stages. This strongly suggests that the 218 bp 5′ of the region of transcriptional initiation of the Pbs21 gene, which were present in the plasmid, are able to serve as a promoter for gene transcription. However, this limited sequence confers neither the sex nor stage specificity observed for transcription of the chromosomal copy of the Pbs21 gene.
The strong signal for Pbs21 mRNA detected in PbHP/221 parasite preparations may be due to Pbs21 mRNA accumulated in gametocytes (26). Despite a plasmid copy number of 2 to 6 per parasite nucleus, the signal for the Pbs21 transcript in transformed asexual parasites of clone Pb233/221 was not strong. Promoter regions possess important control elements which can significantly affect the rate of transcription. For example, in Plasmodium falciparum, shortening of the 5′ sequence of the DHFR-TS promoter region by as few as 27 bp from position −575 to −548 reduced the level of transcriptional activity by 50% (4). The relative efficiency and specificity of the 218 bp of Pbs21 5′ untranslated region as a putative promoter have yet to be determined, although this sequence has been used to drive the expression of selectable marker genes in P. berghei merozoites (25). Given the proportional intensity of the steady-state RNA levels of the two genes contained on pMD221, it is possible that the transcription of the Pbs21 gene on the plasmid receives a significant contribution from run-through of previously loaded RNA polymerase. However, the observed size of the Pbs21 mRNA in the transgenic parasites is consistent with the accurate use of both the transcription start site and the site of polyadenylation.
Translation.
Despite abundant Pbs21 mRNA in female gametocytes circulating in the vertebrate host, translation normally occurs only after activation of the gametocytes in the invertebrate vector. The normal translation repression of the Pbs21 mRNA exhibited by female gametocytes is absent in these transgenic parasites: all blood stages, including female gametocytes, were able to express the protein. The absence of translational inhibition in transformed blood-stage parasites could arise for the following different reasons: (i) asexual blood stages and male gametocytes which do not normally produce Pbs21 may lack appropriate inhibitors for translation of the Pbs21 mRNA; (ii) in contrast, in the macrogametocyte, where translation is normally repressed, the mechanism may be saturated by the overproduction of Pbs21 mRNA from the plasmid gene copies; (iii) translation may occur as a result of as-yet-unidentified differences between plasmid and genome-derived Pbs21 mRNA. Although translation by different ribosome types is not a feature of the control here, it should be noted that this message is normally translated during the period of transition between A- and S-type ribosomes (32). On the basis of the data obtained here, it is conceivable that the translation of Pbs21 is restricted to A-type ribosomes and that its cessation is associated with completion of the transition to S-type ribosomes. Ribosome type-specific mRNA species might still be a mode of control of gene expression (through translation) in Plasmodium.
Protein trafficking.
The Pbs21 protein is a surface protein, associated with the plasmalemma of zygotes and ookinetes (24, 33). In blood stages of transgenic parasites, Pbs21 protein was generally distributed throughout the cell without edge delineation and was not preferentially exported to the plasmalemma, as it is in the ookinete, but was retained in the cytoplasm despite the fact that the polypeptide is putatively identical to the natural protein, i.e., it possesses both the secretory signal sequence and the GPI anchor addition sites (1). Transgenically expressed Pbs21 was indistinguishable to ookinete-derived Pbs21 in terms of molecular weight and conformation. Furthermore, following phase separation, Pbs21 expressed by transgenic parasites was found exclusively in the detergent phase, as is Pbs21 expressed by ookinetes (1). The hydrophobic properties of Pbs21 expressed by ookinete stages was attributed to the presence of a GPI anchor (1). The data presented here are consistent with a membrane anchor modification of Pbs21 expressed by transformed parasites; its precise nature, however, needs further investigation. The fact that the protein is found predominantly in the cytoplasm of transformed parasites cannot be explained at present. It has recently been shown that in P. berghei parasites transgenic for the P. falciparum merozoite surface protein AMA-1, the precise subcellular location is dependent upon timing of expression. The protein was only correctly distributed to its primary location in the rhoptries when under control of the homologous P. berghei AMA-1 promoter (12). Thus, it would appear that the timing of expression of transgenes and hence the use of the entire homologous promoter sequence in Plasmodium can be crucial for the correct subcellular distribution of the protein produced.
The stability of Pbs21 protein may also play a role in its observed distribution in the transgenic parasite forms. The transgenic protein may have a reduced half-life compared to that normally seen in the female gamete and early mosquito stages, which may account for the predominant expression of Pbs21 protein in the cytoplasm of recombinant parasites. In wild-type parasites, Pbs21 mRNA translation is confined to mosquito midgut stages at 20°C and the protein might be more stable under those conditions than under the conditions in the mammalian host.
Antibodies against proteins of the 25/28-kDa family of late-sexual-stage-specific transmission-blocking antigens have not been found in natural infections (10). The high and apparently unrestricted immunogenicity observed with this type of antigen has been attributed to the fact that they have never been under selective pressure of the vertebrate’s immune system (7). It has been emphasized that antibodies to conformation-dependent epitopes are important for transmission-blocking activity in immune sera (5, 14, 15). Following transgenic expression of Pbs21 in the asexual stages of P. berghei, the structure of the transgenic protein could not be distinguished from that of native Pbs21 produced by the sexual-stage parasite. We therefore anticipated that such a protein would (and did) induce conformation-dependent antibodies that recognized native and recombinant Pbs21 under nonreducing conditions in Western blot analyses. In previous studies, protein-specific antibody 10-fold higher titers than those currently induced by this first series of studies using Pbs21-transgenic parasites were required to prevent parasite transmission (14, 20, 27). Future studies must address the possibility of increasing such titers through alternative infection protocols and different modes of transgene expression.
In addition, studies on the artificial induction of expression of immunologically important, stage-specific proteins by drugs through their interaction with molecules involved in transcriptional or translational control of expression could be facilitated. The work presented here suggests that drugs that unlock the translational repression of female gametocyte mRNA species could represent a valuable new research line in the search for new antimalaria strategies.
ACKNOWLEDGMENT
This work was supported financially by the European Commission, Directorate General XII (INCO-DC program) contract CT950022.
We are grateful to M. C. Rodriguez, Geoff Butcher, and A. Paez for valuable help, Annette Beetsma for useful discussions, and Kai Wengelnik and J. Dessens for critical reading of the manuscript.
REFERENCES
- 1.Alejo-Blanco, A. R., A. Paez, P. Gerold, A. L. Dearsly, G. Margos, R. T. Schwarz, G. Barker, M. C. Rodriquez, and R. E. Sinden. The biosynthesis and post-translational modification of Pbs21, a transmission-blocking ookinete-surface immunogen of Plasmodium berghei. Submitted for publication. [DOI] [PubMed]
- 2.Beetsma A L, van de Wiel T J J M, Sauerwein R W, Eling W M C. Plasmodium berghei ANKA: purification of large numbers of infectious gametocytes. Exp Parasitol. 1998;88:69–72. doi: 10.1006/expr.1998.4203. [DOI] [PubMed] [Google Scholar]
- 3.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 4.Crabb B S, Cowman A F. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:7289–7294. doi: 10.1073/pnas.93.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy P E, Pimenta P, Kaslow D C. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993;177:505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy P E, Kaslow D C. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Good M F, Miller L H, Kumar S, Quakyi I A, Keister D, Adams J H, Moss B, Berzofsky J A, Carter R. Limited immunological recognition of critical malaria vaccine candidate antigens. Science. 1988;242:574–577. doi: 10.1126/science.2902690. [DOI] [PubMed] [Google Scholar]
- 8.Jacobi K, Kretschmar W. Die Milchtherapie der Malaria-Infektion (Plasmodium berghei) bei der Maus. Z Tropenmed Parasitol. 1962;13:286–304. [PubMed] [Google Scholar]
- 9.Jacobs R L. Role of p-aminovenzoic acid in Plasmodium berghei infection in the mouse. Exp Parasitol. 1964;15:213–225. doi: 10.1016/0014-4894(64)90017-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaslow D. Progress toward a transmission-blocking vaccine. In: Saul A, Good M, editors. Molecular immunological considerations in malaria vaccine development, chapter 8. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 209–244. [Google Scholar]
- 11.Kawamoto F, Alejo-Blanco R, Fleck S L, Sinden R E. Plasmodium berghei: ionic regulation and the induction of gametogenesis. Exp Parasitol. 1991;72:33–42. doi: 10.1016/0014-4894(91)90118-g. [DOI] [PubMed] [Google Scholar]
- 12.Kocken, L. H. M., A. M. van der Wel, M. A. Dubbeld, F. M. van de Rijke, G.-J. van Gemert, L. H. Bannister, C. J. Janse, A. P. Waters, and A. W. Thomas. Precise timing of expression of a Plasmodium falciparum derived transgene in P. berghei is a critical determinant of subsequent subcellular location. Submitted for publication. [DOI] [PubMed]
- 13.Kretschmar W. Die Bedeutung der p-Aminobenzoesaeure fuer den Krankheitsverlauf und die Immunitaet bei der Malaria im Tier (Plasmodium berghei) und im Menschen (Pl. falciparum). I. Versuche an NMRI-Maeusen. Z Tropenmed Parasitol. 1966;17:301–320. [PubMed] [Google Scholar]
- 14.Margos G, Kurtenbach K, Posnett E, Barker G C, Matsuoka H, Paton M G, Sinden R E. Expression of the Plasmodium berghei ookinete protein Pbs21 in a baculovirus-insect cell system produces an efficient transmission-blocking immunogen. Parasite Immunol. 1993;17:167–176. doi: 10.1111/j.1365-3024.1995.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka H, Paton M G, Barker G C, Sinden R E. Studies on the immunogenicity of a recombinant ookinete surface antigen Pbs21 from Plasmodium berghei expressed in Escherichia coli. Parasite Immunol. 1994;16:27–34. doi: 10.1111/j.1365-3024.1994.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka H, Kobayashij J, Barker G C, Miura K, Chinzei Y, Miyajima S, Ishii A, Sinden R E. Induction of anti-malarial transmission-blocking immunity with a recombinant ookinete surface antigen of Plasmodium berghei produced in silk-worm larvae using the baculovirus expression vector system. Vaccine. 1996;14:120–126. doi: 10.1016/0264-410x(95)00162-t. [DOI] [PubMed] [Google Scholar]
- 17.Paez, A. Unpublished data.
- 18.Paton M G, Barker G C, Matsuoka H, Ramesar J, Janse C J, Waters A P, Sinden R E. Structure and expression of a posttranscriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol Biol Parasitol. 1993;59:263–276. doi: 10.1016/0166-6851(93)90224-l. [DOI] [PubMed] [Google Scholar]
- 19.Peters W, Howells R E. Chemotherapy. In: Killick-Kendrick R, Peters W, editors. Rodent malaria. London, United Kingdom: Academic Press; 1978. pp. 345–391. [Google Scholar]
- 20.Ranawaka G, Alejo-Blanco R, Sinden R E. Characterization of the modes of action of anti-Pbs21 malaria transmission-blocking immunity: ookinete to oocyst differentiation in vivo. Parasitology. 1994;109:403–411. doi: 10.1017/s0031182000080653. [DOI] [PubMed] [Google Scholar]
- 21.Robson K J, Jennings M W. The structure of the calmodulin gene of Plasmodium falciparum. Mol Biochem Parasitol. 1991;46:19–34. doi: 10.1016/0166-6851(91)90195-c. [DOI] [PubMed] [Google Scholar]
- 22.Shaw M K, Thompson J, Sinden R E. Localisation of ribosomal RNA and Pbs21-mRNA in the sexual stages of Plasmodium berghei using electron microscope in situ hybridisation. Eur J Cell Biol. 1997;71:270–276. [PubMed] [Google Scholar]
- 23.Simonetti A B, Billingsley P F, Winger L A, Sinden R E. Kinetics of expression of two major Plasmodium berghei antigens in the mosquito vector, Anopheles stephensi. J Eukaryot Microbiol. 1993;40:569–576. doi: 10.1111/j.1550-7408.1993.tb06109.x. [DOI] [PubMed] [Google Scholar]
- 24.Sinden R E, Winger L, Carter E H, Hartley R H, Tirawanchai N, Davies C S, Moore J, Sluiters J F. Ookinete antigens of Plasmodium berghei: a light and electron-microscope immunogold study of expression of the 21 kDa determinant recognized by a transmission-blocking antibody. Proc R Soc Lond B. 1987;230:443–458. doi: 10.1098/rspb.1987.0028. [DOI] [PubMed] [Google Scholar]
- 25.Thomas, A. Unpublished data.
- 26.Thompson J, Sinden R E. In situ detection of Pbs21 mRNA during sexual development of Plasmodium berghei. Mol Biol Parasitol. 1994;68:189–196. doi: 10.1016/0166-6851(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 27.Tirawanchai N, Winger L A, Nicholas J, Sinden R E. Analysis of immunity induced by the affinity-purified 21 kD zygote-ookinete surface antigen of Plasmodium berghei. Infect Immun. 1991;59:36–44. doi: 10.1128/iai.59.1.36-44.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dijk M R, McConkey G A, Vinkenoog R, Waters A P, Janse C J. Mechanisms of pyrimethamine resistance in two different strains of Plasmodium berghei. Mol Biochem Parasitol. 1994;68:167–171. doi: 10.1016/0166-6851(94)00163-4. [DOI] [PubMed] [Google Scholar]
- 29.van Dijk M R, Waters A P, Janse C J. Stable transfection of malaria parasite blood stages. Science. 1995;268:1358–1362. doi: 10.1126/science.7761856. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk M R, Winkenoog R, Ramesar J, Vervenne R A W, Waters A P, Janse C J. Replication, expression and segregation of plasmid-borne DNA in genetically transformed malaria parasites. Mol Biol Parasitol. 1997;86:155–162. doi: 10.1016/s0166-6851(97)02843-0. [DOI] [PubMed] [Google Scholar]
- 31.Vervenne R A W, Dirks R W, Ramesar J, Waters A P, Janse C J. Differential expression in blood stages of the gene coding for the 21-kilodalton surface protein of ookinetes of Plasmodium berghei as detected by RNA in situ hybridisation. Mol Biol Parasitol. 1994;68:259–266. doi: 10.1016/0166-6851(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 32.Waters A P, van Spaendonk R M, Ramesar J, Vervenne R A, Dirks R W, Thompson J, Janse C J. Species-specific regulation and switching of transcription between stage-specific ribosomal RNA genes in Plasmodium berghei. J Biol Chem. 1997;272:3583–3589. doi: 10.1074/jbc.272.6.3583. [DOI] [PubMed] [Google Scholar]
- 33.Winger L A, Tirawanchai N, Nicholas J, Carter H E, Smith J E, Sinden R E. Ookinete antigens of Plasmodium berghei. Appearance on the zygote surface of an Mr 21 kD determinant identified by transmission-blocking monoclonal antibodies. Parasite Immunol. 1988;10:193–207. doi: 10.1111/j.1365-3024.1988.tb00214.x. [DOI] [PubMed] [Google Scholar]