Abstract
Elevated concentrations of intracellular calcium ([Ca]i) have been implicated as an important signalling event during attaching and effacing (A/E) lesion formation by enteropathogenic Escherichia coli (EPEC). The highly localized nature of the cytoskeletal and cell surface alterations occurring during A/E lesion formation suggests that there should be equally localized EPEC-induced signalling events. To analyze further the calcium responses to infection of HEp-2 cells by EPEC, we employed calcium-imaging fluorescence microscopy, which allows both temporal and spatial measurements of [Ca]i in live cells. Using this imaging technique, not only were we unable to detect any significant elevation in [Ca]i at sites of A/E EPEC adhesion, but, with several different classical EPEC and enterohemorrhagic E. coli (EHEC) strains and three different infection procedures, each of which resulted in extensive A/E bacterial adhesion, we were unable to detect any significant alterations in [Ca]i in infected cells compared to uninfected cells. In addition, chelation of intracellular free calcium with bis-(aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA) did not, as previously reported, prevent A/E lesion formation. We conclude that increased [Ca]i are not required for A/E lesion formation by EPEC and EHEC.
Enteropathogenic Escherichia coli (EPEC) strains remain an important cause of severe infantile diarrheal disease in many parts of the developing world (8). EPEC colonizes the intestinal mucosa and produces attaching and effacing (A/E) lesions, which are characterized by localized destruction of brush border microvilli, intimate bacterial adhesion, and formation of a complex cytoskeletal structure beneath intimately attached bacteria (23). Accumulation of polymerized actin beneath bacteria results in the formation of cup-like pedestal structures in the apical enterocyte membrane (22). EPEC produces morphologically similar A/E lesions in a variety of cultured cell lines (21). The determinants of A/E lesion formation have been localized to a large pathogenicity island on the EPEC chromosome termed the locus of enterocyte effacement or (LEE) (27). Encoded within the LEE are a type III secretion system (16); an outer membrane protein adhesin, intimin (17); a translocated intimin receptor, Tir (18); and three EPEC secreted proteins (Esps), EspA, EspB, and EspD, each of which has been shown to be required for signal transduction to host cells and A/E lesion formation (12, 20, 25). Enterohemorrhagic E. coli (EHEC) strains, which cause a hemorrhagic colitis, possess a homologous LEE and also produce A/E lesions (27).
In vitro studies employing defined mutants support a three-stage model of A/E lesion formation (10). Stage 1 involves an initial nonintimate bacterial adhesion to epithelial cells, which is thought to be promoted by a plasmid-encoded bundle-forming pilus (BFP). In stage 2 signal transduction in host cells is triggered by Esps, resulting in cytoskeletal rearrangements, microvillous effacement, and tyrosine phosphorylation of Tir following translocation to the host cell membrane (18). Stage 3 involves intimin binding to tyrosine-phosphorylated Tir to produce the characteristic intimate attachment; pedestal formation results from further cytoskeletal reorganization and polymerization beneath intimately attached bacteria.
Early studies of EPEC signal transduction examined second messengers and demonstrated increased concentrations of intracellular calcium ([Ca]i) in EPEC-infected epithelial cells (2, 11). The subsequent demonstration of inositol phosphate fluxes, including increased levels of inositol triphosphate (11, 13), suggested possible stimulation of the classical phospholipase C pathway and release of calcium from inositol triphosphate-sensitive intracellular stores. Activation of brush border villin by calcium, disruption of the microvillous actin cytoskeleton, and vesiculation of the microvillous membrane provided a plausible model for EPEC-induced brush border effacement (4). One difficulty with such a model, however, was how such a calcium signal could give rise to the highly localized cytoskeletal changes seen during EPEC infection: microvillous effacement occurs only at sites of intimate bacterial adhesion. We hypothesized that if calcium signalling is important, calcium signals should be equally localized within the cell to sites of A/E lesion formation. To test this hypothesis and analyze further the calcium responses to infection of HEp-2 cells by EPEC (and EHEC), we employed calcium-imaging fluorescence microscopy, which not only allows real-time measurements of [Ca]i in infected cells but also allows the spatial distribution of [Ca]i to be determined and thus correlated with A/E lesion formation. Here we report that not only were we unable to detect any significant elevation in [Ca]i at sites of A/E EPEC adhesion, but we were unable to detect any significant alterations in [Ca]i in EPEC- and EHEC-infected HEp-2 cells compared to uninfected cells.
MATERIALS AND METHODS
Bacterial strains.
The EPEC and EHEC strains used in the study are listed in Table 1. Stock cultures were subcultured into Mueller-Hinton broth and incubated aerobically at 37°C for 18 h.
TABLE 1.
EPEC and EHEC strains used in this study
| Strain | Serotype | Description | Origin |
|---|---|---|---|
| E2348/69 | O127:H6 | EPEC isolate | United Kingdom |
| 20293 | O111: | EPEC isolate | United Kingdom |
| 7958 | O126: | EPEC isolate | France |
| 172/1 | O55:H− | EPEC isolate | Brazil |
| E29962 | O157:H7 | EHEC isolate | United Kingdom |
| 85-170 | O157:H7 | EHEC isolate spontaneously cured of phages encoding Shiga-like toxins | United States |
Infection of cultured HEp-2 cells.
HEp-2 cells were grown on 25-mm-diameter glass coverslips in 199 medium (Sigma) supplemented with 10% fetal calf serum and 5% glutamine. Subconfluent cultures were briefly washed in phosphate-buffered saline (PBS) and transferred to six-well tissue culture plates containing 4 ml of HEPES-buffered Eagle’s minimum essential medium (MEM) containing 2% newborn calf serum (NCS) and 0.5% d-mannose. For calcium-imaging studies three different bacterial infection procedures were used.
(i) “Spindown” infection.
The spindown infection protocol used a method recently developed in our laboratory to achieve rapid synchronous EPEC infection of a cell monolayer (6). Briefly, 30 μl of an overnight bacterial broth culture was subcultured into 3 ml of tissue culture medium in a 30-mm-diameter petri dish for 4 h at 37°C to induce expression of virulence factors, including BFP, Esps, and intimin; BFP expression resulted in spontaneous aggregation of bacteria. A 0.5-ml volume of the aggregated bacterial suspension was placed in a Leiden coverslip chamber containing a cell monolayer preloaded with the fluorescent calcium-imaging dye fura-2 (see below), and the chamber was centrifuged at 700 × g for 2 min. The culture medium was immediately replaced with 0.5 ml of fresh medium, and calcium imaging was performed immediately for 1 h as described below.
(ii) “Settledown” infection.
In the spindown protocol there was a short (up to 5-min) delay between the centrifugation step and commencement of calcium imaging, during which time alterations in cell calcium could have occurred. In order to eliminate the possibility that we might miss rapid alterations in cell calcium, we used a protocol similar to the spindown procedure except that the centrifugation step was omitted and calcium imaging was begun immediately upon addition of bacteria.
(iii) Gradual infection.
The gradual infection protocol essentially reproduced the method originally used by Baldwin et al. (2), in which calcium levels were assayed in cells following infection with EPEC for 1, 2, 3, 4, and 6 h. Coverslips in six-well plates containing 4 ml of MEM plus 2% NCS were inoculated with 80 μl of bacterial broth culture and incubated at 37°C for up to 6 h. Cells were loaded with fura-2 during the 40 min prior to the appropriate imaging time point. Washed coverslips were mounted in the Leiden chamber and transferred to the microscope, and calcium imaging was performed as described below.
Measurement of intracellular free calcium.
Intracellular calcium concentrations were determined by digital fluorescence imaging with the ratiometric, dual-excitation wavelength dye fura-2. Fura-2 was loaded into cells as the acetoxymethyl (AM) ester derivative of fura-2, fura-2 AM (Molecular Probes), which, being more hydrophobic than the dye itself, enters mammalian cells more easily. Once inside the cell, endogenous esterases cleave the AM group, trapping the dye inside the cells. HEp-2 cells were loaded with fura-2 by incubating cell monolayers in loading medium (MEM containing 10% NCS and 2 μg of fura-2 AM per ml for 40 min at 32°C, with the reduced temperature being used to help reduce compartmentalization of the dye. Monolayers were rinsed three times in fresh medium to remove extracellular dye.
The dye-loaded cell monolayers were mounted in Leiden coverslip chambers and transferred to an open perfusion chamber, which was maintained at 37°C and mounted on the stage of a Zeiss Axiovert 135TV inverted microscope, and imaged by using a 40× achrostigmat oil immersion phase-contrast lens. The Ca-free and Ca-bound forms of fura-2 fluoresce maximally at 380 and 340 nm, respectively. These excitation wavelengths were provided by a Zeiss XBO75 Xenon lamp and transmitted through filters mounted in a computer-controlled filter wheel. Emitted fluorescence was detected with a high-sensitivity Extended ISIS Intensified charge-coupled device video camera (Photonic Science Ltd.). The camera and filter wheel were connected to an Apple Macintosh 6100/60 computer running the dedicated calcium image analysis software IonVision III (Image Processing and Vision Company Ltd., Coventry, United Kingdom), which was used to control the experiment, capture the relevant fluorescence images, and calculate both the ratiometric images and the [Ca]i values by using ion standards. Pseudocolor ratio images produced immediately after each image pair was recorded allowed real-time alterations in calcium levels to be monitored directly during an experiment; a more detailed statistical analysis of the data was performed later by using saved image pairs. Complementary phase-contrast images of the same microscope fields used for calcium imaging were recorded with a Hamamatsu C3077 charge-coupled device video camera.
In spindown and settledown infection experiments, one ratio image was taken every minute from the same area of cells for the 60-min duration of the experiment; in the gradual infection experiments, five ratio images from different fields were taken at each time point to obtain a representative value for the whole monolayer. At the end of some experiments, cells were exposed to the calcium ionophore ionomycin (10 μM) or to the calcium agonist histamine (0.1 M) to confirm the responsiveness of the cells and the detection system. At the end of other experiments, cells were fixed and stained for actin to detect A/E lesion formation (22).
Chelation of intracellular calcium.
To assess the ability of EPEC to produce A/E lesions in the absence of elevated intracellular calcium levels, intracellular calcium was chelated by incubating HEp-2 cell monolayers with 10 μM AM bis-(aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM) in MEM–2% NCS for 45 min at 37°C. The spindown protocol was used to infect washed cell monolayers with EPEC for 1 h at 37°C, after which time the cells were washed again and examined by the fluorescence actin-staining (FAS) test for A/E lesion formation (22). The calcium-buffering capacity of BAPTA-loaded cells was assessed by comparing the histamine responses of control and BAPTA-treated HEp-2 cells by using the IonVision system.
Fluorescence staining of filamentous actin (FAS test).
Formalin-fixed and washed cells were permeabilized by treating coverslips with 0.1% Triton X-100 in PBS for 4 min. After three washes in PBS, coverslips were stained with a 5-μg/ml solution of fluorescein isothiocyanate phalloidin (Sigma) in PBS for 20 min to specifically stain filamentous actin (22). Coverslips were washed a further three times in PBS and mounted in glycerol-PBS. Cells were examined by incident-light fluorescence with a Leitz Dialux microscope. Fluorescence and phase-contrast micrographs of the same field were made.
Live-dead assay.
The viability of cells following EPEC infection was assessed by fluorescence microscopy with the commercial EukoLight Viability/Cytotoxicity Kit (Molecular Probes) containing calcein-AM and ethidium homodimer. At the end of appropriate experiments, HEp-2 cell monolayers were incubated with the live-dead kit solution (2 μM calcein-AM and 4 μM ethidium homodimer in PBS) for 45 min at room temperature, washed, and examined by incident-light fluorescence for viable (green) and dead (red) cells.
Statistical analyses.
Results are presented as means ± standard errors of the means. The data were analyzed by Student’s t test, and P < 0.05 was considered statistically significant.
RESULTS
The resting [Ca]i in uninfected HEp-2 cells, determined by averaging [Ca]i in cells from five different microscope fields, was 151 ± 34 nM (n = 7); this value is comparable to basal levels reported by others (2, 11). To confirm that the HEp-2 cells being used were responsive to calcium agonists and that the imaging system was capable of detecting changes in [Ca]i under the conditions employed, fura-2-loaded HEp-2 cells were stimulated with 1 mM histamine during imaging. This resulted in large, though variable, rises in [Ca]i of 435 ± 273% (n = 5).
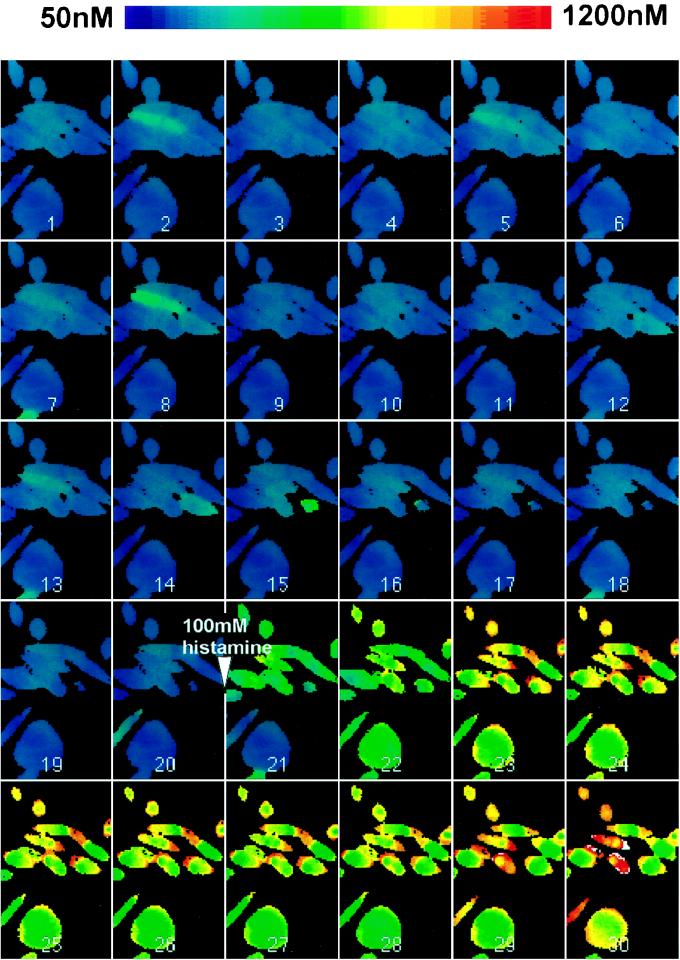
Based on knowledge of BFP and Esp expression in mid-exponential-phase cultures of EPEC grown in tissue culture medium, we developed an infection protocol (spindown infection) which achieved a rapid synchronous EPEC infection of HEp-2 cell monolayers (6). Time course experiments in which coverslips were fixed and examined by using the FAS test showed that A/E lesions were produced after 10 to 20 min (6). We used this rapid infection protocol to measure [Ca]i in HEp-2 cell monolayers infected with EPEC strain E2348/69, with calcium levels being determined in a single microscope field of cells at 1-min intervals for 60 min. In a typical experiment there were ∼15 to 20 cells in a microscope field, and at least 75% of cells possessed adherent microcolonies of A/E bacteria as assessed at the end of an experiment by phase-contrast microscopy and the FAS test (Fig. 1A). Irrespective of whether we measured [Ca]i averaged over all cells in the field, in individual cells, or in specific regions of cells containing attached bacterial microcolonies (identified by comparison with phase-contrast micrographs taken at the end of the experiment), we were unable to detect anything other than minor fluctuations in [Ca]i throughout the experimental period. Results of a typical experiment are illustrated in Fig. 2, which shows pseudocolor ratio images taken at 2-min intervals. Although A/E lesion formation occurred between images 5 and 10, no significant alterations in [Ca]i were detected during this period. However, ∼4- to 5-fold increases in [Ca]i were seen immediately following the addition of 100 mM histamine after 40 min, confirming the responsiveness of the cells and the detection system.
FIG. 1.
FAS tests performed at the end of typical calcium-imaging experiments. The micrographs show fluorescence (left panels) and corresponding phase-contrast images (right panels) of HEp-2 cell monolayers spindown infected with EPEC strain E2348/69 for 1 h (A), gradually infected with EPEC strain E2348/69 for 6 h (B), gradually infected with EHEC strain E29962 for 6 h (C), and treated with BAPTA and then spindown infected with EPEC strain E2348/69 for 1 h (D). Each infection procedure resulted in good colonization of cells with A/E EPEC (A and B), although adhesion by EHEC strains was significantly less than that by EPEC strains (C); characteristic EPEC-induced actin accretion occurred in BAPTA-treated cells (D). Magnification, ×320.
FIG. 2.
Montage of pseudocolor ratio images taken at 2-min intervals, showing [Ca]i in HEp-2 cells spindown infected with E2348/69 for 1 h. The images show no significant alterations in [Ca]i during the period of A/E lesion formation (images 5 to 10). After 40 min (image 21), 100 mM histamine was added to the cells, resulting in a large increase in [Ca]i.
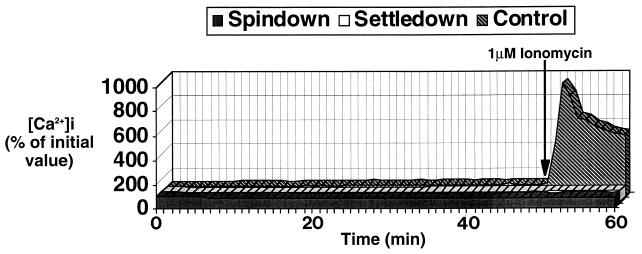
The spindown infection protocol resulted in rapid EPEC adhesion and A/E lesion formation. However, there was a short delay between the centrifugation step and commencement of calcium imaging, during which time rapid calcium responses could have been missed. We therefore used a settledown procedure in which calcium imaging was commenced immediately following the addition of an aggregated bacterial suspension. Although adhesion and A/E lesion formation were not as rapid as with the spindown procedure, by 1 h there was equally good bacterial attachment and A/E lesion formation (data not shown). Using this settledown procedure, we were still unable to detect any significant alteration in [Ca]i throughout the 60-min experimental period. As with the spindown experiments, corresponding phase-contrast and FAS tests confirmed that imaged fields possessed infected cells producing A/E lesions, and addition of histamine or ionomycin confirmed the responsiveness of the cells and the detection system. A graphical representation of [Ca]i in typical spindown and settledown infection experiments in comparison with those in uninfected cells is shown in Fig. 3.
FIG. 3.
Mean [Ca]i (n = 4) in control HEp-2 cell monolayers and HEp-2 cell monolayers infected for 1 h with EPEC strain E2348/69 by the spindown and settledown procedures. No significant alteration in [Ca]i was seen throughout the 1-h infection period, during which extensive A/E adhesion occurred. An ∼10-fold increase in [Ca]i occurred in control cells following the addition of 1 μM ionomycin, confirming the responsiveness of the cells and the detection system.
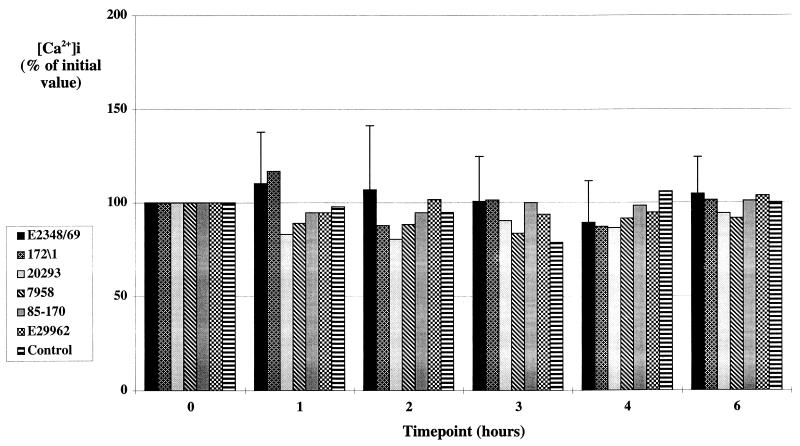
Since we were unable to detect [Ca]i in cells infected by using rapid infection procedures, we used a gradual infection which essentially reproduced the method originally used to demonstrate increased [Ca]i in EPEC-infected HEp-2 cells (2). Cells were infected with an overnight broth culture of EPEC, and [Ca]i was measured after 1, 2, 3, 4, and 6 h. Little bacterial adhesion occurred during the first hour of infection, but adhesion and A/E lesion formation were clearly seen at 2 h, after which adhesion increased rapidly, and by 6 h all cells possessed microcolonies of A/E bacteria (Fig. 1B). Average [Ca]i from five randomly selected microscope fields were determined at each time point, but again we were unable to detect any significant alterations in [Ca]i in cell monolayers infected with EPEC strain E2348/69, with three other classical EPEC strains belonging to serogroups O55, O111, and O126, or with two O157:H7 EHEC strains (Fig. 4). Although the EHEC strains did not adhere to HEp-2 cells as efficiently as EPEC strains, significant levels of A/E bacterial adhesion were apparent after a 6-h incubation (Fig. 1C). The responsiveness of the cells and detection system was again confirmed at the end of the experimental period by using histamine (data not shown).
FIG. 4.
Mean [Ca]i (n = 2) in control HEp-2 cell monolayers and HEp-2 cell monolayers infected for up to 6 h with four different EPEC strains and two different EHEC strains by using the gradual infection procedure. Values are presented as percentages of the mean [Ca]i in the cells at time zero. No significant change in [Ca]i was seen with any of the six strains over the 6-h time course. Error bars are illustrated for strain E2348/69 and are typical of those for the remainder of the strains.
Additional evidence for a role of cell calcium in A/E lesion formation has been the reported inhibition of lesion formation following the buffering of intracellular free calcium with BAPTA. The ability of 10 μM BAPTA to buffer intracellular calcium was confirmed by demonstrating the abrogation of the histamine response in BAPTA-treated cells. Typical calcium responses were seen following sequential additions of 1 mM histamine to control HEp-2 cells monolayers, but such responses were completely inhibited in BAPTA-treated cells (Fig. 5). Comparable A/E lesion formation assessed by FAS occurred in BAPTA-treated (Fig. 1D) compared to untreated (Fig. 1A) HEp-2 cells.
FIG. 5.
Effect of BAPTA on histamine-induced rises in HEp-2 cell [Ca]i. Histamine (H) (1 mM) was added to both control and BAPTA-treated cells after 7 and 18 min (arrows). Histamine-stimulated rises in [Ca]i were completely abrogated in the BAPTA-treated cells, whereas considerable increases in [Ca]i were seen in the untreated cells.
Substantial cell death following EPEC infection of HEp-2 cells has previously been reported (1). Since cell viability is a factor which would affect cell calcium measurements, we assessed HEp-2 cell viability. In our experiments, <5% HEp-2 cell nonviability throughout the 1-h spindown or settledown experiments (data not shown) and throughout the 6-h gradual infection experiments with EPEC strain E2348/69 (Fig. 6) was observed.
FIG. 6.
Mean percent cell death (n = 2) in HEp-2 cell monolayers gradually infected with EPEC strain E2348/69 for 6 h. Less than 5% cell death occurred over the 6-h time period of the experiment.
DISCUSSION
In this study we attempted to measure temporal and spatial alterations in [Ca]i in EPEC- and EHEC-infected HEp-2 cells by using fura-2 ratiometric digital fluorescence microscopy to determine if previously reported increases in [Ca]i were localized to sites of bacterial adhesion and A/E lesion formation. Not only were we unable to detect any significant elevation in [Ca]i at sites of A/E EPEC adhesion, we were unable to detect any significant alterations in [Ca]i in infected cells compared to uninfected cells. In addition, buffering intracellular calcium with BAPTA did not, as previously reported, prevent A/E lesion formation. We therefore conclude that specific calcium signalling and increased [Ca]i are not required for the formation of A/E lesions by EPEC and EHEC.
These findings contradict those of several previous studies which have demonstrated elevated [Ca]i in EPEC- and EHEC-infected cells. Baldwin et al. (2) first described alterations in [Ca]i in EPEC-infected epithelial cells and reported a sustained increase in [Ca]i following EPEC infection of HEp-2 cell monolayers compared to that for control cells or cells infected with pathogenic E. coli strains that did not produce A/E lesions; EPEC infection resulted in a doubling of [Ca]i after 1 h and in an ∼6-fold-higher [Ca]i after a 4-h incubation. Evidence supporting release of calcium from intracellular stores was also presented, and fluorescence micrographs of EPEC-infected cells loaded with fluo-3 were interpreted as showing increased [Ca]i in regions of the cell corresponding to areas of bacterial attachment (2). Dytoc et al. (11), also reported significant increases in [Ca]i in EPEC-infected HEp-2 cells, although their reported increase of 125 ± 40% over 6 h was significantly less than that reported by Baldwin et al. (2). Electron microscopy studies showing precipitation of calcium oxalate subjacent to areas of EPEC attachment were presented as further evidence for localized increases in [Ca]i following EPEC infection. Both groups reported that buffering intracellular free calcium with BAPTA prevented A/E lesion formation (2, 11). Small increases in [Ca]i (46 ± 9%) following infection of HEp-2 cells with O157:H7 EHEC have also been reported (15).
Because we used a ratiometric technique and performed real-time calcium measurements displayed throughout an infection of cells in which we knew extensive A/E adhesion was occurring, we find it hard to believe that we would not have detected significantly increased [Ca]i had they occurred. It is conceivable that we could have missed very rapid and/or highly localized calcium increases (i) by taking images at 1-min intervals rather than at shorter intervals or (ii) due to the resolution of the imaging system, although both possibilities seem unlikely given the hundreds of images examined over many experiments and given that the same imaging system resolved individual bacteria by phase-contrast microscopy; the histamine-ionomycin response data show that our system would have detected sustained increases in [Ca]i had they occurred.
Even though data obtained by using a ratiometric method should be more accurate than the previously reported data, which used nonratiometric methods, methodological differences are unlikely to explain why previous studies have detected significant calcium changes following EPEC infection when we did not. One possible explanation is that calcium changes reported by others reflect cytotoxic effects of EPEC on cells rather than specific calcium signalling events associated with A/E lesion formation. Calcium signalling usually involves highly controlled transient changes in [Ca]i, which fall within a narrow 5- to 10-fold range since high [Ca]i are destructive to cells (3). The sustained high [Ca]i originally reported by Baldwin et al. (2) were associated with high levels of cell death (1), whereas in this study we observed extensive A/E adhesion over 6 h with minimal cell death. Cytotoxic effects could also be an explanation for the observations of Philpott et al. (28), who reported a 115 ± 14% increase in [Ca]i over 3 h with T84 intestinal cells grown on plastic but were unable to provide data for longer periods because of cell death. Significantly, no increases in [Ca]i were observed in the same cells grown on permeable filters.
Increased [Ca]i associated with cytotoxicity and loss of cell viability are unlikely to give rise to localized increases associated with adherent bacteria, yet evidence in support of localized increases in [Ca]i during EPEC infection has been presented (2, 11). Baldwin et al. (2) used fluo-3 to image calcium in EPEC-infected cells and reported increased [Ca]i localized to sites of adherent bacterial microcolonies. Since increased fluorescence was visible throughout the perinuclear region of the cell and not just at the sites of adherent bacterial microcolonies, a more plausible interpretation is that the fluo-3 has preferentially compartmentalized within mitochondria, a major cell compartment for the sequestration of calcium. Such effects are eliminated with ratiometric methods but would be seen with nonratiometric dyes such as fluo-3. Dytoc et al. (11) reported precipitation of calcium oxalate in the apical cytoplasm beneath adherent A/E EPEC as evidence for localized increases in [Ca]i; we attempted, but were unable, to reproduce this effect.
Ratiometric calcium imaging was recently used to show that EPEC eaeA (intimin gene) mutants do not cause elevations in [Ca]i in infected HeLa cells (31). Although eaeA mutants, which lack intimin, do not display intimate attachment or produce A/E lesions, they nevertheless do induce signal transduction in host cells (9), supporting the conclusion that EPEC signal transduction events associated with A/E lesion formation do not involve calcium signalling. Additionally, EPEC-mediated depolarization of HeLa cell membranes, an event dependent on EPEC signal transduction to host cells, was also shown to be independent of an EPEC-mediated elevation in [Ca]i (31).
Several additional host cell factors which are associated with EPEC infection and which would suggest calcium-dependent signalling events have been described, including induction of host cell inositol phosphate fluxes (11, 13), tyrosine phosphorylation of phospholipase C-γ1 (19), and activation of protein kinase C (7, 26). Our data indicate that there is no significant alteration in cell calcium during A/E lesion formation but that alterations in cell calcium may be related to cytotoxic effects of EPEC. If this is the case, then perhaps other reported signalling events (e.g., increased inositol triphosphate levels) may be similarly associated with cytotoxic effects of EPEC and should now be reevaluated in assays in which minimal cell damage occurs.
Based on morphological similarities between the effects of calcium and the effects of EPEC on brush border microvilli, it was originally hypothesized that EPEC stimulates a localized increase in [Ca]i leading to activation of villin, disruption of the microvillous actin core, and vesiculation of the microvillous membrane (4). Neither we nor anybody else has demonstrated the micromolar calcium levels required for villin-mediated microvillous disassembly (4). Thus, if increased calcium is not the mechanism whereby EPEC induces brush border effacement, then what is? It is now clear that a number of enteric pathogens, including Yersinia, Salmonella, and Shigella spp., translocate into host cells via type III secretion system virulence proteins which induce gross cytoskeletal rearrangements. Yersinia spp. translocate the YopE cytotoxin (30) and Salmonella spp. translocate a tyrosine phosphatase, SptP (14), which cause disruption of the host cell actin microfilament structure. In a calcium-independent manner (5), Shigella spp. translocate the invasin IpaA, which rapidly associates with vinculin, a protein linking actin filaments to the plasma membrane to induce highly localized cytoskeletal rearrangements which result in membrane ruffling and bacterial uptake (32). In a similar calcium-independent manner, it seems likely that EPEC and EHEC, which also possess type III secretion systems, translocate into host cells distinct effector proteins which induce the localized cytoskeletal rearrangements involved in A/E lesion formation. Indeed, two type III secreted EPEC proteins, EspB (24, 33) and Tir (18), have recently been shown to be translocated into host cells. Three functions have been ascribed to Tir. Tir functions as the intimate receptor for EPEC on mammalian cell surfaces, Tir nucleates actin following intimin binding, and Tir stimulates signal transduction events following intimin binding (18). EspB or another, as-yet-to-be-identified effector protein could be responsible for localized disruption of the microvillus actin cytoskeleton and micrvillous effacement.
ACKNOWLEDGMENTS
This work was supported by grants from the Wellcome Trust (to C.B. and S.K.) and by a fellowship to R.K. from Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) (Proc. FAPESP no. 95/07033-1).
We are grateful to Henry Smith, Central Public Health Laboratory, London, United Kingdom; Jim Kaper, Center for Vaccine Development, University of Maryland, College Park; and Bernard Joly, Bacteriological Laboratory, University of Clermont-Ferrand, Clermont-Ferrand, France for providing strains used in the study.
REFERENCES
- 1.Baldwin T J, Lee-Delaunay M B, Knutton S, Williams P H. Calcium-calmodulin dependence of actin accretion and lethality in cultured HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1993;61:760–763. doi: 10.1128/iai.61.2.760-763.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin T J, Ward W, Aitken A, Knutton S, Williams P H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge M J. Elementary and global aspects of calcium signalling. J Exp Med. 1997;200:315–319. doi: 10.1242/jeb.200.2.315. [DOI] [PubMed] [Google Scholar]
- 4.Burgess D R, Prum B E. Reevaluation of brush border motility: calcium induces core filament solation and microvillar vesiculation. J Cell Biol. 1982;94:97–107. doi: 10.1083/jcb.94.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerc P L, Berthon B, Claret M, Sansonetti P J. Internalization of Shigella flexneri into HeLa cells occurs without an increase in the cytosolic Ca2+ concentration. Infect Immun. 1989;57:2919–2922. doi: 10.1128/iai.57.9.2919-2922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collington G K, Booth I W, Knutton S. Enteropathogenic Escherichia coli (EPEC) infection rapidly modulates electrolyte transport in Caco-2 cell monolayers. Gut. 1998;42:200–207. doi: 10.1136/gut.42.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane J K, Oh J S. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–3285. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg M S. Enteropathogenic Escherichia coli. In: Blaser M J, et al., editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 709–726. [Google Scholar]
- 9.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 11.Dytoc M, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 12.Foubister V, Rosenshine I, Donnenberg M S, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC) triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Galan J E. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol Microbiol. 1998;27:359–368. doi: 10.1046/j.1365-2958.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 15.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a specialised secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenny B, DeVinney R, Stein M, Reinsheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adhesion into mammalian cells. Cell. 1997;91:511–529. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 19.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-gamma 1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli (EPEC) is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 21.Knutton S, Baldini M M, Kaper J B, McNeish A S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987;55:78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutton S, Baldwin T J, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai L-C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manjarrez-Hernandez H A, Baldin T J, Williams P H, Haigh R, Knutton S, Aitken A. Phosphorylation of myosin light chain at distinct sites and its association with the cytoskeleton during enteropathogenic Escherichia coli infection. Infect Immun. 1996;64:2368–2370. doi: 10.1128/iai.64.6.2368-2370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 29.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 30.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein M A, Mathers D A, Yan H, Baimbridge K G, Finlay B B. Enteropathogenic Escherichia coli markedly decreases resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect Immun. 1996;64:4820–4825. doi: 10.1128/iai.64.11.4820-4825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trau van Nhieu G, Ben-Ze’ev A, Sansonetti P J. Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J. 1997;16:2717–2729. doi: 10.1093/emboj/16.10.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into HeLa cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]