Abstract
Objective
The clinical impact of baseline mitral regurgitation (MR) on the outcomes after transcatheter aortic valve replacement (TAVR) is not clear. This study sought to assess the clinical impact of baseline MR on outcomes after TAVR.
Methods
The study was a retrospective analysis. Data was from 120 consecutive patients with severe aortic stenosis (AS) undergoing TAVR at our center from June 2018 and July 2020. Clinical outcomes were assessed at 30-day, 1- and 2-year follow-up.
Results
The median follow-up was 736.0 (interquartile range, 666.0–965.0) days. Overall survival in patients with nonsignificant and significant baseline MR was not significantly different, while patients from the improved MR group after TAVR demonstrated a significantly higher survival than unchanged or worsened MR group during 2-year follow-up. NYHA functional class had generally improved at 1 year, with only 8.3 % of patients with nonsignificant MR and 17.5 % of patients with significant MR in class III or IV. Patients with improved MR at 1 year after TAVR had a significantly higher LVEF, smaller LVEDD and LVESD than those with unchanged or worsened MR. Among the significant baseline MR group, 70.4 % and 80.0 % of patients had improved to nonsignificant MR at 30-day and 1-year follow-up after TAVR, respectively.
Conclusions
Significant baseline MR was not associated with the increased risk of all-cause mortality 2 years after TAVR. Significant baseline MR was improved in most patients at 1 year after TAVR. Patients with unchanged or worsened MR had an increased all-cause mortality.
Keywords: Aortic stenosis, Transcatheter aortic valve replacement, Mitral regurgitation, Clinical outcome, Follow-up
1. Introduction
Among general patients suffering from heart valve diseases, 25 % show involvement of both the aortic and mitral valves [1]. Aortic stenosis (AS) and mitral regurgitation (MR) accounts for roughly 75 % of all valve diseases in patients over the age of 65 years [2]. And they are often found concomitantly: up to one-third of patients with severe AS also suffer from a certain degree of MR [3]. Over the last decade, transcatheter aortic valve replacement (TAVR) has evolved to a clinical standard for the treatment of severe AS in patients with high or prohibitive surgical risk [4]. But in this setting, relevant concomitant MR is typically left untreated [5]. So far, however, the clinical impact of significant baseline MR in patients undergoing TAVR has not been fully evaluated. Several studies have showed an association between moderate or greater MR and mortality within 30-day and 1-year follow-up [6], [7], [8], [9]. Conversely, other studies have failed to demonstrate this difference in mortality outcomes after adjustment between moderate or greater MR and less-than moderate MR at long-term follow-up [10], [11], [12].
Therefore, the objectives of our study were to assess the clinical impact of significant baseline MR on outcomes after TAVR, and the changes in MR severity over time and its impact on mortality.
2. Methods
2.1. Study design
The study was a retrospective analysis of prospectively collected data. This study was approved by the Institutional Review Board of Southwest Hospital of Third Military Medical University (Army Medical University) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
A total of 126 consecutive patients who underwent TAVR at our center between June 2018 and July 2020 due to severe AS. All patients were at intermediate or high surgical risk defined as a Society of Thoracic Surgeons score of 4 %. Patients with none or trace MR, severe aortic regurgitation (AR) and mitral valves surgery before or after TAVR were excluded. Of these, 6 patients were excluded because of severe AR (n = 5), or a history of surgical mitral valve replacement (n = 1). The final study population consisted of 120 patients. Patients were classified into 2 groups according to baseline MR grade: I) baseline MR < moderate (nonsignificant MR); and II) baseline MR ≥ moderate (significant MR). For statistical purpose, any decrease of 1 or more grades was considered an improvement of MR severity.
Every patient experienced echocardiography and contrast-enhanced multidetector computed tomography (MDCT) at admission. AS and MR was graded by either transthoracic or transoesophageal echocardiography following the European Guidelines of Echocardiography [13]. Severe AS was defined as the aortic valve area ≤ 1.0 cm2, peak aortic velocity > 4 m/s or mean pressure gradient > 40 mmHg. MR was graded as none, trace, mild, moderate, and severe using a multiparametric integrative approach. MDCT was performed to assess aortic annulus dimensions, coronary ostia height, mitral annular diameters, and degree of mitral apparatus calcification. The severity of mitral apparatus calcification was graded according to a semiquantitative score previously described [14].
Comprehensive clinical and echocardiographic assessments were scheduled at hospital discharge, 30 days, 6 and 12 months, and yearly thereafter. All patients were followed up until July 2022 by direct interview during regular outpatient clinic visits or by telephone inquiry.
2.2. TAVR procedures
The TAVR procedures were performed using the Venus A-Valve system (Venus Medtech, Hangzhou, China). The diameter of an aortic annulus for sizing the prosthesis was calculated on the perimeter and area of the native aortic annulus. Pre-procedure aorta-iliac-femoral computed tomography was performed to evaluate the size of vessel caliber and feasibility of transfemoral approach. If it was not viable, transiliac, transapical, or transaortic approaches were considered.
2.3. Study endpoints
The primary endpoint was all-cause mortality. Secondary endpoints included procedure- and valve-related complications, and echocardiographic assessment of the valve and cardiac function. Clinical events were recorded and defined according to the VARC-2 (Valve Academic Consortium) criteria [15].
2.4. Statistical analysis
Continuous variables are shown as mean ± standard deviation or as median (interquartile range: 25th to 75th percentile) in cases of skewed distribution. Categorical variables are presented as raw counts and percentages. Assessment of normality was performed using the Shapiro-Wilk test. For normally distributed continuous data, two-tailed unpaired Student’s t tests were used for comparisons between groups. For non-normally distributed data, Mann-Whitney U test was used. Categorical variables were compared using the chi-square test. Survival was estimated with the Kaplan-Meier method, and differences were compared with a log-rank test. A P value < 0.05 was considered significant. Analyses were performed using the statistical packages SAS version 9.4 (SAS Institute, Cary, North Carolina).
3. Results
3.1. Patient baseline characteristics
In total, 65/120 (54.2 %) patients were classified as having nonsignificant baseline MR, and 55/120 (45.8 %) as significant baseline MR (Table 1). Those with significant baseline MR exhibited a higher New York Heart Association (NYHA) functional class, a higher Society of Thoracic Surgeons (STS) risk score, a lower left ventricle ejection fraction (LVEF), smaller aortic valve area, and more frequent mitral annular calcification than those with nonsignificant baseline MR.
Table 1.
Baseline Characteristics.
| Nonsignificant MR (n = 65) |
Significant MR (n = 55) |
P value | |
|---|---|---|---|
| Clinical variables | |||
| Age (years) | 75.5 ± 8.1 | 76.2 ± 8.3 | 0.966 |
| Male | 47 (72.3) | 40 (72.7) | 0.692 |
| Body mass index (kg/m2) | 26.0 ± 5.5 | 26.5 ± 5.0 | 0.573 |
| NYHA functional class III or IV | 18 (27.7) | 26 (47.3) | < 0.001 |
| STS risk score (%) | 5.6 ± 3.8 | 7.7 ± 3.9 | < 0.001 |
| Diabetes mellitus | 19 (29.2) | 17 (30.9) | 0.558 |
| Hypertension | 33 (50.8) | 29 (52.7) | 0.723 |
| COPD | 36 (55.4) | 33 (60.0) | 0.129 |
| Pulmonary hypertension | 12 (18.5) | 12 (21.8) | 0.225 |
| CKD (eGFR > 60 ml/min/m2) | 24 (36.9) | 22 (40.0) | 0.184 |
| Peripheral artery disease | 15 (23.1) | 13 (23.6) | 0.883 |
| Coronary artery disease | 21 (32.3) | 18 (32.7) | 0.936 |
| Previous myocardial infarction | 14 (21.5) | 13 (23.6) | 0.784 |
| Previous atrial fibrillation | 27 (41.5) | 26 (47.3) | 0.064 |
| Previous PCI | 8 (12.3) | 8 (14.5) | 0.387 |
| Previous CABG | 6 (9.2) | 5 (9.1) | 0.905 |
| Previous cerebrovascular accident | 9 (13.8) | 9 (16.4) | 0.491 |
| Echocardiographic variables | |||
| Mean aortic gradient (mmHg) | 46.9 ± 6.6 | 47.6 ± 5.6 | 0.501 |
| Aortic valve area (cm2) | 0.80 ± 0.14 | 0.55 ± 0.12 | < 0.001 |
| LVEF (%) | 52.4 ± 5.9 | 43.6 ± 4.6 | < 0.001 |
| MDCT variables | |||
| Perimeter-derived aortic annulus (mm) | 22.5 ± 2.2 | 22.7 ± 2.2 | 0.988 |
| Mitral annulus calcification (2–3) | 10 (15.4) | 17 (30.9) | < 0.001 |
| Mitral leaflets calcification (2–3) | 20 (30.8) | 19 (34.5) | 0.381 |
| Mitral annulus diameter (4c) | 33.5 ± 2.7 | 33.6 ± 2.9 | 0.617 |
Values are expressed as mean ± standard deviation or number (%). MR, mitral regurgitation; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; MDCT, multidetector computed tomography; 4c, 4-chamber view.
3.2. Procedural and 30-day outcomes
Transfemoral and nontransfemoral approaches of TAVR were used in 88 patients (73.3 %) and 32 patients (26.7 %), respectively (Table 2). The procedural outcomes in the 2 groups were similar except for acute kidney injury requiring dialysis, which occurred at a higher rate, length of hospital stay, which was longer, and post-procedural effective orifice area, which was smaller, in those with significant baseline MR. Also, patients with significant baseline MR displayed a higher percentage of residual moderate-to-severe aortic regurgitation (AR) at hospital discharge. However, the 30-day outcomes did not differ between the 2 groups.
Table 2.
Procedural and 30-Day Outcomes.
| Nonsignificant MR (n = 65) |
Significant MR (n = 55) |
P value | |
|---|---|---|---|
| Procedural variables | |||
| Prosthesis size ≤ 24 mm | 33 (50.8) | 29 (52.7) | 0.606 |
| Access routes | |||
| Transfemoral | 47 (72.3) | 41(74.5) | 0.612 |
| Nontransfemoral | 18 (27.7) | 14 (25.5) | 0.784 |
| Post-procedural variables | |||
| Acute kidney injury requiring dialysis | 5 (7.7) | 10 (18.2) | 0.022 |
| Disabling stroke | 2 (3.1) | 2 (3.6) | 0.191 |
| New permanent pacemaker | 3 (4.6) | 3 (5.5) | 0.321 |
| Length of hospital stay (days) | 5.8 ± 2.2 | 7.3 ± 2.5 | 0.023 |
| 2-valve implantation | 2 (3.1) | 3 (5.5) | 0.663 |
| Major vascular complication | 1 (1.5) | 1 (1.8) | 0.534 |
| Coronary occlusion | 0 | 0 | – |
| Myocardial infarction | 0 | 0 | – |
| Major bleeding | 0 | 0 | – |
| Post-procedural echocardiography | |||
| Mean aortic gradient (mmHg) | 8.9 ± 4.2 | 8.5 ± 4.7 | 0.899 |
| Aortic valve area (cm2) | 1.65 ± 0.58 | 1.62 ± 0.59 | 0.827 |
| Residual moderate to severe AR | 3 (4.6) | 6 (11.0) | 0.006 |
| 30-day outcomes | |||
| All-cause mortality | 1 (1.5) | 1 (1.8) | 0.542 |
| Cardiovascular mortality | 0 | 0 | – |
Values are expressed as mean ± standard deviation or number (%). MR, mitral regurgitation; AR, aortic regurgitation.
3.3. Follow-up outcomes
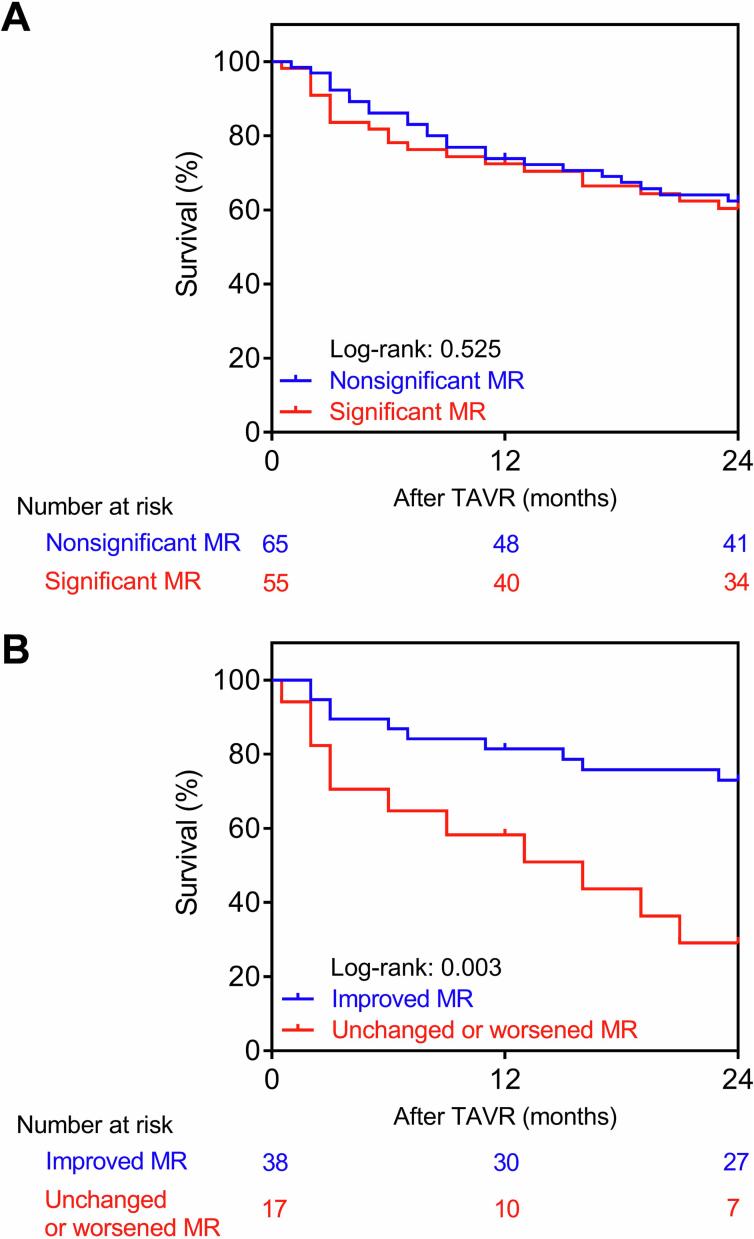
The median follow-up was 736.0 (interquartile range, 666.0–965.0) days. Overall survival in patients with nonsignificant and significant baseline MR was not significantly different (log-rank p = 0.525, Fig. 1A). Next, we compared survival between the improved MR and unchanged or worsened MR subgroups after TAVR within the significant baseline MR group of patients. Patients from the improved MR group demonstrated a significantly higher survival (log-rank p = 0.003, Fig. 1B).
Fig. 1.
(A) Kaplan-Meier estimates of overall survival after TAVR in patients with nonsignificant versus significant baseline MR. (B) Kaplan-Meier estimates of overall survival after TAVR in patients with improved versus unchanged or worsened MR. MR, mitral regurgitation; TAVR, transcatheter aortic valve replacement.
3.4. Changes in NYHA functional class and MR grade over time
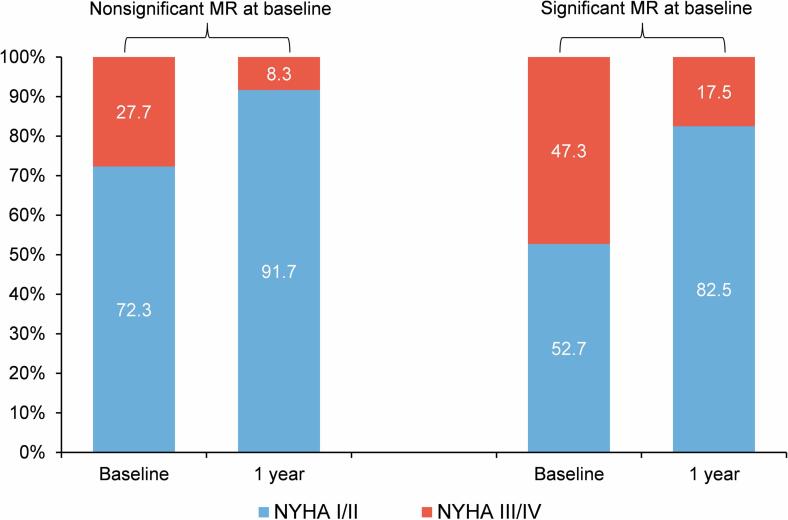
At baseline, NYHA functional class III/IV were present in 27.7 % and 47.3 % of the nonsignificant and significant baseline MR groups, respectively. At 1 year, NYHA functional class had generally improved, with only 8.3 % of patients with nonsignificant MR and 17.5 % of patients with significant MR in class III or IV (Fig. 2).
Fig. 2.
NYHA functional class at baseline and 1-Year follow-up after TAVR in patients with nonsignificant versus significant baseline MR. MR, mitral regurgitation; TAVR, transcatheter aortic valve replacement.
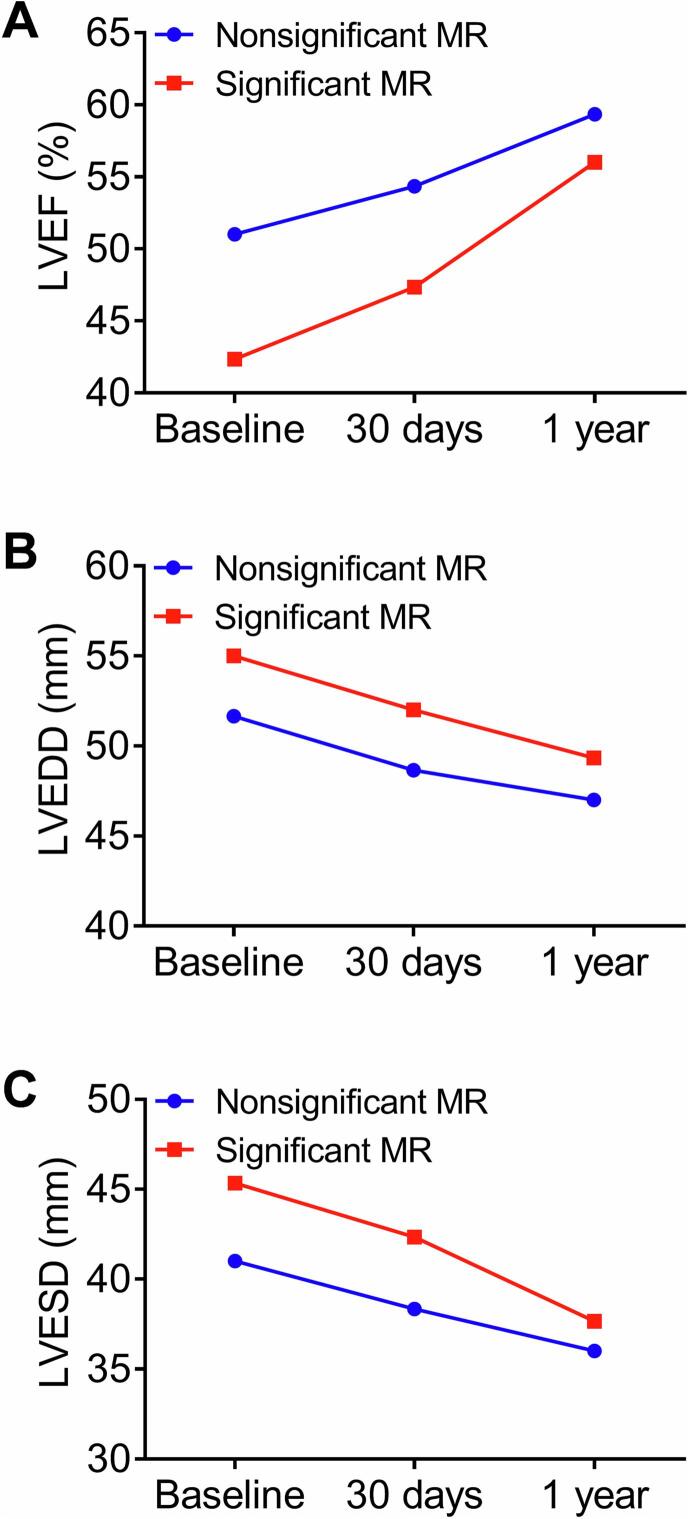
Changes in LVEF, left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD) over time among 1-year survivors are shown in Fig. 3. Patients with improved MR at 1 year after TAVR had a significantly higher LVEF, smaller LVEDD and LVESD than those with unchanged or worsened MR.
Fig. 3.
Changes in left ventricular ejection fraction (LVEF; A), left ventricular end-diastolic diameter (LVEDD; B) and left ventricular end-systolic diameter (LVESD; C) over time (baseline, 30 days, and 1 year after TAVR) in patients with nonsignificant versus significant baseline MR. MR, mitral regurgitation; TAVR, transcatheter aortic valve replacement.
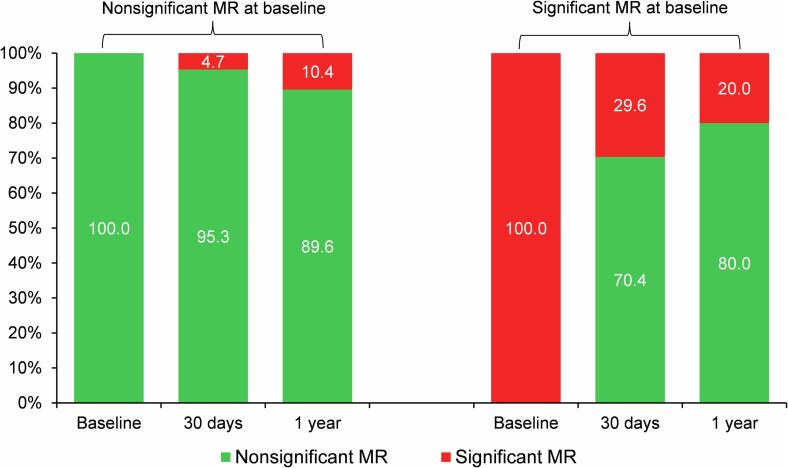
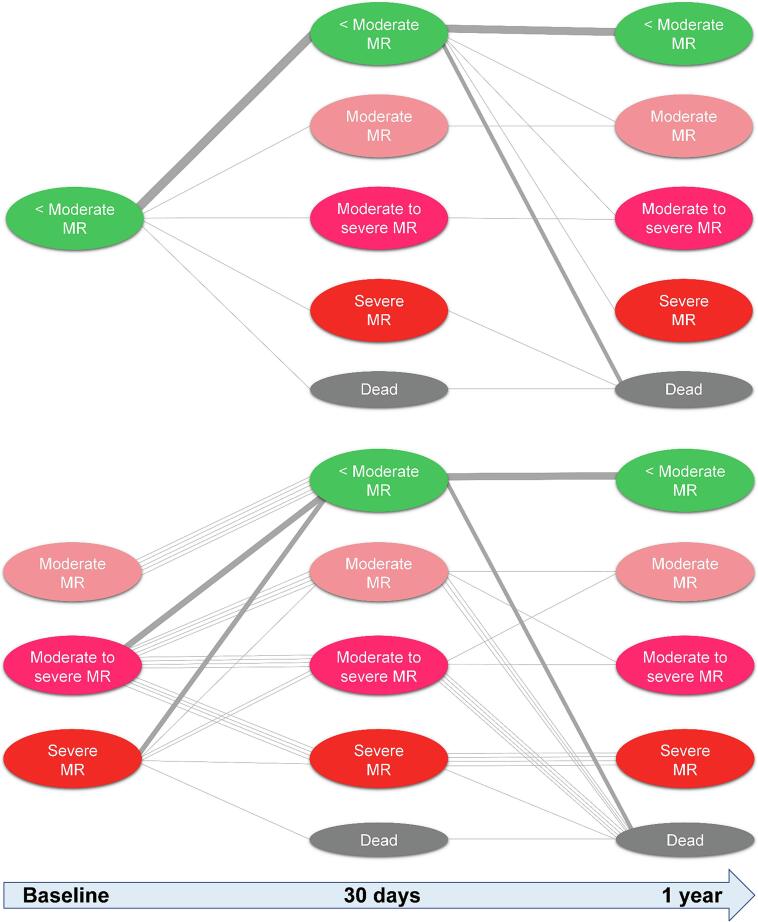
Changes over time in MR severity after TAVR are shown in Fig. 4. No patients underwent transcatheter mitral valve repair during the follow-up. In patients with nonsignificant baseline MR, MR worsened to significant MR in 4.7 % and 10.4 % at 30-day and 1-year follow-up, respectively. Among the significant baseline MR group, 70.4 % and 80.0 % of patients had improved to nonsignificant MR at 30-day and 1-year follow-up, respectively. The data shown in Fig. 5 showed the dynamic outcomes of MR after TAVR in individual patients.
Fig. 4.
Changes in mitral regurgitation (MR) over time (baseline, 30 days, and 1 year after TAVR) in patients with nonsignificant versus significant baseline MR. MR, mitral regurgitation; TAVR, transcatheter aortic valve replacement.
Fig. 5.
Tracked outcomes of individual patients over time (baseline, 30 days, and 1 year after TAVR) in patients with nonsignificant versus significant baseline MR. MR, mitral regurgitation; TAVR, transcatheter aortic valve replacement.
4. Discussion
This single-center study demonstrated the presence of significant baseline MR is very common in patients with TAVR for severe AS. The presence of significant baseline MR did not associate with greater mortality, while improvement in baseline MR (demonstrated in approximately 70 % of individuals) after TAVR did significantly reduce all-cause mortality.
The “2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease” does not provide clear recommendations related to the treatment of patients with mixed AS and MR [16]. Double-valve surgery has long been the mainstay therapy. However, it predicts high morbidity and mortality, with an average surgical mortality rate of 9 % for concomitant aortic and mitral valve replacement [17]. Typically, once the downstream myocardial mismatch is resolved by aortic valve replacement, the severity of MR Is reduced and mitral valve replacement is not necessary. Several meta-analyses have shown that concomitant baseline MR improved in approximately 50 % to 60 % of patients after TAVR [18], [19]. In our study, approximately 70 % of patients with significant baseline MR exhibited a significant improvement in MR severity after TAVR.
A number of physiological changes may contribute to reducing baseline MR severity. In the short term, acute improvement after TAVR may be explained by a decrease in left ventricular volumes and improved coaptation of the leaflets [20]. In the long term, TAVR is associated with reverse cardiac remodeling, which may also lead to an improvement in baseline MR severity. This process encompasses several morphological and hemodynamic changes, such as regression of left ventricular hypertrophy and diffuse fibrosis, reduction in left ventricular volumes and mitral tethering forces, improvement of LVEF, and normalization of diastolic function [21]. However, the possible mechanisms in persistence or worsening of baseline MR include concealed organic etiology, distortion of the mitral aortic curtain of the aortic stent, persistence of pulmonary hypertension, and atrial fibrillation [22].
There are conflicting data on whether baseline MR in patients with severe AS independently affects clinical outcomes in patients undergoing TAVR. Some studies reported significant baseline MR as an independent predictor of all-cause mortality at 1-year follow-up [23], while others did not note this [24]. Our study also found that all-cause mortality during 2-year follow-up after TAVR was not associated with significant baseline MR. Despite the clinical risk profile of patients with significant baseline MR which predisposes them to cardiovascular events compared with those with nonsignificant baseline MR [25], it cannot be efficiently predicted by baseline MR grade even if combined with these other baseline parameters. Some studies showed that patients with “true” high risk for mortality are those patients with post-procedural, rather than pre-procedural significant MR [26]. Swedish national registry-based study indicated that patients whose significant baseline MR remained unchanged or worsened after TAVR had approximately a 1.7-fold and 2-fold increase in 5-year mortality, respectively [27]. In our study, patients from the improved MR group after TAVR demonstrated a significantly higher survival than unchanged or worsened MR group. In view of these results, presence of MR grade ≥ moderate after TAVR should not be ignored, as it is associated with poor clinical outcomes.
As a result, evaluation of MR severity should be performed early after TAVR. Once significant MR is noticed, medical therapy or re-synchronization coupled with invasive modalities should not to postpone. Transcatheter mitral valve repair devices are emerging as treatment options for patients with significant MR and prohibitive surgical risk. A recent study demonstrated that, in patients with persistent significant MR, transcatheter mitral valve repair played a role in reducing the risk of mortality [28].
5. Study limitations
The study included a relatively small number of patients. The follow-up period was relatively short. Future studies in larger populations with longer follow-up are needed to clarify the impact of baseline MR for patients undergoing TAVR.
6. Conclusions
Significant baseline MR was not associated with the increased risk of all-cause mortality 2 years after TAVR. Significant baseline MR was improved in most patients at 1 year after TAVR. Patients with unchanged or worsened MR had an increased all-cause mortality.
CRediT authorship contribution statement
Hua-Jie Zheng: Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Xin Liu: Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization. De-Qing Lin: . Yong-Bo Cheng: Resources, Software, Supervision, Validation, Visualization. Chao-Jun Yan: Software, Validation, Visualization. Jun Li: Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Wei Cheng: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Chongqing Science and Health Joint Medical Research Project (No. 2023MSXM110) and the Science and Technology Innovation Capacity Improvement Project of University (No. 2019XYY13).
Ethical Statement.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Southwest Hospital of Army Medical University. The informed consent was signed by all patients to allow the intervention and data recording. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Contributor Information
Jun Li, Email: lijun10461046@163.com.
Wei Cheng, Email: yjchw@126.com.
References
- 1.Iung B., Delgado V., Rosenhek R., et al. Contemporary presentation and management of valvular heart disease: the eurobservational research programme valvular heart disease II survey. Circulation. 2019;140:1156–1169. doi: 10.1161/CIRCULATIONAHA.119.041080. [DOI] [PubMed] [Google Scholar]
- 2.Coffey S., Roberts-Thomson R., Brown A., et al. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021;18:853–864. doi: 10.1038/s41569-021-00570-z. [DOI] [PubMed] [Google Scholar]
- 3.Witberg G., Codner P., Landes U., et al. Effect of transcatheter aortic valve replacement on concomitant mitral regurgitation and its impact on mortality. J. Am. Coll. Cardiol. Intv. 2021;14:1181–1192. doi: 10.1016/j.jcin.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Hagar A., Wei X., et al. The relationship of mitral annulus shape at CT to mitral regurgitation after transcatheter aortic valve replacement. Radiology. 2021;301:93–102. doi: 10.1148/radiol.2021210267. [DOI] [PubMed] [Google Scholar]
- 6.Khawaja M.Z., Williams R., Hung J., et al. Impact of preprocedural mitral regurgitation upon mortality after transcatheter aortic valve implantation (TAVI) for severe aortic stenosis. Heart. 2014;100:1799–1803. doi: 10.1136/heartjnl-2014-305775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Assa E., Biner S., Banai S., et al. Clinical impact of post procedural mitral regurgitation after transcatheter aortic valve replacement. Int. J. Cardiol. 2020;299:215–221. doi: 10.1016/j.ijcard.2019.07.092. [DOI] [PubMed] [Google Scholar]
- 8.Bedogni F., Latib A., De Marco F., et al. Interplay between mitral regurgitation and transcatheter aortic valve replacement with the CoreValve Revalving System: a multicenter registry. Circulation. 2013;128:2145–2153. doi: 10.1161/CIRCULATIONAHA.113.001822. [DOI] [PubMed] [Google Scholar]
- 9.Cortes C., Amat-Santos I.J., Nombela-Franco L., et al. Mitral regurgitation after transcatheter aortic valve replacement: prognosis, imaging predictors, and potential management. J. Am. Coll. Cardiol. Intv. 2016;9:1603–1614. doi: 10.1016/j.jcin.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Miura M., Yamaji K., Shirai S., et al. Clinical Impact of Preprocedural Moderate or Severe Mitral Regurgitation on Outcomes After Transcatheter Aortic Valve Replacement. Can. J. Cardiol. 2020;36:1112–1120. doi: 10.1016/j.cjca.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Chew P.G., Dobson L.E., Garg P., et al. CMR quantitation of change in mitral regurgitation following transcatheter aortic valve replacement (TAVR): impact on left ventricular reverse remodeling and outcome. Int. J. Cardiovasc. Imaging. 2019;35:161–170. doi: 10.1007/s10554-018-1441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbanti M., Webb J.G., Hahn R.T., et al. Impact of preoperative moderate/severe mitral regurgitation on 2-year outcome after transcatheter and surgical aortic valve replacement: insight from the Placement of Aortic Transcatheter Valve (PARTNER) Trial Cohort A. Circulation. 2013;128:2776–2784. doi: 10.1161/CIRCULATIONAHA.113.003885. [DOI] [PubMed] [Google Scholar]
- 13.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Rev Esp Cardiol (engl Ed). 2022;75:524. doi: 10.1016/j.rec.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Amat-Santos I.J., Revilla A., Lopez J., et al. Value of CT in patients undergoing self-expandable TAVR to assess outcomes of concomitant mitral regurgitation. J. Am. Coll. Cardiol. Img. 2015;8:226–227. doi: 10.1016/j.jcmg.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Kappetein A.P., Head S.J., Genereux P., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document (VARC-2) Eur. J. Cardiothorac. Surg. 2012;42:S45–S60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 16.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 17.Jneid H., Suffredini J.M., Plana J.C. Transcatheter therapies for severe aortic stenosis and mitral regurgitation: a tale of 2 cities. J. Am. Coll. Cardiol. Intv. 2021;14:1193–2115. doi: 10.1016/j.jcin.2021.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Nombela-Franco L., Eltchaninoff H., Zahn R., et al. Clinical impact and evolution of mitral regurgitation following transcatheter aortic valve replacement: a meta-analysis. Heart. 2015;101:1395–1405. doi: 10.1136/heartjnl-2014-307120. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqi T.J., Usman M.S., Ahmed J., Shahid I., Ahmed W., Alkhouli M. Evaluating the effect of multivalvular disease on mortality after transcatheter aortic valve replacement for aortic stenosis: a meta-analysis and systematic review. Future Cardiol. 2022 doi: 10.2217/fca-2021-0061. [DOI] [PubMed] [Google Scholar]
- 20.Shibayama K., Harada K., Berdejo J., et al. Effect of transcatheter aortic valve replacement on the mitral valve apparatus and mitral regurgitation: real-time three-dimensional transesophageal echocardiography study. Circ. Cardiovasc. Imaging. 2014;7:344–351. doi: 10.1161/CIRCIMAGING.113.000942. [DOI] [PubMed] [Google Scholar]
- 21.Treibel T.A., Kozor R., Schofield R., et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J. Am. Coll. Cardiol. 2018;71:860–871. doi: 10.1016/j.jacc.2017.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashida K., Lefevre T., Chevalier B., et al. Impact of post-procedural aortic regurgitation on mortality after transcatheter aortic valve implantation. J. Am. Coll. Cardiol. Intv. 2012;5:1247–1256. doi: 10.1016/j.jcin.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Zahn R., Gerckens U., Linke A., et al. Predictors of one-year mortality after transcatheter aortic valve implantation for severe symptomatic aortic stenosis. Am. J. Cardiol. 2013;112:272–1229. doi: 10.1016/j.amjcard.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Mavromatis K., Thourani V.H., Stebbins A., et al. Transcatheter Aortic Valve Replacement in Patients with Aortic Stenosis and Mitral Regurgitation. Ann. Thorac. Surg. 2017;104:1977–1985. doi: 10.1016/j.athoracsur.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 25.Nappi F., Nenna A., Timofeeva I., Mihos C., Gentile F., Chello M. Mitral regurgitation after transcatheter aortic valve replacement. J. Thorac. Dis. 2020;12:2926–2935. doi: 10.21037/jtd.2020.01.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter M.P., Bartko P.E., Krickl A., et al. Adaptive development of concomitant secondary mitral and tricuspid regurgitation after transcatheter aortic valve replacement. Eur. Heart J. Cardiovasc. Imaging. 2021;22:1045–1053. doi: 10.1093/ehjci/jeaa106. [DOI] [PubMed] [Google Scholar]
- 27.Feldt K., De Palma R., Bjursten H., et al. Change in mitral regurgitation severity impacts survival after transcatheter aortic valve replacement. Int. J. Cardiol. 2019;294:32–36. doi: 10.1016/j.ijcard.2019.07.075. [DOI] [PubMed] [Google Scholar]
- 28.Stone G.W., Lindenfeld J., Abraham W.T., et al. Transcatheter mitral-valve repair in patients with heart failure. N. Engl. J. Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]