Abstract
A weak correlation between diffusing capacity of the lung for carbon monoxide (DLCO) and emphysema has been reported. This study investigated whether impaired DLCO in chronic obstructive pulmonary disease (COPD) is associated with increased risk of acute exacerbation independent of the presence or extent of emphysema. This retrospective cohort study included patients with COPD between January 2004 and December 2019. The participants were divided into four groups based on visually detected emphysema and impaired DLCO. Among 597 patients with COPD, 8.5% had no emphysema and impaired DLCO whereas 36.3% had emphysema without impaired DLCO. Among the four groups, patients with impaired DLCO and emphysema showed a higher risk of moderate-to-severe or severe exacerbation than those with normal DLCO. Impaired DLCO was an independent risk factor for severe exacerbation (hazard ratio, 1.524 [95% confidence interval 1.121–2.072]), whereas the presence of emphysema was not. The risk of moderate-to-severe or severe exacerbation increases with the severity of impaired DLCO. After propensity-score matching for the extent of emphysema, impaired DLCO was significantly associated with a higher risk of moderate-to-severe (p = 0.041) or severe exacerbation (p = 0.020). In patients with COPD and heterogeneous parenchymal abnormalities, DLCO can be considered an independent biomarker of acute exacerbation.
Subject terms: Diseases, Respiratory tract diseases, Chronic obstructive pulmonary disease
Introduction
The diffusion capacity of the lungs for carbon monoxide (DLCO) is a physiological indicator of parenchymal, alveolar, or capillary injury in chronic obstructive pulmonary disease (COPD). Impaired DLCO is considered a poor prognostic factor in patients with COPD. Current smokers with impaired DLCO had a higher risk of progression to COPD1. Impaired DLCO is associated with worse respiratory symptoms, lower quality of life, decreased exercise performance, and a higher risk of severe exacerbation in COPD2. A prospective study reported that DLCO was positively correlated with survival in patients with COPD3. Even in patients with mild COPD, DLCO < 60% was a risk factor for all-cause mortality4. A previous meta-analysis showed that impaired DLCO in COPD was associated with emphysema dominance and adverse clinical outcomes including exacerbation and mortality5.
Impaired DLCO is believed to be primarily caused by emphysema in patients with COPD. The extent of emphysema is associated with the severity of DLCO reduction6–8. Currently, the mechanism of how low diffusing capacity is related to poor prognosis in COPD patients has been explained by parenchymal destruction and loss of the pulmonary capillary bed due to emphysema3,6. As the amount of oxygen in the blood decreases with low DLCO, inflammatory mediators such as hypoxia-inducible factor are more likely to be expressed, which increases the risk of acute exacerbation (AE) of COPD9,10. However, the correlation coefficient between DLCO and extent of emphysema was not sufficient to insist that emphysema is a major contributor to poor prognosis in patients with impaired DLCO7,11. Even in patients without emphysema, DLCO may play an important role as a physiological indicator that reflects parenchymal, alveolar, or capillary injury and as a prognostic factor related to worse clinical outcomes in COPD.
Therefore, our study aimed to investigate whether impaired DLCO in COPD patients is associated with increased risk of AE of COPD independent of emphysema.
Materials and methods
This study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement12.
Study design and participants
We analyzed patients who were diagnosed with COPD and followed up for 5 years in a teaching hospital between January 2004 and December 2019. Eligible patients had (1) post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < 0.7, with potential risk factors for COPD; (2) baseline and follow-up spirometric evaluation, including FEV1 and DLCO; and (3) baseline chest computed tomography (CT). The included patients were classified into four groups: no emphysema without impaired DLCO (Group 1), no emphysema with impaired DLCO (Group 2), emphysema without impaired DLCO (Group 3), and emphysema with impaired DLCO (Group 4). We excluded the patients who had asthma or severe anemia (hemoglobin < 8.0 g/dL).
This study was conducted according to the principles of the Declaration of Helsinki. The Institutional Review Board of Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center waived the requirement for written informed consent and approved this study (20-2022-80). It was confirmed that all procedures adhered to the relevant guidelines and regulations.
Definition
Emphysema was defined as emphysema visually detected by an experienced radiologist (K.N.J.) on baseline chest CT. The extent of emphysema was evaluated using quantitative CT analysis of the percentage of lung voxels with attenuation of < − 950 Hounsfield units (%LAA-950). Impaired DLCO was defined as DLCO < 80%. We defined the severity of DLCO as follows: normal, DLCO ≥ 80%; mild, DLCO ≥ 60% and < 80%; moderate, DLCO ≥ 40% and < 60%; and severe, DLCO < 40%13. Moderate exacerbation is defined as an increase in or new onset of respiratory symptoms requiring treatment with antibiotics and/or systemic steroid. Severe exacerbation is defined as an increase in or new onset of respiratory symptoms requiring hospitalization14.
Variables
Baseline information including age, sex, body mass index (BMI), smoking history, Charlson comorbidity index (CCI), and respiratory morbidities was obtained. Clinical features including symptoms, previous history of exacerbation, Global Initiative for Chronic Obstructive Lung Disease (GOLD) group, blood test results, spirometric test results, radiologic findings, and inhaled treatments were collected.
Outcomes
The study outcomes were moderate-to-severe and severe exacerbations in patients with COPD, classified according to emphysema and DLCO. Subgroup analyses were performed to evaluate the risk of moderate-to-severe or severe exacerbations according to DLCO severity. For sensitivity analysis, a propensity score-matched analysis was performed to evaluate the risk of moderate-to-severe or severe exacerbation according to the severity of impaired DLCO.
Statistical analyses
Analysis of variance or Kruskal–Wallis analysis was conducted to compare continuous variables. The chi-squared test or Fisher’s exact test was used to compare categorical variables. Cox regression analyses with backward elimination based on likelihood ratio tests were performed to identify clinical variables independently related to AE. The Kaplan–Meier (K–M) curve and log-rank test were used to compare the time to the first moderate-to-severe and severe exacerbation according to emphysema and the severity of impaired DLCO. For the sensitivity analysis, we performed 1:1 propensity score matching to evaluate the adjusted effect of DLCO on AE. The propensity score was calculated using age, sex, BMI, smoking status, smoking amount (pack-years), CCI, moderate-to-severe exacerbation history, post-bronchodilator FEV1, and %LAA-950. A variance inflation factor > 4.0 was determined as significant multicollinearity. Statistical significance was set at two-tailed p < 0.05. R statistical software (version 4.1.2; R Foundation, Vienna, Austria) was used for statistical analyses.
Ethics approval and consent to participate
This study was conducted according to the principles of the Declaration of Helsinki. The Institutional Review Board of Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center waived the requirement for written informed consent and approved this study (20-2022-80).
Results
A total of 614 patients with COPD were followed for 5 years. After excluding 17 patients without DLCO results or without baseline chest CT, the remaining 597 patients were divided into four groups based on visually detected emphysema and impaired DLCO (Supplementary Fig. S1 online). In total, 115 (19.3%) patients had normal DLCO without emphysema, 51 (8.5%) had impaired DLCO without emphysema, 217 (36.3%) had normal DLCO with emphysema, and 214 (35.8%) had impaired DLCO with emphysema. Low correlations were found between %LAA-950 and emphysema (R2 = 0.144), %LAA-950 and DLCO (R2 = 0.139), and emphysema and DLCO (R2 = 0.024).
Baseline characteristics and clinical features
Group 3 and 4 showed a higher age, more males, more ever-smokers, a higher pack-year, less history of asthma, less bronchiectasis, and higher %LAA-950 than group 1 and 2 (Table 1). Group 2 were younger, more likely to be female, and had less history of smoking than the other groups. In addition, group 2 stands out for a higher prevalence of tuberculosis and bronchiectasis compared to the other groups. There was significantly more sputum production (48.3% vs. 35.3%; p = 0.001) and dyspnea symptoms (COPD Assessment Test ≥ 10 or modified Medical Research Council score ≥ 2, 77.1% vs. 84.9%; p = 0.018) in group 2 and 4 than in group 1 and 3. Cough did not differ according to DLCO or presence of emphysema (Table 2). Blood eosinophil counts did not differ among the four groups. The post-bronchodilator FEV1 was lower in group 2 and 4 than in group 1 and 3. Group 2 had lower post-bronchodilator FVC and higher post-bronchodilator FEV1/FVC than group 4. Regular inhalation treatment was used more frequently in group 2 and 4 than in group 1 and 3, whereas there was no difference in regular inhalation treatment when comparing group 1 and 2 to group 3 and 4.
Table 1.
Baseline characteristics of COPD patients in unadjusted entire study population.
| Variable | No emphysema without impaired DLCO Group 1 (n = 115) |
No emphysema with impaired DLCO Group 2 (n = 51) |
Emphysema without impaired DLCO Group 3 (n = 217) |
Emphysema with impaired DLCO Group 4 (n = 214) |
p-value |
|---|---|---|---|---|---|
| Age, year, mean (SD) | 62.1 (10.9) | 59.8 (13.3) | 67.7 (10.0) | 66.2 (9.4) | < 0.001 |
| ≥ 65, n (%) | 55 (47.8) | 20 (39.2) | 141 (65.0) | 122 (57.0) | 0.001 |
| ≤ 50, n (%) | 15 (13.0) | 11 (21.6) | 13 (6.0) | 8 (3.7) | < 0.001 |
| Male, n (%) | 87 (79.8) | 29 (61.7) | 209 (97.7) | 201 (95.3) | < 0.001 |
| Body mass index, kg/m2, mean (SD) | 23.1 (3.1) | 22.4 (3.3) | 22.5 (3.3) | 21.6 (3.5) | 0.001 |
| Smoking status, n (%) | |||||
| Never smoker | 37 (32.5) | 21 (41.2) | 12 (5.5) | 12 (5.6) | < 0.001 |
| Ex-smoker | 43 (37.7) | 15 (29.4) | 107 (49.3) | 106 (49.5) | |
| Current smoker | 34 (29.8) | 15 (29.4) | 98 (45.2) | 96 (44.9) | |
| Pack-years in ever smoker, mean (SD) | 23.5 (23.9) | 18.3 (21.3) | 42.1 (26.0) | 41.0 (25.4) | < 0.001 |
| Comorbidities | |||||
| CCI, category, n (%) | |||||
| 0–1 | 80 (69.6) | 38 (74.5) | 154 (71.0) | 136 (63.6) | 0.601 |
| 2–3 | 29 (25.2) | 12 (23.5) | 53 (24.4) | 67 (31.3) | |
| ≥ 4 | 6 (5.2) | 1 (2.0) | 10 (4.6) | 11 (5.1) | |
| History of asthma, n (%) | 38 (33.0) | 17 (33.3) | 63 (29.2) | 44 (20.6) | 0.043 |
| History of tuberculosis, n (%) | 28 (24.3) | 24 (47.1) | 43 (19.9) | 64 (29.9) | 0.001 |
| Radiologic findings | |||||
| Bronchiectasis, n (%) | 42 (36.5) | 21 (41.2) | 45 (20.7) | 43 (20.1) | < 0.001 |
| Interstitial lung disease, n (%) | 3 (2.6) | 0 (0.0) | 2 (0.9) | 4 (1.9) | 0.564 |
| %LAA-950, mean (SD) | 2.6 (4.4) | 4.0 (5.4) | 7.4 (7.7) | 14.0 (10.8) | < 0.001 |
Data are expressed as mean (± standard deviation) or number (percentage).
CCI Charlson comorbidity index, COPD chronic obstructive pulmonary disease, DLco diffusing capacity for carbon monoxide, %LAA-950 percentage of lung voxels with attenuation < − 950 Hounsfield units, SD standard deviation.
Table 2.
Clinical features of COPD patients in unadjusted entire study population.
| Variable | No emphysema without impaired DLCO Group 1 (n = 115) |
No emphysema with impaired DLCO Group 2 (n = 51) |
Emphysema without impaired DLCO Group 3 (n = 217) |
Emphysema with impaired DLCO Group 4 (n = 214) |
p-value |
|---|---|---|---|---|---|
| Symptoms and quality of life, n (%) | |||||
| Cough | 8 (7.0) | 8 (15.7) | 14 (6.5) | 19 (8.9) | 0.172 |
| Sputum | 37 (32.2) | 25 (49.0) | 75 (34.7) | 104 (48.6) | 0.003 |
| CAT ≥ 10 or mMRC ≥ 2 | 85 (78.0) | 42 (89.4) | 164 (76.6) | 177 (83.9) | 0.094 |
| Previous exacerbation history, n (%) | |||||
| Moderate-to-severe | 16 (14.7) | 11 (23.4) | 51 (23.8) | 64 (30.3) | 0.022 |
| GOLD group, n (%) | |||||
| A | 19 (17.4) | 3 (6.4) | 37 (17.3) | 20 (9.5) | 0.028 |
| B | 74 (67.9) | 33 (70.2) | 126 (58.9) | 127 (60.2) | 0.248 |
| C | 5 (4.6) | 2 (4.3) | 13 (6.1) | 14 (6.6) | 0.852 |
| D | 11 (10.1) | 9 (19.1) | 38 (17.8) | 50 (23.7) | 0.030 |
| Blood test, mean (SD) | |||||
| White blood cell, /uL | 7750 (2359) | 8343 (3690) | 7745 (2936) | 8774 (3832) | 0.009 |
| Neutrophil, /uL | 5035 (2208) | 5529 (3568) | 5012 (2839) | 5963 (3794) | 0.018 |
| Lymphocyte, /uL | 1971 (780) | 1971 (920) | 1956 (668) | 2001 (970) | 0.960 |
| Neutrophil–lymphocyte ratio | 3.22 (2.94) | 3.96 (4.17) | 3.06 (2.62) | 4.14 (6.21) | 0.077 |
| Eosinophil, /uL | 239 (240) | 235 (324) | 211 (195) | 233 (286) | 0.720 |
| ≥ 300, n (%) | 29 (25.7) | 12 (23.5) | 45 (21.2) | 55 (25.8) | 0.691 |
| Protein, g/dL | 6.9 (0.6) | 6.9 (067) | 6.9 (0.5) | 6.7 (0.6) | 0.106 |
| Albumin, g/dL | 4.0 (0.4) | 3.9 (0.5) | 4.0 (0.4) | 3.9 (0.4) | 0.005 |
| Spirometric test, mean (SD) | |||||
| Post-bronchodilator FEV1, L | 1.82 (0.49) | 1.31 (0.44) | 1.80 (0.52) | 1.50 (0.54) | < 0.001 |
| Post-bronchodilator FEV1, % | 71.78 (14.67) | 56.44 (16.41) | 72.23 (17.14) | 59.29 (18.75) | < 0.001 |
| Post-bronchodilator FVC, L | 2.98 (0.78) | 2.24 (0.76) | 3.36 (0.70) | 3.16 (0.85) | < 0.001 |
| Post-bronchodilator FVC, % | 83.64 (16.23) | 67.75 (17.28) | 93.51 (15.56) | 86.49 (19.47) | < 0.001 |
| Post-bronchodilator FEV1/FVC, % | 61.03 (8.54) | 60.12 (13.65) | 53.10 (11.57) | 47.79 (13.07) | < 0.001 |
| Regular inhaled treatment, n (%) | 100 (87.0) | 48 (94.1) | 179 (82.5) | 199 (93.0) | 0.004 |
| LABA | 2 (1.7) | 2 (3.9) | 4 (1.9) | 7 (3.3) | 0.596 |
| LAMA | 20 (17.4) | 3 (5.9) | 23 (10.6) | 21 (9.8) | 0.121 |
| ICS/LABA | 25 (21.7) | 10 (19.6) | 27 (12.5) | 21 (9.8) | 0.014 |
| LABA/LAMA | 40 (34.8) | 18 (35.3) | 84 (38.9) | 75 (35.0) | 0.824 |
| ICS/LABA/LAMA | 13 (11.3) | 15 (29.4) | 41 (19.0) | 75 (35.0) | < 0.001 |
| Use of ICS | 38 (33.0) | 25 (49.0) | 68 (31.5) | 96 (44.9) | 0.007 |
Data are expressed as mean (± standard deviation) or number (percentage).
CAT COPD assessment test, COPD chronic obstructive pulmonary disease, DLco diffusing capacity for carbon monoxide, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, GOLD global initiative for chronic obstructive lung disease, ICS inhaled corticosteroid, LABA long-acting beta-agonist, LAMA long-acting muscarinic antagonist, mMRC modified medical research council, SD standard deviation.
Moderate-to-severe exacerbation
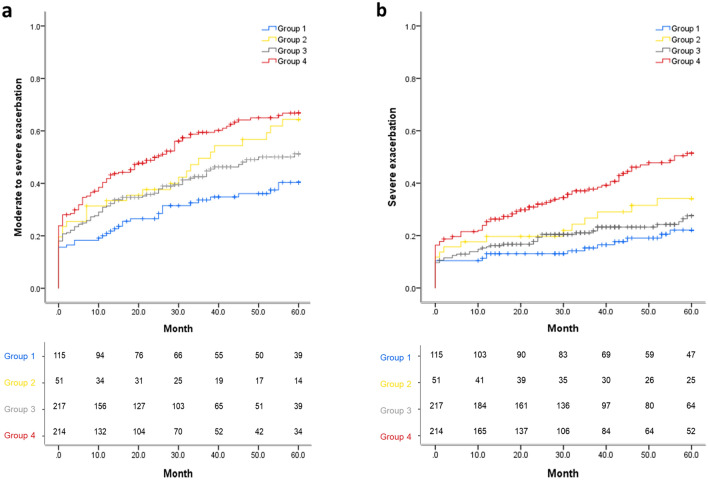
Moderate-to-severe exacerbation events occurred in 36.5% of group 1, 58.8% of group 2, 45.6% of group 3, and 60.3% of group 4. The time to the first moderate-to-severe exacerbation analyzed by K-M curve and log-rank test significantly differed among the four groups according to emphysema and DLCO (log-rank p < 0.001; Fig. 1). Group 4 showed a higher risk of moderate-to-severe exacerbations than group 3 (log-rank p = 0.002). There was a significant difference in moderate-to-severe exacerbation between group 1 and 2 (log-rank p = 0.012). In the univariate Cox regression analysis, impaired DLCO (p < 0.001) or emphysema (p = 0.013) was significantly associated with an increased risk of moderate-to-severe exacerbation (Table 3). However, this relationship disappeared in multivariate Cox regression analyses.
Figure 1.
The time to the first (a) moderate to severe and (b) severe exacerbation analyzed by Kaplan–Meier curve and log-rank test according to emphysema and DLCO in unadjusted entire study population. The included patients were classified into four groups: no emphysema without impaired DLCO (Group 1), no emphysema with impaired DLCO (Group 2), emphysema without impaired DLCO (Group 3), and emphysema with impaired DLCO (Group 4). Group 1 ( ) vs. Group 2 (
) vs. Group 2 ( ), Log-rank p-value = 0.012. Group 1 (
), Log-rank p-value = 0.012. Group 1 ( ) vs. Group 3 (
) vs. Group 3 ( ), Log-rank p-value = 0.058. Group 1 (
), Log-rank p-value = 0.058. Group 1 ( ) vs. Group 4 (
) vs. Group 4 ( ), Log-rank p-value < 0.001. Group 2 (
), Log-rank p-value < 0.001. Group 2 ( ) vs. Group 3 (
) vs. Group 3 ( ), Log-rank p-value = 0.250. Group 2 (
), Log-rank p-value = 0.250. Group 2 ( ) vs. Group 4 (
) vs. Group 4 ( ), Log-rank p-value = 0.375. Group 3 (
), Log-rank p-value = 0.375. Group 3 ( ) vs. Group 4 (
) vs. Group 4 ( ), Log-rank p-value = 0.002. Group 1 (
), Log-rank p-value = 0.002. Group 1 ( ) vs. Group 2 (
) vs. Group 2 ( ), Log-rank p-value = 0.109. Group 1 (
), Log-rank p-value = 0.109. Group 1 ( ) vs. Group 3 (
) vs. Group 3 ( ), Log-rank p-value = 0.270. Group 1 (
), Log-rank p-value = 0.270. Group 1 ( ) vs. Group 4 (
) vs. Group 4 ( ), Log-rank p-value < 0.001. Group 2 (
), Log-rank p-value < 0.001. Group 2 ( ) vs. Group 3 (
) vs. Group 3 ( ), Log-rank p-value = 0.392. Group 2 (
), Log-rank p-value = 0.392. Group 2 ( ) vs. Group 4 (
) vs. Group 4 ( ), Log-rank p-value = 0.055. Group 3 (
), Log-rank p-value = 0.055. Group 3 ( ) vs. Group 4 (
) vs. Group 4 ( ), Log-rank p-value < 0.001.
), Log-rank p-value < 0.001.
Table 3.
Cox regression model for acute exacerbation of COPD patients in unadjusted entire study population.
| Variable | Moderate-to-severe exacerbation | Severe exacerbation | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Age | 1.014 (1.004–1.025) | 0.007 | 1.022 (0.008–1.036) | 0.002 | 1.022 (1.005–1.039) | 0.009 | ||
| Male | 0.902 (0.622–1.309) | 0.588 | 0.923 (0.574–1.486 | 0.742 | ||||
| Body mass index | 0.938 (0.907–0.970) | < 0.001 | 0.905 (0.867–0.945) | < 0.001 | 0.956 (0.911–1.002) | 0.063 | ||
| Smoking status (ref.: never smoker) | ||||||||
| Ex-smoker | 1.201 (0.852–1.691) | 0.296 | 1.283 (0.811, 2.031) | 0.287 | ||||
| Current smoker | 1.008 (0.708–1.434) | 0.966 | 1.185 (0.742, 1.892) | 0.478 | ||||
| Charlson comorbidity index (ref.: 1) | ||||||||
| 2–3 | 0.915 (0.706–1.184) | 0.498 | 1.234 (0.896–1.700) | 0.198 | 1.131 (0.816, 1.569) | 0.459 | ||
| ≥ 4 | 1.270 (0.763–2.112) | 0.357 | 2.048 (1.153–3.637) | 0.014 | 2.120 (1.163–3.865) | 0.014 | ||
| History of asthma | 1.227 (0.964–1.563) | 0.096 | 1.191 (0.874–1.623) | 0.268 | ||||
| History of tuberculosis | 1.183 (0.926–1.512) | 0.178 | 1.142 (0.832–1.568) | 0.412 | ||||
| Previous moderate-to-severe exacerbation history | 8.892 (6.858–11.528) | < 0.001 | 13.893 (7.361–26.221) | < 0.001 | 7.887 (5.814, 10.700) | < 0.001 | 6.577 (4.794–9.022) | < 0.001 |
| GOLD group (Ref.: GOLD A) | ||||||||
| GOLD B | 1.877 (1.119–3.148) | 0.017 | 1.733 (0.955–3.144) | 0.070 | 1.548 (0.743–3.229) | 0.244 | ||
| GOLD C | 11.655 (6.362–21.354) | < 0.001 | 0.722 (0.479–1.089) | 0.120 | 14.117 (6.414–31.075) | < 0.001 | ||
| GOLD D | 17.452 (10.120–30.095) | < 0.001 | 10.823 (5.222–22.432) | < 0.001 | ||||
| Use of ICS | 1.732 (1.384–2.168) | < 0.001 | 1.708 (1.261–2.313) | 0.001 | ||||
| Neutrophil–lymphocyte ratio | 1.037 (1.022–1.053) | < 0.001 | 1.050 (1.035–1.064) | < 0.001 | ||||
| Eosinophil ≥ 300/uL | 1.159 (0.900–1.493) | 0.254 | 0.881 (0.624, 1.242) | 0.469 | ||||
| Albumin, g/dL | 0.495 (0.385–0.637) | < 0.001 | 0.634 (0.483–0.834) | 0.001 | 0.331 (0.248–0.442) | < 0.001 | 0.461 (0.326–0.651) | < 0.001 |
| Post-bronchodilator FEV1/FVC % | 0.969 (0.960–0.979) | < 0.001 | 0.986 (0.976–0.996) | 0.008 | 0.967 (0.955–0.979) | < 0.001 | ||
| Emphysema (ref. No emphysema) | 1.399 (1.074–1.822) | 0.013 | 0.989 (0.704–1.390) | 0.951 | 1.662 (1.162–2.378) | 0.005 | 1.017 (0.647–1.598) | 0.941 |
| With impaired DLCO (ref. without impaired DLco) | 1.585 (1.266–1.985) | < 0.001 | 1.116 (0.861–1.447) | 0.407 | 2.032 (1.514–2.727) | < 0.001 | 1.524 (1.121–2.072) | 0.007 |
CAT COPD assessment test, CI confidence interval, COPD chronic obstructive pulmonary disease, CT computed tomography, DLCO diffusing capacity for carbon monoxide, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity.
Severe exacerbation
The time to first severe exacerbation analyzed by K–M curve and log-rank test was significantly different among the four groups (log-rank p < 0.001; Fig. 1). Group 4 showed a higher risk of severe exacerbation than group 1 and 3 (log-rank p < 0.001). In univariate Cox regression analysis, impaired DLCO or emphysema was associated with a higher risk of severe exacerbation (Table 3). Even in multivariate Cox regression analysis, impaired DLCO was associated with severe exacerbation (hazard ratio, 1.524; 95% confidence interval 1.121–2.072; p = 0.007).
Exacerbation and severity of impaired DLCO
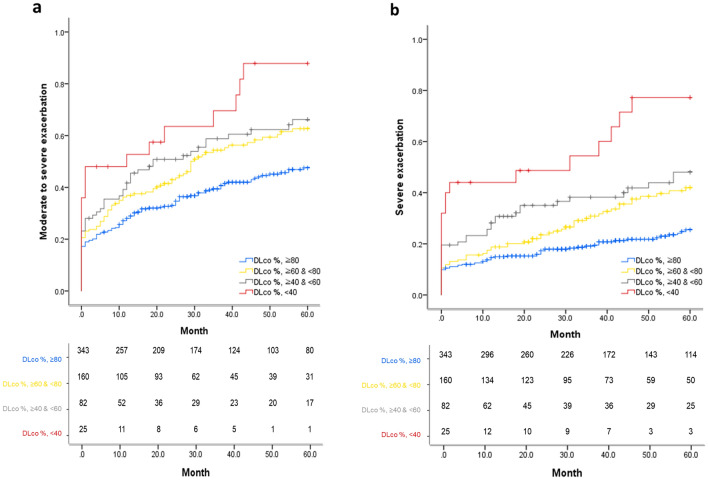
The time to the first moderate-to-severe or severe exacerbation analyzed by K-M curve and log-rank test was significantly different among the four groups according to the severity of impaired DLCO (log-rank p < 0.001; Fig. 2). The risk of moderate-to-severe or severe exacerbation was significantly lower in patients with normal DLCO than in those with any severity of impaired DLCO (log-rank p < 0.005). In addition, the time to the first moderate-to-severe or severe exacerbation was significantly shorter in patients with severe DLCO impairment than in those with mild or moderate impairment of DLCO (log-rank p < 0.05) (Supplementary Fig. S2 online).
Figure 2.
The time to the first (a) moderate-to-severe and (b) severe exacerbation analyzed by Kaplan–Meier curve and log-rank test according to DLCO severity in unadjusted entire study population. DLCO %, ≥ 80 ( ) vs. DLCO %, ≥ 60 & < 80 (
) vs. DLCO %, ≥ 60 & < 80 ( ), Log-rank p-value = 0.004. DLCO %, ≥ 80 (
), Log-rank p-value = 0.004. DLCO %, ≥ 80 ( ) vs. DLCO %, ≥ 40 & < 60 (
) vs. DLCO %, ≥ 40 & < 60 ( ), Log-rank p-value = 0.002. DLCO %, ≥ 80 (
), Log-rank p-value = 0.002. DLCO %, ≥ 80 ( ) vs. DLCO %, < 40 (
) vs. DLCO %, < 40 ( ), Log-rank p-value < 0.001. DLCO %, ≥ 60 & < 80 (
), Log-rank p-value < 0.001. DLCO %, ≥ 60 & < 80 ( ) vs. DLCO %, ≥ 40 & < 60 (
) vs. DLCO %, ≥ 40 & < 60 ( ), Log-rank p-value = 0.430. DLCO %, ≥ 60 & < 80 (
), Log-rank p-value = 0.430. DLCO %, ≥ 60 & < 80 ( ) vs. DLCO %, < 40 (
) vs. DLCO %, < 40 ( ), Log-rank p-value = 0.012. DLCO %, ≥ 40 & < 60 (
), Log-rank p-value = 0.012. DLCO %, ≥ 40 & < 60 ( ) vs. DLCO %, < 40 (
) vs. DLCO %, < 40 ( ), Log-rank p-value = 0.080. DLCO %, ≥ 80 (
), Log-rank p-value = 0.080. DLCO %, ≥ 80 ( ) vs. DLCO %, ≥ 60 & < 80 (
) vs. DLCO %, ≥ 60 & < 80 ( ), Log-rank p-value = 0.002. DLCO %, ≥ 80 (
), Log-rank p-value = 0.002. DLCO %, ≥ 80 ( ) vs. DLCO %, ≥ 40 & < 60 (
) vs. DLCO %, ≥ 40 & < 60 ( ), Log-rank p-value < 0.001. DLCO %, ≥ 80 (
), Log-rank p-value < 0.001. DLCO %, ≥ 80 ( ) vs. DLCO %, < 40 (
) vs. DLCO %, < 40 ( ), Log-rank p-value < 0.001. DLCO %, ≥ 60 & < 80 (
), Log-rank p-value < 0.001. DLCO %, ≥ 60 & < 80 ( ) vs. DLCO %, ≥ 40 & < 60 (
) vs. DLCO %, ≥ 40 & < 60 ( ), Log-rank p-value = 0.207. DLCO %, ≥ 60 & < 80 (
), Log-rank p-value = 0.207. DLCO %, ≥ 60 & < 80 ( ) vs. DLCO %, < 40 (
) vs. DLCO %, < 40 ( ), Log-rank p-value < 0.001. DLCO %, ≥ 40 & < 60 (
), Log-rank p-value < 0.001. DLCO %, ≥ 40 & < 60 ( ) vs. DLCO %, < 40 (
) vs. DLCO %, < 40 ( ), Log-rank p-value = 0.011.
), Log-rank p-value = 0.011.
Propensity score-matched analysis
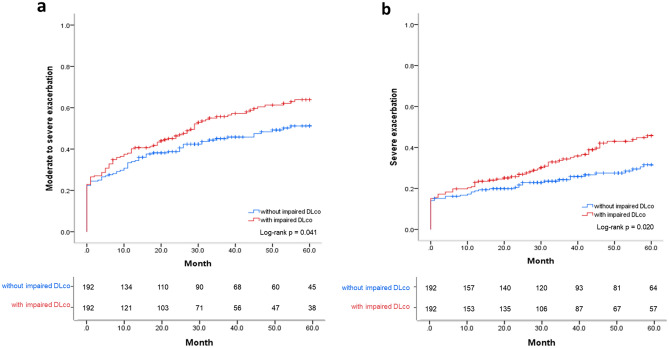
After 1:1 propensity-score matching, the baseline severity of dyspnea, post-bronchodilator FEV1, previous moderate-to-severe exacerbation, and %LAA-950 were balanced between the groups with normal and impaired DLCO (n = 192; Supplementary Tables S1 and S2 online). By using K-M curve and log-rank test, we found a significant difference in the time to the first moderate-to-severe (log-rank p = 0.041) and severe exacerbations (log-rank p = 0.020) between patients with normal and those with impaired DLCO in the propensity score-matched population (Fig. 3).
Figure 3.
The time to the first (a) moderate to severe exacerbation or (b) severe exacerbation analyzed by Kaplan–Meier curve and log-rank test according to the group without impaired DLCO and the group with impaired DLCO in propensity score matched adjusted population. The propensity score was calculated using age, sex, BMI, smoking status, smoking amount (pack-years), CCI, moderate-to-severe exacerbation history, post-bronchodilator FEV1, and %LAA-950. DLCO diffusing capacity for carbon monoxide, FEV1 forced expiratory volume in 1 s, %LAA-950 percentage of lung voxels with attenuation < − 950 Hounsfield units.
Discussion
Patients with COPD were classified into four groups based on visually detected emphysema and impairment of DLCO. Approximately half of patients with emphysema had normal DLCO, whereas 8.5% had impaired DLCO without emphysema. Patients who had impaired DLCO with emphysema showed a higher risk of moderate-to-severe or severe exacerbations than those with normal DLCO. In the multivariate analyses, impaired DLCO was significantly associated with a higher risk of severe exacerbation, whereas the presence of emphysema was not. The risk of moderate-to-severe or severe exacerbation increases with the severity of impaired DLCO. In the propensity score-matched population, impaired DLCO was significantly associated with a higher risk of moderate-to-severe or severe exacerbation. Therefore, DLCO needs to be considered as a promising biomarker for the risk of future exacerbation in COPD patients with heterogeneous etiotypes.
Our study showed that impaired DLCO was independently associated with moderate-to-severe or severe exacerbations, regardless of the presence of visually detected emphysema on chest CT. Impaired DLCO is reportedly associated with an increased risk of AE in COPD. A previous study showed a significant association between impaired DLCO (%) and severe exacerbation in multivariable analysis, which is consistent with our results2. Our study augmented existing knowledge by categorizing patients into four groups based on the presence of DLCO impairment and emphysema. This categorization allowed us to contribute additional insights to the current understanding. By presenting the differences in baseline characteristics and clinical features among these groups, our study facilitated the development of plausible explanations for observed group differences. Through this stratification, we effectively excluded the potential correlation between DLCO and emphysema. Subsequently, we presented HRs for AE in COPD using a Cox regression analysis. This approach is considered more robust for handling censoring data and provides an intuitive interpretation of the relationship between observed time and the occurrence of events. Furthermore, employing propensity score matching with clinical variables including the extent of emphysema (%LAA-950), our study revealed a significant difference in the time to AE between the normal and impaired DLCO groups. These findings strongly suggest that impaired DLCO may serve as a critical and independent risk factor for AE of COPD, regardless of the presence of emphysema.
The risk of AE was further increased when impaired DLCO and chronic bronchitis were combined15. In a meta-analysis, a lower DLCO was associated with a higher risk of exacerbation and mortality5. However, the mechanism by which impaired DLCO is related to AE is not well identified. One plausible hypothesis is that DLCO can accurately reflect the actual severity of emphysema and exercise tolerance16,17. Considering that low DLCO is related with progression of airflow limitation even in healthy smokers with normal spirometric profiles, it is speculated that DLCO can more sensitively detect the progression of small airway disease compared to other conventional spirometric parameters1. In addition, inflammatory mediators such as hypoxia-inducible factor are more likely to be expressed in hypoxemic conditions with impaired DLCO, which increases the risk of AE of COPD9,10. Based on our results, it could be assumed that impaired DLCO and emphysema have different mechanisms on AE of COPD.
Although emphysema is believed to be the main contributor to impaired DLCO in patients with COPD, we found a discrepancy between visually detected emphysema and impaired DLCO in 45% of patients. In addition, the correlation between emphysema and DLCO is weak. Several studies have also reported a weak correlation between DLCO and extent of emphysema. Among spirometric parameters, DLCO had the highest correlation with the Visual Emphysema Score, but it was still a weak correlation (R2 = 0.438)18. The DLCO corrected for alveolar volume (DLCO/VA) had a weak correlation with %LAA-950 (R2 = 0.417) and visual extent of emphysema (R2 = 0.411)7. DLCO/VA was better correlated with emphysema in COPD patients compared to healthy smokers, but the correlation between DLCO/VA and %LAA-950 is still suboptimal (R2 = 0.48)19. In fact, DLCO can be impaired by bronchiectasis or tuberculosis-destroyed lung as well as emphysema. Impaired DLCO was associated with an increasing number of bronchiectatic lobes20. The mean value of DLCO in patients with pulmonary sequelae of tuberculosis was 74.1–78.8%21,22. Therefore, it would be better to understand the natural course of COPD with heterogeneous features by using DLCO as a holistic index of parenchymal destruction rather than the extent of emphysema.
Patients with impaired DLCO without emphysema tended to be younger, female, and had less of a smoking history compared to those with emphysema. Considering that they had a history of tuberculosis or bronchiectasis and a lower FVC, early life events of pneumonia or tuberculosis could be major contributing factors for the development of COPD. Therefore, impaired DLCO without emphysema would be more likely found in young patients with COPD or COPD due to infections (COPD-I). The term “young COPD” has been suggested for those under 50 years of age with risk factors of COPD23. Young COPD is related with an increased risk of clinical COPD, hospitalization due to respiratory disease24, and mortality25. COPD-I is a currently proposed taxonomy for those with a history of early-life respiratory infection or tuberculosis. Especially in never-smokers, COPD-I is one of the major etiotypes of COPD26,27. Our study showed a significant difference in moderate-to-severe exacerbation between impaired DLCO and normal DLCO in patients without emphysema. Therefore, DLCO may be a useful biomarker of AE in young patients with COPD or COPD-I.
This study has several limitations. First, our results cannot be generalized to a wider COPD population owing to its retrospective design. As we included patients from a single teaching hospital, there is likely selection bias, such as COPD patients with a higher symptom burden or more severe lung parenchymal destruction. Although we conducted a multivariate analysis and propensity-score matching, unmeasurable confounding variables may not have been sufficiently controlled. Second, emphysema was defined as emphysema visually detected by an experienced radiologist, which may have caused inter-observer variability. Although several studies have suggested the optimal cut-off of %LAA-950 to determine clinically relevant emphysema, we could not use it because various optimal cut-off values of %LAA-950 have been reported and other parenchymal abnormalities such as bronchiectasis or bulla can contribute to a larger %LAA-950. Third, DLCO is affected by clinical factors beyond alveolar destruction, such as pulmonary vascular disease or obesity28. Indeed, DLCO has been reportedly associated with pulmonary hypertension29. Considering the known association between pulmonary hypertension and an increased risk of severe exacerbations in patients with COPD, it is plausible that pulmonary hypertension may act as a mediating factor in the relationship between impaired DLCO and an increased risk of exacerbation30. One of the limitations of the present study is the absence of an analysis on pulmonary hypertension.
Conclusion
DLCO may be an independent biomarker of AE in COPD patients with heterogeneous parenchymal abnormalities, regardless of the presence of emphysema.
Supplementary Information
Author contributions
Conceptualization: H.P., H.W.L. Data curation: H.P., H.W.L. Formal analysis: H.P., H.W.L., H.J.L., J.L., T.Y.P., K.N.J., E.Y.H., D.K.K. Investigation: H.P., H.W.L. Methodology: H.W.L., H.J.L., J.L., T.Y.P., K.N.J., E.Y.H., D.K.K. Project administration: H.W.L. writing—original draft: H.P., H.W.L. writing—review and editing: H.W.L., H.J.L., J.L., T.Y.P., K.N.J., E.Y.H., D.K.K.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-51593-8.
References
- 1.Harvey B-G, et al. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur. Respir. J. 2015;46:1589–1597. doi: 10.1183/13993003.02377-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramanian A, et al. Diffusing capacity of carbon monoxide in assessment of COPD. Chest. 2019;156:1111–1119. doi: 10.1016/j.chest.2019.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutou AK, et al. Lung function indices for predicting mortality in COPD. Eur. Respir. J. 2013;42:616–625. doi: 10.1183/09031936.00146012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de-Torres, J. P. et al. Clinical and prognostic impact of low diffusing capacity for carbon monoxide values in patients with global initiative for obstructive lung disease I COPD. Chest160, 872–878 (2021) [DOI] [PMC free article] [PubMed]
- 5.Ni Y, Yu Y, Dai R, Shi G. Diffusing capacity in chronic obstructive pulmonary disease assessment: A meta-analysis. Chron. Respir. Dis. 2021;18:14799731211056340. doi: 10.1177/14799731211056340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Anna SE, et al. High-resolution computed tomography quantitation of emphysema is correlated with selected lung function values in stable COPD. Respiration. 2012;83:383–390. doi: 10.1159/000329871. [DOI] [PubMed] [Google Scholar]
- 7.Nambu A, et al. Relationships between diffusing capacity for carbon monoxide (DLCO), and quantitative computed tomography measurements and visual assessment for chronic obstructive pulmonary disease. Eur. J. Radiol. 2015;84:980–985. doi: 10.1016/j.ejrad.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Criner, R. et al. Relationship between diffusion capacity and small airway abnormality in COPDGene. in A18. COPD: EARLY DISEASE AND HOW TO FIND IT A1032–A1032 (American Thoracic Society, 2018). [DOI] [PMC free article] [PubMed]
- 9.Nakazawa S, Shimizu K, Mogi A, Kuwano H. Low diffusing capacity, emphysema, or pulmonary fibrosis: Who is truly pulling the lung cancer strings? J. Thorac. Dis. 2018;10:600–602. doi: 10.21037/jtd.2017.12.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trudzinski FC, et al. Combined effects of lung function, blood gases and kidney function on the exacerbation risk in stable COPD: Results from the COSYCONET cohort. Respir. Med. 2019;154:18–26. doi: 10.1016/j.rmed.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu K, et al. Transfer coefficients better reflect emphysematous changes than carbon monoxide diffusing capacity in obstructive lung diseases. J. Appl. Physiol. 2018;125:183–189. doi: 10.1152/japplphysiol.01062.2017. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham, B. L. et al. DLCO: adjust for lung volume, standardised reporting and interpretation. Eur. Respir. J. 50 (2017). [DOI] [PubMed]
- 14.Jones P, Higenbottam T. Quantifying of severity of exacerbations in chronic obstructive pulmonary disease: Adaptations to the definition to allow quantification. Proc. Am. Thorac. Soc. 2007;4:597–601. doi: 10.1513/pats.200707-115TH. [DOI] [PubMed] [Google Scholar]
- 15.Lee HY, et al. Lower diffusing capacity with chronic bronchitis predicts higher risk of acute exacerbation in chronic obstructive lung disease. J. Thorac. Dis. 2016;8:1274–1282. doi: 10.21037/jtd.2016.04.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grydeland TB, et al. Quantitative CT measures of emphysema and airway wall thickness are related to D(L)CO. Respir. Med. 2011;105:343–351. doi: 10.1016/j.rmed.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Díaz AA, et al. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir. Med. 2015;109:882–889. doi: 10.1016/j.rmed.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Park HJ, Hwang JH. Quantification of emphysema with a three-dimensional chest CT scan: Correlation with the visual emphysema scoring on chest CT, pulmonary function tests and dyspnea severity. Korean J. Radiol. 2011;65:247–256. doi: 10.3348/jksr.2011.65.3.247. [DOI] [Google Scholar]
- 19.Zhang D, Guan Y, Fan L, Xia Y, Liu SY. Quantitative analysis of emphysema and air trapping at inspiratory and expiratory phase multi-slice spiral CT scan in smokers: correlation with pulmonary function test. Zhonghua Yi Xue Za Zhi. 2018;98:1467–1473. doi: 10.3760/cma.j.issn.0376-2491.2018.19.003. [DOI] [PubMed] [Google Scholar]
- 20.Guan W-J, et al. Characterization of lung function impairment in adults with bronchiectasis. PLoS One. 2014;9:e113373. doi: 10.1371/journal.pone.0113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes AJ, Camilo GB, de Menezes SLS, Guimarães FS. Impact of different etiologies of bronchiectasis on the pulmonary function tests. Clin. Med. Res. 2015;13:12–19. doi: 10.3121/cmr.2014.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishi MP, et al. Pulmonary functional assessment: Longitudinal study after treatment of pulmonary tuberculosis. Rev. Inst. Med. Trop. Sao Paulo. 2021;63:e65. doi: 10.1590/s1678-9946202163065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir. Med. 2023;11:18. doi: 10.1016/S2213-2600(22)00494-5. [DOI] [PubMed] [Google Scholar]
- 24.Çolak, Y., Afzal, S., Nordestgaard, B. G., Vestbo, J. & Lange, P. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease. The Copenhagen general population study. Am. J. Respir. Crit. Care Med.201, 671–680 (2020). [DOI] [PMC free article] [PubMed]
- 25.Divo MJ, et al. Comorbidities and mortality risk in adults younger than 50 years of age with chronic obstructive pulmonary disease. Respir. Res. 2022;23:267. doi: 10.1186/s12931-022-02191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomsen M, Nordestgaard BG, Vestbo J, Lange P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: A prospective population study. Lancet Respir Med. 2013;1:543–550. doi: 10.1016/S2213-2600(13)70137-1. [DOI] [PubMed] [Google Scholar]
- 27.Yakar HI, Gunen H, Pehlivan E, Aydogan S. The role of tuberculosis in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:323–329. doi: 10.2147/COPD.S116086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanojevic, S. et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J.60, (2022) [DOI] [PubMed]
- 29.Frost, A. et al. Diagnosis of pulmonary hypertension. Eur. Respir. J.53, (2019) [DOI] [PMC free article] [PubMed]
- 30.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur. Respir. J. 2008;32:1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.