Abstract
Background
Cadonilimab is a bispecific antibody that simultaneously targets programmed cell death receptor-1 and cytotoxic T lymphocyte-associated antigen-4. This study aimed to assess the safety and efficacy of cadonilimab plus anlotinib for the first-line treatment of advanced non-small cell lung cancer (NSCLC) without sensitizing EGFR/ALK/ROS1 mutations.
Methods
Patients received cadonilimab 15 mg/kg and 10 mg/kg every three weeks (Q3W) plus anlotinib at doses of 10 or 12 mg once daily for two weeks on a one-week-off schedule. The primary endpoints included safety and objective response rate (ORR).
Results
Sixty-nine treatment-naïve patients received cadonilimab 15 mg/kg Q3W combination (n = 49) and 10 mg/kg Q3W combination (n = 20). Treatment-related adverse events (TRAEs) were reported in 48 (98.0%) and 19 (95.0%) patients, with grade ≥3 TRAEs occurring in 29 (59.2%) and five (25.0%) patients, respectively. TRAEs leading to cadonilimab discontinuation occurred in eight (16.3%) and one (5.0%) patients in the cadonilimab 15 mg/kg Q3W and 10 mg/kg Q3W dosing groups. The confirmed ORRs were 51.0% (25/49) and 60.0% (12/20) accordingly.
Conclusions
Cadonilimab 10 mg/kg Q3W plus anlotinib showed manageable safety and promising efficacy as a first-line chemo-free treatment for advanced NSCLC.
ClinicalTrials.gov identifier
Subject terms: Non-small-cell lung cancer, Cancer immunotherapy
Introduction
Lung cancer is a common malignant tumor with high morbidity and mortality worldwide, with non-small cell lung cancer (NSCLC) accounting for 85% of all lung cancers [1]. Approximately 70% of NSCLC patients are diagnosed at an advanced stage (stage IIIB/IV) [2]. In recent years, chemotherapy combined with programmed cell death receptor-1 (PD-1)/PD-L1 inhibitors has become the standard treatment for advanced NSCLC without driver gene mutations, which results in toxicity. Several chemo-free therapies, including pembrolizumab, atezolizumab, and cemiplimab monotherapy, and dual chemo-free immunotherapy with nivolumab plus ipilimumab have been approved as first-line therapies for advanced NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 50% or ≥1% and without driver gene mutations [3–7]. Only patients with TPS ≥ 50% benefit from approved chemo-free monotherapies. Accordingly, novel and effective chemo-free regimens are needed to treat advanced NSCLC.
Numerous studies have demonstrated the synergistic effects of immunotherapy and antiangiogenic therapy in advanced solid tumors. Clinical studies of anti-PD-1/PD-L1 antibodies in combination with anti-angiogenic drugs have shown promising efficacy and manageable safety in treating advanced NSCLC. Sintilimab plus anlotinib showed promising antitumour activity with a tolerable safety profile as a first-line therapy in patients with advanced NSCLC [8]. Camrelizumab plus apatinib showed encouraging antitumour activity and acceptable toxicity in chemotherapy-pretreated patients with advanced non-squamous NSCLC [9]. A phase Ib/II trial of lenvatinib plus pembrolizumab showed promising clinical activity and a manageable safety profile in previously treated patients with NSCLC, although this combination regimen showed no improvement in overall survival (OS) compared with pembrolizumab monotherapy in first-line treatment of advanced NSCLC with PD-L1 TPS ≥ 1% in LEAP-007 [10, 11].
Cadonilimab is a humanized immunoglobulin G1 bispecific antibody with fragment crystallizable (Fc) mutations that eliminate Fc receptor- and complement-mediated cytotoxic effects. Cadonilimab binds to PD-1 and CTLA-4 and blocks PD-1/PD-L1, PD-1/PD-L2, CTLA-4/B7.1, and CTLA-4/B7.2 interactions [12]. Anlotinib, a new orally administered tyrosine kinase inhibitor that targets vascular endothelial, fibroblast, and platelet-derived growth factor receptors, has been approved as a third-line therapy for advanced lung cancer in China [13]. We report a phase Ib/II study conducted to evaluate the safety and efficacy of a combination of cadonilimab plus anlotinib in the treatment of previously untreated advanced NSCLC and to explore the optimal dosing for this combination.
Materials and methods
Study design and patients
This was a multicentre, open-label, phase Ib/II trial of cadonilimab combined with anlotinib in previously untreated patients with advanced NSCLC, and was conducted across 13 centers in China.
The inclusion criteria were as follows: age 18–75; histologically confirmed irresectable locally advanced (IIIB/C phase) or metastatic (IV phase) NSCLC according to AJCC 8th edition without sensitizing EGFR/ALK/ROS1 mutations; treatment-naïve; one measurable lesion as defined by the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1; an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, a life expectancy of ≥3 months, and adequate organ function. Patients with a history of active autoimmune disease, concurrent use of immunosuppressive medications or immunosuppressive doses of systemic corticosteroids (>10 mg daily prednisone equivalent), untreated brain metastases, active interstitial lung disease, central squamous histology carcinoma with a cavity or a high risk of pulmonary hemorrhage, uncontrolled hypertension, or clinically significant cardiovascular or cerebrovascular disease were excluded.
The trial was conducted in accordance with the International Conference on Good Clinical Practice Standards and the Declaration of Helsinki. The institutional review boards and independent ethics committees of all participating centers approved the study protocol and amendments, and all patients provided written informed consent.
Procedures
This Ib/II trial included the safety run-in and expansion phases. Patients received intravenous cadonilimab at doses of 10 mg/kg every two weeks (Q2W) and 15 mg/kg every three weeks (Q3W) in the safety run-in phase, along with oral anlotinib at 10 or 12 mg/day for two weeks on a one-week off schedule, to preliminarily assess the toxicities, tolerability and efficacy of this combination. Owing to the poor tolerance and low efficacy observed with the cadonilimab 10 mg/kg Q2W combination (shown in the results section), patients received cadonilimab at doses of 15 mg/kg and 10 mg/kg Q3W plus anlotinib at 10 or 12 mg/day for two weeks on a one-week off schedule in the expansion phase. Treatment was continued until disease progression, unacceptable toxicity, withdrawal of consent, investigator’s decision, or a maximum of 35 doses (approximately two years), whichever occurred first.
Radiographic tumor assessment was performed at baseline, every six weeks during the first 48 weeks, and every 12 weeks thereafter, according to RECIST v1.1. Patients who discontinued treatment for any reason other than disease progression or death were followed-up for tumor radiological assessment until disease progression, initiation of new anticancer treatment, loss to follow-up, withdrawal of informed consent, or death, whichever occurred first. PD-L1 expression was assessed by immunohistochemistry using PD-L1 IHC 22C3 pharmDx (Dako Omnis), and PD-L1 positivity was defined as a PD-L1 TPS ≥ 1%.
Adverse events (AEs) were monitored for 30 days, and serious AEs were followed up for 90 days after the last dose or the start of a new anticancer treatment, whichever occurred first. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. During follow-up, patients were contacted every three months to assess their survival.
Endpoints
The primary endpoints were safety and objective response rate (ORR), defined as the proportion of patients who achieved a confirmed complete response (CR) or partial response (PR), as assessed by investigators per RECIST v1.1. The secondary endpoints included duration of response (DOR; the time from the first recorded complete or partial response to disease progression or death), disease control rate (DCR; the proportion of patients who achieved a complete response, partial response, or stable disease as the best overall response), progression-free survival (PFS; the time from the first dose of study medication to radiographic disease progression or death), and OS (the time from the first dose of study medication to death due to any cause). The exploratory endpoint was the correlation between the PD-L1 TPS and efficacy.
Sample size estimation and statistical analysis
Approximately 12 subjects (6 subjects per dosing regimen, cadonilimab 10 mg/kg Q2W combination and 15 mg/kg Q3W combination) were enrolled in the safety run-in phase to preliminarily assess the toxicities, tolerability and efficacy of this combination regimen. The sample size for this part was not based on statistical test. For the phase II part (expansion phase), approximately 76 patients (38 patients per dosing regimen, cadonilimab 15 mg/kg Q3W combination and 10 mg/kg Q3W combination) were enrolled with a power of 82% to detect a 25% increase in ORR compared with the historical control ORR of 25%, under a 2-sided α of 0.05 and a 20% dropout rate of subjects.
The full analysis set was the primary analysis set for efficacy analysis, defined as all patients who had received at least one dose of cadonilimab (15 or 10 mg/kg Q3W) plus anlotinib and had measurable disease (as per RECIST v1.1) at baseline. The safety set included all patients who received at least one dose of cadonilimab (15 or 10 mg/kg Q3W) plus anlotinib. The analysis of ORR and DCR was based on a full analysis set and two-sided 95% confidence intervals (CI) using the Clopper–Pearson exact method. The Kaplan–Meier product limit method was used to estimate survivorship function for time-to-event endpoints such as DOR, PFS, and OS. Median time-to-event endpoints and 95% CI were calculated. PFS and OS analyses were based on the safety analysis set. A spider plot of percent change in tumor burden from baseline over time, swimmer plot of treatment duration and best overall response, and waterfall plot of the best percent change in tumor burden from baseline were generated. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
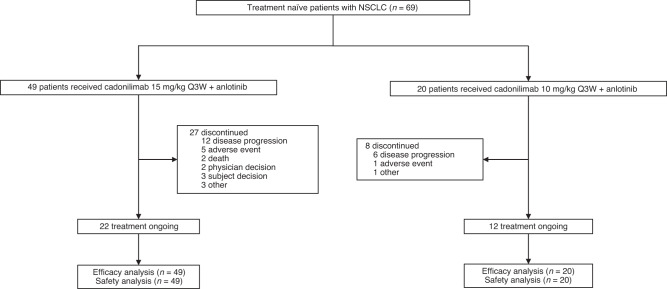
As of May 17, 2022 (cut-off date), 76 patients were enrolled and treated. Owing to poor tolerance and low efficacy, only seven patients were enrolled and administered cadonilimab 10 mg/kg Q2W plus anlotinib in the safety run-in phase. For the seven patients, the occurrence of grade ≥3 treatment-related adverse events (TRAEs) was 42.9% (n = 3). Meanwhile, an unsatisfying ORR of 14.3% (95% CI, 0.4, 57.9) was observed. These patients were not included in the safety and efficacy analyses and are not reported here. Cadonilimab 15 mg/kg Q3W plus anlotinib (n = 49) or cadonilimab 10 mg/kg Q3W plus anlotinib (n = 20) was administered to 69 patients (Fig. 1). Anlotinib was administered at an initial dose of 10 or 12 mg, and no significant differences in efficacy were observed between the two anlotinib doses; therefore, they were pooled together for analysis. Of the 69 patients treated with cadonilimab 15 mg/kg or 10 mg/kg Q3W plus anlotinib with a median age of 63.5 years, 58 (84.1%) were men, 59 (85.5%) had an ECOG performance status of 1, 53 (76.8%) had stage IV NSCLC, and 30 (43.5%) had squamous cell carcinoma. PD-L1 expression was assessed in all 69 patients; 43 (62.3%) were PD-L1 positive (defined as PD-L1 TPS ≥ 1%), of whom eight (11.6%) had PD-L1 TPS ≥ 50%. Demographic and disease characteristics at baseline are shown in Table 1. At a median follow-up of 8.8 months (range, 3.0–17.2 months), 34 (49.3%) of the 69 patients were still under treatment, 22 (44.9%) of 49 received 15 mg/kg cadonilimab Q3W, and 12 (60.0%) of 20 patients who received 10 mg/kg cadonilimab Q3W remained on study therapies (Fig. 1).
Fig. 1. Trial profile.
The enrolled subjects were shown according to two cadonilimab dosing groups. The treatment discontinued reasons were categoried.
Table 1.
Patient demographics and characteristics at baseline.
| Cadonilimab 15 mg/kg Q3W | Cadonilimab 10 mg/kg Q3W | Overall | |
|---|---|---|---|
| (N = 49) | (N = 20) | (N = 69) | |
| Age (year) | |||
| Median (Min, Max) | 64.0 (40, 74) | 58.5 (45, 74) | 63.5 (40, 74) |
| <65, n (%) | 29 (59.2) | 15 (75.0) | 44 (63.8) |
| >=65, n (%) | 20 (40.8) | 5 (25.0) | 25 (36.2) |
| Sex, n (%) | |||
| Male | 40 (81.6) | 18 (90.0) | 58 (84.1) |
| Female | 9 (18.4) | 2 (10.0) | 11 (15.9) |
| ECOG, n (%) | |||
| 0 | 5 (10.2) | 5 (25.0) | 10 (14.5) |
| 1 | 44 (89.8) | 15 (75.0) | 59 (85.5) |
| Smoking Status, n (%) | |||
| Never | 11 (22.4) | 4 (20.0) | 15 (21.7) |
| Former | 32 (65.3) | 14 (70.0) | 46 (66.7) |
| Current | 6 (12.2) | 2 (10.0) | 8 (11.6) |
| Clinical Stage at | |||
| Study Entry, n (%) | |||
| IIIB/IIIC | 14 (28.6) | 2 (10.0) | 16 (23.2) |
| IV | 35 (71.4) | 18 (90.0) | 53 (76.8) |
| Histological Type, n (%) | |||
| Squamous Cell | 24 (49.0) | 6 (30.0) | 30 (43.5) |
| Carcinoma | |||
| Non-squamous | 25 (51.0) | 14 (70.0) | 39 (56.5) |
| Cell Carcinoma | |||
| PD-L1 TPS, n (%) | |||
| <1% | 24 (49.0) | 2 (10.0) | 26 (37.7) |
| >=1% | 25 (51.0) | 18 (90.0) | 43 (62.3) |
| 1–49% | 22 (44.9) | 13 (65.0) | 35 (50.7) |
| >=50% | 3 (6.1) | 5 (25.0) | 8 (11.6) |
| Brain Metastasis, n (%) | 2 (4.1) | 4 (20.0) | 6 (8.7) |
| Liver Metastasis, n (%) | 3 (6.1) | 1 (5.0) | 4 (5.8) |
ECOG Eastern Cooperative Oncology Group, PD-1 programmed cell death receptor-1, PD-L1 programmed cell death ligand-1, TPS tumor proportion score, Q3W every 3 weeks.
Safety
Among the 69 patients who received cadonilimab 15 mg/kg and 10 mg/kg, TRAEs were reported in 67 (97.1%), with grade ≥3 TRAEs occurring in 34 (49.3%). Serious TRAEs were reported in 35 (50.7%), and TRAEs leading to permanent discontinuation of cadonilimab were reported in nine patients (13.0%). TRAEs leading to death occurred in one (1.4%) patient (Table 2). The safety profile was also compared between the dosing groups. TRAEs were reported in 48 (98.0%) and 19 (95.0%) patients in the cadonilimab 15 mg/kg and cadonilimab 10 mg/kg dosing groups, respectively; grade ≥3 TRAEs occurred in 29 (59.2%) and five (25.0%) patients, serious TRAEs were reported in 28 (57.1%) and seven (35.0%) patients, respectively; TRAEs leading to permanent discontinuation of cadonilimab were reported in eight (16.3%) and one (5.0%) patient, respectively. One TRAE leading to death was recorded in the cadonilimab 15 mg/kg dosing group, which was sudden cardiac death, compared with no TRAEs leading to death in the cadonilimab 10 mg/kg dosing group. The most common TRAEs of any grade were hypothyroidism (n = 27, 39.1%), increased aspartate aminotransferase (n = 23, 33.3%), and decreased appetite (n = 19, 27.5%). The most common grade ≥ 3 TRAEs were hypertension (n = 5, 7.2%) and abnormal hepatic function (n = 5, 7.2%) (Table S1).
Table 2.
Overview of adverse events (AEs).
| Cadonilimab 15 mg/kg Q3W | Cadonilimab 10 mg/kg Q3W | Overall | |
|---|---|---|---|
| (N = 49) | (N = 20) | (N = 69) | |
| TRAEs, n (%) | 48 (98.0) | 19 (95.0) | 67 (97.1) |
| Grade ≥3 TRAEs, n (%) | 29 (59.2) | 5 (25.0) | 34 (49.3) |
| Serious TRAEs, n (%) | 28 (57.1) | 7 (35.0) | 35 (50.7) |
| TRAEs leading to cadonilimab and anlotinib discontinuation, n (%) | 3 (6.1) | 1 (5.0) | 4 (5.8) |
| TRAEs leading to cadonilimab discontinuation, n (%) | 8 (16.3) | 1 (5.0) | 9 (13.0) |
| TRAEs leading to anlotinib discontinuation, n (%) | 7 (14.3) | 1 (5.0) | 8 (11.6) |
| TRAEs leading to death, n (%) | 1 (2.0) | 0 | 1 (1.4) |
TRAEs treatment-related adverse events, Q3W every 3 weeks.
Fourteen (20.3%) patients experienced immune-related adverse events (irAEs) (Table S2). Grade ≥3 irAEs accounted for 5.8% (n = 4), with two (4.1%) and two (10.0%) patients receiving cadonilimab at 15 mg/kg Q3W and 10 mg/kg Q3W, respectively. Grade ≥3 irAEs were hypopituitarism, immune-mediated arthritis, autoimmune hepatitis and ketoacidosis (one patient each) (Table S3).
In terms of specific AEs related to anlotinib, hypertension occurred in 17 (24.6%) patients, of whom five had grade ≥3 hypertension. Proteinuria also occurred in 17 (24.6%) patients; all were grade 1, 2 (Table S1).
Efficacy
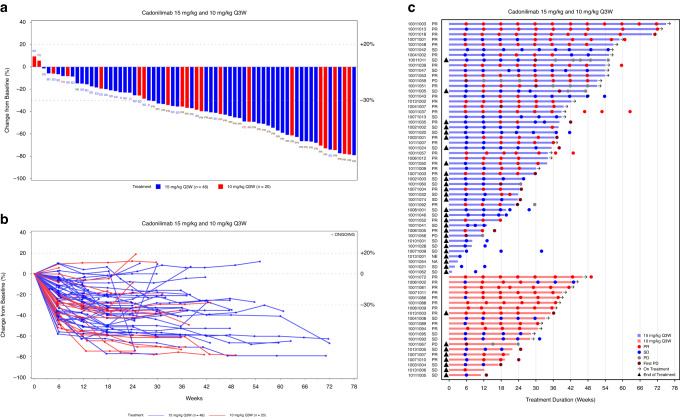
Regarding the cut-off date, among the 69 patients treated with cadonilimab 15 mg/kg or 10 mg/kg Q3W plus anlotinib, 37 achieved confirmed PR, the overall ORR was 53.6% (95% CI, 41.2–65.7) and the DCR was 92.8% (95% CI, 83.9–97.6). The ORR and DCR were 51.0% and 91.8% and 60.0% and 95.0% for patients receiving 15 mg/kg Q3W and 10 mg/kg Q3W, respectively (Table 3). Among the 69 patients analyzed by PD-L1 TPS and histology, the ORRs were 60.5% and 42.3% among PD-L1-positive versus PD-L1-negative patients and 60.0% and 48.7% among squamous cell carcinoma versus non-squamous cell carcinoma patients, respectively (Table S4). Subgroup analysis of ORR among patients treated with 15 mg/kg and 10 mg/kg Q3W is shown in Fig. S1. A decrease in the target lesion size was observed in 66 (97.1%) of the 68 patients (Fig. 2a, b). The responses were durable, with a median DOR that was not reached (NR) (95% CI, 5.8–NR) (Fig. 2c, Table 3).
Table 3.
Tumor response.
| Cadonilimab 15 mg/kg Q3W | Cadonilimab 10 mg/kg Q3W | Overall | |
|---|---|---|---|
| (N = 49) | (N = 20) | (N = 69) | |
| ORR, % (95% CI) | 51.0 (36.3, 65.6) | 60.0 (36.1, 80.9) | 53.6 (41.2, 65.7) |
| DCR, % (95% CI) | 91.8 (80.4, 97.7) | 95.0 (75.1, 99.9) | 92.8 (83.9, 97.6) |
| Best Overall Response, n (%) | |||
| CR | 0 | 0 | 0 |
| PR | 25 (51.0) | 12 (60.0) | 37 (53.6) |
| SD | 20 (40.8) | 7 (35.0) | 27 (39.1) |
| PD | 2 (4.1) | 1 (5.0) | 3 (4.3) |
| NE | 1 (2.0) | 0 | 1 (1.4) |
| NA | 1 (2.0) | 0 | 1 (1.4) |
| DOR, Median (months), (95% CI) | NR (5.2, NR) | NR (7.0, NR) | NR (5.8, NR) |
ORR objective response rate, DCR disease control rate, CI confidence interval, NE not evaluable, NA no post-baseline tumor assessment, DOR duration of response, NR not reached, Q3W every 3 weeks.
Fig. 2. Antitumour activity and treatment duration.
a Waterfall plot of best percentage change from baseline of sum of target lesion diameters. b Spider plot of percentage change from baseline of sum of target lesion diameters. c Swimmer plot of treatment duration and best overall response.
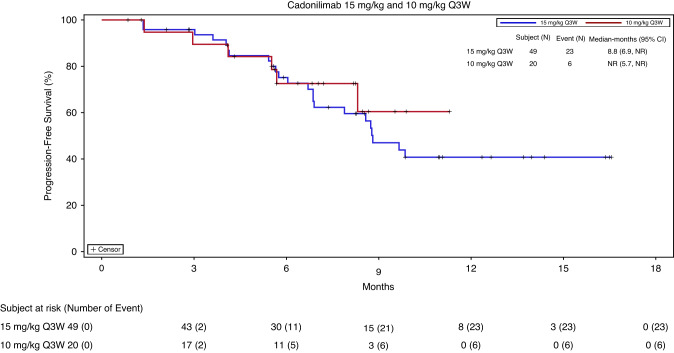
Among the 69 patients, the median PFS was 8.8 months (95% CI, 6.9–NR) in the cadonilimab 15 mg/kg Q3W dosing group and NR (95% CI, 5.7–NR) in the 10 mg/kg Q3W dosing group (Fig. 3). PFS benefits were observed in the subgroups defined according to PD-L1 TPS and histology (Fig. S2). The median PFS was NR (95% CI, 7.9–NR) and 8.8 months (95% CI, 5.7–NR) among PD-L1-positive versus PD-L1-negative patients, and 8.8 months (95% CI, 6.9–NR) and 9.9 months (95% CI, 6.9–NR) among patients with squamous cell versus non-squamous cell carcinoma, respectively. The OS data were immature at the time of the cut-off date.
Fig. 3. Progression-free survival (PFS) in patients treated with cadonilimab 15 mg/kg and 10 mg/kg every 3 weeks (Q3W).
PFS was estimated by Kaplan–Meier product limit method. The two cadonilimab dosing groups were distinguished by curves of different colours.
Discussion
In this phase Ib/II study, the cadonilimab 10 mg/kg Q3W plus anlotinib combination regimen achieved promising and potent efficacy and a manageable safety profile in the first-line treatment setting for NSCLC. With this study, we are the first to report a chemo-free combination strategy involving anti-PD-1 and anti-CTLA4 bispecific antibodies and antiangiogenic agents for the first-line treatment of advanced NSCLC worldwide.
Cadonilimab is a humanized immunoglobulin G1 bispecific antibody with Fc-engineering that is designed based on the Akeso Tetrabody platform; and does not exhibit Fc effector functions including antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity. Cadonilimab targets PD-1 and CTLA-4 simultaneously and blocks PD-1/PD-L1, PD-1/PD-L2, CTLA-4/B7.1, and CTLA-4/B7.2 interactions. Preclinical studies have indicated that tumor tissue preferential retention of cadonilimab over conventional anti-PD-1 and anti-CTLA-4 antibodies may lead to a better safety profile [12, 14]. Early data from a phase 1 study of cadonilimab suggested that cadonilimab may be associated with improved tolerance compared to the combination of a PD-1 inhibitor and a CTLA-4 inhibitor [15, 16]. Furthermore, cadonilimab was approved for marketing in China in June 2022 for treating patients with relapsed/metastatic cervical cancer who progressed on or after platinum-based chemotherapy. Approval was based on positive results across a pivotal phase II clinical study [17].
This trial was designed as a dose-finding study, and the primary objective was to assess the safety and efficacy and to explore the optimum dosing schedule for the combination of cadonilimab and anlotinib. Cadonilimab was administered to 69 patients at doses of 15 mg/kg and 10 mg/kg Q3W, and an overall ORR of 53.6% was observed (ORR of 51.0% vs. 60.0% for 15 mg/kg Q3W vs. 10 mg/kg Q3W, respectively). Regarding the safety profile, grade ≥3 TRAEs occurred in 29 (59.2%) of the 49 patients administered cadonilimab at a dose of 15 mg/kg Q3W and in five (25.0%) of the 20 patients treated with cadonilimab at a dose of 10 mg/kg Q3W. Although this was not a randomized controlled dose-response trial, the results indicated that the dosing schedule of cadonilimab at a dose of 10 mg/kg Q3W plus anlotinib should be recommended as a phase 3 dose. A comparison of the safety profile of cadonilimab plus anlotinib to other similar regimens, such as sintilimab plus anlotinib in a phase 1b trial and pembrolizumab plus lenvatinib in LEAP-007, showed that cadonilimab at a dose of 10 mg/kg Q3W plus anlotinib had a more manageable safety profile [8, 10].
Currently, PD-1/PD-L1 inhibitors combined with chemotherapy are the standard of care for treatment-naïve patients with advanced NSCLC without driver gene mutations. In the KEYNOTE-189 study, an ORR of 47.6% and a median PFS of 8.8 months were reported for the pembrolizumab combination group [18]. In the KEYNOTE-407 study, an ORR of 62.6% and a median PFS of 6.4 months were reported for the pembrolizumab combination group [19]. In this study, the cadonilimab 15 mg/kg Q3W regimen achieved an ORR of 51.0% and median PFS of 8.8 months, and the cadonilimab 10 mg/kg Q3W regimen achieved an ORR of 60.0% and median PFS of NR, which were comparable to those of the pembrolizumab plus chemotherapy combination regimen. Our data showed potent and consistent ORR and PFS benefits in subgroups of patients with PD-L1 positive and negative and squamous and non-squamous cell carcinoma. Moreover, the safety profile of the combination of the cadonilimab 10 mg/kg Q3W dosing schedule was much better than that of the pembrolizumab combination regimen in KEYNOTE-189 and KEYNOTE-407, in which grade ≥3 TRAEs occurred in 67.2% and 69.8% of the patients, respectively.
Previous studies have demonstrated that angiogenesis inhibition normalizes abnormal tumor vessels to regulate the tumor microenvironment, leading to increased infiltration of immune effector cells into tumors and promotion of anti-PD-1/PD-L1 monoclonal antibody efficacy [20–23]. Atezolizumab combined with bevacizumab plus chemotherapy (ABCP) in IMpower150 achieved an ORR of 63.5% and a median PFS of 8.3 months for first-line treatment of metastatic non-squamous NSCLC. A value of 58.5% grade ≥3 TRAEs was consistently reported for the ABCP group, indicating high toxicity [24]. The non-head-to-head comparison between these studies indicated that the cadonilimab-anlotinib combination regimen might achieve efficacy comparable to that of standard first-line PD-1/PD-L1 inhibitors combined with chemotherapy but presents a better and more manageable safety profile.
In recent years, pembrolizumab, atezolizumab, and cemiplimab monotherapies and dual immunotherapy with nivolumab plus ipilimumab have been approved as first-line treatments for advanced NSCLC with PD-L1 TPS ≥ 1% or high PD-L1 expression (PD-L1 TPS ≥ 50% or IC ≥ 10%). However, less than 40% (ORR 27–44.8%) of the patients in the corresponding studies were responsive to these approved chemo-free monotherapies or dual immunotherapy, which was much lower than the response rate achieved in the current study (ORR of 60.5% in PD-L1 positive patients). The much higher response rate achieved by the combination regimen of cadonilimab plus anlotinib suggested that this chemo-free regimen could benefit more patients compared to already approved therapies and is worth validation in the future.
Moreover, PD-L1-negative patients could hardly benefit from the approved pembrolizumab, atezolizumab, and cemiplimab monotherapies. Dual immunotherapy with nivolumab plus ipilimumab achieved an ORR of 27.3% and a median PFS of 5.1 months among PD-L1-negative patients in CheckMate 227. In this study, the combination of cadonilimab and anlotinib showed potent efficacy, with an ORR of 42.3% and a median PFS of 8.8 months among PD-L1 negative patients, suggesting a promising chemo-free regimen in a first-line treatment setting regardless of PD-L1 expression.
Although several chemo-free immunotherapies have been approved, their efficacy has yet to be improved. The KEYNOTE-598 study examined whether adding ipilimumab to pembrolizumab improved its efficacy over pembrolizumab alone in the first-line treatment of NSCLC with high PD-L1 expression. However, the results showed no improvement in efficacy [25]. In LEAP-007, the combination regimen of lenvatinib plus pembrolizumab showed no improvement in OS compared with pembrolizumab monotherapy in the first-line treatment of advanced NSCLC with PD-L1 TPS ≥ 1% [10]. Recently, Roche reported results from a phase III SKYSCRAPER-01 study, which evaluated the anti-TIGIT (T cell immunoglobulin and ITIM domain) immunotherapy tiragolumab plus atezolizumab versus atezolizumab alone as a first-line treatment for NSCLC with high PD-L1 expression. This study did not meet the co-primary endpoint of PFS. In addition, other immune combination therapies such as eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein, plus pembrolizumab in the first-line treatment of NSCLC exhibited promising efficacy in early phase trials but need to be validated in confirmatory trials [26]. There are many obstacles, and careful design and proper combination regimens are vital to further improve the efficacy of approved chemo-free therapies. Our results in the current study have shown promise.
This study had some limitations. This was a non-randomized trial with a relatively small sample size. The results obtained in this study require further validation in well-conducted randomized controlled trials with larger sample sizes. Moreover, OS data were not yet mature at the time of publication.
In conclusion, this phase Ib/II study showed that the chemo-free cadonilimab of 10 mg/kg Q3W plus anlotinib combination regimen had favorable clinical efficacy and a manageable safety profile in the first-line treatment of advanced NSCLC patients and might be a promising chemo-free treatment option for them.
Supplementary information
Acknowledgements
This work was funded by Akeso Biopharma Inc. We thank all patients and their families who participated in this study, along with all investigators and site personnel. We would like to thank Springer Nature (www.springernature.com) for English language editing.
Author contributions
BC, WY, XL, and GL: Formal analysis, Investigation, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing. QC, HL, YD, JL, HD, HW, ZX, HS, LL, LX, YX, FX, YK, XP, KL, QW, and JL: Investigation, Resources, Data curation, Writing - Review & Editing. BL, and YX: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Project administration. LW: Conceptualization, Methodology, Investigation, Resources, Data curation, Writing - Review & Editing, Supervision, Project administration, Funding acquisition.
Funding
This work was supported by Akeso Biopharma, Inc. and The Science and Technology Innovation Program of Hunan Province (2023SK4024).
Data availability
All data related to the study are included in the article or the supplementary materials. Individual participant data will not be available.
Ethics approval and consent to participate
This study was approved by the ethics committee of Hunan Cancer Hospital (approval number: 2018L02552) and the institutional review boards or independent ethics committees of all other participating centers. The trial was conducted in accordance with the International Conference on Good Clinical Practice Standards and the Declaration of Helsinki. All patients provided written informed consent before study participation.
Competing interests
LW reported personal fees from AstraZeneca, Roche, Bristol-Myers Squibb, MSD, Pfizer, Lilly, Johnson and Johnson, GSK, Bayer, Sanofi, Boehringer Ingelheim, Merck, Innovent, and Hengrui outside the submitted work. No disclosures were reported by the other authors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bolin Chen, Wenxiu Yao, Xingya Li, Gen Lin.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02519-0.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17:362–87. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med. 2020;383:1328–39. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 6.Sezer A, Kilickap S, Gumus M, Bondarenko I, Ozguroglu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 7.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–31. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 8.Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16:643–52. doi: 10.1016/j.jtho.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wang Y, Zhao J, Chen G, Liu Z, Gu K, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy. Clin Cancer Res. 2021;27:1296–304. doi: 10.1158/1078-0432.CCR-20-3136. [DOI] [PubMed] [Google Scholar]
- 10.Yang J-H, Luft A, Jiménez EDLM, Lee J, Koralewski P, Karadurmus N, et al. Pembrolizumab (Pembro) with or without lenvatinib (Lenva) in first-line metastatic NSCLC with PD-L1 TPS≥ 1%(LEAP-007): a phase III, randomized, double-blind study. Ann Oncol. 2021;32:S1429–S30. doi: 10.1016/j.annonc.2021.10.139. [DOI] [Google Scholar]
- 11.Brose MS, Vogelzang NJ, DiSimone C, Jain SK, Richards DA, Encarnacion CA, et al. A phase Ib/II trial of lenvatinib plus pembrolizumab in non-small cell lung cancer. J Clin Oncol. 2019;37:16. doi: 10.1200/JCO.2019.37.8_suppl.16. [DOI] [Google Scholar]
- 12.Li B, Huang Z, Pang X, Zhong T, Chen N, Wang M, et al. Bispecific antibodies with an anti-PD-1 backbone for cancer therapy generate enhanced immune activity. Cancer Res. 2018;78:3827. doi: 10.1158/1538-7445.AM2018-3827. [DOI] [Google Scholar]
- 13.Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4:1569–75. doi: 10.1001/jamaoncol.2018.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z, Pang X, Zhong T, Chen N, He X, Xia D, et al. Cadonilimab, an anti-PD1/CTLA4 bi-specific antibody with Fc effector null backbone. J ImmunoTher Cancer. 2021;9:A313–4. doi: 10.1136/jitc-2021-SITC2021.289. [DOI] [Google Scholar]
- 15.Markman B, Tran B, Gan H, Prawira A, Coward J, Jin X, et al. A Phase 1 study of AK104, a tetrameric bispecific antibody that targets PD-1 and CTLA-4 in patients with advanced solid tumors. J Immunother Cancer. 2019;7:283. [Google Scholar]
- 16.Millward M, Frentzas S, Gan H, Prawira A, Tran B, Coward J, et al. Safety and antitumor activity of AK104, a bispecific antibody targeting PD-1 and CTLA-4, in patients with mesothelioma which is relapsed or refractory to standard therapies. Ann Oncol. 2020;31:S705–S06. doi: 10.1016/j.annonc.2020.08.1141. [DOI] [Google Scholar]
- 17.Wu X, Ji J, Lou H, Li Y, Feng M, Xu N, et al. Efficacy and safety of cadonilimab, an anti-PD-1/CTLA4 bi-specific antibody, in previously treated recurrent or metastatic (R/M) cervical cancer: a multicenter, open-label, single-arm, phase II trial. Gynecol Oncol. 2022;166:S47–S48. doi: 10.1016/S0090-8258(22)01293-8. [DOI] [Google Scholar]
- 18.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–51. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 2019;7:630–43. doi: 10.1158/2326-6066.CIR-17-0640. [DOI] [PubMed] [Google Scholar]
- 21.Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877. doi: 10.3389/fimmu.2020.598877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Y, Luo B, Lu Y, Wang D, Yan J, Zheng J, et al. Anlotinib induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint blockade in neuroblastoma. Clin Cancer Res. 2022;28:793–809. doi: 10.1158/1078-0432.CCR-21-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai C, Zhang X, Ren L, You L, Pan Q, Pan H, et al. The efficacy and safety of anlotinib combined with PD-1 antibody for third-line or further-line treatment of patients with advanced non-small-cell lung cancer. Front Oncol. 2020;10:619010. doi: 10.3389/fonc.2020.619010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 25.Boyer M, Şendur MA, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. 2021;39:2327–38. doi: 10.1200/JCO.20.03579. [DOI] [PubMed] [Google Scholar]
- 26.Felip E, Majem M, Doger B, Clay TD, Carcereny E, Bondarenko I, et al. A phase II study (TACTI-002) in first-line metastatic non–small cell lung carcinoma investigating eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab: Updated results from a PD-L1 unselected population. J Clin Oncol. 2022;40:9003. doi: 10.1200/JCO.2022.40.16_suppl.9003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to the study are included in the article or the supplementary materials. Individual participant data will not be available.