Abstract
In this study, three mutants, dsbA::kan, dsbC-kan, and dsbD-kan, of Shigella flexneri serotype 5 were constructed and characterized to investigate the role of the periplasmic thiol:disulfide oxidoreductases in pathogenicity. In gentamicin protection assays and the Serény test, the dsbA mutant showed reduced virulence while the dsbC and dsbD mutants were similar to the wild type. That inactivation of dsbA was responsible for the reduced virulence was verified by complementation with the cloned wild-type gene in in vitro and in vivo assays. Despite the changed virulence behavior, the dsbA mutant could penetrate HeLa cells 15 min postinfection, consistent with the fact that it actively secretes Ipa proteins upon Congo red induction. Furthermore, the dsbA mutant was able to produce actin comets and protrusions, indicating its capacity for intra- and intercellular spread. However, a kinetic analysis of intracellular growth showed that the dsbA mutant barely grew in HeLa cells during a 4-h infection whereas the wild type had a doubling time of 41 min. Electron microscopy analysis revealed that dsbA mutant bacteria were trapped in protrusion-derived vacuoles surrounded by double membranes, resembling an icsB mutant reported previously. Moreover, the trapped bacteria appeared to be lysed simultaneously with the double membranes, resulting in characteristic empty vacuoles in the host cell cytosol. Thus, the attenuation mechanism for dsbA mutant appears to be more complicated than was previously suggested.
Invasion of Shigella flexneri into epithelial cells involves a multistep process—phagocytosis, lysis of the phagocytic vacuole, multiplication in the cytosol, intracellular spreading, and passage into adjacent cells (28). The entry of the bacterium depends on the Ipa invasins, IpaB, IpaC, and IpaD (22), which are secreted upon contact with the epithelial cell surface. The secreted Ipa proteins are able to induce actin polymerization at the site of bacterial attachment on the cytoplasmic side of the host cell membrane (23, 36). This causes massive rearrangements of the cell cytoskeleton and localized membrane ruffling that mediates bacterial uptake (1, 23). A few minutes after internalization, the bacterium destroys the phagocytic vacuole through the hemolytic activity of IpaB and escapes into and multiplies in the cell cytosol (16). Unipolar expression of IcsA enables the bacterium to polymerize cellular actin filaments, which generates forward movement for the bacterium to spread (13). ics, which is responsible for asymmetrically generated movement, is also responsible for the formation of protrusions by which bacteria pass into an adjacent cell. The bacterial IcsB protein can lyse the double-membrane-bounded protrusion, which allows the organism to escape into the cell cytosol again to complete an infection cycle (3).
The secretion of Ipa proteins is apparently a prerequisite for their becoming functional. IpaB and IpaC are kept apart in the bacterial cytoplasm by their specific molecular chaperon IpgC, but they form a complex when they are secreted into the extracellular milieu (24). The secretion of Ipa proteins relies entirely upon the type III secretion apparatus encoded by spa-mxi at the locus adjacent to the ipa operon on the 220-kb virulence plasmid. A number of genes at the mxi locus have been individually demonstrated to play a role in Ipa protein secretion (2, 4, 5), and the spa32 gene has also been shown to be necessary for the secretion of Ipa proteins (36). Most interestingly, the secretion of Ipa proteins has been reported to be impaired in a Shigella strain in which DsbA, the dominant periplasmic disulfide bond catalyst, has been inactivated (37). When Spa32 failed to form its single intramolecular disulfide bond in the dsbA mutant, it was no longer presented on the outer membrane, in turn leading to failure of Ipa protein secretion.
In recent years, studies of protein folding have revealed that the periplasm of gram-negative bacteria contains a set of redox proteins, DsbA, DsbB, DsbC, DsbD, DsbE, DsbF, and DsbG, involved in disulfide bond exchange and in the balance of the redox potential (25). These proteins belong to the thioredoxin superfamily, and each of them possesses a Cys-X-X-Cys active site. The more N-terminal cysteine is more active than the other one and can form a mixed disulfide bond with a target protein, which on resolution results in an oxidized target protein and a reduced Dsb protein. The same mechanism also appears to be the route by which Dsb proteins interact. Through their integrated action, DsbA and DsbB form a catalytic pathway. DsbA catalyzes oxidative protein folding, while DsbB, a membrane protein, recycles reduced DsbA to the active oxidized form (9, 18). Based on the evidence that a mutation in dsbD or trxA (thioredoxin) causes the same phenotype as a mutation in dsbC, i.e., defective in formation of proteins with multiple disulfide bonds, a second (isomerization) pathway has also been proposed. DsbC reshuffles misfolded multiple disulfide bonds, while DsbD, together with thioredoxin, maintains the activity of DsbC (31). Missiakas and Raina (26) have proposed that DsbE and DsbF also belong to this second pathway and depend on DsbD for their activity. Recently, a new member of the Dsb family, DsbG, has been identified. This protein appears to play a vital role in maintenance of the proper redox balance between the two pathways (6). It has also been reported that the respiratory electron transfer chain participates in the oxidation of DsbA, primarily by acting on DsbB (19).
The involvement of DsbA in pathogenicity, primarily through catalysis of oxidative protein folding in virulence factors, has been demonstrated in a number of bacterial pathogens. For example, DsbA is known to be necessary for the biogenesis of enterotoxin and toxin-coregulated pili in Vibrio cholerae (29, 38). In enteropathogenic Escherichia coli, DsbA is required for the stability of bundle-forming pili (39). In uropathogenic E. coli, DsbA catalyzes disulfide bond formation in a pilin-specific molecular chaperone, PapD, which in turn is required for the assembly of P pili (17). A role for DsbC in the biogenesis of secreted virulence factors with multiple disulfide bonds has also been reported for the plant pathogen Erwinia chrysanthemi (34).
The present investigation into the role of Dsb proteins in pathogenicity has been initiated because of a striking feature of Shigella invasion. The bacterium escapes into and multiplies in the host cell cytosol, a highly reducing environment that is maintained by high concentrations of reduced glutathione (10 mM) (20). Since the major porins in the outer membrane of gram-negative bacteria allow the entry of substances up to 650 Da, reduced glutathione (molecular weight, 307.33) can readily reach the periplasm (20). Clearly, Shigella must have a mechanism to deal with such changes in the redox environment encountered on entering the eukaryotic cytosol. Dsbs are hypothesized to play a vital role at this stage. It has been demonstrated in vitro that a mutation in all of the dsb genes except dsbE renders E. coli sensitive to reducing reagents such as dithiothreitol (25). Dsbs may directly oxidize the reductants or, alternatively, catalyze the formation of other proteins that accomplish detoxification.
To test this hypothesis in S. flexneri, three mutants, dsbA, dsbC, and dsbD, were constructed and characterized in this study. Reduced virulence was observed in the dsbA mutant but not in the dsbC and dsbD mutants. However, the manner in which DsbA affects virulence appears to involve not only prevention of Ipa protein secretion, as previously reported (37), but also other mechanisms connected to bacterial intracellular growth.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. S. flexneri strains were routinely grown at 37°C overnight on tryptic soy agar (TSA) plates containing 0.01% Congo red. Red colonies were inoculated into tryptic soy broth (TSB) and grown to an appropriate turbidity at 37°C with shaking (180 rpm). E. coli strains were routinely grown at 37°C in Luria-Bertani medium (L broth or 1.5% L agar). Dithiothreitol (10 mM) or penicillin G (50 μg/ml) was added as a supplement to TSA to confirm the presence of dsb mutants. Antibiotic supplementation when necessary was to the following final concentrations: streptomycin, 100 μg/ml; ampicillin, 200 μg/ml; kanamycin, 50 μg/ml; and trimethoprim, 100 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Geno/pheno type | Source or reference |
|---|---|---|
| Shigella flexneri | ||
| M90TS | Wild type, Strr | 3 |
| Sh4 | dsbA::kan mutant, Strr Kanr | This study |
| Sh16 | dsbC-kan mutant, Strr Kanr | This study |
| Sh12 | dsbD-kan mutant, Strr Kanr | This study |
| E. coli | ||
| JCB571 | MC1000 phoR zih12::Tn10 dsbA::kan1 | 8 |
| XL-1 Blue | recA1 lac endA1 gyrA96 thi hsdR17 supE44 rclA1 (F′, proAB lacIqlacZΔM15 Tn10) | Pharmacia |
| CC118 λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1 λpir phage lysogen | 16 |
| Plasmids | ||
| pGEM-T | Vector for cloning of PCR products | Promega |
| pCVD442 | Suicide plasmid with R6K origin and the sacB gene of Bacillus subtilis | 11 |
| pJYU2 | Derivative of pCVD442 for delivery of the dsbD-kan mutation | This study |
| pJYU3 | Derivative of pCVD442 for delivery of the dsbC-kan mutation | This study |
Plasmid pMAW205 (carrying the dsbA gene from S. flexneri 2a), was kindly provided by C. Sasakawa, University of Tokyo, Tokyo, Japan (37).
PCR cloning and DNA sequencing.
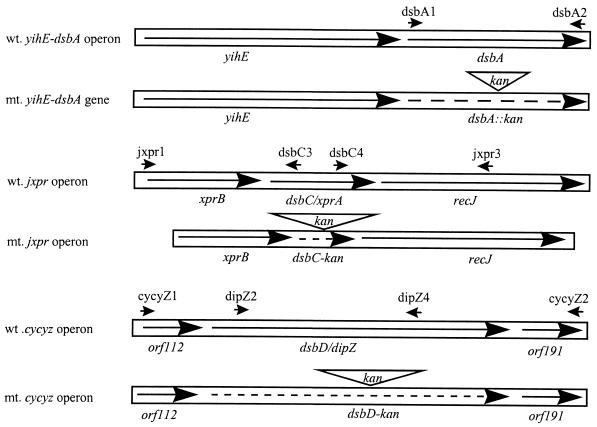
The oligonucleotide primers used were as indicated in Fig. 1A. dsbA1 (5′-GGAATTCGGAGAGAGTAGATCATGAA-3′) and dsbA2 (5′-CCCGGATCCTTTTTTCTCGGACAGATAT-3′) correspond to S. flexneri dsbA (accession no. D38253) bases 401 to 420 and 1042 to 1021 (antisense), respectively. jxpr1 (5′-CTTCTCGATTCTGCTGT-3′), dsbC3 (5′-CGGGATCCCAACATAAAACCTTTCTTCAT-3′), dsbC4 (5′-CGGGATCCCCTCGACGAACACCAAAA-3′), and jxpr3 (5′-CGGGATCCTGTTGCAGCAGACGGAAA-3′) correspond to E. coli jxpr operon (accession no. M54884) bases 3 to 20, 1048 to 1028 (antisense), 1699 to 1716, and 3270 to 3152 (antisense), respectively. cycyZ1 (5′-GGGTACTCTGGGTGTT-3′), cycyZ2 (5′-TAAACCGTAATCTGCAC-3′), dipZ2 (5′-GTTGTTGATCGGTATTGG-3′), and dipZ4 (5′-ACTGCAAGTGTCGTTCA-3′) correspond to E. coli cycyz operon (accession no. X77707) bases 1 to 16, 2897 to 2881 (antisense), 1003 to 1020, and 2188 to 2005 (antisense) respectively.
FIG. 1.
Schematic representation (not to scale) of wild-type (wt., solid arrows) dsbA, dsbC, and dsbD genes and their respective isogenic mutants (mt., broken arrows). Primers used in the study are indicated (small arrows). dsbA has its own strong promoter but can be cotranscribed with the preceding gene, yihE, from a weak promoter regulated by CpxAR (10, 30). Downstream of the stop codon of the dsbA is a strong terminator. dsbC/xprA is the second gene in the jxpr operon. It is likely to be cotranscribed with jxprB and recJ (accession no. M54884). dsbD/dipZ, possessing a typical promoter consensus sequence, is adjacent to orf112 and orf119 and may be cotranscribed with the latter gene (accession no. X77707).
Red-Hot DNA polymerase (Advanced Biotechnologies) was used for PCR amplification. PCR cloning was carried out with a pGEM-T system (Promega). In vitro mutagenesis was carried out on cloned dsbC and dsbD genes by insertion of a kanamycin cassette (kan) from pUC4K into appropriate sites. The mutated genes were subcloned into pCVD442 to generate suicide plasmids for the delivery of dsb mutations to the M90TS chromosome. DNA sequencing was done with a model ABI373 automated sequencing machine. Restriction enzymes were from Boehringer Mannheim, Lewes, United Kingdom.
Genetic manipulation.
P1 transduction was performed by the method of Silhavy et al. (35) with a phage lysate prepared from an E. coli dsbA::kan strain, JCB571 (8). The wild-type dsbC and dsbD genes were replaced by allelic exchange, using plasmid pJYU3 and pJYU2, respectively, by the method of Donnenberg and Kaper (11). The suicide vector pCVD422 carries a sacB gene, so that the use of L agar containing 5% sucrose offers a positive selection for a double-crossover event (11). The dsbC mutant was first constructed in pGEM-T with a kan cassette of 1.2 kb from pUC4K (Pharmacia) replacing a 651-bp internal fragment of the dsbC gene. The dsbC-kan insert was subcloned into pCVD442 as a SacI-SphI fragment. This gave rise to plasmid pJYU3, which was used to deliver the dsbC mutation to the M90TS chromosome. The dsbD-kan insert was constructed after the PCR product of the dsbD region (by using primers cycyZ1 and cycyZ2) was cloned into pGEM-T. The dsbD-kan insert was later subcloned into pCVD442 as a SacI-SphI fragment to give rise to plasmid pJYU2, which was used to deliver the dsbD mutation to the M90TS chromosome.
Infection of HeLa cells by S. flexneri.
HeLa cells were cultured in minimal essential medium (GIBCO BRL) supplemented with 10% fetal calf serum at 37°C under 5% CO2. Gentamicin protection assays were carried out by the method of Sansonetti et al. (32). Infection was stopped by extensive washing with phosphate-buffered saline (PBS), and the cells were either fixed with 3.7% paraformaldehyde for Giemsa staining or lysed with 1 ml of 0.5% sodium deoxycholate for assessing bacterial viable counts (by plating out a serial dilution on TSA plates).
For kinetic analysis of bacterial growth within HeLa cells and immunofluorescent labelling, infection was performed in the same way as for gentamicin protection assays but stopped at set time points.
Immunoblotting.
Bacteria were grown in TSB at 37°C with aeration. Congo red was added to 0.01% (wt/vol) 0, 1.5, 3, and 5 h. For sample preparation, equal numbers of bacteria from each culture were taken during the late exponential phase by adjusting the optical density at 600 nm to 1. Supernatants were passed through a 0.45-μm-pore-size filter. Proteins were precipitated with 10% trichloroacetic acid, washed with 70% ethanol, dissolved in 100 μl of sample buffer, and boiled for 5 min. A 25-μl volume (approximately 10 μg of protein) was loaded on the gel. Bacterial pellets were washed once with PBS, resuspended in 100 μl of sample buffer, and boiled for 5 min. A 10-μl volume (approximately 30 μg of protein) was loaded onto the gel. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. IpaB and IpaC were detected with monoclonal antibodies H16 (anti-IpaB) and J22 (anti-IpaC) followed by incubation with goat anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (7). Immunoblots were visualized by chemiluminescence (Amersham).
Sereny test.
One full loop of bacteria was taken from each overnight culture on Congo red-TSA and instilled into the conjunctival sac of a healthy guinea pig. The animals were observed every day for 14 days for the development of keratoconjunctivitis.
Immunofluorescence labelling. (i) Double labelling of intra- and extracellular bacteria.
At 15 min postinfection, the HeLa cell monolayers were washed extensively with PBS and fixed with 3.7% (wt/vol) paraformaldehyde for 30 min. Extracellular bacteria were labelled in red; the primary antibody was mouse monoclonal antibody against S. flexneri serotype 5 lipopolysaccharide (LPS), and the secondary antibody was donkey anti-mouse IgG conjugated with Texas red. Intracellular bacteria were labelled in green; cells were permeabilized with 0.1% Triton X-100 for 20 min, and the intracellular bacteria were captured with rabbit anti-serum against S. flexneri serotype 5 LPS followed by secondary labelling with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (27).
(ii) Detection of actin comets and protrusions.
Infected HeLa cell monolayers were fixed with 3.7% (wt/vol) paraformaldehyde for 30 min. The bacteria were labelled in red; rabbit antiserum against S. flexneri serotype 5 LPS was used to capture bacteria followed by secondary labelling with goat anti-rabbit polyclonal IgG conjugated with Texas red (27). Polymerized actin was labelled in green by using bodify-phallacidin (Sigma). Preparations were studied by fluorescence microscopy (BH2-RFCA; Olympus Optical Co. Ltd.).
Transmission electron microscopy.
Transmission electron microscopy was carried out by the method of Sansonetti et al. (32). Infected HeLa cell monolayers were fixed with 4% (wt/vol) glutaraldehyde for 1 h at room temperature and then overnight at 4°C. The cells were then scraped loose, recovered by centrifugation, and concentrated in 1% agar. They were further treated with 1% osmium tetroxide, stained with uranyl acetate, embedded, thin sectioned, and treated with Reynold’s lead citrate contrast agent. Microscopy observations were made with a Philips 400 T electron microscope.
RESULTS
Construction of dsbA, dsbC, and dsbD mutants of S. flexneri 5.
A schematic illustration of the wild-type dsb genes and their respective mutants constructed in this study is shown in Fig. 1. The dsbA mutation was transduced into S. flexneri M90TS from E. coli JCB571 (dsbA::kan) (8). A candidate transductant, Sh4, which is unable to grow on TSA containing 10 mM dithiothreitol, was confirmed by PCR as well as DNA sequencing (data not shown). dsbC and dsbD mutants were obtained by allelic exchange, substituting the respective wild-type genes with in vitro-constructed dsbC-kan and dsbD-kan as described in Materials and Methods. Both the dsbC (Sh16) and dsbD (Sh12) mutants were sensitive not only to dithiothreitol (10 mM) but also, characteristically, to penicillin G (50 μg/ml), indicating that the respective dsb genes were inactivated (23). The interrupted dsbC and dsbD genes were confirmed by PCR (data not shown).
Virulence analysis: only Sh4, the dsbA mutant, showed reduced virulence.
In three separate experiments, Sh4 showed a 100-fold fall in recovered viable counts 2 h postinfection compared to M90TS (Table 2). However, examination of Giemsa-stained samples under the light microscope indicated that Sh4 invaded to the same extent as the wild type but that there were fewer bacteria in infected cells (Table 2). In this it differed from a dsbA mutant of S. flexneri serotype 2a, reported to be less invasive than the wild type (37). The dsbC and dsbD mutants did not show any difference from the wild type in recovered viable counts or on inspection of Giemsa-stained samples (Table 2).
TABLE 2.
Virulence characterization of S. flexneri serotype 5 strains
| Strain/plasmid | Gentamicin protection assaya
|
Sereny test results | ||
|---|---|---|---|---|
| Viable countsb | % of infected cells | Mean no. of bacteria per cellc | ||
| M90TS | (6.8 ± 0.07) × 106 | 45 ± 2 | 100 ± 20 | Positive: conjunctival edema and hyperemia occurred in 24 h; keratoconjunctivitis became obvious at 48 h. |
| Sh4 | (4.8 ± 0.11) × 104 | 49 ± 3 | 0.6 ± 0.1 | Negative: normal appearance throughout 14 days |
| Sh4/pMAW205 | (6.5 ± 0.20) × 106 | 43 ± 2 | 100 ± 30 | Positive: conjunctival edema and hyperemia occurred on day 3; keratoconjunctivitis became clear on day 4 |
| Sh16 | (6.9 ± 0.02) × 106 | 45 ± 3 | NDd | Positive: same as M90TS |
| Sh12 | (6.9 ± 0.15) × 106 | 44 ± 4 | NDd | Positive: same as M90TS |
Results of three separate experiments were collected and analyzed.
Viable counts (CFU) recovered from bacterially infected HeLa cell monolayers 2 h after infection.
The mean number of intracellular bacteria per infected cell was calculated by using the equation of Sansonetti et al. (32): (number of bacteria per plate)/(number of HeLa cells × percentage of infected cells).
ND, not determined.
To assess whether the invasion of HeLa cells correlated with the ability to cause infection in vivo, the Sereny test was used. As shown in Table 2, all the strains except Sh4 induced characteristic keratoconjunctivitis in healthy guinea pigs.
Inactivation of dsbA is responsible for reduced virulence.
Since the dsbC and dsbD mutants had the same virulence properties as the wild-type strain in the in vitro and in vivo models used, no further studies were performed on these strains. Attention was focused on the dsbA mutant. trans complementation was used to confirm that the reduced virulence observed in Sh4 was indeed due to the dsbA::kan mutation. For this, plasmid pMAW205 was introduced into Sh4 by electroporation. This plasmid carries the entire dsbA sequence followed by a strong transcriptional terminator and the distal 397-bp upstream yihE gene (its total coding sequence is 986 bp) (37). As shown in Table 2, Sh4/pMAW205 produced comparable viable counts to M90TS 2 h postinfection and induced keratoconjuctivitis on day 4. On the other hand, the transformant retained one abnormal property of Sh4 in vitro. Although it grew as well as M90TS in TSB when appropriate aeration was provided, it formed smaller colonies on TSA and grew poorly on MacConkey agar (data not shown). This, as well as the altered DsbA expression, may have contributed to the delay in causing keratoconjunctivitis. It therefore seemed reasonable to conclude that the change in virulence behaviour was due to the inactivation of dsbA.
The dsbA mutant can efficiently penetrate HeLa cells.
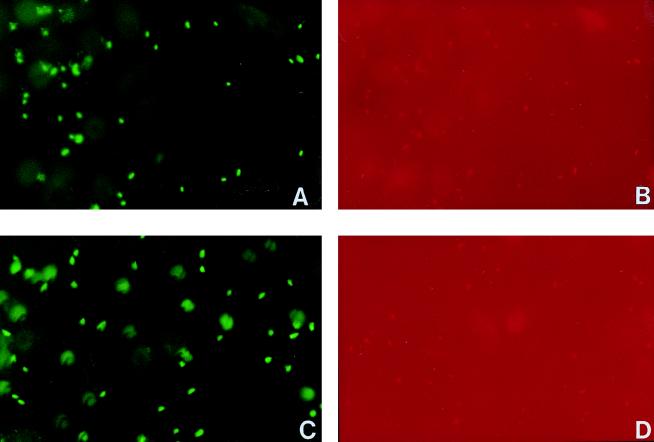
The fact that Sh4 can invade HeLa cells to a similar extent as that of M90TS during a 2-h infection suggested that the it did not have a defect that compromised entry. This was confirmed by a double-immunofluorescence labelling assay. Infection of HeLa cells was arrested 15 min after contact of M90TS or Sh4 with the cell monolayer. Intra- and extracellular bacteria were labelled in green and red, respectively, and examined by immunofluorescence microscopy. It was quite clear from the comparability of fields in each case that penetration of the mutant was as efficient as that of the wild type (Fig. 2).
FIG. 2.
Double-immunofluorescence microscopy analysis of bacterial entry. Infection of HeLa cells was stopped 15 min after infection. Intra- and extracellular bacteria are green and red, respectively. (A and B) Same field of M90TS-infected HeLa cells. (C and D) Same field of Sh4-infected cells.
The dsbA mutant actively secretes Ipa proteins.
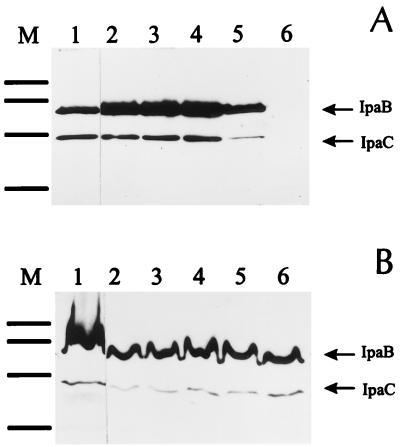
Since the secretion of Ipa proteins is a prerequisite for Shigella invasion but there was no difference between M90TS and Sh4 in efficiency of entry, this suggested that Ipa protein secretion from the mutant was not impaired. This was confirmed by an immunoblot analysis with specific monoclonal antibodies against IpB and IpaC (Fig. 3). Because the secretion of Ipa proteins by S. flexneri is known to be efficient during the exponential phase (7), cultures were collected at late exponential phase. To obtain comparable quantities of protein, equal numbers of bacteria were taken from each culture by adjusting the optical density at 600 nm to 1. Also, because the secretion can be induced by Congo red, all the cultures, except the negative control, were supplemented with this compound (0.01%). To ensure that the response of Sh4 to Congo red induction was unchanged during growth, the compound was added to the cultures at different time points. As shown in Fig. 3A (lanes 2 to 5), Sh4 secreted Ipa proteins to the same extent as did the wild type (lane 1) under the conditions used. No Ipa proteins were detected in the supernatant of Sh4 when Congo red was not added to the culture (lane 6), but they were present in the cell lysates (Fig. 3B, lane 6). Therefore, it may be concluded that secretion of Ipa proteins from Sh4 was not impaired.
FIG. 3.
Immunoblot analysis of Ipa protein secretion from S. flexneri serotype 5. The bacteria were grown in TSB. Congo red was added to 0.01% at 0 h (lane 1 and 2), 1.5 h (lane 3), 3 h (lane 4), and 5 h (lanes 5). Lane 6 contains a noninduction control. Protein samples were prepared and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. IpaB and IpaC were detected with specific monoclonal antibodies. (A) Culture supernatants. Approximately 10 μg of protein was loaded in each lane. (B) Cell lysates. Approximately 30 μg of protein was loaded in each lane. Lanes: 1 M90TS; 2 to 6 Sh4. M, molecular mass marker (96, 68, 43, and 29 kDa from the top to the bottom).
The dsbA mutant retains the capacity for icsA-mediated movement.
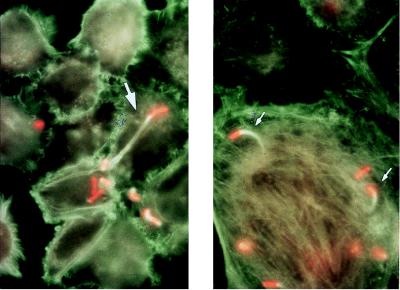
Cellular movement is an important virulence property of S. flexneri. IcsA/VirG protein expressed at one pole of the bacterium elicits actin polymerization, enabling bacterial intracellular movement and cell-to-cell spread (13). The fact that Sh4 appeared in as many host cells as did the wild type after 2 h (see above) suggested that the mutant retained normal ics movement. This was confirmed by immunofluorescence labelling, which enabled detection of the association of intracellular bacteria with polymerized actin. Sh4 produced “comets,” consisting of an associated bacterium (red head) and polymerase actin filaments (green tail), and “protrusions” (membrane-wrapped comets) for intercellular spreading (Fig. 4), which were indistinguishable from those produced by M90TS (data not shown). In addition, immunoblotting analysis with anti-IcsA antibody indicated that the expression and processing of IcsA protein were unchanged in Sh4 compared to M90TS (data not shown).
FIG. 4.
Fluorescence microscopy analysis of bacterial cellular movement. Bacterial infection was stopped after 2 h. Bacteria are shown in red, and polymerized actin filaments are shown in green. Comets (small arrows) and protrusion (large arrow) produced by Sh4 are indicated. They are indistinguishable from those produced by M90T (data not shown).
The dsbA mutant is deficient in intracellular growth.
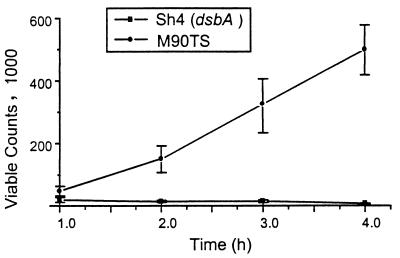
To determine whether DsbA plays a role in intracellular survival and multiplication, kinetic analysis of bacterial intracellular growth was carried out by the method of Sansonetti et al. (32). M90TS grew steadily with a doubling time of about 41 min, close to that reported previously (32), whereas Sh4 barely grew (Fig. 5). This suggested that the dsbA mutant was indeed defective either in survival or in multiplication in the reducing host cell cytosol.
FIG. 5.
Kinetic analysis of bacterial growth in HeLa cells. The multiplicities of infection for M90T and Sh4 were 10 and 100, respectively. At the time points shown, infection was stopped and viable counts were determined.
The dsbA mutant is trapped in double-membrane-bounded vacuoles and lysed.
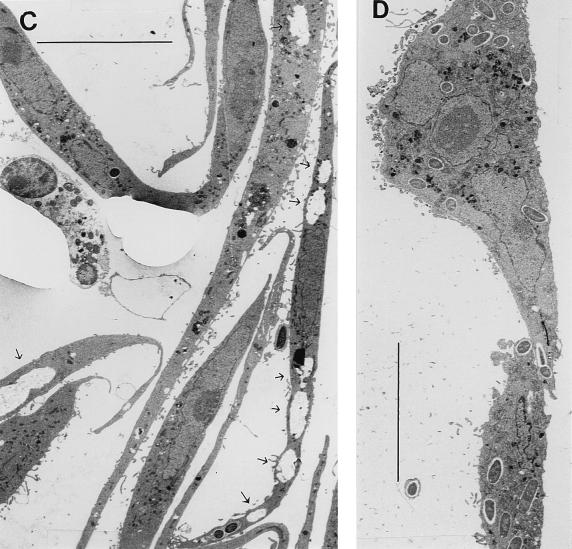
To better understand the attenuation of the dsbA mutant, transmission electron microscopy of infected HeLa cells was carried out by the method of Sansonetti et al. (32). This revealed striking features in cells infected by Sh4. As shown in Fig. 6A, several bacteria were enclosed in a vacuole limited by double membranes. In some areas, the membranes had been destroyed. Inside the compartment, there was a loose network, possibly composed of actin filaments (3), as well as completely deserted areas. The structure of the compartment to some extent resembled that seen on invasion of an icsB mutant (3). In the latter case, the vacuoles were surrounded by intact double membranes containing a large space loosely packed with actin filaments and bacteria but lacking the empty areas seen here. Another double-membrane-bounded vacuole is shown in Fig. 6B. In this case, the vacuole contained only two bacteria and the membranes seemed to have undergone lysis at one pole. Interestingly, the bacterium near the pole had apparently begun to lose its own membranes. In contrast, at the other pole the vacuole membranes as well as the bacterial membranes were clearly intact. A second vacuole shown in Fig. 6B contained no bacteria but just debris with no clear structure. There was no membrane boundary to the cell cytosol. These kinds of empty vacuoles were common in some host cells, as shown in Fig. 6C. In contrast, on infection with M90TS, all the organisms were seen free in the host cell cytosol and no empty vacuoles were observed (Fig. 6D). The trans-complemented strain Sh4/pMAW205 behaved like M90TS in this analysis (data not shown).
FIG. 6.
Transmission electron microscopy analysis of HeLa cells infected with S. flexneri. (A) One double-membrane-bounded vacuole containing four bacteria of Sh4. In some areas, the vacuole membranes have undergone lysis (arrows). N, nucleus. Bar, 1 μm. (B) A double-membrane-bounded vacuole containing two bacteria is shown on the left. At one pole of the vacuole, the membranes have undergone lysis and the bacterium near to this pole has begun to lose its own membrane (thick arrowhead). The bacterium near the other pole has membranes intact (small arrowhead). An empty vacuole (E) similar in size to that containing bacteria is shown on the right. It does not have a membrane boundary, and it contains debris with no clear structure. Bar, 1 μm. (C) Empty vacuoles (arrows) are abundant in some HeLa cells. Bar, 10 μm. (D) M90TS-infected HeLa cells. All bacteria are free in the host cell cytosol. Bar, 10 μm.
DISCUSSION
While a contribution of DsbA to the virulence of S. flexneri has been demonstrated in this study, the mechanism(s) revealed seems different from that reported previously. Watarai et al. (37) showed that in a dsbA mutant of an S. flexneri 2a strain, an outer membrane protein, Spa32, a component of the type III secretion apparatus, failed to form its single disulfide bond, leading to impaired Ipa protein secretion. However, in the present study, an immunoblotting analysis showed that Sh4, the dsbA mutant of S. flexneri serotype 5, did not secrete smaller amounts of Ipa proteins than the wild type did under the growth conditions used. Because the appearance of Ipa proteins in the supernatant was clearly a response to Congo red, nonspecific leakage is not the explanation (Fig. 3). It is not known why the two dsbA mutants behave differently in their Ipa protein secretion. However, an immunoblot analysis suggests that the Ipa protein secretion is not completely impaired in the serotype 2a dsbA mutant constructed by Watarai et al. (37a).
In accordance with the finding that the dsbA mutant actively secretes Ipa proteins, the bacterium penetrates HeLa cells by 15 min postinfection (Fig. 2). The fact that the mutant produces actin comets and protrusions (Fig. 4) further supports the conclusion that Ipa protein secretion is not impaired in this strain; IpaB is responsible for lysis of the phagocytic vacuole, which is a prerequisite for the bacterium to polymerize cellular actin filaments for the formation of these structures (16).
Kinetic analysis of bacterial intracellular growth showed that the wild-type M90TS had a double time of 41 min whereas the dsbA mutant barely grew during a 4-h infection (Fig. 5). This strongly suggests that DsbA is crucial for S. flexneri to survive or proliferate in the host cell cytosol, and a possible mechanism has been revealed by transmission electron microscopy. The dsbA mutant was trapped in double-membrane-bounded vacuoles and was apparently lysed simultaneously with the vacuole (Fig. 6B). This bacterial killing (Fig. 6B and C) must be a major factor slowing the growth of the proliferation of organisms within host cells. Formation of double-membrane-bounded vacuoles can occur only after the bacteria pass into adjacent cells via protrusions. Wild-type strains transiently enclosed in this way cannot be easily observed by electron microscopy, because the bacteria destroy the membranes rapidly. An icsB mutant cannot escape from the protrusion and is permanently trapped in the vacuole, but there is no evidence in this case of lysis of the microorganism (3), as apparently occurs in the case of the dsbA mutant. It is unknown why the dsbA mutant is deficient in lysis of the double membranes, similar to the icsB mutant, but one hypothesis was offered. Although IcsB does not have a typical leader peptide, it has been tentatively localized in the periplasm based on fractionation and alkaline phasphatase fusion analysis (3). If this localization is correct, IcsB, possessing four cysteine residues, may well need functional DsbA to form crucial disulfide bonds. When formation of disulfide bonds is severely impaired, as in the dsbA mutant, very little correctly folded IcsB may be produced. An investigation to address the dependency of IcsB biogenesis on DsbA is under way.
What is the molecular basis for lysis of the protrusion vacuoles in the dsbA mutant-infected host cells, and why do bacteria apparently undergo lysis together with the vacuoles? In rabbit polymorphonuclear leukocytes, S. flexneri is trapped and killed in the phagocytic vacuole (21). It has been suggested that the changed microenvironment leads to the failure of IpaB secretion. This results in organisms remaining in the vacuoles (21), where oxygen-independent bactericidal agents, bactericidal/permeability protein in particular, are responsible for bacterial killing. In HeLa cells, which lack such killing mechanisms, wild-type S. flexneri can multiply up to 300 bacteria per cell and no bacterial lysis is observed (32). Since there is no evidence that HeLa cells themselves have cytolytic activity directed at double membranes, low levels of IcsB activity possessed by the dsbA mutant may be responsible for the disintegration of the vacuole membranes. In protrusions, the bacteria must be in a different physiological state from its state elsewhere. The bacteria may have to express some proteins that need functional DsbA to form crucial disulfide bonds. Presumably, these proteins are important for the bacteria to survive upon reentry into the host cell cytosol. This might explain why the dsbA mutant apparently dies on lysis of the protrusion but not on lysis of phagocytic vacuoles.
In summary, while inactivation of DsbC and DsbD of S. flexneri serotype 5 did not adversely affect virulence, inactivation of DsbA caused pleiotropic effects. The most prominent mechanism has been revealed by electron microscopy; bacteria were trapped and lysed in protrusion vacuoles. This not only effectively slows the proliferation of the bacteria but also prevents further spread within the host cell monolayer. Whether other abnormal behavior observed in vitro, such as formation of small colonies on agar plates, also plays a subtle role in causing the reduced virulence is certainly an interesting question. The dsbA clone, pMAW205 expressing dsbA but not yihE, made a virtually full complementation on virulence (Table 2) but does not effectively restore Sh4 to normal growth on solid media. This implies that an effect on expression of the upstream yihE gene may be responsible for the latter defect. Since yihE can be cotranscribed only with dsbA (10), interruption of the latter gene may shorten the half-life of the polycistronic mRNA, leading to reduced expression of yihE. As a final tentative hypothesis, it may be that YihE is important for S. flexneri to grow on solid media and possibly fine-tunes Shigella infection. A full understanding of the attenuation mechanism awaits not only identification of the target proteins for DsbA but also a study of the role of YihE in bacterial infection.
ACKNOWLEDGMENTS
I am grateful to J. S. Kroll for his enthusiastic support for this project and for critical reading of the manuscript and to P. J. Sansonetti and his staff, especially J. Mounier, at Institut Pasteur, where much of the characterization of the mutant strains was carried out. I thank C. Sasakawa, University of Tokyo, for kindly providing pMAW205; G. Collar for electron microscopy service; C. Piper and B. Edwards-Jones for excellent technical support; and P. Lanford for useful discussion throughout the study and during the preparation of the manuscript.
J. Yu is a Wellcome Trust Career Development research fellow (U.K.) and is supported by grant COND/7/94.
REFERENCES
- 1.Adam T, Giry M, Boquet P, Sansonetti P J. Rho-dependent membrane folding causes Shigella entry into epithelial cells. EMBO J. 1996;15:3315–3321. [PMC free article] [PubMed] [Google Scholar]
- 2.Allaoui A, Sansonetti P J, Pasort C. MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J Bacteriol. 1992;174:7661–7669. doi: 10.1128/jb.174.23.7661-7669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaoui A, Mounier J, Prevost M C, Sansonetti P J, Parsot C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol Microbiol. 1992;6:1605–1616. doi: 10.1111/j.1365-2958.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 4.Allaoui A, Sansonetti P J, Pasort C. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol Microbiol. 1993;7:59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 5.Allaoui A, Sansonetti P J, Menard R, Barzu S, Mounier J, Phalipon A, Pasort C. MxiG, a membrane protein required for secretion of Shigella spp. Ipa invasins: involvement in entry into epithelial cells and intercellular dissemination. Mol Microbiol. 1995;17:461–470. doi: 10.1111/j.1365-2958.1995.mmi_17030461.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson C L, Matthey-Dupraz A, Missakas D, Raina S. A new Escherichia coli gene, dsbG, encodes a periplasmic protein involved in disulphide bond formation, required for recycling DsbA/DsbB and DsbC redox proteins. Mol Microbiol. 1997;26:121–132. doi: 10.1046/j.1365-2958.1997.5581925.x. [DOI] [PubMed] [Google Scholar]
- 7.Bahrani F K, Sansonetti P J, Parsot C. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect Immun. 1997;65:4005–4010. doi: 10.1128/iai.65.10.4005-4010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardwell J C A, McGovern K, Beckwith J. Identification of a protein required for disulphide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 9.Bardwell J C A, Lee J O, Jander G, Martin N, Berlin D, Beckwith J. A pathway for disulphide bond formation in vivo. Proc Natl Acad Sci USA. 1993;90:1038–1042. doi: 10.1073/pnas.90.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belin P, Boquet P L. The Escherichia coli dsbA gene is partly transcribed from the promoter of a weekly expressed upstream gene. Microbiology. 1994;140:3337–3348. doi: 10.1099/13500872-140-12-3337. [DOI] [PubMed] [Google Scholar]
- 11.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enetropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Prada C M, Hoover D L, Tall B D, Venkatesan M M. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infect Immun. 1997;65:1486–1496. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg M B, Arzu O, Parsot C, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993;175:2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale T L, Formal S B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981;32:137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic-resistant selection markers for cloning and stable chromosomal insertion of gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.High N, Mounier J, Prevost M C, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultgren S J, Abraham S, Caparon M, Falk P, St. Geme III J W, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 18.Kishigami S, Kanaya E, Kikuchi M, Ito K. DsbA-DsbB interaction through their active site cysteines. J Biol Chem. 1995;270:17072–17074. doi: 10.1074/jbc.270.29.17072. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Kishigami S, Sone M, Inokuchi H, Mogi T, Ito K. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc Natl Acad Sci USA. 1997;94:11857–11862. doi: 10.1073/pnas.94.22.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodish H, Baltimore D, Berk A, Zipursky S L, Matsudaira P, Darnell J. Molecular cell biology. 3rd ed. New York, N.Y: Scientific American Books, Inc.; 1995. [Google Scholar]
- 21.Mandic-Mulec I, Weiss J, Zychlinsky A. Shigella flexneri is trapped in polymorphonuclear leukocyte vacuoles and efficiently killed. Infect Immun. 1997;65:110–115. doi: 10.1128/iai.65.1.110-115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menard R, Sansonetti P J, Pasort C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menard R, Sansonetti P J, Pasort C. The secretion of the Shigella flexneri Ipa invasin is induced by the epithelial cell and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menard R, Sansonetti P J, Pasort C, Vasselo T. Extracellular association and cytoplasmic partioning of the IpaB and IpaC invasins of Shigella flexneri. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 25.Missiakas D, Schwager F, Raina S. Identification and characterisation of a new disulphide isomerase-like protein (DsbD) in Escherichia coli. EMBO J. 1995;14:3415–3424. doi: 10.1002/j.1460-2075.1995.tb07347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missiakas D, Raina S. Protein folding in the bacterial periplasm. J Bacteriol. 1997;179:2465–2471. doi: 10.1128/jb.179.8.2465-2471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mounier J, Bahrani F K, Sansonetti P J. Secretion of Shigella flexneri Ipa invisins on contact with epithelial cells and subsequent entry of the bacterium into cells are growth stage dependent. Infect Immun. 1997;65:774–782. doi: 10.1128/iai.65.2.774-782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasort C, Sansonetti P J. Invasion and the pathogenesis of Shigella infections. Curr Top Microbiol Immunol. 1996;209:25–41. doi: 10.1007/978-3-642-85216-9_2. [DOI] [PubMed] [Google Scholar]
- 29.Peek J A, Taylor R K. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factor of Vibrio cholerae. Proc Natl Acad Sci USA. 1992;89:6210–6214. doi: 10.1073/pnas.89.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pogliano J, Lynch A S, Belin D, Lin E C C, Beckwith J. Regulation of Escherichia coli cell envelop proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 31.Rietsch A, Belin D, Martin N, Beckwith J. An in-vivo pathway for disulphide bond isomerisation in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:13048–13053. doi: 10.1073/pnas.93.23.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sansonetti P J, Ryter A, Clerc P, Maurelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. Infect Immun. 1993;175:2334–2346. doi: 10.1128/jb.175.8.2334-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shevchik V E, Bortoligerman I, Robertbaudouy J, Robinet S, Barras F, Condemine G. Differential effect of DsbA and DsbC mutations on extracellular enzyme-secretion in Erwinia chrysanthemi. Mol Microbiol. 1995;16:745–753. doi: 10.1111/j.1365-2958.1995.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 35.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 36.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Disulphide oxidoreductase activity of Shigella flexneri is required for release of Ipa proteins and invasion of epithelial cells. Proc Natl Acad Sci USA. 1995;92:4927–4931. doi: 10.1073/pnas.92.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Yu, J. Unpublished observation.
- 38.Yu J, Webb H, Hirst T R. A homologue of the Escherichia coli DsbA protein involved in disulphide bond formation is required for enterotoxin biogenesis in Vibrio cholerae. Mol Microbiol. 1992;6:1949–1958. doi: 10.1111/j.1365-2958.1992.tb01368.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H Z, Donnenberg M S. DsbA is required for stability of the type-IV pilin of enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:787–797. doi: 10.1046/j.1365-2958.1996.431403.x. [DOI] [PubMed] [Google Scholar]