TO THE EDITOR:
Sickle cell disease (SCD) is a hereditary blood disorder impacting around 100 000 Americans, predominantly those who are from socioeconomically disadvantaged backgrounds.1,2 Among adults living with SCD, there is a high prevalence of depression, affecting 20% to 57% of the population, which is even higher than the general population, affecting only ∼8% of adults.3, 4, 5, 6, 7, 8 Depression among adults with SCD adversely affects the overall quality of life, acute visits, SCD outcomes, and survival.9, 10, 11, 12 National guidelines recommend annual screening for depression in adults with SCD.13 However, many people are reluctant to seek help and underreport symptoms of depression due to stigma.14, 15, 16, 17 In SCD, stigma is accentuated due to other compounding factors related to both their race and the need for opioid medications to treat pain,18,19 which may further limit depression symptom disclosure. Therefore, without regular and effective screening strategies, depression might remain unrecognized and untreated and contribute to poor outcomes in adults with SCD.
One standard screening tool for depression is the 9-item Patient Health Questionnaire (PHQ-9), which can be self-administered or conducted by health care providers or clinic staff.20 The PHQ-9 is a 9-item questionnaire scored from 0 to 27 with scores ≥5, ≥10, ≥15, and ≥20 representing mild, moderate, moderately severe, and severe depression, respectively.20 However, the most effective method of administering this screening tool to individuals with SCD is unknown, and different methods of administration might result in more false-negative depression symptoms screens. Thus, this study aimed to identify rates of depression in an adult SCD cohort receiving routine outpatient PHQ-9 screening before a visit with a primary care provider and test the hypothesis that self-administered PHQ-9 screening would yield more positive screenings for depression than verbal PHQ-9 administration by clinic nurses.
This study was part of a more extensive, single-site, prospective study to evaluate the impact of a patient-centered medical home with an integrated primary care provider for adults with SCD. This study was conducted at the Ohio State University Wexner Medical Center Comprehensive Sickle Cell Clinic and was approved by the Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki. Adults aged ≥18 years with any type of SCD were recruited between 1 January 2021 and 31 March 2023. Participants were given computer tablets to self-administer the demographic surveys and the PHQ-9 and were also verbally asked the PHQ-9 by the clinic nursing staff. McNemar tests were computed for differences among the proportion who screened positive for depression between the 2 strategies, and exact binomial tests were used for discordant individual item scores on the self-administered vs interviewer-administered questionnaires. A paired t test was calculated to determine the differences in PHQ-9 scores by the mode of administration. Analyses were performed using R (version 4.3.0).
Although 52 adults completed both the self-administered PHQ-9 and interviewer-administered PHQ-9, a total of 14 were excluded because they did not complete both modes of assessment on the same day. Of the 38 included participants, 36 (95%) received the interviewer-administered PHQ-9 first. Mean participant age was 30.66 years (standard deviation [SD], 10.06 years); 58% were females; 95% were Black (95%); 53% had hemoglobin (Hb) SS or HbSβ0thalassemia, and 47% had HbSC or HbSβ+thalassemia. Of these 38 participants, 18 (47%) scored ≥5 (a positive screen for depression) on the self-administered PHQ-9, whereas 8 (21%) scored ≥5 on the interviewer-administered PHQ-9 (P < .01). Everyone (n = 8) who scored ≥5 on the interviewer-administered PHQ-9, also scored ≥5 on the self-administered PHQ-9.
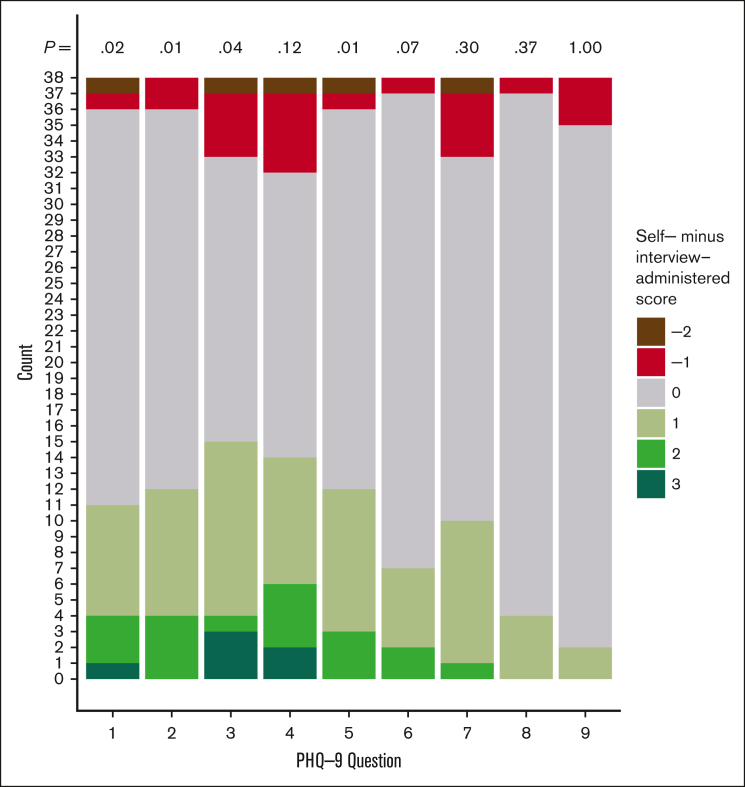
Self-administered questionnaires indicated higher rates of positive depression screening and more severe depression (Table 1). Mean PHQ-9 scores were higher with the self-administered PHQ-9 (mean, 5.71; SD, 6.09) than with interviewer-administered (mean, 3.47; SD, 4.91) (difference, 2.24; 95% confidence interval, 1.13-3.35; P < .001). On the first 2 questions of the PHQ-9, which are necessary for the diagnosis of major depression, more participants reported higher frequency of having little interest or pleasure in doing things on the self-administered than on the interviewer-administered questionnaire (n = 11 [29%] vs 2 [5%]; P = .02), and more reported higher frequency of feeling down, depressed, or hopeless (n = 12 [32%] vs 2 [5%], P = .01) The last PHQ-9 question, which asks about suicidal thoughts, had similar scores between the 2 routes (P = 1.0) (Figure 1). The minimal difference in the suicide item is likely due to the rarity of suicide in SCD, which was also evident from the very low scores among our participants (mean score, 0.10). Results were consistent in the corresponding analyses for the 14 excluded participants; there was a similar proportion of discordant answers between administration modes, and the direction trend of discordance was unchanged.
Table 1.
Distribution of scores and severities between the self-administered PHQ-9 and the interviewer-administered PHQ-9 among the 38 participants
|
PHQ-9 question |
Self-administered (n = 38) |
Interviewer-administered (n = 38) |
|---|---|---|
| Mean score (SD) | Mean score (SD) | |
| Over the last 2 weeks, how often have you been bothered by any of the following problems? | ||
| 1: Little interest or pleasure in doing things | 0.76 (± 1.02) | 0.42 (± 0.83) |
| 2: Feeling down, depressed, or hopeless | 0.71 (± 1.01) | 0.34 (± 0.74) |
| 3: Trouble falling or staying asleep, or sleeping too much | 0.97 (± 1.01) | 0.55 (± 0.89) |
| 4: Feeling tired or having little energy | 1.21 (± 1.23) | 0.81 (± 1.01) |
| 5: Poor appetite or overeating | 0.60 (± 0.85) | 0.29 (± 0.77) |
| 6: Feeling bad about yourself or that you are a failure or have let yourself or your family down | 0.50 (± 0.95) | 0.29 (± 0.77) |
| 7: Trouble concentrating on things, such as reading the newspaper or watching television | 0.71 (± 1.01) | 0.58 (± 0.98) |
| 8: Moving or speaking so slowly that other people could have noticed. Or the opposite being so fidgety or restless that you have been moving around a lot more than usual | 0.13 (± 0.34) | 0.05 (± 0.23) |
| 9: Thoughts that you would be better off dead, or of hurting yourself | 0.10 (± 0.51) | 0.13 (± 0.41) |
| Total | 5.71 (± 6.09) | 3.47 (± 4.91) |
| PHQ-9 score: depression severity, n (%) | ||
| 0-4: none-minimal | 20 (53%) | 30 (79%) |
| 5-9: mild | 10 (26%) | 4 (10%) |
| 10-14: moderate | 3 (8%) | 2 (5%) |
| 15-19: moderately severe | 3 (8%) | 1 (3%) |
| 20-24: severe | 2 (5%) | 1 (3%) |
Figure 1.
Distributions of score difference (self-administered minus interview-administered) by PHQ-9 item.
Although PHQ-9 screening in a primary care setting for the general population suggests that self-administered and interviewer-administered depression screening provide equivalent results,21 our results suggest that verbal administration of depression screening among adults with SCD may identify fewer adults with depression symptoms and less severe symptoms than via self-administration. Potential reasons for why this might have occurred is that self-administration might promote a deeper self-awareness of symptoms and less concern about the stigma, especially because stigma for mental health is more common among African Americans.22 Our study suggests that identifying an optimal strategy to screen for depression, potentially by self-administration only or by a combination of self-administration with follow-up, is crucial in this population with a high burden of depression, because depression can ultimately affect the quality of life, SCD outcomes, and mortality. Optimal screening is critical because medical professionals can offer further support, including referrals to behavioral health and more effective treatment for those with depressive symptoms, and potentially improve their outcomes.
This study was limited as it was a single-center study, and only those who presented in the SCD clinic were included, which could limit generalizability. Another limitation is that the ordering of interviewer-administered vs self-administered was not randomized and based on clinic flow. Because the majority received the self-administered PHQ-9 after the interviewer-administered PHQ-9, the additional time, readministration, and reflection of their symptoms may have contributed to score differences. However, multiple studies on the validation of the PHQ-9 demonstrated that the test-retest reliability of the PHQ-9 is excellent20,23,24, meaning that asking the questionnaires at different times within 2 weeks resulted in similar scores on the PHQ-9. Therefore, asking these questions at other time points is unlikely to make a difference, and the results we found were unlikely related to the order in which they were administered but by the administration method. Finally, although some individuals may have screened positive for depression, it is essential to note that the PHQ-9 test is a screening test, and not all individuals may be ultimately diagnosed with depression. The diagnosis should be confirmed using the Diagnostic and Statistical Manual of Mental Disorders, fifth edition, criteria if the screening is positive. However, a lack of a positive screening may miss this crucial diagnosis. Future multicenter studies for optimal strategies to screen, diagnose, and manage depression among adults with SCD are needed.
In summary, this study is 1 of the first to demonstrate that the method of depression screening administration is important to consider when screening for depression symptoms in adults with SCD so that all of those with symptoms can be identified and receive appropriate mental health services.
Conflict-of-interest disclosure: P.D. reports being a consultant for Global Blood Therapeutics for grant review; advisory board member for Forma; and a consultant and speaker for Novartis. R.M.C. and S.C. report grant funding from Formabridge. The remaining authors declare no competing financial interests.
Acknowledgments
Contribution: R.M.C. designed the study; N.Q., K.L., R.D.C., P.D., and R.M.C. collected the data; P.M.S. and M.L. performed the analyses; P.M.S., S.C., R.D.C., P.D., and R.M.C. interpreted the results; R.M.C., M.L., and P.M.S. wrote the first version of the manuscript; and all authors reviewed and edited the manuscript before submission.
Footnotes
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 9-12 December 2023.
Data are available upon reasonable request from the corresponding author, Robert M. Cronin (robert.cronin@osumc.edu).
References
- 1.Michlitsch J, Azimi M, Hoppe C, et al. Newborn screening for hemoglobinopathies in California. Pediatr Blood Cancer. 2009;52(4):486–490. doi: 10.1002/pbc.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motulsky AG. Frequency of sickling disorders in U.S. blacks. N Engl J Med. 1973;288(1):31–33. doi: 10.1056/NEJM197301042880108. [DOI] [PubMed] [Google Scholar]
- 3.Jonassaint CR, Jones VL, Leong S, Frierson GM. A systematic review of the association between depression and health care utilization in children and adults with sickle cell disease. Br J Haematol. 2016;174(1):136–147. doi: 10.1111/bjh.14023. [DOI] [PubMed] [Google Scholar]
- 4.Osunkwo I, Andemariam B, Minniti CP, et al. Impact of sickle cell disease on patients' daily lives, symptoms reported, and disease management strategies: results from the International Sickle Cell World Assessment Survey (SWAY) Am J Hematol. 2021;96(4):404–417. doi: 10.1002/ajh.26063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Major Depression . 2021. National Institute of Mental Health. [Google Scholar]
- 6.Hasan SP, Hashmi S, Alhassen M, Lawson W, Castro O. Depression in sickle cell disease. " J Natl Med Assoc. 2003;95(7):533–537. [PMC free article] [PubMed] [Google Scholar]
- 7.Jenerette C, Funk M, Murdaugh C. Sickle cell disease: a stigmatizing condition that may lead to depression. Issues Ment Health Nurs. 2005;26(10):1081–1101. doi: 10.1080/01612840500280745. [DOI] [PubMed] [Google Scholar]
- 8.Comer EW. Integrating the health and mental health needs of the chronically ill: a group for individuals with depression and sickle cell disease. Soc Work Health Care. 2004;38(4):57–76. doi: 10.1300/J010v38n04_04. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi S, Ghafuri DL, Jordan N, Kassim A, Rodeghier M, DeBaun MR. Clustering of end-organ disease and earlier mortality in adults with sickle cell disease: a retrospective-prospective cohort study. Am J Hematol. 2018;93(9):1153–1160. doi: 10.1002/ajh.25202. [DOI] [PubMed] [Google Scholar]
- 10.Adam SS, Flahiff CM, Kamble S, Telen MJ, Reed SD, De Castro LM. Depression, quality of life, and medical resource utilization in sickle cell disease. Blood Adv. 2017;1(23):1983–1992. doi: 10.1182/bloodadvances.2017006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerrell JM, Tripathi A, McIntyre RS. Prevalence and treatment of depression in children and adolescents with sickle cell disease: a retrospective cohort study. Prim Care Companion CNS Disord. 2011;13(2) doi: 10.4088/PCC.10m01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reese FL, Smith WR. Psychosocial determinants of health care utilization in sickle cell disease patients. Ann Behav Med. 1997;19(2):171–178. doi: 10.1007/BF02883334. [DOI] [PubMed] [Google Scholar]
- 13.Siu AL, US Preventive Services Task Force USPSTF, Bibbins-Domingo K, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(4):380–387. doi: 10.1001/jama.2015.18392. [DOI] [PubMed] [Google Scholar]
- 14.Hunt M, Auriemma J. Self-report bias and underreporting of depression on the BDI-II. J Pers Assess. 2003;80(1):26–30. doi: 10.1207/S15327752JPA8001_10. [DOI] [PubMed] [Google Scholar]
- 15.Smith SK, Johnston J, Rutherford C, Hollowell R, Tanabe P. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43(5):444–450. doi: 10.1016/j.jen.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Barney Lisa J, Griffiths KM, Jorm AF, Christensen H. Stigma about depression and its impact on help-seeking intentions. Aust N Z J Psychiatry. 2006;40(1):51–54. doi: 10.1080/j.1440-1614.2006.01741.x. [DOI] [PubMed] [Google Scholar]
- 17.Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58(1):55–61. doi: 10.1001/archpsyc.58.1.55. [DOI] [PubMed] [Google Scholar]
- 18.Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;39(8):675–686. doi: 10.1080/01612840.2018.1443530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102(11):1050–1055. doi: 10.1016/s0027-9684(15)30732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke Kurt, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto-Meza A, Serrano-Blanco A, Peñarrubia MT, Blanco E, Haro JM. Assessing depression in primary care with the PHQ-9: can it be carried out over the telephone? J Gen Intern Med. 2005;20(8):738–742. doi: 10.1111/j.1525-1497.2005.0144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsey MA, Joe S, Nebbitt V. Family matters: the role of mental health stigma and social support on depressive symptoms and subsequent help seeking among African American boys. J Black Psychol. 2010;36(4):458–482. doi: 10.1177/0095798409355796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuithoff NPA, Vergouwe Y, King M, et al. The Patient Health Questionnaire-9 for detection of major depressive disorder in primary care: consequences of current thresholds in a crosssectional study. BMC Fam Pract. 2010;11(1):98. doi: 10.1186/1471-2296-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Fu Z, Bo Q, Mao Z, Ma X, Wang C. The reliability and validity of PHQ-9 in patients with major depressive disorder in psychiatric hospital. BMC Psychiatry. 2020;20(1):474. doi: 10.1186/s12888-020-02885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]