Abstract
Low-stress handling methods have been studied in detail in mice, but relatively little research exists concerning preferred handling methods in rats. Most recommendations for low-stress handling of rats have been extrapolated from the mouse literature, despite known differences in handler interaction between the 2 species. The goal of the current study was to evaluate common methods of handling in rats, including application of recognized, low-stress handling methods from other species to rats, in order to determine relative stress levels associated with the handling methods. Seventy male and 70 female, 8-wk-old, Crl:CDSD rats, were housed either individually or in pairs, and were handled weekly or daily using one of the following methods: encircling of the torso (standard thoracic hold), handled using a tunnel, handled using a protective bite glove, handled using a soft paper towel, or tickled prior to being handled by the torso (n = 10 per sex per treatment group). Body weight and clinical observations were scored at each handling session, abbreviated functional observation batteries were performed every other week, and an interaction test and hematology were conducted prior to study and on the day of study termination. Rats that were socially housed and handled weekly using the standard thoracic hold showed the least evidence of stress, while those that were singly housed and handled weekly using a protective bite glove or tunnel showed the highest level of stress. These effects were predominantly seen in males. This study suggests that standard low-stress handling methods used for other species may not be optimal for rats, and that additional research is needed to identify alternative methods to the standard thoracic hold that would further reduce stress during handling in rats.
Introduction
The use of low-stress handling methods has gained significant attention in the animal world over the past several decades, with the goal of decreasing potential fear and anxiety experienced by animals in the care of humans. Fear and anxiety, in addition to being disconcerting to the animal, can also cause significant physiologic and behavioral changes.24,29, 30 In the research setting, these may act as extraneous variables, potentially affecting study data. In some extreme cases, animals may respond to fear and anxiety by showing self-defense and becoming aggressive to conspecifics and/or those individuals working with them.24, 27, 31
Over the past several years, significant energy has been devoted to investigating procedures for low stress handling in mice to reduce the fear associated with being handled. Current data suggest that mice that are picked up by the tail experience significantly more anxiety than mice that are picked up using a tunnel.12 Additional studies suggest ‘cupping,’ or scooping mice up using an open hand, can also be a less aversive method for handling.12 As a result, the use of tunnel handling or cupping is now being recommended for handling of mice by a variety of animal welfare advocates.9,12
Rats, however, do not appear to have received the same attention. Little scientific literature currently exists on the preferred handling techniques of rats, with many of the recommendations for handling rats being extrapolated from the data already collected in mice. A 2013 report reviewed a variety of handling techniques, but none of these were actually evaluated for stress20; a similar publication was published in 2012.17 A 2005 study compared common methods for lifting and handling rats, using telemetry to measure various cardiovascular parameters.3 All methods measured, including holding by the scruff, encircling the animal around the thorax, lifting and holding by the tail, and handling using a soft plastic restraint cone, resulted in significant increases in mean arterial pressure and heart rate compared with baseline.3 The authors concluded that being lifted in a restraint cone appeared to be the most disturbing handling method for the rats, followed by the tail method, as determined by prolonged duration of increased cardiovascular parameters as compared with the encircling or scruffing methods.3 This suggests that, like mice, tail handling may be aversive, while cone handling may not be the preferred handling method for rats. ‘Cupping’ or scooping rats has not been examined to our knowledge, possibly because it can be more difficult to perform with larger rats. A similar process in which the rat is encircled around the thorax to be lifted from the cage is commonly the preferred handling method, although minimal literature is available on this method aside from the study referenced above.3
More information is available on ways to mitigate handling stress in rats apart from modifications of direct handling methods. Social housing alone appears to reduce the fear and anxiety associated with basic handling; one study showed that when rats were handled and weighed, those that were individually housed had significantly higher increases in heart rate than those that were socially housed30. Another study showed that rats kept in social isolation weighed less than did socially-housed animals, and also had anhedonia and higher corticosterone and ACTH levels within 2 to 4 wk, all suggestive of increased stress.19 However, daily handling of singly housed rats mitigated these responses, resulting in corticosterone and ACTH levels similar to those of socially housed rats, with no differences in weight gain or development of anhedonia.19 Another paper also demonstrated that frequent handling mitigates the stress response to handling in rats; this study reported that handling rats twice per week was associated with lower anxiety-related scores in an open-field test as compared with rats that had not been handled.11 Tickling, in which the rough and tumble play of rats is mimicked by the human handler, has been touted as a method to improve the interactions between rats and their handlers.6,15 Studies examining tickling have demonstrated increased emittance of positive ultrasonic vocalizations, decreased anxiety measures, improved latency to approach responses, and thus reduction in the overall fear of handling.6,15 However, these approaches cannot themselves be viewed as handling techniques and they does not address the issue of how best to remove the animal from their cage (Figure 1).
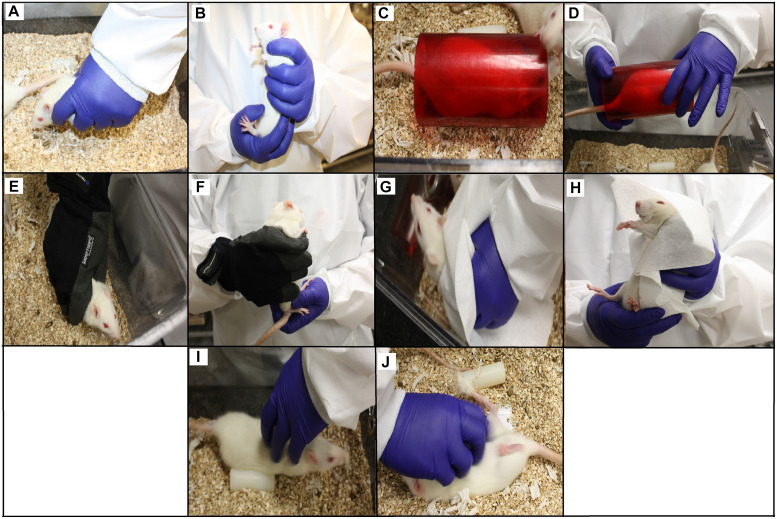
Figure 1.
Handling methods used in the home cage and after removal from the home cage. (A, B) encircling the torso and securely grasping the rat around the thorax; (C, D) using the tunnel; (E, F), using a protective glove; (G, H) placement of a Wypall over the rat; (I, J) tickling prior to removing the rat from the home cage via the thorax.
While the above-described methods address maintaining an environment that promotes low stress handling, the issue of how best to handle rats that show extreme stress or aggression has received little scientific attention. In most cases, recommendations appear to come from health and safety groups, which recommend the use of additional protective equipment for the handler, without consideration of the effect that this may have on the animal.28,36 Protective gloves made of thick leather or Para-aramid synthetic fiber are commonly recommended, but these gloves can reduce the handler’s dexterity, making the rat feel less secure.27,39 Safe animal handling practices have been well-studied in companion animals, with processes such as counter-conditioning, the use of head collars, and the use of towel wraps being highly recommended.10,39 The use of a towel wrap has great potential for handling aggressive rats; the additional protective layer between the rat and the handler protects the handler, perhaps without impairing dexterity, acting as a visual barrier between the handler and the rat.1,22,39 This method is easily implemented and requires minimal training of both the animal and personnel as compared with the counter conditioning or head collar methods.
In the toxicology setting, study design is often dictated by the type of test article being studied and the requirements of the applicable regulatory agency. As a result, animal care and veterinary staff have less control over factors such as social housing and frequency of rat manipulation. The main focus of the current study was to improve animal welfare and reduce stress and anxiety in our rats during handling. The study was designed to identify possible methods for handling of rats to minimize handling stress, particularly in studies that require rats to be individually-housed and/or handled on a limited basis (weekly), as well as to identify the best methods to use for rats that show signs of aggression. We compared these approaches with our site standards of social housing and removing rats from their cages by encircling the thorax. We also evaluated use of a tunnel, use of a soft, absorbent paper towel (Wypall, Kimberly Clark, Irving, TX), and use of a protective glove. We additionally evaluated the effect of tickling prior to using the standard thoracic hold to determine whether a potentially positive experience paired with standard handling would reduce handling stress.
We hypothesized that rats handled using a paper towel or the standard thoracic method would be less fearful than those handled via other methods. We also hypothesized that socially-housed rats would be less anxious about handling than those that were housed individually, and that rats that were handled more frequently would be less anxious than those handled less frequently.
Materials and Methods
Ethical statement.
The study was approved by Charles River Mattawan’s Institutional Animal Care and Use Committee (IACUC). Charles River Mattawan is an AAALAC-accredited institution.
Animals.
One hundred and 40 (70 female and 70 male), 8-wk-old Crl:CDSD rats (Charles River Laboratories, Raleigh, NC) were placed on study. The rats were housed in open-topped, transparent polycarbonate cages (916 cm2 × 18.4 cm in height; Allentown, Allentown, NJ), initially in groups of 2 to 3 prior to study, and then singly or in pairs, depending on treatment group, when the study began. Bedding was aspen woodchips (Northeastern Products Corp, Warrensburg, NY); municipal tap water and feed (Lab Diet Certified Rodent Diet #5002, PMI Nutrition International, St. Louis, MO) were both available ad libitum. The drinking water was treated with 0.5 to 2 ppm chlorine dioxide and filtered to 5 μm. Water was tested quarterly for coliform bacteria and heterotrophic plate count, and annually for all primary contaminants included in the United States Environmental Protection Agency National Primary Drinking Water Regulations.37
All cages contained one rat tunnel (BioServ, Flemington, NJ), one Diamond Twist (Envigo, Madison, WI), and a chewable nylon rod (Total Plastics, Kalamazoo, MI). Temperature and relative humidity were monitored and recorded daily, and maintained between 70 and 77 °F (21 and 25 °C) and 27 to 73%, respectively. Fluorescent lighting was provided on a 12 h:12-h light:dark cycle (lights on at 0600 and off at 1800), with all functions performed during the light cycle. Rats were ordered to be free of Hantaan Virus, Kilhams Rat Virus, Lymphocytic Choriomeningitis Virus, Mouse Adenovirus, Pneumonia Virus of Mice, Rat Minute Virus, Rat Parvovirus 1, Rat Theliovirus, Respiratory Enteric Virus III, Sendai Virus, Sialodacryoadenitis Virus, Toolan H-1 Parvovirus, Bordetella bronchiseptica, Pneuomocystis carinii, CAR bacillus, Clostridium piliforme, Corynebacterium kutscheri, Klebsiella oxytoca, Mycoplasma pulmonis, Pasteurella penumotropica, Pseudomonas aeruginosa, Salmonella spp., Staphylococcus aureus, Streptobacillus moniliforis, Streptococcus pneumoniae, and Encephalitozoon cuniculi, as well as endo- and ectoparasites. Colony health surveillance was performed quarterly using sentinel rats housed in open-topped caging. Seroconversion to Pneuomocystis carinii occurred, and PCR testing was positive for Helicobacter ganmani, Beta Streptococcus sp. (group B), Entamoeba spp., and Syphacia muris; rats remained free of all other diseases. Only essential husbandry functions were performed for the first 7 d after arrival to allow acclimation for all rats but those assigned to the “tickling” group; those rats were habituated to the tickling procedure for 3 d prior to study start (see below for additional details). All rats survived until study termination (18 wk after the start of the study) with no adverse events. After study completion, the rats were transferred to the facility’s training colony and used for various training purposes.
Study groups.
Ten females and 10 males were assigned to each of the 7 study groups using a standard, weight-based (± 20% of the mean body weight) randomization procedure that was performed using Provantis 9 (Instem, Philadelphia, PA). This number was chosen as a commonly used group size in toxicology studies of similar type and duration (10 main study animals per sex per group if not performing recovery testing and not collecting TK samples). Formal sample size calculations were not conducted as these fell within facility and standard toxicology study guidelines.
All rats were handled by using nitrile gloves. A total of 20 handlers participated in the study, alternating between groups and never handing the same group twice, to avoid handler bias. All individuals who performed handling and other functions had completed our formal training program for each function, were deemed proficient in the function by an approved trainer or veterinarian, and regularly performed the function on other studies in the facility. Table 1 describes the housing and handling parameters of each of the study groups. A soft, absorbent paper towel (Wypall) was used in place of a towel as it was more conducive to maintaining cleanliness and preventing test-article cross-contamination in the toxicology laboratory environment. All data collected was recorded in Provantis 9 (Instem, Philadelphia, PA).
Table 1.
Study group design
| Treatment Group (abbreviation)a | Housing Type | Handling Frequency | Handling Methods (Figure 1) |
|---|---|---|---|
| A (So/W/Et) | Pair | Weekly | Encircling of torso (standard thoracic hold) |
| B (Si/D/Et) | Single | Daily | Encircling of torso |
| C (Si/W/Et) | Single | Weekly | Encircling of torso |
| D (Si/W/Tu) | Single | Weekly | Tunnel |
| E (Si/W/Pg) | Single | Weekly | Protective glove |
| F (Si/W/Wy) | Single | Weekly | Soft, absorbent paper towel (Wypall) |
| G (Si/W/Ti) | Single | Weekly | Tickling followed by encircling of torso |
So/W/Et, socially housed, handled weekly via encircling the thorax; Si/D/Et, singly housed, handled daily via encircling the thorax; Si/W/Et, singly housed, handled weekly via encircling the thorax; Si/W/Tu, singly housed, handled weekly via a tunnel; Si/W/Pg, singly housed, handled weekly using a protective glove; Si/W/Wy, singly housed, handled weekly using a Wypall; Si/W/Ti, singly housed, handled weekly and tickled.
Tickling procedure.
All rats in group 8 were subjected to three 15-s tickling sessions for 3 consecutive days, as described previously,14 in the week prior to study start to habituate them to the tickling process. After this habituation, rats received one 15-s tickling session immediately before each handling session for basic evaluations and other associated tests.
Detailed clinical observations and body weights.
Detailed clinical observations were performed weekly concurrent with handling. Observations included, but were not limited to, evaluation of the skin, fur, eyes, ears, nose, oral cavity, thorax, abdomen, external genitalia, limbs and feet, respiratory and circulatory systems, and the nervous system, and included any evidence of unusual reactivity to handling, vocalization, or other unusual behaviors. To allow a complete examination to be performed, rats handled with the tunnel were removed by gently tipping the tunnel toward the handler’s hand; rats were restrained via the thorax. Body weights were measured weekly concurrent with handling. Individuals performing these activities were not blind to handling methods due to the need to use the appropriate handling method handling. Handling, weighing, and observation sessions required approximately 2 to 4 min per session.
Interaction test.
An interaction test that was based on a previously described test was performed the day before study start to establish a baseline and again on the day of study termination to evaluate the willingness of the rats to voluntarily interact with their handler and any handling-associated devices.12 After one minute of standing motionless in front of the cage, the handler placed a gloved hand (Treatment groups A through C and G) or a gloved hand with a tunnel, bite glove, or Wypall (Treatment groups D through F, respectively) into the cage for 1 min while keeping the hand and associated device motionless. The duration of the following behaviors was measured: 1) sniffing the hand or associated device, 2) entry into or under the hand of handling device, 3) climbing on the hand or handling device, and 4) peeking into or under hand or handling device and retreating immediately. The rat was then handled for scheduled basic evaluations, and then the test was repeated once the rat was returned to the cage. Individuals performing the functions were not blind to handling methods due to need to use the appropriate handling method handling to perform the study evaluations. To assess rats that were socially housed, the conspecific was removed from the cage before evaluation of the rat of interest.
Abbreviated functional observation battery examinations.
Every other week, starting on the first day of handling, all rats were evaluated using an abbreviated functional observation battery. Rats were removed from the home cage using the assigned handling method and were observed for reactions to handling; pair-housed animals were removed one at a time, with each animal scored independently and only at the time of handling. Handling reactivity was scored using the descriptions included in Table 2. Rats were then placed in the corner of a standard open-field testing box (length: 53.0 cm, width: 53.0 cm, height: 19.1 cm) and observed. The number of grids entered with both forefeet over one minute were counted and recorded (motor activity grid). The box was rinsed with tepid water and then dried using a clean paper towel between rats. Individuals performing the tests were not blind to handling methods due to the need to use the appropriate handling method handling to perform study activities.
Table 2.
Scoring system for reactivity to handling (component of the abbreviated functional observational battery testing)
| Score | Behavioral Reactivity |
|---|---|
| 0 | Very low (rat is totally limp or otherwise unresponsive) |
| 1 | Low (no resistance, easy to handle) |
| 2 | Moderately low (slight resistance, with or without vocalization) |
| 3 | Moderately high (may freeze, be tense, or rigid in hand, with or without vocalization) |
| 4 | High (squirming, twisting, or attempting to bite, with or without vocalizations) |
Hematology measurements.
All rats had 1.0 mL of whole blood collected via the sublingual vein into a Vacuette K2EDTA blood collection tube (Greiner Bio-One, Monroe, NC) 2 d before study start and on the day of study termination. Parameters were assessed using the Advia2120i hematology system (Siemens, Malvern, PA) and included red blood cell counts, red blood cell distribution width, absolute reticulocyte count, and counts of white blood cells, basophils, eosinophils, lymphocytes, neutrophils, and monocytes, measurement of hematocrit, hemoglobin, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, mean corpuscular volume, platelet counts, and mean platelet volume. All analyses were completed by individuals who were blind to treatment groups.
Statistical analyses.
All statistical tests were completed in SAS 9.4 and SAS/STAT 15.1 (Statistical Analysis Software, Cary, NC). Treatment groups A and C were considered to be control groups, with group A representing our standard handling practice for handling pair-housed rats on restricted-handling studies, and group C representing our standard handling practices for singly-housed rats that are on restricted-handling studies. Body weight, body weight change, hematologic parameters, and continuous abbreviated functional observational battery (FOB) endpoints were compared using one-way ANOVAs. Assumptions of normality and homogeneity of variances were tested using the Shapiro–Wilk and Levene tests, respectively, to determine if a log transformation or rank transformation was needed. A Dunnett adjustment was used to correct for multiple comparisons to single control groups.21 For the continuous abbreviated FOB endpoints, the data were not normally distributed and therefore were analyzed using a nonparametric Kruskal–Wallis test with a Dunn adjustment for P values.21 A general linear model was constructed for the motor activity FOB data and a simulated adjustment method was applied to the P values.
Hematology data were analyzed with a one-way ANOVA with an adjustment applied. Logistic regression was used for the interaction test data with adjustment applied. Cochran–Mantel–Haenszel tests were used to compare mean scores and standard deviations for detailed clinical observations. If differences in observations were significant, pairwise tests were conducted comparing each treatment group to the control groups; adjustments to the P value for multiple comparisons were made using the Stepdown Sidak method.
A significance level of P ≤ 0.05 was used for all statistical tests. In all statistical analyses, the individual animal was considered to be the experimental unit; however, for detailed clinical observations, additional analyses were conducted using each type of observation rather than each individual animal as the experimental unit, because one animal could repeatedly display an individual observation over time. These have been reported in the applicable sections as “total number of animals affected” and “total number of observations made” or “total observations”, respectively. This allowed the determination of whether observations could be attributed to individual rats or appeared equally in all rats.
Results
Body weights and detailed clinical observations.
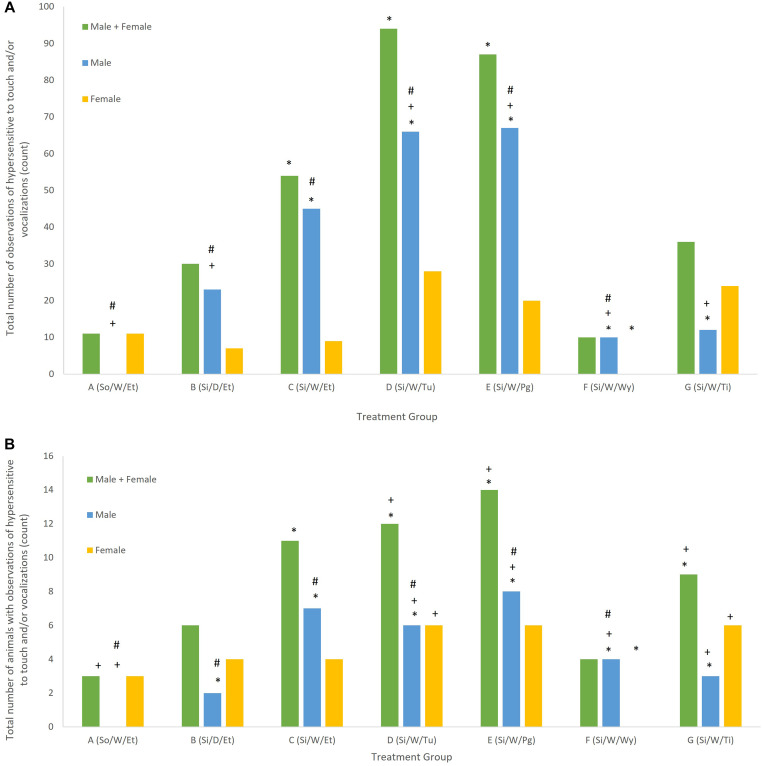
No significant changes were detected in body weights or body weight changes between groups. The behavioral observations of “hypersensitivity to touch” (defined as “an exaggerated response when touched”) and “vocalization” were recorded under the “Detailed Clinical Observations” category; these responses differed significantly from controls with regard to both the total number of observations made (where an observation may be repeated by the same rat) and the total number of rats affected. When combining all data across time, rats that were pair-housed and handled weekly via the torso were significantly less hypersensitive to touch (P < 0.01) and vocalized significantly less (P < 0.01) than did singly housed rats that were handled weekly either via the torso, by using a tunnel, or with a protective glove. Similar statistically significant trends were detected in individual rats rather than the total number of events. Vocalizations from individual rats were not significantly different as compared with pair-housed rats, but all groups that were significantly more hypersensitive to touch were also significantly different when evaluated as individuals (P < 0.01). Rats that were pair-housed and handled weekly were also significantly less hypersensitive to touch as compared with rats that were singly housed and handled daily, or singly housed and tickled weekly (P < 0.01), while vocalization was not different between these groups. Rats that were singly housed and handled using the tunnel or the protective glove vocalized significantly more (P < 0.01) than singly housed rats with weekly torso handling when assessed based on the total number of events or the total number of rats. Rats that were singly housed and handled using a Wypall were significantly less hypersensitive to touch and vocalized significantly less than rats that were singly housed and handled weekly via the torso (P < 0.01); they did not differ significantly from rats that were pair-housed and handled weekly via the torso (Figure 2).
Figure 2.
(A) Total overall number of observations of “hypersensitive to touch” and/or “vocalization”; (B) Total overall number of rats noted with observations of “hypersensitive to touch” and/or “vocalization”. *Significantly different from group A (pair housed, handled weekly by encircling the torso) (P < 0.01). +Significantly different from group C (singly housed, handled weekly by encircling the torso) (P < 0.05). #Males significantly different from females (P < 0.01).
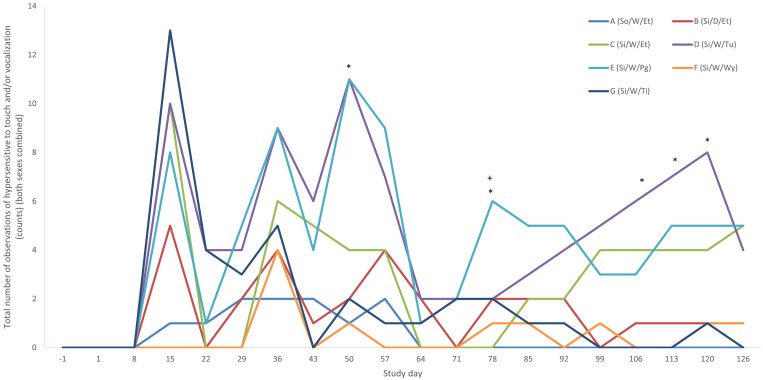
When examining the data at each time point, individual animal differences were not detected until day 15 of the study. At that time, singly housed rats that were tickled were significantly more hypersensitive to touch than were rats that were pair-housed and handled via the torso (P < 0.01). Additional differences between groups were detected on day 78, when rats that were singly housed and handled using the glove were significantly more vocal (P < 0.05) than rats that either were singly- or pair-housed and handled weekly via the torso. When the combined total number of observations made from all animals in each group were examined (Figure 3), no significant differences between treatment groups were observed until day 15, where results were the same as when individual animals were examined. However, significant differences were detected prior to day 78, with rats that were singly housed and handled using either the glove or the tunnel were significantly more hypersensitive to touch and vocalized more than did pair-housed rats that were handled by the torso on day 50 (P < 0.01). By day 78, rats handled by tunnel were also significantly more hypersensitive to touch or vocalized more than either singly- or pair-housed, torso-handled rats (P < 0.05). These significant effects were lost and then but reappeared in 3 of the 4 final weeks of the study (days 106, 113, and 120), with rats that were singly housed and handled using the tunnel being significantly more hypersensitive to touch or significantly more likely to vocalize than were pair-housed rats handled via the torso (P < 0.05).
Figure 3.
Total number of observations of “hypersensitivity to touch” and/or “vocalization” at each time point (both sexes combined). *Significantly different from group A (pair housed, handled weekly by encircling the torso) (P < 0.05). +Significantly different from group C (singly housed, handled weekly by encircling the torso) (P < 0.05).
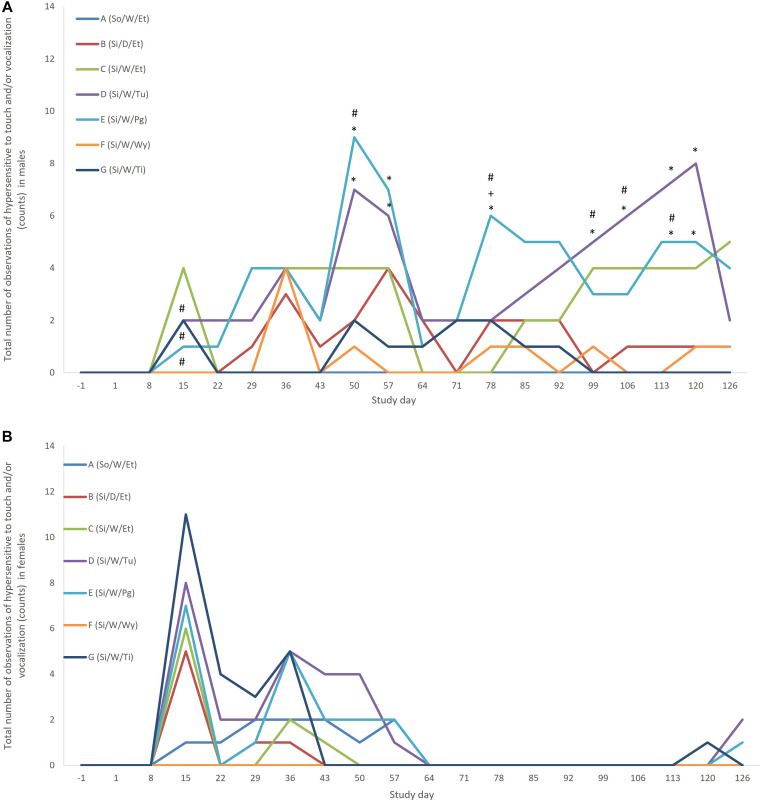
Overall, males were significantly more hypersensitive to touch and vocalized significantly more than as compared with females. When total numbers of observations were pooled across time, singly housed males handled by the torso daily, by the torso once weekly, by the tunnel, by protective glove (P < 0.01 for all), and by the Wypall (P < 0.05), vocalized significantly more and/or were significantly more hypersensitive to touch than were females. The only case where males were less vocal and/or less reactive than females was when they were pair-housed and handled weekly via the torso (P < 0.01). Similar patterns were seen in individual rats, but males were significantly less reactive or less vocal than females that were singly housed and handled daily via the torso, suggesting that the aforementioned significant difference was likely due to a small number of individual rats. Unlike males, females were significantly less reactive to touch or vocalized less when singly housed and handled with a Wypall than when they were pair-housed and handled via the torso regardless of whether individual rats or total observations were assessed (P < 0.05). Over time, only males contributed to the significant differences discussed above (Figure 4).
Figure 4.
(A) Total number of observations of “hypersensitive to touch” and/or “vocalization” at each time point in males; (B) Total number of observations of “hypersensitive to touch” and/or “vocalization” at each time point in females. *Significantly different from group A (pair housed, handled weekly by encircling the torso) (P < 0.05). +Significantly different from group C (singly housed, handled weekly by encircling the torso) (P < 0.05). #Males significantly different from females (P < 0.05).
Interaction test.
Significant effects in the interaction test were detected only for the interaction of sex and handling (P < 0.01), both before and after the study (Table 3). Prior to study start, pair housed males handled weekly were less likely to interact with their handler and handling equipment than were pair housed females handled weekly (P < 0.01), as were tunnel-handled males compared to tunnel-handled females (P < 0.01). While this effect did not persist throughout the study for the pair housed males, it continued for the tunnel-handled males (P < 0.01) and developed for the males handled weekly using the glove (P < 0.01) and the Wypall (P < 0.05). Aside from when female rats were pair housed and handled weekly, all handling paradigms resulted in female rats being significantly more willing to interact with their handler and handling equipment on the final day of study as compared to before the study (P < 0.05); males, however, only increased interactions if pair housed and handled weekly (P < 0.01) or if singly housed and handled via a Wypall (P < 0.05).
Table 3.
Interaction test values before and after study and end of study (least squares mean ± SEM)
| Time of Testing | Treatment group | A (So/W/Et) | B (Si/W/Et) | C (Si/W/Et) | D (Si/W/Tu) | E (Si/W/Pg) | F (Si/W/Wy) |
|---|---|---|---|---|---|---|---|
| Before study | Male | 0.1 ± 0.7 | 2.4 ± 0.3 | <0.1 ± >0.0 | 2.0 ± 0.4 | 1.7 ± 0.4 | 2.0 ± 0.3 |
| Female | 2.5 ± 0.3* | 1.9 ± 0.4 | 1.1 ± 0.5 | 3.1 ± 0.3* | 2.7 ± 0.3 | 2.5 ± 0.3 | |
| After study | Male | 2.8 ± 0.2 | 3.0 ± 0.1 | 3.0 ± 0.2 | 2.4 ± 0.2* | 2.4 ± 0.2* | 2.8 ± 0.2* |
| Female | 3.2 ± 0.1 | 3.3 ± 0.1 | 3.2 ± 0.1 | 3.7 ± 0.1 | 3.6 ± 0.1 | 3.4 ± 0.1 |
Males significantly different from females (P < 0.05).
Abbreviated functional observational battery.
When both sexes were combined, reactivity to handling was significantly higher in rats handled using a tunnel as compared with pair-housed rats handled via the torso on days 43 (P < 0.01) and 85 (P < 0.05); this also occurred on day 85 for rats handled using the glove (P < 0.05). Overall, all handing reactivity scores remained relatively low regardless of handling or housing type. Activity on the grid was significantly lower on day 57 in rats handled using the glove as compared with pair-housed rats handled via the torso (P < 0.05). By day 99, all versions of handling were associated with significantly less motor activity as compared with pair housed rats that were handled via the torso (P < 0.01). By day 113, all handling methods were associated with significantly less motor activity as compared with the pair housed rats (P < 0.01).
No significant differences were detected overall between handling groups in males for either motor activity or handling reactivity (Tables 4 and 5). However, when examining effects over time, differences were detected in males starting day 71. Males that were singly housed and handled via the torso and using a Wypall were significantly more active in the open field than were rats that were pair housed and handled via the torso (P < 0.05). Males handled using either a tunnel or the protective glove were significantly less active than rats that were singly housed and handled via the torso (P < 0.01). Males that were singly housed and handled via the torso, a tunnel and Wypall were significantly less active on day 99 than pair-housed males that were handled via the torso (P < 0.05). On the final day of study, males handled using a tunnel, Wypall, or that were tickled before handling all had significantly less activity in the open field than did pair-housed rats handled via the torso (P < 0.01). The only significant difference over time for handling reactivity in males occurred on day 85 when rats handled using the glove had a higher reactivity score than did pair-housed rats that were handled via the torso (P < 0.01)
Table 4.
Overall open-field activity scores (mean ± SD)
| Treatment Group | A (So/W/Et) | B (Si/W/Et) | C (Si/W/Et) | D (Si/W/Tu) | E (Si/W/Pg) | F (Si/W/Wy) | G (Si/W/Ti) |
|---|---|---|---|---|---|---|---|
| Male | 17.2 ± 8.7 | 18.3 ± 5.6 | 16.9 ± 5.1 | 15.8 ± 6.2 | 17.4 ± 7.1 | 15.9 ± 6.5 | 16.9 ± 6.4 |
| Female | 25.2 ± 7.6 | 23.8 ± 6.3 | 23.1 ± 6.2 | 23.4 ± 5.9 | 20.0* ± 6.3 | 24.7 ± 8.6 | 22.5 ± 5.3 |
Significantly different from group A (P < 0.01).
Table 5.
Number of animals scoring at each interaction test score overall
| Sex | Score | A (So/W/Et) | B (Si/W/Et) | C (Si/W/Et) | D (Si/W/Tu) | E (Si/W/Pg) | F (Si/W/Wy) | G (Si/W/Ti) |
|---|---|---|---|---|---|---|---|---|
| Males | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 84 | 86 | 73 | 54 | 63 | 80 | 83 | |
| 2 | 6 | 4 | 15 | 30 | 24 | 9 | 7 | |
| 3 | 0 | 0 | 2 | 5 | 2 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | |
| Females | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 77 | 88* | 86 | 60* | 73 | 77 | 72 | |
| 2 | 13 | 2 | 4 | 30 | 17 | 12 | 17 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Significantly different from group A (P < 0.05).
In females, overall open-field activity was significantly lower for rats handled using the protective glove than for pair-housed rats handled via the torso (P < 0.01) (Table 4). Handling reactivity scores were also more frequently lower for singly-housed females that were handled daily (P < 0.05), and were more frequently higher for females that were handled using the tunnel (P < 0.05), compared with females that were pair-housed and handled via the torso (Table 5). Similar to males, these and additional differences developed as the study progressed. Starting on day 57, females handled using a protective glove had significantly less activity in the open field than did those that were pair housed and handled via the torso (P < 0.01). This difference persisted until Day 71, when it was again lower than in pair and singly housed rats handled via the torso (P < 0.05 and < 0.01, respectively). On Day 99, activity was lower in singly housed rats handled via the torso (P < 0.05), the protective glove (P < 0.01), and the Wypall (P < 0.01), as compared with pair housed females handled via the torso. Females handled via the tunnel were significantly more active than were singly housed females handled via the torso (P < 0.05). On the final day of study, females handled via the tunnel, the protective glove, and the Wypall were significantly less active than pair housed females that were handled via the torso (P < 0.01). Females handled via the protective glove and the Wypall were significantly less active than singly housed females that were handled via the torso (P < 0.01).
Hematology measurements.
Significant group differences (P < 0.05) were detected between groups before the study in mean corpuscular hemoglobin (pg), mean corpuscular hemoglobin concentration (g/dL), mean corpuscular volume (fL), and absolute reticulocyte and neutrophil counts, but all values were within the normal historical range for Crl:CDSD rats of the applicable age group housed at Charles River Mattawan. No significant differences were noted between groups in any of the values measured at the end of study.
Discussion
The current study assessed both commonly used and novel handling methods to identify potential low stress handling options in rats and to protect the handler in the case of aggression. Our study also compared the effects of handling frequency and pair housing on handling.
Overall, pair housing and handling the rat by the torso appeared to attenuate reactivity and vocalization in response to handling as compared with single housing and other types and frequencies of handling. Rats that were individually housed but handled using the same method and at the same frequency showed greater hypersensitivity to handling as compared with pair-housed rats, indicating stress reduction. A similar effect also developed with regard to open-field activity, although significant effects were sporadic early in in the study. Because low activity in an open-field test can be attributed to increased anxiety,29 the greater total locomotor activity seen in pair housed rats, combined with the lower hypersensitivity to touch and vocalization, suggests an overall lower anxiety level in these rats. The frequency of handling appeared to have less effect on anxiety than did pair housing did. The protective effects of pair housing on stress and anxiety, also known as “social buffering,” have been supported by the literature for several decades and reduce both physiologic and behavioral parameters related to stress.2,8,13,19,30 Therefore, our data suggests that pair housing reduces handling-related stress and anxiety to a greater degree than does more frequent handling.
Handling using the protective glove or the tunnel appeared to be most aversive to the rats as compared with the other handling methods assessed. The negative effect of the protective glove is not surprising, as protective gloves can cause additional stress, especially as the handler’s dexterity is limited.27 In the case of cats and dogs, the use of protective gloves is recommended when no less stressful alternatives are not available.39 Our data suggest a similar approach should be taken for rats. The negative effect of using a tunnel was somewhat surprising given the anxiolytic effect of tunnel handling in mice9,12. Rats and mice are both prey species for which burrowing is a naturalistic behavior for protection and evasion of predators, and the tunnel could potentially mimic the burrow, thus providing a shelter and a feeling of safety.7,16,18 The fact that the tunnel was not associated with anxiety until later in the study, and was associated with anxiety more frequently in males may provide some insight into why the tunnel did not perform as anticipated. A possible factor may be tunnel size relative to animal size. The growth curve of rats is rapid during the first 10 to 15 wk of life, particularly for Crl:CDSD rats, with males growing at a much higher rate than females.4,5,35 Therefore, over time, the ratio of the size of the rat to the available space in the tunnel visibly decreased, especially in males. Mice, on the other hand, show relatively shallow growth curves after 5 to 6 wk of age,5,32,33 suggesting that available space in the tunnel is less compromised as the mice age. The soft, plastic restraint cone used in a previous study also provided minimal free space available the rats.3 Technicians working on the current study noted that toward the end of the study, rats became more resistant to voluntarily entering the tunnel and more difficult to extract from the tunnel. Therefore, the tunnel itself may not have been aversive, but space limitations in the tunnel made entering and/or exiting the tunnel more difficult for the rats and therefore more stressful. Future studies could compare tunnels of larger diameters that maintain the rat:space ratio in the tunnel to test this idea.
Although tickling did not seem to cause any meaningful change in anxiety levels in females, singly housed males appeared to react to tickling with more signs of anxiety and stress as compared with pair housed male rats that were handled via the torso. The successes of tickling have primarily been reported in young, singly housed rats, with social housing minimizing the effect.15 Sex differences in both conspecific play, which tickling is supposed to mimic, and in response to actual tickling, have been reported.26,34 Play is thought to become “rougher” after males reach sexual maturity,25 and thus tickling may begin to elicit a more defensive, and therefore stress-inducing, response from males as they age, such that tickling may be less beneficial to sexually-mature rats, especially males.15 In fact, the 3Rs Collaborative recommends caution when exposing older rats to tickling for the first time, and indicates that older females tend to respond better than older males.23 Combined with the results of our study, this suggests that tickling may not be helpful in reducing stress and anxiety in mature male rats, and may actually promote stress responses.
The use of a Wypall had variable effects in the current study. Although this method seemed to be better overall in terms of reducing hypersensitivity to touch and vocalizations of singly housed rats that were handled via the torso, open-field activity was more variable. Early in the study, open-field activity was higher in males handled with the Wypall, suggesting less anxiety. However, activity was later lower in both males and females as compared with torso handling in pair housed rats and in females as compared with singly housed, torso-handled rats, suggesting a potential increase in anxiety toward Wypall handling over time.
Although our study provided insight into handling preferences of rats, it nonetheless has several limitations with regards to its general application to all rats. Due to the need to perform animal evaluations in association with handling in most cases, evaluators could not be blind to the handling treatment group of each rat. Because a large number of individuals participated in performing these evaluations and never evaluated the same parameters for the same group, our expectation was that observational bias would likely be reduced due to the number of individuals performing the assessments, but a blinded study would be preferable. The current study also exclusively examined Crl:CDSD rats. However, Sprague–Dawley rats may show more depressive-like behaviors and isolation-induced anxiety than do Wistar and Lister hooded rats.38 In addition, because our study was directed toward stress reduction in specific toxicology protocols, all novel handling methods were compared using singly housed rats. As both the current study and the literature suggest, social housing will likely alter the response of rats to handling. Finally, the majority of differences between the groups didn’t begin to emerge until later in the study. Extended studies could evaluate handling preferences of rats as they age.
Acknowledgments
We thank Charles River Mattawan’s operational staff for their technical skills in performing the handling functions, behavioral assays, and blood collection and analysis, Charles River Mattawan’s Environmental Health and Safety Services Team who encouraged and supported this study, and the animals who made this study possible.
References
- 1. Anseeuw E, Apker C, Ayscue C, Barker L, Blair D, Brennan J, Brooks S, Case-Pall D, Caspersen H, Clark J, Colson L, Covill A, DeLong J, Dickey D, Harr K, Heine N, Krishna G, Lynch K, Maki J, Malamed R, McAuliffe M, McLucas B, Mengering C, Mulligan L, Nicholson M, Rodan I, Schobert-Nichols H, Steele A, Young R. 2006. Handling cats humanely in the veterinary hospital. J Vet Behav 1:84–88. 10.1016/j.jveb.2006.06.003. [DOI] [Google Scholar]
- 2. Baker S, Bielajew C. 2007. Influence of housing on the consequences of chronic mild stress in female rats. Stress 10:283–293. 10.1080/10253890701265362. [DOI] [PubMed] [Google Scholar]
- 3. Baturaite Z, Voipio HM, Ruksenas O, Luodonpää M, Leskinen HK, Apanaviciene N, Nevalainen T. 2005. Comparison of and habituation to four common methods of handling and lifting of rats with cardiovascular telemetry. Scand J Lab Anim Sci 32:137–148. [Google Scholar]
- 4. Brower M, Grace M, Kotz CM, Koya V. 2015. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources. Lab Anim Res 31:166–173. 10.5625/lar.2015.31.4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles River Laboratories. [Internet]. 2022. CD® (Sprague Dawley) IGS rat. [Cited 10 December 2022]. Available at: https://www.criver.com/products-services/find-model/cd-sd-igs-rat?region=3611.
- 6. Cloutier S, Wahl K, Baker C, Newberry RC. 2014. The social buffering effect of playful handling on responses to repeated intraperitoneal injections in laboratory rats. J Am Assoc Lab Anim Sci 53:168–173. [PMC free article] [PubMed] [Google Scholar]
- 7. Deacon RM. 2009. Burrowing: A sensitive behavioural assay, tested in five species of laboratory rodents. Behav Brain Res 200:128–133. 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 8. Gornicka-Pawlak E, Jabłońska A, Chyliński A, Domańska-Janik K. 2009. Housing conditions influence motor functions and exploratory behavior following focal damage of the rat brain. Acta Neurobiol Exp (Warsz) 69:62–72. [DOI] [PubMed] [Google Scholar]
- 9. Gouveia K, Hurst JL. 2013. Reducing mouse anxiety during handling: effect of experience with handling tunnels. PLoS One 8:e66401. 10.1371/journal.pone.0066401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herron ME, Shreyer T. 2014. The pet-friendly veterinary practice: A guide for practitioners. Vet Clin North Am Small Anim Pract 44:451–481. 10.1016/j.cvsm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 11. Holson RR, Scallet AC, Ali SF, Turner BB. 1991. “Isolation stress” revisited: Isolation-rearing effects depend on animal care methods. Physiol Behav 49:1107–1118. 10.1016/0031-9384(91)90338-O. [DOI] [PubMed] [Google Scholar]
- 12. Hurst JL, West RS. 2010. Taming anxiety in laboratory mice. Nat Methods 7:825–826. 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- 13. Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. 2004. Partner’s stress status influences social buffering effects in rats. Behav Neurosci 118:798–804. 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- 14. LaFollette MR, O’Haire ME, Cloutier S, Gaskill BN. 2018. Practical rat tickling: Determining an efficient and effective dosage of heterospecific play. Appl Anim Behav Sci 208:82–91. 10.1016/j.applanim.2018.08.005. [DOI] [Google Scholar]
- 15. LaFollette MR, O’Haire ME, Cloutier S, Blankenberger WB, Gaskill BN. 2017. Rat tickling: A systematic review of applications, outcomes, and moderators. PLoS One 12:e0175320. 10.1371/journal.pone.0175320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: A review and prospectus. Can J Zool 68:619–640. 10.1139/z90-092. [DOI] [Google Scholar]
- 17. Machholz E, Mulder G, Ruiz C, Corning BF, Pritchett-Corning KR. 2012. Manual restraint and common compound administration routes in mice and rats. J Vis Exp 67:e2771. 10.3791/2771-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makowska IJ, Weary DM. 2016. The importance of burrowing, climbing and standing upright for laboratory rats. R Soc Open Sci 3:160136. 10.1098/rsos.160136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manouze H, Ghestem A, Poillerat V, Bennis M, Ba-M’hamed S, Benoliel JJ, Becker C, Bernard C. 2019. Effects of single cage housing on stress, cognitive, and seizure parameters in the rat and mouse pilocarpine models of epilepsy. eNeuro 6:ENEURO.0179-18.2019. 10.1523/ENEURO.0179-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manzoor M, Raza S, Chaudhry B. 2013. Proficient handling and restraint of the laboratory animal rat (Rattus Norvegicus) facilitate essential biochemical and molecular level studies in biomedical sciences. J Pharmacol Biol Sci 6:21–33. [Google Scholar]
- 21. Milliken GA, Johnson DE. 1992. Analysis of messy data, Vol 1: Designed experiments. Boca Raton (FL): Chapman & Hall/CRC. [Google Scholar]
- 22. Moffat K. 2008. Addressing canine and feline aggression in the veterinary clinic. Vet Clin North Am Small Anim Pract 38:983–1003. 10.1016/j.cvsm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 23.North American 3Rs Collaborative. [Internet]. 2022. Rat tickling background & FAQ. [Cited 28 November 2022]. Available at: https://www.na3rsc.org/rat-tickling-faq/.
- 24. Patki G, Atrooz F, Alkadhi I, Solanki N, Salim S. 2015. High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci Lett 584:308–313. 10.1016/j.neulet.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pellis SM. 2002. Sex differences in play fighting revisited: Traditional and nontraditional mechanisms of sexual differentiation in rats. Arch Sex Behav 31:17–26. 10.1023/A:1014070916047. [DOI] [PubMed] [Google Scholar]
- 26. Pellis SM, Pellis VC. 1990. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol 23:215–231. 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- 27. Riemer S, Heritier C, Windschnurer I, Pratsch L, Arhant C, Affenzeller N. 2021. A review on mitigating fear and aggression in dogs and cats in a veterinary setting. Animals (Basel) 11:158. 10.3390/ani11010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmitt JM, Wilson DE, Raber JM. 2018. Occupational safety and health, chapter 14. In: Weichbrod RH, Thompson GAH, Norton JN, editors. Management of Animal Care and Use Programs in Research, Education, and Testing , 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- 29. Sestakova N, Puzserova A, Kluknavsky M, Bernatova I. 2013. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol 6:126–135. 10.2478/intox-2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharp J, Zammit T, Azar T, Lawson D. 2003. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci 42:9–18. [PubMed] [Google Scholar]
- 31. Steimer T. 2002. The biology of fear- and anxiety-related behaviors. Dialogues Clin Neurosci 4:231–249. 10.31887/DCNS.2002.4.3/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taconic Biosciences. [Internet]. 2022. Swiss Webster. [Cited 10 December 2022]. Available at: https://www.taconic.com/mouse-model/swiss-webster.
- 33.The Jackson Laboratory. [Internet]. 2022. Body weight information for C57BL/6J. [Cited 10 December 2022]. Available at: https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000664.
- 34. Tivey EKL, Martin JE, Brown SM, Bombail V, Lawrence AB, Meddle SL. 2022. Sex differences in 50 kHz call subtypes emitted during tickling-induced playful behaviour in rats. Sci Rep 12:15323. 10.1038/s41598-022-19362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turner KM, Burne TH. 2014. Comprehensive behavioural analysis of Long Evans and Sprague-Dawley rats reveals differential effects of housing conditions on tests relevant to neuropsychiatric disorders. PLoS One 9:e93411. 10.1371/journal.pone.0093411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United States Department of Health and Human Services. 2020. Centres for disease control and prevention, & National Institutes of Health. In: Biosafety in Microbiological and Biomedical Laboratories , 6th ed. Washington (DC): U.S. Government Printing Office. [Google Scholar]
- 37.United States Environmental Protection Agency. [Internet]. 2023. National ground water and drinking water - Primary drinking water regulations. [Cited DD Month YYYY]. Available at: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations.
- 38. Weiss IC, Di Iorio L, Feldon J, Domeney AM. 2000. Strain differences in the isolation-induced effects on prepulse inhibition of the acoustic startle response and on locomotor activity. Behav Neurosci 114:364–373. 10.1037/0735-7044.114.2.364. [DOI] [PubMed] [Google Scholar]
- 39. Yin S. 2009. Low stress handling, restraint and behavior modification of dogs & cats. Davis (CA): CattleDog Publishing. [Google Scholar]