Abstract
Background and Aim:
Captive animals are susceptible to parasitic diseases due to the stress and confinement they experience. In addition, they can serve as reservoirs of zoonotic parasites that have the potential to infect humans. To investigate this possibility, we estimated the prevalence of gastrointestinal (GI) parasites in captive mammals at Khon Kaen Zoo, Thailand.
Materials and Methods:
One hundred and forty-seven individual mammals (37 primates, 43 carnivores, 62 herbivores, and 5 rodents) were examined for parasitic infections by fecal examination daily for 3 consecutive days using the formalin-ethyl acetate concentration technique (FECT) and the agar plate culture method.
Results:
According to FECT, the overall prevalence of GI parasites was 62.6% (92/147). Within animal groups, the numbers were as follows: 67.6% (25/37) in primates, 23.3% (10/43) in carnivores, 85.5% (53/62) in herbivores, and 80.0% (4/5) in rodents. Using the agar plate culture method, 21.43% (27/126) were positive for Strongyloides spp. and hookworm infections. The GI parasites identified belonged to three categories: protozoa (including Entamoeba histolytica species complex, Entamoeba coli, Giardia spp., coccidia, and ciliated protozoa), trematodes (minute intestinal flukes and rumen flukes), and nematodes (strongyle/hookworm, Strongyloides spp., Ascarididae, and Trichuris spp.).
Conclusion:
The findings of this study indicate the prevalence of several GI parasites in zoo animals with the potential for transmission to humans, given the animals’ close proximity to both visitors and animal caretakers.
Keywords: captive mammals, gastrointestinal parasites, zoo, zoonotic parasites
Introduction
Zoonotic parasitic diseases can lead to substantial health complications for captive animals [1] and those responsible for their care, such as animal keepers. Exposure to contaminated feces, soil, and plants can put individuals at risk of infection [2, 3]. Helminth infections in captive wild animals can be fatal [4]. Moreover, prolonged captivity can amplify the interaction among parasite species, animals, and humans, increasing the chances of transmission. Protozoa with zoonotic potential that have been detected in captive animals include Giardia duodenalis, Balantioides coli, Cryptosporidium spp., and Entamoeba histolytica/Entamoeba dispar complex [5, 6]. Toxoplasma gondii was recently detected in humans working in a zoo [7]. Many studies have shown that nematode parasites can spread from animals to humans in shared habitats [8]. In particular, non-human primates (NHPs) have a close phylogenetic relationship with humans and can share nematodes such as Necator americanus, Ancylostoma duodenale, Ascaris lumbricoides, Strongyloides stercoralis, S. fuelleborni, Trichuris trichiura, and Enterobius vermicularis with nearby humans [9–12].
Animal reservoirs frequently release zoonotic parasites into the environment as oocysts, eggs, and larvae in feces [13–16]. Humans can become infected with GI parasites by consuming contaminated food and water containing oocysts or eggs [17–21]. Moreover, direct transmission can occur through contact with the feces of reservoir animals that retain infective larval stages [22]. “One Health” is a worldwide philosophy primarily concerned with the overlooked zoonotic transmission of parasites between animals and humans [23, 24].
Regrettably, despite the risk of zoonotic transmission, little research has addressed the GI parasites in captive wildlife mammals residing within Thailand’s zoos [25, 26]. In response to this knowledge gap, we estimated the prevalence of GI parasites across a range of captive mammals at Khon Kaen Zoo.
Materials and Methods
Ethical approval
All animal experiments were approved by the Animal Ethics Committee of the Zoological Park Organization of Thailand (No.2301638) and the Animal Ethics Committee of the Faculty of Medicine, Khon Kaen University (AEMDKKU 004/2022).
Study period and location
The study was conducted from March to June 2022 in Khon Kaen Zoo, located in Suan Kwang Mountain in Northeastern Thailand (16° 50’ 42.4” N, 102° 53’ 48.1” E). Khon Kaen Zoo was established as an ecotourism and research center for the conservation of rare and threatened species. Enclosures typically contain a covered part, with completely or partially finished floors, and an area exposed to the environment such as a grassy meadow. The animals are fed daily and the enclosures and grounds are cleaned daily, generally in the morning.
Sample collection and fecal examination
Fecal samples were collected from 147 individual captive mammals housed in Khon Kaen Zoo (37 primates, 43 carnivores, 62 herbivores, and 5 rodents: Table-1). To maximize sensitivity, fecal samples from 3 consecutive days were examined. For 69 individual animals (10 primates, 29 carnivores, and 30 herbivores: Table-2), fecal samples were collected on each of 3 consecutive days. Fresh feces were collected directly from the floor of the enclosures. The sample was retrieved from the center of the fecal mass, packed in plastic bags with the name of the host species, and weighed before being transported in an insulated box (at approximately 15°C) to the laboratory of the Parasitology Department, Faculty of Medicine, Khon Kaen University.
Table-1.
The numbers of captive mammals from which feces were collected and used for parasite identification in Khon Kaen Zoo, Thailand.
| Common name | Species | Number of individuals |
|---|---|---|
| Primate | ||
| Chimpanzee | Pan troglodytes | 4 |
| Bornean Orangutan | Pongo pygmaeus | 3 |
| Red-shanked Douc Langur | Pygathrix nemaeus | 3 |
| Hamadryas baboon | Papio hamadryas | 3 |
| Ring-tailed Lemur | Lemur catta | 8 |
| Tenasserim Lutung | Trachypithecus barbei | 1 |
| Common Squirrel Monkey | Saimiri sciureus | 3 |
| Bengal Slow Loris | Nycticebus bengalensis | 1 |
| Geoffoy’s Marmoset | Callithrix geoffroyi | 4 |
| Common Marmoset | Callithrix jacchus | 6 |
| Golden-handed Tamarin | Saguinus midas | 1 |
| Total | 37 | |
| Carnivore | ||
| White Lion | Panthera leo | 2 |
| Lion | Panthera leo | 4 |
| Malayan Sun Bear | Helarctos malayanus | 2 |
| Asiatic Black Bear | Ursus thibetanus | 4 |
| Binturong | Arctictis binturong | 4 |
| White Tiger | Panthera tigris | 1 |
| Indo-Chinese Tiger | Panthera tigris corbetti | 2 |
| Leopard Cat | Prionailurus bengalensis | 5 |
| Spotted Hyaena | Crocuta crocuta | 3 |
| Tanuki | Nyctereutes procyonoides viverrinus | 4 |
| Small-clawed Otter | Aonyx cinereus | 2 |
| Asiatic Jackal | Canis aureus | 4 |
| Common Palm Civet | Paradoxurus hermaphroditus | 1 |
| Ferret | Mustela putorius furo | 2 |
| Meerkat | Suricata suricatta | 1 |
| Fennec fox | Vulpes zerda | 1 |
| South American Fur Seal | Arctocephalus australis | 1 |
| Total | 43 | |
| Herbivore | ||
| Red Kangaroo | Macropus rufus | 2 |
| white Bennett’s Wallaby | Macropus rufogriseus | 1 |
| Pygmy Hippopotamus | Choeropsis liberiensis | 5 |
| Southern White Rhinoceros | Ceratotherium simum simum | 1 |
| Oryx | Oryx gazella | 2 |
| Springbok | Antidorcas marsupialis | 1 |
| Barasingha | Rucervus duvaucelii | 3 |
| Barbary Sheep | Ammotragus lervia | 3 |
| Ankole-Watusi | Bos taurus indicus | 7 |
| Common Barking Deer | Muntiacus muntjak | 5 |
| Hog Deer | Axis porcinus | 16 |
| Nyala | Tragelaphus angasii | 1 |
| Dromedary Camel | Camelus dromedarius | 1 |
| Nilgai | Boselaphus tragocamelus | 1 |
| Chinese Serow | Capricornis milneedwardsii | 1 |
| Burchell’s Zebra | Equus quagga burchellii | 2 |
| Giraffe | Giraffa camelopardalis | 2 |
| Spotted Deer | Axis axis | 1 |
| Rusa Deer | Rusa timorensis | 2 |
| Elephant | Elephas maximus | 1 |
| Brow-Antlered Deer | Rucervus eldii thamin | 3 |
| Sika Deer | Cervus nippon | 1 |
| Total | 62 | |
| Rodentia | ||
| Malayan Porcupine | Hystrix brachyura | 2 |
| Capybara | Hydrochoerus hydrochaeris | 3 |
| Total | 5 | |
| All samples | 147 |
Table-2.
The numbers of captive mammals from which feces were collected on each of 3 consecutive days and used for parasite identification in Khon Kaen Zoo, Thailand.
| Common name | Species | Number of individual |
|---|---|---|
| Primate | ||
| Bornean Orangutan | Pongo pygmaeus | 3 |
| Red-shanked Douc Langur | Pygathrix nemaeus | 1 |
| Ring-tailed Lemur | Lemur catta | 3 |
| Tenasserim Lutung | Trachypithecus barbei | 1 |
| Common Marmoset | Callithrix jacchus | 1 |
| Golden-handed Tamarin | Saguinus midas | 1 |
| Total | 10 | |
| Carnivore | ||
| White Lion | Panthera leo | 1 |
| Lion | Panthera leo | 4 |
| Malayan Sun Bear | Helarctos malayanus | 2 |
| Asiatic Black Bear | Ursus thibetanus | 4 |
| Binturong | Arctictis binturong | 1 |
| White Tiger | Panthera tigris | 1 |
| Indo-Chinese Tiger | Panthera tigris corbetti | 2 |
| Leopard Cat | Prionailurus bengalensis | 1 |
| Spotted Hyaena | Crocuta crocuta | 3 |
| Tanuki | Nyctereutes procyonoides viverrinus | 4 |
| Asiatic Jackal | Canis aureus | 4 |
| Common Palm Civet | Paradoxurus hermaphroditus | 1 |
| South American Fur Seal | Arctocephalus australis | 1 |
| Total | 29 | |
| Herbivore | ||
| Red Kangaroo | Macropus rufus | 2 |
| White Bennett’s Wallaby | Macropus rufogriseus | 1 |
| Pygmy Hippopotamus | Choeropsis liberiensis | 1 |
| Southern White Rhinoceros | Ceratotherium simum simum | 1 |
| Oryx | Oryx gazella | 2 |
| Springbok | Antidorcas marsupialis | 1 |
| Ankole-Watusi | Bos taurus indicus | 7 |
| Common Barking Deer | Muntiacus muntjak | 5 |
| Hog Deer | Axis porcinus | 2 |
| Nyala | Tragelaphus angasii | 1 |
| Dromedary Camel | Camelus dromedarius | 1 |
| Nilgai | Boselaphus tragocamelus | 1 |
| Chinese Serow | Capricornis milneedwardsii | 1 |
| Burchell’s Zebra | Equus quagga burchellii | 1 |
| Giraffe | Giraffa camelopardalis | 2 |
| Elephant | Elephas maximus | 1 |
| Total | 30 | |
| All samples | 69 |
Formalin-ethyl acetate concentration technique (FECT)
All fecal samples were analyzed using the FECT [27] with 3 consecutive days’ examinations of each sample (a total of 354 samples). Two grams of feces were mixed with 10 mL of 10% formalin solution, filtered into a 15 mL centrifuge tube using two layers of gauze, and centrifuged at 500× g for 5 min. After removing the supernatant, the debris was mixed with 3 mL of ethyl acetate solution and 7 mL of 10% formalin solution and centrifuged at 500 g for 5 min. After removing the supernatant, 1 mL of 10% formalin solution was added to the sediment. Two drops of the three aliquots were stained with 1% iodine and examined under a light microscope at 10× and 40× magnifications (Olympus, Japan). Parasites were identified based on eggs’ color, shape, and content or the anatomy of trophozoites, larvae, or other propagules [28, 29].
Agar plate culture technique (APCT)
Strongyloides spp. and hookworms were detected using an APCT. A total of 126 fecal samples (each approximately 2 g) were available for examination by APCT. Filariform larvae of Strongyloides, hookworms, and free-living adults of Strongyloides were investigated after 4-5 days of culture at room temperature (27°C–35°C).
Statistical analysis
The percentage of individuals infected with each species of parasite was calculated. McNemar’s Chi-square test was used to compare proportions from paired samples [30] and to determine whether the ability to detect a parasite from a single fecal sample was significantly different from that based on samples from 3 consecutive days. Statistical analysis was considered significant at p < 0.05.
Results
The overall prevalence of GI parasites was 62.6% (92/147) in captive mammals at Khon Kaen Zoo, Thailand, according to the FECT. Corresponding values for different groups of mammals were as follows: primates, 67.6% (25/37); carnivores, 23.3% (10/43); herbivores, 85.5% (53/62); and rodents, 80.0% (4/5) (Table-3). In addition, 126 fecal samples were examined using the APCT and 21.43% (27/126) were positive for Strongyloides or hookworm (Table-4).
Table-3.
The overall prevalence of GI parasites in captive mammals according to the FECT.
| Type | Number of animals | Parasite positive (%) |
|---|---|---|
| Primate | 37 | 25 (67.6) |
| Carnivore | 43 | 10 (23.3) |
| Herbivore | 62 | 53 (85.5) |
| Rodents | 5 | 4 (80.0) |
| Total | 147 | 92 (62.6) |
GI=Gastrointestinal, FECT=Formalin-ethyl acetate concentration technique
Table-4.
The prevalence of GI parasites in captive mammals according to the APCT.
| Types | Number | Strongyloides spp. (%) | Hookworm (%) | Mixed infection (%) |
|---|---|---|---|---|
| Primate | 21 | 4 (19.0) | 4 (19.0) | 4 (19.0) |
| Carnivore | 38 | - | 3 (7.9) | - |
| Herbivore | 62 | 3 (4.9) | 7 (11.3) | - |
| Rodent | 5 | 2 (40.0) | - | - |
| Total | 126 | 9 (7.1) | 14 (11.1) | 4 (3.2) |
GI=Gastrointestinal, APCT=Agar plate culture technique
The prevalence of GI parasites in 69 individual animals was determined by examination of fecal samples collected on 3 consecutive days. The prevalence rates were 55.1% (38/69), 49.28% (34/69), and 52.17% (36/69) based on the 1st-, 2nd-, and 3rd-day examination, respectively. One new infected individual was detected on the 2nd day of examination, and three on the 3rd day (Table-5). The McNemar test showed no statistically significant differences between day 2 (p > 0.05) and the cumulative 3 consecutive days (p > 0.05). The GI parasites found in captive primates included Giardia spp., E. histolytica species complex, Entamoeba coli, minute intestinal trematodes, ciliated protozoa, hookworm, Strongyloides spp., and Trichuris spp. In carnivores, the GI parasites included ciliated protozoa, Ascarididae, hookworm, and Strongyloides spp. In herbivores, the feces yielded E. histolytica species complex, E. coli, Giardia spp., coccidia cysts, ciliated protozoa, rumen flukes, Ascarididae, Trichuris spp., strongyles, and Strongyloides spp. (Figures-1–3). Rodents had E. histolytica species complex, hookworms, Strongyloides spp., and Trichuris spp.
Table-5.
The frequency of detection of GI parasites in captive mammals on 3 consecutive days according to the FECT.
| Type/day | Number | Parasite positive on each day (%) | New individual discovery |
|---|---|---|---|
| Mammal | |||
| Day 1 | 69 | 38 (55.07) | - |
| Day 2 | 69 | 34 (49.28) | 1 |
| Day 3 | 69 | 36 (52.17) | 3 |
| Total | 69 | 42 (60.87) | 4 |
GI=Gastrointestinal, FECT=Formalin-ethyl acetate concentration technique
Figure-1.
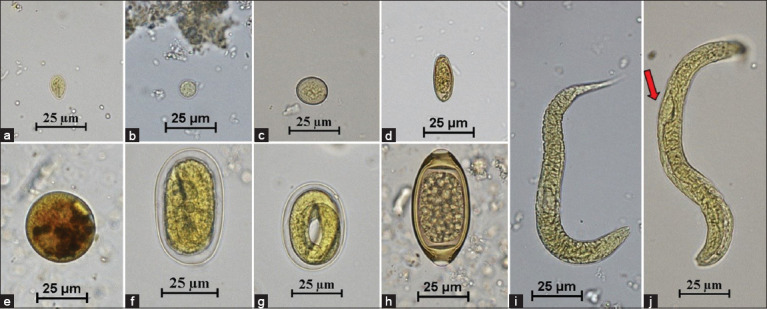
Figures of gastrointestinal parasites in fecal samples of captive primates. (a) Giardia spp. (40×); (b) Entamoeba histolytica species complex (40×); (c) Entamoeba coli (40×); (d) Minute intestinal trematode (40×); (e) Ciliated protozoa (40×); (f) Hookworm (40×); (g) Strongyloides spp. (40×); (h) Trichuris spp. (40×); (i) Hookworm rhabditiform larva (40×); and (j) Strongyloides spp. rhabditiform larva (40×). Red arrow = Prominent genital primordium.
Figure-3.
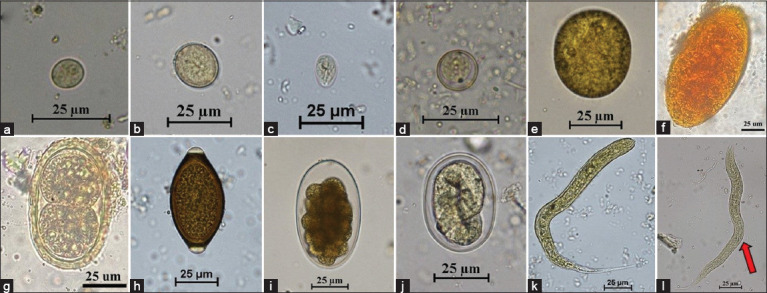
Figures of gastrointestinal parasites in fecal samples of captive herbivores. (a) Entamoeba histolytica species complex (60×); (b) Entamoeba coli (40×); (c) Giardia spp. (40×); (d) Coccidia cyst (60×); (e) Ciliated protozoa (40×); (f) Rumen fluke (40×); (g) Ascarididae (40×); (h) Trichuris spp. (40×); (i) Strongyle (40×); (j) Strongyloides spp. (40×); (k) Strongyle rhabditiform larva (40×); and (l) Strongyloides spp. rhabditiform larva (40×). Red arrow = prominent genital primordium.
Figure-2.
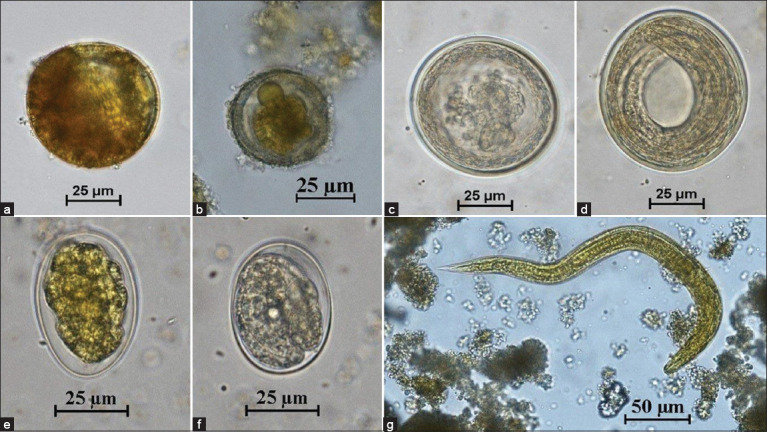
Figures of gastrointestinal parasites in fecal samples of captive carnivores. (a) Ciliated protozoa (40×); (b-d) Ascarididae (40×); (e) Hookworm (40×); (f) Strongyloides spp. (40×); and (g) Hookworm rhabditiform larva (40×).
Discussion
In our study, fecal samples were collected directly from the floor of zoo animal enclosures. This non-invasive approach eliminates the need for chemical or mechanical restraint of the animals, thus reducing stress and not affecting their welfare [31, 32]. The significant finding of a considerable range of GI parasitic infections (62.6% of animals infected) among captive mammals at Khon Kaen Zoo raises concerns. Comparable prevalence values have been reported from zoo populations in other countries, such as Nepal (19.5%) [33], Malaysia (56.3%) [34], and Bangladesh (60.5%) [35]. Higher prevalence has been reported in some cases, such as 68.3% in the Rio de Janeiro Zoo [36], 72.5% in Spain [5], and 71.8% and 74.2% in Brazil [37, 38].
Identifying protozoans within the captive animal in our study reveals the potential for easy transmission among hosts due to the environmental resilience of cysts and oocysts, capacity for passive oral transmission, and lack of requirement for intermediate hosts [5, 6]. Most parasite infections in wild animals are asymptomatic [39], but stress from captivity can make them symptomatic, resulting in severe clinical symptoms of diarrhea [40–42]. We detected Giardia spp., E. histolytica species complex, hookworms, Strongyloides spp., Ascarididae, and Trichuris spp., all of which have the potential for transmission in the zoo environment. GI parasites can spread to animal keepers, who may not always be aware of the risk [43–45]. Prevention of transmission requires a multifaceted approach encompassing suitable medications, food-handling practices, and heightened sanitation to enhance animal and worker welfare. Contaminated food and water are the major sources of GI parasite infections and are likely the transmission routes of infections that we detected in this study.
The quantity of stool samples adequate to detect intestinal parasites in epidemiologic research is still uncertain [46]. Parasites can produce eggs or cysts intermittently, which means that a single fecal examination may not detect all cases. Although traditional fecal examination techniques, including the APCT, FECT, Baermann technique, and direct smear, have been the main reference procedures for diagnosing strongyloidiasis, these techniques have low sensitivity and are unreliable due to irregular larval excretion in humans [47, 48] and the high fluctuation in larval excretion from animals [49]. Hence, examination of fecal samples collected on multiple days could improve the accuracy of detecting parasites, making it possible to provide adequate treatment in a timely manner. It is typically recommended to examine stool samples collected on 3 different days [50], an approach demonstrated to improve the detection of organisms such as E. histolytica/E. dispar [51, 52]. Collection of fecal samples in the zoo is quite easy due to the high compliance of organization, routine cleaning, and regular use of anthelmintic treatments.
A previous study by Moustafa [53] showed that using three consecutive daily examinations, sensitivity of the agar plate method increased from 70.3% to 96.2%. Another study revealed a significantly higher cumulative positive rate of S. stercoralis from 13.3% to 22% by examining fecal samples daily for 3 consecutive days [54]. Repeated fecal examinations clearly increase the evaluation of the prevalence of strongyloidiasis, which is an important disease in humans.
However, the findings of this study imply that the prevalence of GI parasites acquired through a single stool examination using the FECT technique could be equally reliable when compared to the results from the analysis of fecal samples collected over 3 consecutive days. Importantly, it should be noted that the parasites identified in this investigation potentially have the capacity for zoonotic transmission due to their hosts’ close proximity to humans.
One limitation of this study was the problem of fecal collection from known individuals of herd animals such as many herbivores. It can be difficult to identify the feces of each animal in a group, particularly if they are free-ranging or have access to shared feeding and watering areas. In these circumstances, the collection process can be time-consuming and labor-intensive, especially when dealing with a large group. Furthermore, this procedure may induce stress among the animals, leading to potential alterations in their behavior and defecation patterns. Finally, variations might increase as some animals defecate more frequently or in different locations than others, consequently challenging the accuracy of individual fecal collection to reflect the overall herd prevalence. The results from this study will provide information on the prevalence of parasitic infection in captive mammals and hence inform zoo management to improve animal welfare and health. It is important to minimize the dangers of zoonotic infections to tourists, researchers, animal keepers, and veterinarians.
Conclusion
To the best of our knowledge, this study is the first to investigate the prevalence of GI parasites in captive mammals kept in the Khon Kaen Zoo (includes NHPs, carnivores, herbivores, and rodents) based on examination of fecal samples on each of 3 consecutive days. The zoo animals served as important reservoir hosts for several zoonotic GI parasites such as Giardia spp., E. histolytica species complex, hookworms, Strongyloides spp., Ascarididae, and Trichuris spp. These parasites possess the capacity to propagate among animal hosts, potentially triggering disease, and representing a hazard to zookeepers, veterinarians, and visitors at Khon Kaen Zoo. The key is to implement the prevention and control of these GI parasites. This calls for a One Health approach to ensure the well-being of animals, caretakers, and visitors.
Authors’ Contributions
JS: Methodology, investigation, data analysis, and writing-original draft. CE, NH, and AA: Methodology, validation, and writing-review and editing. CC, KP, and SK: Investigation and writing-review and editing. NL: Validation and writing-review and editing. TB and PS: Conceptualization, methodology, and writing-review and editing. OP: Conceptualization, methodology, validation, investigation, data analysis, and writing-original draft. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
This study was supported by the Fundamental Fund of Khon Kaen University, which has received funding support from the National Science, Research and Innovation Fund or NSRF, Thailand and Jirawat Sangpeng was also supported by a postgraduate study support grant from the Faculty of Medicine, Khon Kaen University. We would like to thank the Khon Kaen Zoo staff for providing the facility for our study. Finally, we would like to thank Professor David Blair for editing the manuscript through Publication Clinic KKU, Thailand.
Footnotes
This study was supported by the Fundamental Fund of Khon Kaen University, which has received funding support from the National Science, Research and Innovation Fund or NSRF, Thailand and Jirawat Sangpeng was also supported by a postgraduate study support grant from the Faculty of Medicine, Khon Kaen University.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Cibot M, Guillot J, Lafosse S, Bon C, Seguya A, Krief S. Nodular worm infections in wild non-human primates and humans living in the Sebitoli area (Kibale National Park, Uganda):Do high spatial proximity favor zoonotic transmission? PLoS Negl. Trop. Dis. 2015;9(10):e0004133. doi: 10.1371/journal.pntd.0004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slifko T.R, Smith H.V, Rose J.B. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 2000;30(12–13):1379–1393. doi: 10.1016/s0020-7519(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 3.Panayotova-Pencheva M.S. Parasites in captive animals:A review of studies in some European zoos. Der Zool. Garten. 2013;82(1–2):60–71. [Google Scholar]
- 4.Borghare A.T, Bagde V.P, Jaulkar A.D, Katre D.D, Jumde P.D, Maske D.K, Bhangale G.N. Incidence of gastrointestinal helminthiasis in captive deers at Nagpur. Vet. World. 2009;2(9):337–338. [Google Scholar]
- 5.Cordon G.P, Prados A.H, Romero D, Moreno S.M, Pontes A, Osuna A, Rosales M.J. Intestinal parasitism in the animals of the zoological garden “Pena escrita”(Almunecar, Spain) Vet. Parasitol. 2008;156(3–4):302–309. doi: 10.1016/j.vetpar.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Levecke B, Dorny P, Geurden T, Vercammen F, Vercruysse J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet. Parasitol. 2007;148(3–4):236–246. doi: 10.1016/j.vetpar.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Echarte G.V, Fernández Y.E.S, Augusto A.M, Santos A.L.C, Dantas M.M.L, Iraola R.C, Amendoeira M.R.R. Assessment professional competence and risk factors perception of Toxoplasma gondii at the Cuba national zoo park and zoo garden of Rio de Janeiro, Brazil. Rev. Ciên. Vet. Saúde Públ. 2019;6(1):16–29. [Google Scholar]
- 8.Ashford R.W, Reid G.D, Butynski T.M. The intestinal faunas of man and mountain gorillas in a shared habitat. Ann. Trop. Med. Parasitol. 1990;84(4):337–340. doi: 10.1080/00034983.1990.11812477. [DOI] [PubMed] [Google Scholar]
- 9.Mbaya A.W, Udendeye U.J. Gastrointestinal parasites of captive and free-roaming primates at the Afi mountain primate conservation area in Calabar, Nigeria and their zoonotic implications. Pak. J. Biol. Sci. 2011;14(13):709–714. doi: 10.3923/pjbs.2011.709.714. [DOI] [PubMed] [Google Scholar]
- 10.Levecke B, Dorny P, Vercammen F, Visser L.G, Van Esbroeck M, Vercruysse J, Verweij J.J. Transmission of Entamoeba nuttalli and Trichuris trichiura from nonhuman primates to humans. Emerg. Infect. Dis. 2015;21(10):1871–1872. doi: 10.3201/eid2110.141456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thanchomnang T, Intapan P.M, Sanpool O, Rodpai R, Tourtip S, Yahom S, Kullawat J, Radomyos P, Thammasiri C, Maleewong W. First molecular identification and genetic diversity of Strongyloides stercoralis and Strongyloides fuelleborni in human communities having contact with long-tailed macaques in Thailand. Parasitol. Res. 2017;116(7):1917–1923. doi: 10.1007/s00436-017-5469-z. [DOI] [PubMed] [Google Scholar]
- 12.Medkour H, Amona I, Laidoudi Y, Davoust B, Bitam I, Levasseur A, Akiana J, Diatta G, Pacheco L, Gorsane S, Sokhna C, Hernandez-Aguilar R.A, Barciela A, Fenollar F, Raoult D, Mediannikov O. Parasitic infections in African humans and non-human primates. Pathogens. 2020;9(7):561. doi: 10.3390/pathogens9070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naz S, Arooj S, Ali Z, Farooq Z. Potential consequences of captivity and environmental pollution in endoparasitic prevalence in different antelopes kept at wildlife parks. Environ. Sci. Pollut. Res. Int. 2021;28(13):16308–16313. doi: 10.1007/s11356-020-11561-x. [DOI] [PubMed] [Google Scholar]
- 14.Shusterman L, Marsh A.E, Joyner P.H, Habing G. Detection of Trichuris eggs in feces and soil from giraffe (Giraffa camelopardalis) and other hoofstock enclosures under human care in the USA. Int. J. Parasitol. Parasites Wildl. 2021;15(3):208–213. doi: 10.1016/j.ijppaw.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavallero S, Montalbano Di Filippo M, Rondón S, De Liberato C, D'Amelio S, Friedrich K.G, Berrilli F. Nuclear and mitochondrial data on Trichuris from Macaca fuscata support evidence of host specificity. Life (Basel) 2020;11(1):18. doi: 10.3390/life11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vonfeld I, Prenant T, Polack B, Guillot J, Quintard B. Gastrointestinal parasites in non-human primates in zoological institutions in France. Parasite. 2022;29:43. doi: 10.1051/parasite/2022040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai W, Ryan U, Xiao L, Feng Y. Zoonotic giardiasis:An update. Parasitol. Res. 2021;120(12):4199–4218. doi: 10.1007/s00436-021-07325-2. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes-Santos R.C, Medici E.P, Testa-José C, Micheletti T. Health assessment of wild lowland tapirs (Tapirus terrestris) in the highly threatened Cerrado biome, Brazil. J. Wildl. Dis. 2020;56(1):34–46. [PubMed] [Google Scholar]
- 19.Dixon B.R. Giardia duodenalis in humans and animals-transmission and disease. Res. Vet. Sci. 2021;135:283–289. doi: 10.1016/j.rvsc.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 20.Santin M. Cryptosporidium and Giardia in ruminants. Vet. Clin. North Am. Food Anim. Pract. 2020;36(1):223–238. doi: 10.1016/j.cvfa.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Dessì G, Tamponi C, Varcasia A, Sanna G, Pipia A.P, Carta S, Salis F, Díaz P, Scala A. Cryptosporidium infections in sheep farms from Italy. Parasitol. Res. 2020;119(12):4211–4218. doi: 10.1007/s00436-020-06947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayinmode A.B, Obebe O.O, Olayemi E. Prevalence of potentially zoonotic gastrointestinal parasites in canine faeces in Ibadan, Nigeria. Ghana Med. J. 2016;50(4):201–206. doi: 10.4314/gmj.v50i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson R.C.A. Parasite zoonoses and wildlife:One Health, spillover and human activity. Int. J. Parasitol. 2013;43(12–13):1079–1088. doi: 10.1016/j.ijpara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Feng Y, Santin M. Host specificity of Enterocytozoon bieneusi and public health implications. Trends Parasitol. 2019;35(6):436–451. doi: 10.1016/j.pt.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Mahannop A, Keittivuti A, Mahannop P. Survey of Intestinal Parasitism in the Exotic Animals at Dusit Zoo, Thailand. Eleventh Annual Veterinary Conference Bangkok, Thailand. 1984 [Google Scholar]
- 26.Kositamongkol T, Nithiuthai S, Chungpivat S, Prechatangkit B, Pattanarangsan R. Study on gastrointestinal parasites of the captive wildlife and birds in Khao Kheow open zoo, Chonburi province. Wildl. J. Thai. 1996;5(2):117–125. [Google Scholar]
- 27.Elkins D.B, Haswell-Elkins M, Anderson R.M. The epidemiology and control of intestinal helminths in the Pulicat lake region of Southern India. I. Study design and pre- and post-treatment observations on Ascaris lumbricoides infection. Trans. R. Soc. Trop. Med. Hyg. 1986;80(5):774–792. doi: 10.1016/0035-9203(86)90384-6. [DOI] [PubMed] [Google Scholar]
- 28.Flynn R.J. Parasites of Laboratory Animals. Ames, IO: The Iowa State University Press; 1973. [Google Scholar]
- 29.Hasegawa H, Chapman C.A, Huffman M.A. Useful Diagnostic References and Images of Protozoans, Helminths, and Nematodes Commonly Found in Wild Primates. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 30.Eliasziw M, Donner A. Application of the McNemar test to non-independent matched pair data. Stat. Med. 1991;10(12):1981–1991. doi: 10.1002/sim.4780101211. [DOI] [PubMed] [Google Scholar]
- 31.Schilling A.K, Mazzamuto M.V, Romeo C. A review of non-invasive sampling in wildlife disease and health research:What's new? Animals (Basel) 2022;12(13):1719. doi: 10.3390/ani12131719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dib L.V, Palmer J.P.S, de Souza Carvalho Class C, Pinheiro J.L, Ramos R.C.F, Dos Santos C.R, Fonseca A.B.M, Rodriguez-Castro K.G, Goncalves C.F, Galetti P.M, Jr, Bastos O.M.P, Uchoa C.M.A, Correa L.L, Bastos A.C.M.P, Amendoeira M.R.R, da Silva Barbosa A. Non-invasive sampling in Itatiaia National Park, Brazil:Wild mammal parasite detection. BMC Vet. Res. 2020;16(1):295. doi: 10.1186/s12917-020-02490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhakal P, Sharma H.P, Shah R, Thapa P.J, Pokheral C.P. Copromicroscopic study of gastrointestinal parasites in captive mammals at Central Zoo, Lalitpur, Nepal. Vet. Med. Sci. 2023;9(1):457–464. doi: 10.1002/vms3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim Y.A, Ngui R, Shukri J, Rohela M, Mat Naim H.R. Intestinal parasites in various animals at a zoo in Malaysia. Vet. Parasitol. 2008;157(1–2):154–159. doi: 10.1016/j.vetpar.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Ferdous S, Chowdhury J, Hasan T, Dutta P, Rahman M.M, Hassan M.M, Faruque M.R, Alim M.A. Prevalence of gastrointestinal parasitic infections in wild mammals of a Safari park and a zoo in Bangladesh. Vet. Med. Sci. 2023;9(3):1385–1394. doi: 10.1002/vms3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbosa A.D.S, Pinheiro J.L, Dos Santos C.R, de Lima C.S.C.C, Dib L.V, Echarte G.V, Augusto A.M, Bastos A.C.M.P, Antunes Uchoa C.M, Bastos O.M.P, Santos F.N, Fonseca A.B.M, Amendoeira M.R.R. Gastrointestinal parasites in captive animals at the Rio de Janeiro Zoo. Acta Parasitol. 2020;65(1):237–249. doi: 10.2478/s11686-019-00145-6. [DOI] [PubMed] [Google Scholar]
- 37.De Freitas M.F.L, De Oliveira A.B, Cavalcanti M.D.B, Oliveira R.A, Sobrinho A.E. Coproparasitologic profile of captive wild mammals in Pernambuco state, Brazil. Parasitol. Día. 2001;25(3–4):121–125. [Google Scholar]
- 38.Moreira R.M.P, Aires C.G, Alves-Sobrinho A.V, de Sa Moraes I, Moreira C.N, Amaral A.V.C.D, Saturnino K.C, Braga Í.A, Pacheco R.D.C, Ramos D.G.D.S. Gastrointestinal parasites of wild carnivores from conservation institutions in the Cerrado of Goiás, Brazil. Rev. Bras. Parasitol. Vet. 2023;32(3):e004823. doi: 10.1590/S1984-29612023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma H.P, Achhami B. Gastro-intestinal parasites of sympatric red panda and livestock in protected areas of Nepal. Vet. Med. Sci. 2022;8(2):568–577. doi: 10.1002/vms3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mir A.Q, Dua K, Singla L.D, Sharma S, Singh M.P. Prevalence of parasitic infection in captive wild animals in Bir Moti Bagh mini zoo (Deer Park), Patiala, Punjab. Vet. World. 2016;9(6):540–543. doi: 10.14202/vetworld.2016.540-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Köster P.C, Lapuente J, Pizarro A, Prieto-Pérez L, Pérez-Tanoira R, Dashti A, Bailo B, Muadica A.S, González-Barrio D, Calero-Bernal R, Ponce-Gordo F, Carmena D. Presence and genetic diversity of enteric Protists in captive and semi-captive non-human primates in cote d'Ivoire, Sierra Leone, and Peru. Int. J. Parasitol. Parasites Wildl. 2022;17:26–34. doi: 10.1016/j.ijppaw.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao W, Zhou H, Jin H, Liu M, Qiu M, Li L, Yin F, Chan J.F.W, Lu G. Molecular prevalence and subtyping of Cryptosporidium hominis among captive long-tailed macaques (Macaca fascicularis) and Rhesus macaques (Macaca mulatta) from Hainan Island, Southern China. Parasit. Vectors. 2019;12(1):192. doi: 10.1186/s13071-019-3449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akinboye D.O, Ogunfetimi A.A, Fawole O, Agbolade O, Ayinde O.O, Atulomah N.O.S, Amosu A.M, Livingstone R. Control of parasitic infections among workers and inmates in a Nigerian zoo. Niger. J. Parasitol. 2010;31(1):35–38. [Google Scholar]
- 44.Labes E.M, Hegglin D, Grimm F, Nurcahyo W, Harrison M.E, Bastian M.L, Deplazes P. Intestinal parasites of endangered orangutans (Pongo pygmaeus) in Central and East Kalimantan, Borneo, Indonesia. Parasitology. 2010;137(1):123–135. doi: 10.1017/S0031182009991120. [DOI] [PubMed] [Google Scholar]
- 45.Köster P.C, Martínez-Nevado E, González A, Abelló-Poveda M.T, Fernández-Bellon H, de la Riva-Fraga M, Marquet B, Guéry J.P, Knauf-Witzens T, Weigold A, Dashti A, Bailo B, Imana E, Muadica A.S, Gonzalez-Barrio D, Ponce-Gordo F, Calero-Bernal R, Carmena D. Intestinal protists in captive non-human primates and their handlers in six European zoological gardens. Molecular evidence of zoonotic transmission. Front. Vet. Sci. 2021;8:819887. doi: 10.3389/fvets.2021.819887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gyorkos T.W, MacLean J.D, Law C.G. Absence of significant differences in intestinal parasite prevalence estimates after examination of either one or two stool specimens. Am. J. Epidemiol. 1989;130(5):976–980. doi: 10.1093/oxfordjournals.aje.a115430. [DOI] [PubMed] [Google Scholar]
- 47.Requena-Mendez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Munoz J. The laboratory diagnosis and follow up of strongyloidiasis:A systematic review. PLoS Negl. Trop. Dis. 2013;7(1):e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schar F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, Marti H, Kramme S. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop. 2013;126(2):89–92. doi: 10.1016/j.actatropica.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Oliveira-Junior S.D, Barcante J.M.P, Barcante T.A, Dias S.R.C, Lima W.S. Larval output of infected and re-infected dogs with Angiostrongylus vasorum (Baillet, 1866) Kamensky, 1905. Vet. Parasitol. 2006;141(1–2):101–106. doi: 10.1016/j.vetpar.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Wolfe M.S. Management of the returnee from exotic places. J. Occup. Med. 1979;21(10):691–695. [PubMed] [Google Scholar]
- 51.Lincicome D.R. Fluctuation in numbers of cysts of Endamoeba histolytica and Endamoeba coli in the stools of Rhesus monkeys. Am. J. Epidemiol. 1942;36(3):321–337. [Google Scholar]
- 52.Sawitz W.G, Faust E.C. The probability of detecting intestinal protozoa by successive stool examinations. Am. J. Trop. Med. Hyg. 1942;22(2):131–136. [Google Scholar]
- 53.Moustafa M.A. An evaluation of the modified agar plate method for diagnosis of Strongyloides stercoralis. J. Egypt. Soc. Parasitol. 1997;27(2):571–579. [PubMed] [Google Scholar]
- 54.Ruantip S, Eamudomkarn C, Kopolrat K.Y, Sithithaworn J, Laha T, Sithithaworn P. Analysis of daily variation for 3 and for 30 days of parasite-specific IgG in urine for diagnosis of strongyloidiasis by enzyme-linked immunosorbent assay. Acta Trop. 2021;218(5):105896. doi: 10.1016/j.actatropica.2021.105896. [DOI] [PubMed] [Google Scholar]


