Abstract
Diabetes is associated with an increased risk and progression of Alzheimer’s (AD) and Parkinson’s (PD) diseases. Conversely, diabetes may confer neuroprotection against amyotrophic lateral sclerosis (ALS). It has been posited that perturbations in glucose and insulin regulation, cholesterol metabolism, and mitochondrial bioenergetics defects may underlie the molecular underpinnings of diabetes effects on the brain. Nevertheless, the precise molecular mechanisms remain elusive. Here, we discuss the evidence from molecular, epidemiological, and clinical studies investigating the impact of diabetes on neurodegeneration and highlight shared dysregulated pathways between these complex comorbidities. We also discuss promising antidiabetic drugs, molecular diagnostics currently in clinical trials, and outstanding questions and challenges for future pursuit.
Keywords: Alzheimer’s disease, amyotrophic lateral sclerosis, diabetes, insulin resistance, Parkinson’s disease
Diabetes: a metabolic disease with devastating effects on brain health.
Diabetes is a chronic metabolic disease characterized by the incapacity of the pancreas to produce enough insulin and the inability of the body to efficiently use the insulin it produces, known as insulin resistance. These perturbations lead to hyperglycemia (See Glossary), which could deteriorate various organ systems, particularly the nerves and blood vessels, leading to blindness, peripheral neuropathy, peripheral vascular disease, kidney disease, cardiovascular disease, and cognitive impairment. According to the World Health Organization (WHO), approximately 422 million people worldwide have diabetes, causing nearly 1.5 million deaths yearlyi. Different forms of diabetes exist, including type 2 diabetes mellitus (T2DM), accounting for more than 90% of the cases occurring primarily in adults, and type 1 diabetes (T1D), commonly present in children and adolescents.
Mounting evidence from epidemiological studies indicate that T2DM has a detrimental impact on brain structure and function and contributes to different degrees of cognitive deterioration, ranging from subtle cognitive decline to overt dementia [1,2]. Moreover, insulin resistance and T2DM have been associated with smaller brain volumes and poorer cognitive functions in subjects free of cerebrovascular disease and dementia [3]. A meta-analysis indicated that T2DM is associated with greater brain atrophy over time and suggested that these changes may start early in adulthood and correlate with disease duration [4]. Brain atrophy due to T2DM has been primarily observed in the ventral striatum, cerebellum, putamen, caudate, and pre-motor cortex, brain areas commonly affected in neurodegenerative diseases [5].
A growing number of studies indicate that T2DM is involved in the pathogenesis of Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) (Box 1). Specifically, T2DM is associated with an increased risk of AD and PD [6–11]. Intriguingly, a neuroprotective role of T2DM has been reported in ALS [12–15]. The question arises; why is T2DM a precursor to AD and PD but protective against ALS? There is no clear answer, but it may be found in how genetics and environmental factors influence how the body processes metabolic precursors used in cellular bioenergetics pathways [16–18] (Figure 1, Key Figure). In the following sections, we will discuss the studies investigating the impact of T2DM on brain health and its pathogenic role in neurodegenerative diseases as well as the neuroprotective potential of antidiabetic drugs currently being tested in clinical trials of neurodegenerative diseases.
Box 1. Neurodegenerative diseases linked to diabetes.
AD, PD, and ALS are sporadic neurodegenerative diseases in which the interplay of genetic and environmental factors contributes to disease pathogenesis. AD is the most common cause of dementia, and it is characterized by a gradual decline in memory and behavior, known as mild cognitive impairment (MCI), that over time progresses to frank dementia. Pathological features of AD include the accumulation and clumping of extracellular amyloid β plaques (Aβ) and intraneuronal neurofibrillary tangles (NFTs) [129]. With age being the greatest risk factor, most people with AD are 65 and older. A smaller number of cases present as early-onset familial AD, caused by mutations in amyloid precursor protein (APP) and presenilin 1 and 2 (PSEN1, PSEN2) [130]. Genome-wide association studies (GWAS) have unveiled more than 56 common genetic loci associated with AD risk [131].
PD affects nearly 8.5 million people worldwideii. It is categorized as a movement disorder with tremors, postural instability, rigidity, and bradykinesia. Dopaminergic cell dysfunction and cell death in the substantia nigra underlies the observed motor dysfunction in PD patients. Accumulation of misfolded α-synuclein (SNCA) in Lewy bodies is a pathological hallmark of PD. Like AD, most cases are sporadic but highly penetrant mutations in SNCA, leucine-rich repeat kinase 2 (LRRK2), vacuolar sorting protein 35 (VPS35), parkin RBR E3 ubiquitin-protein ligase (PRKN), PTEN induced kinase 1(PINK1), parkinsonism associated deglycase (PARK7), and glucosylceramidase beta (GBA) have been linked to familial PD [129]. GWAS has identified a total of 90 risk loci associated with PD risk [132].
ALS is characterized by the progressive degeneration of brain and spinal cord motor neurons. Typical cases of ALS present with simultaneous upper and lower motor neuron symptoms at disease onset but varying phenotypes with either predominance of upper or lower motor neuron symptoms may also exist [133]. Genetic forms account for approximately 10% of the cases, presenting with autosomal dominant mutations in genes encoding Cu/Zn superoxide dismutase (SOD1), TAR-DNA binding protein 43 (TDP-43), C9ORF72, fused in sarcoma (FUS) and less frequently in optineurin (OPN), valosin-containing protein (VCP) and ubiquilin 2 (UBQLN2) [134]. Unfortunately, patients commonly die from paralysis of respiratory muscles within 2–5 years of disease onset [133].
Figure 1 (Key Figure). Diabetes: a tipping point in neurodegeneration.
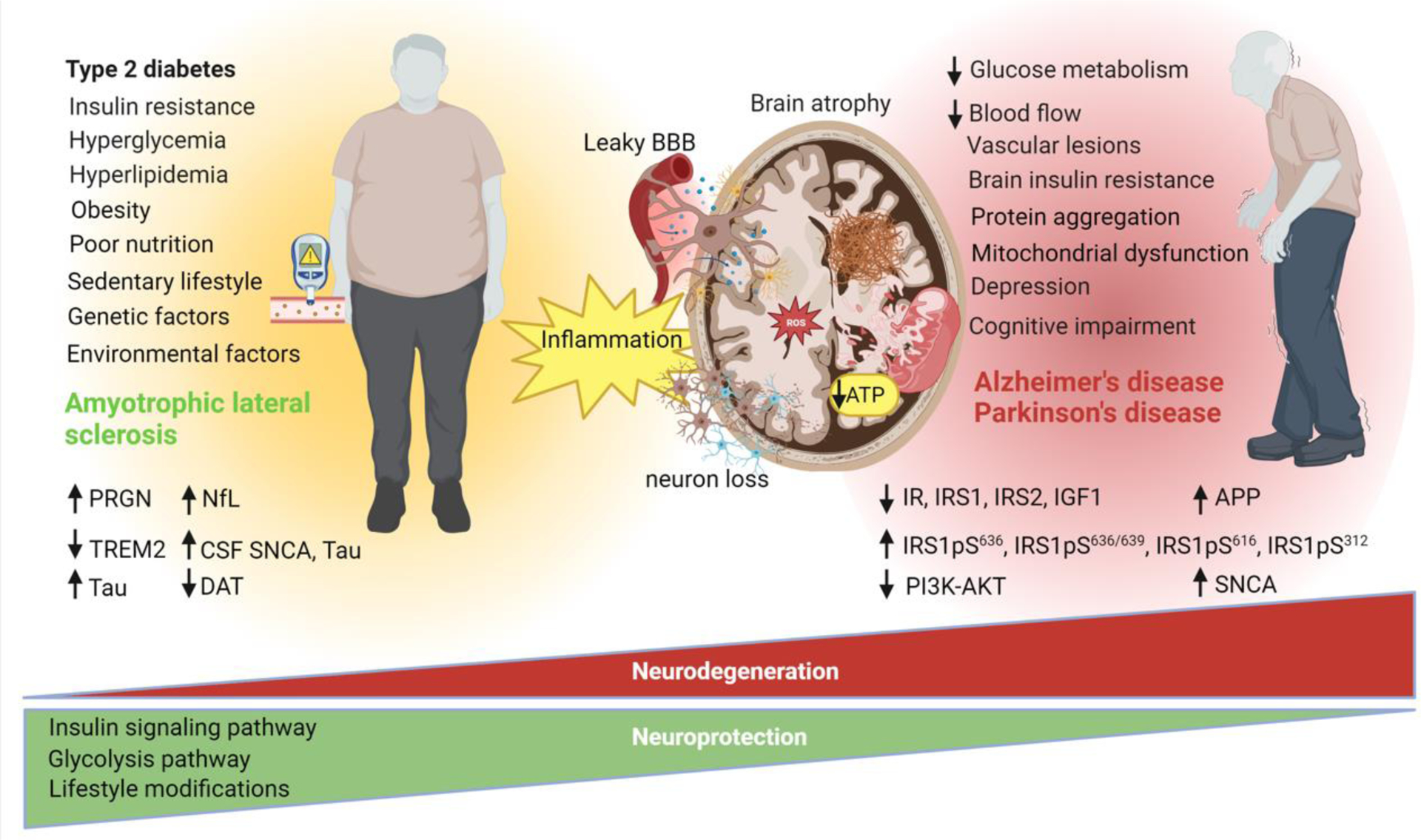
T2DM has many detrimental effects on brain structure and function. T2DM is associated with smaller brain volumes, brain atrophy, decreased blood flow, vascular lesions, disruption of the blood brain barrier, mitochondrial dysfunction, brain insulin resistance, depression, and cognitive impairment. Increasing evidence indicates that T2DM is a risk factor for AD and PD. Metabolic abnormalities associated with T2DM and the metabolic syndrome including insulin resistance, hyperglycemia, hyperlipidemia, and inflammation are implicated in the pathogenesis of neurodegenerative diseases. Intriguingly, these same metabolic abnormalities including T2DM, hyperglycemia, and hyperlipidemia are associated with a decreased risk of ALS in elderly individuals. Emerging research suggests that perturbations in glucose metabolism, insulin signaling, and bioenergetics defects could underlie the relationship between T2DM and neurodegenerative diseases. Several biomarkers, including progranulin (PRGN), triggering receptor on myeloid cells 2 (TREM2), tau, neurofilament light chain (NfL), cerebrospinal fluid (CSF) α-synuclein (SNCA), CSF tau, dopamine binding transporter (DAT), insulin signaling proteins (IR, IRS1, IRS2, IGF1, IRS1pS616, IRS-1 pS636/639, IRS1pS312, PI3K-AKT) are implicated in T2DM and neurodegeneration and may be useful for therapeutic development. Targeting the insulin pathway with antidiabetic drugs and the glycolysis pathway have elicited neuroprotection in several studies. To date, no disease modifying therapy is available for AD, PD, and ALS. Lifestyle modifications including diet and physical activity may help mitigate symptoms and possibly confer neuroprotection. This figure was created using BioRender (https://biorender.com/).
Diabetes and Alzheimer’s disease dementia
Evidence from epidemiological studies suggest that T2DM increases the risk of AD in several populations globally (Table 1). An earlier systematic review reported a higher incidence of any dementia type, including AD and vascular dementia, among patients with T2DM [19]. Vascular complications and impaired glucose, insulin, and amyloid metabolism were suggested to underlie the link between both diseases. Larger, multicenter meta-analyses have obtained similar findings indicating that T2DM increases the risk of cognitive impairment, AD, and other dementias [2,20]. Further, T2DM had an additional 35% increased risk of dementia in individuals carrying mutations in APOE4, the strongest genetic risk factor in AD [21].
Table 1.
Epidemiological studies investigating the association between diabetes and Alzheimer’s disease dementia.
| Study, year | Country | Study design | Sample size | Statistics | Main outcome | Refs. |
|---|---|---|---|---|---|---|
| Cheng et al., 2012. | Multiple | Meta-analysis | N: 44,714 T2DM: 6,184 HC: 38,530 |
RR:1.46 [1.20–1.77] | T2DM increases the risk for AD and related dementias. | [20] |
| Chatterjee et., al 2016. | Multiple | Meta-analysis | N: 2,310,330 T2DM: 70,575 Dementia: 102,174 |
Women RR: 1.62 [1.45–1.80] Men RR: 1.58 [1.38–1.81] |
T2DM is associated with a 60% increased risk of dementia in both sexes. | [145] |
| Ng et al., 2016. | Singapore | Prospective cohort | N:1519 MetS: 340 T2DM: 120 |
HR: 2.47 [1.92–4.19] | T2DM was associated with an increased risk of MCI progression to dementia. | [31] |
| Kivimaki et al., 2019. | Multiple | Meta-analysis | N: 404,840 T2DM: 2,196 Dementia: 94,739 |
Follow-up <10 years HR:1.61 [0.82–3.17] Follow-up > 10 years HR: 1.55 [0.95–2.53] |
T2DM is associated with an increased risk of dementia. Excess dementia risk was observed among physically inactive subjects with cardiometabolic disease. | [146] |
| Xue et al., 2019. | Multiple | Meta-analysis | N: 9,359,005 | Prediabetes and the risk
of: Dementia RR: 1.18 [1.02–1.36] AD 1.36 [1.09–1.70] T2DM and risk of: MCI 1.49 [1.26–1.77] AD 1.43 [1.25–1.62] |
Prediabetes and T2DM were associated with an increased risk of MCI, dementia, and AD. Elevated FPG, HbA1c levels, and hypoglycemia were associated with an increased risk of dementia. | [2] |
| Li et al., 2020. | Multiple | Meta-analysis | N: 16,200 | RR: 1.35 [1.13–1.63] | T2DM conferred a 35 % increased risk of dementia, AD, and vascular dementia. An additional 35% increased risk for those with APOEε4. | [21] |
| Barbiellini et al., 2021. | United Kingdom | Prospective | N: 10,095 T2DM: 1,710 Dementia: 639 |
T2DM onset (HR): >10 yrs, 2.12 [1.50–3.00] 6–10 yrs, 1.49 [0.05–2.32], < 5 yrs 1.11[0.70–1.76] |
Younger age at the onset of diabetes increases the risk of dementia. | [27] |
| Zhang et al., 2017. | Multiple | Meta-analysis | N: 1,746,777 | RR: 1.52 [1.42–1.63] | T2DM is associated with a higher incidence of AD. | [147] |
| Rawlings et al., 2014. | USA | Prospective cohort | N: 13,351 T2DM: 1,779 |
Adjusted Z score −0.15 [−0.22–0.08] | T2DM was associated with a 19% greater cognitive decline in a 20-year follow-up. | [26] |
| Zheng et al., 2018. | United Kingdom | Prospective cohort | N: 5,189 HC: 3553 Prediabetes: 1190 T2DM: 446 |
Z-score SD/year, 95% CI: global cognitive −0.0009 [−0.0014, −0.0003], memory −0.0005 [−0.0009, −0.0001] |
A 1 mmol/mol increment in HbA1c was associated with an increased decline in cognitive scores, memory, and executive functions. | [138] |
HC: healthy controls; HR: hazard ratio; FPG: fasting plasma glucose; RR: risk ratio; MetS: metabolic syndrome; N: number of participants
In contrast, several studies have reported conflicting results. For example, T2DM increased the risk of vascular dementia but not AD in several populations [22,23]. Another cross-sectional study indicated that T2DM was not associated with AD neuropathology, including neuritic plaques and intraneuronal neurofibrillary tangles (NFTs) [24]. Additionally, the Framingham Study showed that T2DM did not increase the risk of incident dementia [25].
Several factors may explain these discrepancies. For instance, these studies did not analyze the age of T2DM onset, duration of T2DM, and other comorbidities. Age of onset, for example, is an important consideration since longitudinal studies have reported that T2DM diagnosed in midlife was associated with a 19% greater cognitive decline in a 20-year follow-up period [26] and that younger age of T2DM onset was associated with a higher risk of developing dementia [27,28]. Likewise, the duration of T2DM is associated with an increased incidence of dementia [29,30]. Notably, individuals in the Framingham Study were predominantly white, and non-fasting glucose levels, instead of fasting, were used to define diabetes [25]. Thus, the cohort used was not representative of the general population, and subjects with impaired glucose tolerance may have been misclassified as non-diabetic.
The impact of other comorbidities and their temporal association with T2DM and dementia are not clearly explained in most epidemiological studies. Patients with T2DM commonly present with hyperlipidemia and hypertension, a cluster of conditions collectively known as the ‘metabolic syndrome,’ also implicated in neurodegeneration. Indeed, a study cohort indicated that T2DM, metabolic syndrome, and obesity were associated with an increased risk of mild cognitive impairment (MCI) progression to dementia [31]. Therefore, a thorough analysis of other comorbidities frequently associated with T2DM will be critical in determining metabolic risk factors in neurodegenerative diseases.
Diabetes and Parkinson’s disease
Like AD, most prospective studies have reported an increased risk of PD among T2DM patients in several populations (Table 2). For example, an increased risk of PD was observed in Finnish men and women with T2DM during an 18-year follow-up period [32]. Similarly, patients with T2DM for more than ten years showed a 40% increased risk of PD in a prospective study in the United States [33]. Similar associations between PD and T2DM have been observed in other populations worldwide [6–10]. These results have been reinforced by a meta-analysis comprising seven population-based cohorts that found a 38% increased risk of PD among T2DM patients [34].
Table 2.
Epidemiological studies investigating the association between diabetes and Parkinson’s disease.
| Study | Country | Study design | Sample size | Statistics | Main outcome | Refs. |
|---|---|---|---|---|---|---|
| Xue et al., 2010. | USA | Prospective cohort | N: 288,662 Diabetes: 21,611 Non-diabetic: 267,051 PD: 1565 |
OR:1.41 [1.20–1.66] | T2DM increased the risk of PD by 40%. | [33] |
| Palacios et al., 2011. | USA | Prospective cohort | N: 147,096 PD: 656 |
RR: 0.88 [0.62–1.25] | A diagnosis of T2DM at baseline was not associated with PD risk. | [148] |
| Schernhammer et al., 2011. | Denmark | Prospective cohort | N: 11,582 PD: 1,931 |
OR: 1.36 [1.08–1.71] | T2DM was associated with a 36% increased risk of PD. | [6] |
| Savica et al., 2012. | USA | Case-control | N:392 PD:196 |
OR: 0.77 [0.37–1.57] | T2DM, or the use of antidiabetic drugs, was not associated with the risk of PD. | [38] |
| Sun et al., 2012. | China | Retrospective, case-control | N: 1,075,604 T2DM: 603,416 |
HR: Men 21–40 years, 2.10 [1.01–4.42]; women 41–60 yrs, 2.05 [1.82–2.30]; older women >60 yrs, 1.65 [1.58–1.73] |
T2DM was associated with an increased risk of PD. | [7] |
| Skeie et al., 2013. | Norway | Case-control | N: 387 PD: 212 |
OR: 1.94 [0.82–4.57] | T2DM is not associated with PD. | [39] |
| Lu et al., 2014. | Multiple | Meta-analysis | N:105974 PD: 21,395 |
OR: 0.75 [0.58–0.98] | T2DM is associated with a decreased risk of PD. | [40] |
| De Pablo Fernandez et al., 2018. | United Kingdom | Prospective cohort | N: 6,173,208 T2DM: 2,017,115 |
HR: 1.32 [1.29–1.35] | T2DM increases the risk of PD. A greater increase is observed in those with complicated T2DM. | [44] |
| Yang et al., 2017. | Taiwan | Case-control | N: 145,176 T2DM: 36,294 |
HR: 1.19 [1.08–1.32] | The incidence of PD was 1.36 higher in T2DM than in non-diabetic controls. | [9] |
| Wahlqvist et al., 2012. | Taiwan | Case-control | N: 800,000 T2DM: 64,166 |
HR: 0.78 [0.61–1.01] | Metformin-sulfonylurea reduced the risk of PD. | [8] |
| Liu et al., 2021. | Multiple | Meta-analysis | N: 3,845,660 PD:26,654 T2DM: 3,819,006 |
Cohort RR: 1.29 [1.15–1.45] Case-controls OR: 0.74, [0.51–1.09] |
T2DM is associated with an increased risk of PD in cohorts but not in case-control studies. | [149] |
| Sanchez-Gomez et al., 2021. | Spain | Retrospective cohort | N: 2,556,928 T2DM: 281,153 Pre-diabetes: 26,379 |
T2DM HR: 1.19 [1.13–1.15] Prediabetes HR: 1.07 [1.00–1.14] |
T2DM and prediabetes were associated with a higher risk of PD. Both prediabetes and T2DM increase the odds of PD, predominantly in women. | [41] |
| Deischinger et al., 2021. | Austria | Cross-sectional | N: 2,173,441 PD: 235,268 |
Men-T2DM OR: 1.46 [1.38–1.54] Women-T2DM OR:1.71 [1.11–1.30] |
Men with T2DM had an increased risk of PD than non-diabetes men. Women with T2DM showed a greater risk of PD than men with T2DM. | [45] |
| Jacobs et al., 2020. | United Kingdom | Case-control | N: 501,780 PD: 1,276 |
OR: 1.27 [1.03–1.57] | T2DM is associated with an increased risk of PD. | [10] |
| Cereda et al., 2012. | Italy | Case-control | N: 961 PD: 783 T2DM: 89 |
UPDRS motor, 22.3 ±9.0 vs. 19.3
± 7.9; Hoehn and Yahr staging, p=0.009 |
The onset of T2DM before the onset of PD was associated with higher UPDRS motor scores and higher Hoehn and Yahr staging. | [51] |
| De Pablo Fernandez et al., 2017. | Spain | Case-control | N: 4,998 PD: 79 |
PD and T2DM OR: 1.89 [0.90–3.98] Long-T2DM duration OR: 3.27 [1.21–8.85] |
No association between PD and T2DM. A positive association in those with long T2DM duration. | [35] |
| Pagano et al., 2018. | USA | Case-control | N: 53 PD-T2DM: 25 T2DM: 14 HC:14 |
Motor progression HR: 4.52 [1.46–13.92] Cognitive decline HR: 9.31 [1.16–74.51] |
T2DM was associated with faster motor progression and cognitive decline. | [44] |
| Chohan et al., 2021. | Multiple | Meta-analysis | T2DM specific meta-analysis Total: ~10 million Any diabetes meta-analysis Total: ~1.3 million |
PD, OR 1.21 [1.07–1.36] motor symptoms, SMD 0.55 [0.39–0.72], causal effect, OR 1.08 [1.02–1.14] |
T2DM was associated with an increased risk of PD and faster progression of motor symptoms. A causal effect of T2DM on PD risk. | [50] |
| Athauda et al., 2022. | United Kingdom | Case-control | N: 1,930 PD-T2DM: 167 PD:1,763 |
UPDRS-III, 25.8 [0.9] vs. 22.5
[0.3]; non-motor symptoms scale, 38.4 [2.5] vs. 31.8 [0.7] |
T2DM patients had more severe motor symptoms assessed by UPDRS-III and non-motor symptoms scales. | [52] |
| Pezzoli et al., 2022. | Italy | Retrospective and prospective study | N: 9,862 PD: 8,380 PD-T2DM: 673 |
HR: 1.64 [1.33–2.02] | Occurrence of T2DM before PD onset was associated with a worse prognosis. | [54] |
| Zhong et al., 2023. | Multiple | Systematic review and meta-analysis | Total: 12,505,077 PD: 306,513 |
Risk of PD OR/RR: 1.23 [1.12–1.35] Motor progression RR: 1.85 [1.47–2.34] Cognitive decline OR/RR: 1.92 [1.45–2.55] |
T2DM is associated with a higher risk of PD, motor progression, and cognitive decline. | [49] |
HR: hazard ratio; FPG: fasting plasma glucose; OR: odds ratio; RR: risk ratio; N: number of participants; SMD: standardized mean difference
Conversely, case-control studies investigating the T2DM-PD linkage have shown conflicting results (Table 2). One study found a positive association between T2DM and PD [6], and another study indicated an increased risk of PD only among those subjects with a long duration of T2DM [35]. Other groups have reported a decreased risk of PD among T2DM patients [36,37] or no significant association [38,39]. In addition, a meta-analysis of 14 case-control studies observed an inverse association between T2DM and PD [40].
A closer inspection of the case-control studies revealed smaller sample sizes and retrospective exposure assessments. Additionally, some studies relied on self-report diagnoses for T2DM. Another consideration is that the distinction between T1D and T2DM was not documented in all the studies. Case-control studies used prevalent PD cases, and the exposure time is sometimes insufficient or not specified. Notwithstanding, most of the more extensive prospective studies, less prone to recall and selection biases, support a positive association between T2DM and PD.
Recently, findings from larger cohort studies indicate that prediabetes and T2DM were associated with a higher risk of PD [41–43]. Interestingly, analysis stratified by sex showed that prediabetes was a risk factor for subsequent PD only in women, and women with T2DM had a higher risk of PD than men [41]. Supporting these findings, recent extensive prospective studies reported that women with T2DM have a higher risk of subsequent PD than men [9,44,45]. This is interesting since most epidemiological studies indicate a higher risk of PD in men [46]. Sex-specific hormonal differences may explain this discrepancy. For instance, estrogen has been suggested to be neuroprotective [47]; thus, its deficiency during menopause and a concurrent T2DM disease process may confer a higher risk of PD in women than men.
Several investigations have explored the impact of T2DM on PD progression and cognitive decline [48–50]. Patients with T2DM who developed PD had greater Unified Parkinson’s Disease Rating Scale (UPDRS) and Hoenh Yahr scores, standard motor scales useful for assessing disease progression [51]. PD patients with comorbid T2DM displayed more severe motor and non-motor symptoms, including worsening gait impairment, cognitive decline, and depression [52]. An important consideration raised in a study [52] is that PD patients with comorbid T2DM were older, had a later disease onset, and had a higher BMI than the PD group. Several studies have reported that T2DM patients with longer disease duration are more likely to develop PD [44,53], which may explain the later PD onset. Moreover, the duration of T2DM increases mortality in PD patients who develop T2DM before PD onset [54]. Additionally, the sample size of PD with comorbid T2DM analyzed in these studies was relatively small, and diagnosis mainly relied on self-report and medication data. Finally, and perhaps more challenging to assess, is the impact of other comorbidities associated with T2DM, as these may also affect PD risk and progression.
Interestingly, an increased risk of PD has been reported in patients with complicated T2DM and younger individuals with T2DM [41,44]. It has been suggested that younger individuals with T2DM may have a greater genetic liability to PD than older individuals, which may be explained by a greater number of shared genetic factors associated with PD and T2DM [41,44]. More studies are needed to clarify the genetic interactions with age in PD and T2DM. Nevertheless, the impact of T2DM on worsening symptoms, disease progression, and increased mortality indicates that T2DM could trigger a more aggressive PD phenotype. It remains unclear whether the effects of T2DM on PD are strictly causal or if there is a synergistic effect of T2DM on PD risk and progression. Because age, age of onset of PD, duration of T2DM, and comorbidities have been shown to influence PD risk and progression, further evaluation in larger, well-characterized, and phenotyped T2DM cohorts will be critical to dissect the linkage between both diseases.
Diabetes and amyotrophic lateral sclerosis
Intriguingly, epidemiological studies suggest a neuroprotective role of T2DM in ALS. A case-control study determined that premorbid T2DM was associated with reduced risk and 4-years later onset of ALS [55]. Another case-control study showed a protective role of T2DM in ALS [56], while other studies did not find a significant association [57,58]. Nevertheless, increasing evidence from case-control and cohort studies from different countries has observed a protective role of T2DM in ALS [12–15] (Table 3).
Table 3.
Epidemiological studies investigating the association between diabetes and ALS.
| Study | Country | Study design | Sample size | Statistics | Main outcome | Refs. |
|---|---|---|---|---|---|---|
| Jawaid et al., 2010. | USA | Case-control | N: 2,371 | ALS-T2DM: years vs. controls 56.3 years, p< 0.05 | T2DM was associated with a 4-year later onset of ALS and motor symptoms. | [55] |
| Zhang et al., 2019. | China | Cohort | N:1,331 ALS-DM: 100 ALS: 1,231 |
Mean age (SD) ALS-T2DM: 57 (9.6) ALS: 52.6 (10.3) |
ALS-T2DM patients showed a 4.4-year delay in disease onset compared to ALS patients without T2DM. | [62] |
| Mitchell et al., 2015. | USA | Case-control | N: 8,849 ALS: 1,288 Controls: 7,561 |
OR: 0.47 [0.38–0.58] | T2DM was associated with a decreased risk of ALS. | [56] |
| Hollinger et al., 2016. | USA | Case-control | N: 1,439 ALS-DM: 129 ALS: 1,310 |
OR: 0.79 [0.526–1.121] | No association between diabetes and ALS. | [58] |
| D’Ovidio et al., 2018. | Italy | Prospective cohort | N=727,977 | HR: 0.30 [0.19–0.45] | T2DM decreases the risk of ALS. | [12] |
| Tsai et al., 2018. | Taiwan | Case-control | ALS:705 Controls: 14,100 |
OR: 0.7 (p<0.05) | T2DM was associated with a lower risk of ALS. | [13] |
| Mariosa et al., 2015. | Sweden | Case-control | ALS: 5,108 Controls: 25,540 |
OR: 0.66 [0.53–0.81] | T2DM decreases the risk of ALS mostly among subjects older >70. | [61] |
| Tsai et al., 2019. | Taiwan | Retrospective cohort | T2DM: 2,135,427 | T2DM onset ≥55 years HR: 0.72 [0.55–0.95] Unstratified analysis HR: 0.87 [0.7–1.07] |
T2DM was negatively associated with ALS only in subjects >55 years at T2DM diagnosis but not in the unstratified analysis. | [63] |
| Sun et al., 2015. | Taiwan | Retrospective cohort | Diabetes: 615,492 controls: 614,835 |
HR: 1.35 [1.10–1.67] | Diabetes increases the risk of ALS, especially in men. There is no distinction between T1D and T2DM. | [59] |
| Kioumourtzoglou et al., 2015. | Denmark | Case-control | ALS: 3,650 Controls:365,000 |
T2DM OR: 0.61 [0.46–0.80] |
T2DM but not T1D is protective against ALS. | [14] |
| Zhang et al., 2022 | European and East Asian | Meta-analysis | ALS:1234 Controls: 2850 |
OR: 0.83 [0.700.992] | T2DM was associated with lower odds of ALS in European and East Asian populations. | [150] |
| Mariosa et al., 2017. | Sweden | Prospective cohort | N: 636,132 | HR: 0.62 [0.42–0.93] | High glucose level (>6.1 mmol/L) was associated with a lower incidence of ALS. | [15] |
| Mariosa et al., 2020. | Sweden | Case-control | ALS:2475 Controls: 12,375 |
OR: 0.76 [0.65–0.90] | ALS patients were less likely to be prescribed antidiabetic medications than controls. | [151] |
| Paganoni et al., 2015. | USA | Retrospective cohort | N: 1,322 ALS-T2DM: 71 ALS: 1,251 |
Log-rank test, p=0.98 | No association between T2DM and ALS prognosis. | [57] |
| Wei et al., 2017. | China | Prospective cohort | ALS:450 | HbA1c, 5.76.4%, HR:1.4
[1.02–1.99]; HbA1c >6.5, HR: 2.06 [1.07–3.96] |
Higher blood HbA1c levels were associated with increased mortality in ALS. | [60] |
HC: healthy controls; HR: hazard ratio; FPG: fasting plasma glucose; OR: odds ratio; PD: RR: risk ratio; N: number of participants; SMD: standardized mean difference; SD: standard deviation
In contrast, some studies have found a positive association between T2DM and ALS. A retrospective cohort study in Taiwan reported a greater risk of ALS among patients with diabetes [59]. Notably, this study did not distinguish between T1D and T2DM, which may have affected the results. A prospective cohort in China found that higher blood levels of glycated hemoglobin (HbA1c), a surrogate marker of T2DM, were associated with increased mortality in ALS [60]. However, a larger prospective cohort in Sweden reported that high glucose levels (≥6.1 mmol/L) were associated with a lower incidence of ALS [15].
Other intriguing findings are related to the interaction between T2DM and the age of onset of ALS. Two European case-control studies showed that T2DM is protective against ALS only in subjects aged 70 or older but increases the risk in younger individuals (<65 years) [14,61]. These age-specific findings are consistent with those reported in two Asian population-based studies [62,63] and a case-control study in the USA [55].
In this regard, aging is associated with a slower metabolism, decreased insulin sensitivity, muscle loss, and weight gain [64]. Metabolic abnormalities, including hyperlipidemia and increased body mass index (BMI), frequently reported in T2DM patients, are considered protective factors in ALS patients [65–67]. Furthermore, hypermetabolism occurs in more than half of ALS patients [68] and is associated with faster functional decline and greater mortality [69]. Although speculative, T2DM may paradoxically confer neuroprotection against ALS by supplying a surplus of glucose and lipids critical for maintaining key cellular and metabolic functions of the motor neuron and respiratory system in older individuals with an ongoing motor neuron degenerative process or increased susceptibility to ALS. Younger individuals, however, are presumably more likely to exhibit a faster metabolism with greater energetic demand, more consistent with the hypermetabolic phenotype observed in ALS patients.
Since metabolic abnormalities and energetic defects have been observed in ALS patients, high-caloric diets have been explored for increasing survival. Preclinical models have reported that a high-caloric diet, either high in fats or carbohydrates, mitigates the energy deficits in motor neurons and improves prognosis in ALS [70], but results from clinical trials have proven futile [71].
Defective insulin signaling, glucose metabolism and cellular bioenergetics in neurodegeneration.
Impaired insulin signaling, glucose metabolism, and defective cellular bioenergetics may underlie the association between T2DM and neurodegeneration. Insulin, a polypeptide hormone secreted in the bloodstream by pancreatic β cells, plays a central role in glucose homeostasis and is critical in neuronal development, survival, and proliferation [72]. How insulin interacts with the blood-brain barrier (BBB) and regulates brain function is the subject of extensive investigation.
Insulin receptors (IRs) are widely expressed in different brain regions, including the hypothalamus, hippocampus, cerebral cortex, striatum, cerebellum, choroid plexus, and olfactory bulb [73,74]. Decreased expression of IRs has been reported in postmortem brain studies of AD and PD patients. For example, there is reduced expression of IR, IR substrates (IRS1, IRS2), insulin-like growth factor 1 (IGF-1), and its receptor (IGF1R) in the brain of AD patients [75,76]. Additionally, there is decreased expression of downstream insulin signaling factors, phosphatidylinositol 3-kinase (PI3K), and activated Akt/PKB in AD [75]. Furthermore, there is increased expression of brain insulin resistance markers, IRS-phosphorylated at serine 616 (IRS-1 pS616) and IRS-1 pS636/639, in the hippocampus and cerebellar cortex of AD patients without T2DM [77]. Brain insulin resistance markers correlated positively with oligomeric Aβ plaques and negatively with episodic and working memory [77]. Impaired insulin signaling is implicated in the accumulation of neurotoxic Aβ and hyperphosphorylated tau via decreased PI3K-AKT signaling and increased activation of glycogen synthase kinase 3β (GSK3β) [78].
Similar patterns of brain insulin resistance are documented in PD. For example, there is decreased expression of IR mRNA and increased expression of IRS-1pS312 in nigral dopaminergic neurons in PD patients and in a rodent model of A53Tmutated SNCA [79,80]. Insulin resistance biomarkers correlated with decreased brain volume in the prefrontal cortex and medial temporal regions in AD and increased in PD patients’ parietal region [81].
Peripheral insulin resistance and impaired glucose metabolism have been reported in several PD studies [82–85]. Though insulin resistance is thought to be underdiagnosed in PD patients [82], it remains unclear whether it is more prevalent in PD than in the general population. Noteworthy, impaired insulin signaling, mitochondrial dysfunction, and inflammation are intimately related processes implicated in neurodegeneration. Insulin resistance promotes lipolysis, producing cytotoxic ceramides that activate proinflammatory molecules, contributing to oxidative stress, mitochondrial dysfunction, apoptosis, DNA damage, and endoplasmic reticulum stress, ultimately leading to neurodegeneration [11].
Recently, a preclinical study demonstrated that human and murine cerebral IRs were concentrated in microvessels rather than the brain parenchyma [86]. Interestingly, the concentration of IRs was lower in microvessels in the parietal cortex of AD subjects and correlated positively with cognitive scores and negatively with Aβ plaques. Surprisingly, activation of IRs was restricted to microvessels. This study indicated for the first time that defective IR signaling at the BBB contributes to brain insulin resistance in AD.
Tight glucose control is essential for maintaining cellular metabolic processes and vital organ functions, including the brain, which relies upon glucose as its primary energy source. Contrary to AD and PD, hyperglycemia has been paradoxically associated with a lower incidence of ALS [15]. Defective glucose metabolism has been reported in mutant SOD1 mouse models and ALS patients [87,88]. Pre-clinical models in Caenorhabditis elegans and Drosophila models showed that increasing glucose availability reduces protein misfolding, improves locomotor function, and increases lifespan [89,90]. Strikingly, overexpression of phosphofructokinase (PFK), the rate-limiting enzyme in glycolysis, rescued motor deficits induced by TDP43 proteinopathy [90]. Similarly, targeting phosphoglycerate kinase 1 (PGK1), the first glycolytic enzyme to produce ATP in glycolysis, with terazosin, a commonly prescribed drug for benign prostatic hyperplasia and hypertension, improved motor function and increased motor neuron number in vitro and in vivo models of ALS [17]. Likewise, terazosin stimulation of glycolysis through PGK1 activation increased brain ATP and dopamine levels and improved motor function in toxin-induced and genetic models of PD [18]. Furthermore, terazosin use was associated with slower disease progression and a lower frequency of PD diagnoses. These findings suggest that targeting bioenergetics pathways through modulation of glycolysis and mitochondrial respiration is a promising therapeutic target in ALS and PD [91].
Shared genetic links between diabetes and neurodegenerative diseases
Several studies have uncovered shared genetic factors between T2DM and neurodegeneration. For example, PD-related genes, PINK1 and PARKIN, are involved in mitochondrial dysfunction, glucose homeostasis, glycolysis, and autophagy, processes implicated in both PD and T2DM [92–94]. In addition, PARK7, also known as DJ-1, an enzyme mutated in autosomal recessive PD, prevents cellular damage by destroying the glycolytic enzyme 1,3-biphosphoglycerate involved in carbohydrate metabolism [95]. Additionally, DJ-1 preserves mitochondrial integrity via the modulation of reactive oxygen species and plays a role in glucose homeostasis in T2DM [96,97]. Another genetic link between PD and T2DM is the downregulation of insulin secretion resulting from the interaction between SNCA and the KATP channels in pancreatic β cells [98]. Similarly, loss of TDP43 linked to sporadic and familial ALS inhibited early-phase insulin secretion by pancreatic β cells through downregulating Cav1.2 calcium channels [99]. Additionally, nuclear depletion of TDP43 in the pancreatic islets mediated the impaired insulin secretion and glucose intolerance observed in early-stage ALS patients [99]. Thus, key genes and proteins linked to pathogenesis in PD and ALS are linked to mechanisms associated with T2DM.
T2DM partly accelerates cognitive decline in AD through interactions with APOE [100,101]. Specifically, T2DM contributed to cognitive decline in APOE3 and APOE2 but not APOE4 carriers {Shinohara, 2020 #175}. Interestingly, T2DM was associated with increased infarcts or lacunes in APOE3 carriers. In contrast, the carrying of APOE4 was associated with lower cognition in T2DM and T2DM-MCI subjects {Zhen, 2018 #176}. Given the functional role of APOE in dyslipidemia and atherosclerosis [101], it is plausible to speculate that T2DM may trigger neurodegeneration in AD through atherosclerosis-induced vascular impairments. Transcriptomic analysis identified shared molecular networks enriched in PI3K-AKT signaling and atherosclerosis in the blood of AD patients [102]. Collectively, these studies suggest that shared genetic links between T2DM and neurodegenerative diseases are predominantly involved in regulating glucose, insulin, and cholesterol, thereby highlighting the impact of metabolic abnormalities in neurodegeneration.
Despite this progress, finding a causal relationship between T2DM and neurodegenerative diseases has been challenging. In the context of AD, genetic correlation analyses using linkage disequilibrium regression and polygenic risk scores did not find convincing evidence for shared genetic susceptibility [103]. Mendelian randomization (MR) studies have identified causal associations in PD and ALS. For example, an MR study found single nucleotide polymorphisms (SNPs) with a significant causal effect of T2DM on PD risk and PD progression [50]. Likewise, an MR approach using large-scale GWAS identified SNPs supporting a neuroprotective role for T2DM in ALS in a European population [104]. Another MR study reported a negative correlation between genetic variants shared between T2DM and ALS, resulting in approximately 5% reduced risk of ALS [105].
Repurposing T2DM drugs for neurodegenerative diseases
One promising area of research is the investigation of antidiabetic drugs for the treatment of neurodegenerative diseases. Targeting the insulin signaling pathway with commonly prescribed antidiabetic drugs has elicited neuroprotective effects in preclinical studies and clinical trials. A longitudinal study of 5,528 veterans with T2DM showed that metformin therapy for more than four years decreased the risk of AD and PD [106]. Another study showed that metformin alone reduced the risk of PD by 60% in T2DM patients [8]. Although some epidemiological studies have found adverse effects of metformin, including cognitive impairment and increased risk of AD and PD [107–109], clinical trials have yielded encouraging results. Metformin intake for eight months was associated with improved executive function, learning, and memory in non-diabetic individuals with MCI and dementia in a phase 2 randomized clinical trial (RCT) (NCT01965756)iii [110]. Similarly, metformin administration for one year improved total memory recall in overweight amnestic MCI subjects in a phase 2 RCT (NCT00620191)iv [111]. Metformin’s neuroprotective effects are mediated via the modulation of AMP-activated protein kinase (AMPK) activity in regulating several key cellular processes such as autophagy, cell growth, and mitochondrial function [112–114] and inhibition of microglial activation and inflammation [115].
Several investigations have explored the therapeutic potential of intranasal insulin in MCI and AD patients with mixed findings. A small phase 2 double-blind RCT (NCT01595646)v showed that intranasal delivery of regular insulin but not long-acting insulin improved memory, preserved MRI brain volume, and reduced CSF tau-P181/Aβ42 ratio after four months in MCI and AD patients compared to placebo [116]. In contrast, treatment with long-acting insulin resulted in memory improvement in APOE4 carriers and worsening in non-carriers [117]. Another study showed that insulin treatment was dose dependent and may vary by sex and APOE status [118]. Interestingly, only APOE4 negative men showed improved cognition, whereas APOE4 negative women displayed the poorest cognitive outcome. These studies highlight the importance of personalized medicine strategies in treating neurodegenerative diseases.
Glucagon-like peptide 1 (GLP1) agonists such as exenatide and lixisenatide are reported to confer neuroprotection. This class of drugs binds to and stimulates GLP-1 receptors in the pancreas, enhancing insulin release and suppressing glucagon release, thus mediating glucose control, and delaying gastric emptying. A phase 2 double-blind RCT showed that PD patients receiving exenatide displayed improved motor symptoms compared to the placebo group at the 60-week time-point [119]. The Exenatide-PD3 study (NCT04232969)vi, a multicenter phase 3 clinical trial, is underway and will determine if previous findings are maintained in a larger cohort over two years [120]. Furthermore, liraglutide and lixisenatide, both GLP1 mimetics, have elicited neuroprotection in animal models of PD [121]. These drugs can cross the blood-brain barrier, enhance neurogenesis in the hippocampus, and increase Brain-derived neurotrophic factor (BDNF) expression, thereby promoting neuroprotection in AD and PD [122,123]. The ELAD study (NCT01843075)vii and the LixiPark study (NCT03439943)viii are phase 2 double-blind and placebo-controlled trials evaluating the efficacy of liraglutide in AD [124] and lixisenatide in PD patients, respectively. These trials also incorporate several biomarkers as surrogate endpoints in the drug development process (Box 2).
Box 2. Biofluid biomarkers as surrogate endpoints in clinical studies testing antidiabetic drugs.
Finding useful biomarkers for early diagnosis and therapeutic development in neurodegenerative diseases remains an unmet goal. Several biomarkers are being evaluated as surrogate endpoints in clinical trials of antidiabetic drugs in PD. For instance, treatment with exenatide showed increased levels of phosphorylated IRS-1Tyr, IRS-1S616, IRS-1S312, total Akt, Akt S473, and p-mTORS2448 indicating the activation of the insulin, Akt, and mTOR signaling pathways in exosomes extracted from peripheral blood of PD patients enrolled in the Exenatide-PD trial [135]. Likewise, the LixiPark trial is evaluating the expression levels of HNF4A and PTBP1 mRNAs, previously identified as potential biomarkers of PD [85,136,137], in early-stage PD patients treated with lixisenatide. If successful, these biomarkers may be used as objective measures of target engagement, monitor disease progression, and therapeutic effects of drugs in clinical trials.
Other shared biomarkers between neurodegenerative diseases and T2DM may be used for therapeutic development. For example, higher levels of HbA1c were associated with cognitive decline and executive functions in patients with T2DM [138] and nondiabetic subjects [139]. Lower serum TREM2 and increased levels of phosphorylated tau, linked to AD pathogenesis [129], were reported in T2DM patients compared to healthy controls [140]. Further, increased serum levels of neurofilament light chain (Nfl), a marker of axonal degeneration, were associated with worsening cognitive functions in T2DM [141]. T2DM patients exhibited lower striatal dopamine transporter binding and higher cerebrospinal fluid levels of tau and SNCA compared to healthy controls [48]. Additionally, plasma progranulin (PRGN) concentrations are increased in T2DM and correlated with insulin resistance [142]. PRGN deficiency and lysosomal dysfunction are shared features in AD, PD, ALS, and frontotemporal dementia [143]. Furthermore, overexpression of PRGN reversed motor neuron defects in TDP43 and FUS mutant zebrafish models [144]. Finally, GLP-1 agonists have been shown to impact the expression of BAX and BCL2, critical factors involved in apoptosis and mitochondrial function [121]. Evaluation of these biomarkers in pre-diabetic and T2DM patients may inform us about cognitive deterioration and impending neurodegeneration.
Concluding remarks
T2DM poses a significant threat to brain health, and its detrimental effects on cognitive deterioration and neurodegeneration are well documented. Although the precise mechanisms by which T2DM mediates neurodegeneration in AD, PD, or neuroprotection in ALS remain elusive, impaired insulin signaling, glucose metabolism, and defective bioenergetics are linked to the neurodegenerative process (Outstanding Questions). Given the multifactorial nature of T2DM and neurodegenerative diseases, a single shared genetic cause or dysregulated pathway is unlikely to explain this complex comorbidity. The impact of T2DM on neurodegeneration should be analyzed in a much broader context of metabolic abnormalities, including but not limited to glucose, insulin, cholesterol, obesity, and other comorbid conditions clustered within the metabolic syndrome. Pharmacological interventions used in T2DM and drugs that modulate glycolysis, have shown significant progress and provide an amenable alternative for preventing or alleviating cognitive decline and neurodegeneration. GLP-1 mimetics have shown neuroprotective potential in clinical trials. Larger prospective clinical trials testing these drugs in pre-diabetics and at-risk patients will be crucial for advancing treatments. In the absence of disease-modifying therapies, lifestyle modifications, including physical activity and diet, may also help mitigate cognitive impairment and neurodegeneration [125–128](Clinician’s Corner). Multidomain clinical trials evaluating the efficacy of multimodal approaches, including diet, physical activity, cognitive training, and mindfulness, are expected to reduce comorbidities such as T2DM, obesity, depression, and cardiovascular disease and thus be more promising in preventing neurodegeneration.
Outstanding Questions.
Hyperglycemia, prediabetes, insulin resistance, and T2DM have been shown to increase the risk of AD and PD. In parallel, the same conditions are accompanied by significant inflammation. Is the impairment in glucose metabolism and insulin signaling or is the inflammation resulting from these processes the main trigger of neurodegeneration?
Targeting the insulin signaling pathway with antidiabetic drugs such as metformin and GLP-1 mimetics and glycolytic pathways has shown promise in preclinical models and clinical studies in neurodegenerative diseases. Will the repurposing of currently approved drugs such as exenatide, lixisenatide and terazosin, be useful prophylaxis treatments in individuals at risk of neurodegeneration?
In the absence of disease-modifying agents for neurodegenerative diseases will implementation of lifestyle modifications such as high calorie diets in early-stage ALS patients, and Mediterranean diet in those at risk for AD and PD, be useful preventative strategies?
Clinician’s Corner
Recent phase 3 randomized clinical trials (RCTs) using anti-Aβ monoclonal antibodies have shown promise in slowing cognitive decline in early-stage AD, but their efficacy and safety are currently debated (NCT03887455)ix, (NCT04437511)x. Further, current treatments for PD, ALS, and other neurodegenerative diseases provide symptomatic relief, but disease-modifying drugs are lacking. Although common drugs prescribed for the treatment of diabetes have been reported to elicit neuroprotective effects in preclinical models and clinical trials, there are still many challenges to overcome.
WHO declared physical inactivity as the fourth cause of mortality globally. More than 500 million people will develop obesity, T2DM, and cardiovascular disease resulting from a sedentary lifestylexi. To this end, non-pharmacological interventions, including diet, physical activity, cognitive therapy, sleep hygiene, and mindfulness, could be adjunct treatments for neurodegenerative diseases. Engaging in daily physical activity has been shown to provide numerous benefits for brain health, including improved cognition, memory, and motor symptoms in several RCTs of neurodegenerative diseases (NCT01506479)xii (NCT02000583)xiii.
Similarly, healthy eating habits such as those adopted by the Mediterranean diet, Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND), and the Dietary Approaches to Stop Hypertension (DASH), rich in leafy green vegetables, fruits, fish, nuts and olive oil may prevent or slow cognitive decline as demonstrated in an open-label RCT (NCT03020186)xiv.
Cognitive training and mindfulness meditation have been shown to reduce depression and improve sleep and cognition. A RCT demonstrated the positive effects of mindfulness training in improving cognitive performance and strengthening connectivity in brain regions vulnerable to aging (NCT02628548)xv.
Given the multifactorial nature of neurodegenerative diseases, it has become evident that a single pharmacological intervention may fall short in preventing neurodegeneration. Multidomain clinical trials evaluating several lifestyle modifications, including diet and physical activity, are expected to determine whether multimodal approaches are more efficient in preventing neurodegeneration as shown in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (NCT01041989; RCT, active, not recruiting)xv.
Highlights.
Epidemiological studies indicate that diabetes increases the risk of Alzheimer’s (AD) and Parkinson’s (PD) diseases. Conversely, diabetes may confer neuroprotection against amyotrophic lateral sclerosis (ALS) in elderly individuals.
Molecular studies have shown that targeting insulin signaling using commonly prescribed antidiabetic drugs and stimulating enzymes in the glycolysis pathway elicits neuroprotection in AD, PD, and ALS.
Ongoing clinical trials testing antidiabetic drugs have shown promise in improving cognitive decline and motor function deterioration in patients with AD and PD.
Acknowledgments
J.A.S is the founder of NeuroHub Analytics LLC. M.K, M.L, and D.V, perform independent research at NeuroHub Analytics LLC. J.A.P is a Professor in the Center for Neurodegenerative Diseases and Therapeutics at Rosalind Franklin University of Medicine and Science and Discipline Chair of Cellular and Molecular Pharmacology at the Chicago Medical School. J.A.P is funded by the National Institute on Aging (NIA) grant number R01AG062176, The Cure Parkinson’s Trust, and Rosalind Franklin University of Medicine and Science. This work is dedicated to all the people affected by neurodegenerative diseases and to their caregivers.
Glossary
- Case-control study
a study that compares groups of people with the disease (cases) and those who do not have the disease (control).
- Hyperglycemia
blood glucose level that is greater than 125 mg/dL while fasting and greater than 180 mg/dL 2 hours postprandial.
- Mendelian randomization
utilizes variations of genes of known function associated with an exposure of interest to determine the causal effects between the exposure and a disease outcome.
- Metabolic syndrome
describes a cluster of conditions that increase cardiovascular, stroke and diabetes risk. The main components of metabolic syndrome include obesity, high blood pressure, high blood triglycerides, low levels of HDL, and insulin resistance.
- Prospective study
a type of longitudinal study where subjects are observed over a time period and gathers information about the development of outcomes.
- Type 1 diabetes
an autoimmune condition where the pancreas cannot produce insulin due to the destruction of the beta cells, leading to hyperglycemia.
- Type 2 diabetes mellitus
a condition where there are high blood glucose levels due to insulin resistance. Normally, our body breaks down glucose and stores it as energy in cells with the help of insulin. However, in type 2 diabetes, the cells do not respond to the insulin and do not take in glucose, leading to hyperglycemia.
- Vascular dementia
a stepwise decline in executive dysfunction due to conditions that block or reduce blood flow to the brain. It is caused by cerebrovascular disease such as diabetes or impaired cerebral blood flow, such as strokes/embolisms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
J.A.S is the founder of NeuroHub Analytics LLC. J.A.P. will test biomarkers in the LixiPark study RC31/16-8912 (NCT03439943)vii. J.A.P. and J.A.S. are the inventors on United States Patent US 9,970,056 B2, Methods and Kits for Diagnosing, Prognosing and Monitoring Parkinson’s Disease. M.K, M.L, and D.V declares no competing interests.
Resources
References
- 1.Geijselaers SLC et al. (2017) The Role of Hyperglycemia, Insulin Resistance, and Blood Pressure in Diabetes-Associated Differences in Cognitive Performance-The Maastricht Study. Diabetes Care 40, 1537–1547. 10.2337/dc17-0330 [DOI] [PubMed] [Google Scholar]
- 2.Xue M et al. (2019) Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev 55, 100944. 10.1016/j.arr.2019.100944 [DOI] [PubMed] [Google Scholar]
- 3.Tan ZS et al. (2011) Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care 34, 1766–1770. 10.2337/dc11-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang T et al. (2022) Association between Type 2 Diabetes Mellitus and Brain Atrophy: A Meta-Analysis. Diabetes Metab J 46, 781–802. 10.4093/dmj.2021.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antal B et al. (2022) Type 2 diabetes mellitus accelerates brain aging and cognitive decline: Complementary findings from UK Biobank and meta-analyses. Elife 11. 10.7554/eLife.73138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schernhammer E et al. (2011) Diabetes and the risk of developing Parkinson’s disease in Denmark. Diabetes Care 34, 1102–1108. 10.2337/dc10-1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y et al. (2012) Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 35, 1047–1049. 10.2337/dc11-1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahlqvist ML et al. (2012) Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with Type 2 diabetes in a Taiwanese population cohort. Parkinsonism Relat Disord 18, 753–758. 10.1016/j.parkreldis.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 9.Yang YW et al. (2017) Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (Baltimore) 96, e5921. 10.1097/MD.0000000000005921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs BM et al. (2020) Parkinson’s disease determinants, prediction and gene-environment interactions in the UK Biobank. J Neurol Neurosurg Psychiatry 91, 1046–1054. 10.1136/jnnp-2020-323646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santiago JA and Potashkin JA (2013) Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol Med 19, 176–186. 10.1016/j.molmed.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 12.D’Ovidio F et al. (2018) The role of pre-morbid diabetes on developing amyotrophic lateral sclerosis. Eur J Neurol 25, 164–170. 10.1111/ene.13465 [DOI] [PubMed] [Google Scholar]
- 13.Tsai CP et al. (2019) Finding diseases associated with amyotrophic lateral sclerosis: a total population-based case-control study. Amyotroph Lateral Scler Frontotemporal Degener 20, 82–89. 10.1080/21678421.2018.1522354 [DOI] [PubMed] [Google Scholar]
- 14.Kioumourtzoglou MA et al. (2015) Diabetes Mellitus, Obesity, and Diagnosis of Amyotrophic Lateral Sclerosis: A Population-Based Study. JAMA Neurol 72, 905–911. 10.1001/jamaneurol.2015.0910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariosa D et al. (2017) Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: A more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol 81, 718–728. 10.1002/ana.24936 [DOI] [PubMed] [Google Scholar]
- 16.Santiago JA et al. (2022) Physical Activity Rewires the Human Brain against Neurodegeneration. Int J Mol Sci 23. 10.3390/ijms23116223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaytow H et al. (2022) Targeting phosphoglycerate kinase 1 with terazosin improves motor neuron phenotypes in multiple models of amyotrophic lateral sclerosis. EBioMedicine 83, 104202. 10.1016/j.ebiom.2022.104202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai R et al. (2019) Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J Clin Invest 129, 4539–4549. 10.1172/JCI129987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biessels GJ et al. (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5, 64–74. 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- 20.Cheng G et al. (2012) Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 42, 484–491. 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- 21.Li L et al. (2020) Diabetes Mellitus Increases Risk of Incident Dementia in APOEvarepsilon4 Carriers: A Meta-Analysis. J Alzheimers Dis 74, 1295–1308. 10.3233/JAD-191068 [DOI] [PubMed] [Google Scholar]
- 22.Hassing LB et al. (2002) Diabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer’s disease: a population-based study of the oldest old. Int Psychogeriatr 14, 239–248. 10.1017/s104161020200844x [DOI] [PubMed] [Google Scholar]
- 23.MacKnight C et al. (2002) Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord 14, 77–83. 10.1159/000064928 [DOI] [PubMed] [Google Scholar]
- 24.Dos Santos Matioli MNP et al. (2017) Diabetes is Not Associated with Alzheimer’s Disease Neuropathology. J Alzheimers Dis 60, 1035–1043. 10.3233/JAD-170179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akomolafe A et al. (2006) Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol 63, 1551–1555. 10.1001/archneur.63.11.1551 [DOI] [PubMed] [Google Scholar]
- 26.Rawlings AM et al. (2014) Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 161, 785–793. 10.7326/M14-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbiellini Amidei C et al. (2021) Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 325, 1640–1649. 10.1001/jama.2021.4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Secnik J et al. (2017) Diabetes in a Large Dementia Cohort: Clinical Characteristics and Treatment From the Swedish Dementia Registry. Diabetes Care 40, 1159–1166. 10.2337/dc16-2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu WC et al. (2015) Progress of Diabetic Severity and Risk of Dementia. J Clin Endocrinol Metab 100, 2899–2908. 10.1210/jc.2015-1677 [DOI] [PubMed] [Google Scholar]
- 30.Reinke C et al. (2022) Diabetes duration and the risk of dementia: a cohort study based on German health claims data. Age Ageing 51. 10.1093/ageing/afab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng TP et al. (2016) Metabolic Syndrome and the Risk of Mild Cognitive Impairment and Progression to Dementia: Follow-up of the Singapore Longitudinal Ageing Study Cohort. JAMA Neurol 73, 456–463. 10.1001/jamaneurol.2015.4899 [DOI] [PubMed] [Google Scholar]
- 32.Hu G et al. (2007) Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 30, 842–847. 10.2337/dc06-2011 [DOI] [PubMed] [Google Scholar]
- 33.Xu Q et al. (2011) Diabetes and risk of Parkinson’s disease. Diabetes Care 34, 910–915. 10.2337/dc10-1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue X et al. (2016) Risk of Parkinson Disease in Diabetes Mellitus: An Updated Meta-Analysis of Population-Based Cohort Studies. Medicine (Baltimore) 95, e3549. 10.1097/MD.0000000000003549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Pablo-Fernandez E et al. (2017) Association between Parkinson’s disease and diabetes: Data from NEDICES study. Acta Neurol Scand 136, 732–736. 10.1111/ane.12793 [DOI] [PubMed] [Google Scholar]
- 36.D’Amelio M et al. (2009) Diabetes preceding Parkinson’s disease onset. A case-control study. Parkinsonism Relat Disord 15, 660–664. 10.1016/j.parkreldis.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 37.Miyake Y et al. (2010) Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci 293, 82–86. 10.1016/j.jns.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 38.Savica R et al. (2012) Metabolic markers or conditions preceding Parkinson’s disease: a case-control study. Mov Disord 27, 974–979. 10.1002/mds.25016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skeie GO et al. (2013) Parkinson disease: associated disorders in the Norwegian population based incident ParkWest study. Parkinsonism Relat Disord 19, 53–55. 10.1016/j.parkreldis.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 40.Lu L et al. (2014) Diabetes and risk of Parkinson’s disease: an updated meta-analysis of case-control studies. PLoS One 9, e85781. 10.1371/journal.pone.0085781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Gomez A et al. (2021) Prediabetes, type 2 diabetes mellitus and risk of Parkinson’s disease: A population-based cohort study. Parkinsonism Relat Disord 89, 22–27. 10.1016/j.parkreldis.2021.06.002 [DOI] [PubMed] [Google Scholar]
- 42.Aune D et al. (2023) Diabetes mellitus, prediabetes and the risk of Parkinson’s disease: a systematic review and meta-analysis of 15 cohort studies with 29.9 million participants and 86,345 cases. Eur J Epidemiol 38, 591–604. 10.1007/s10654-023-00970-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J et al. (2023) Association between prediabetes and cognitive function in Parkinson’s disease. Brain Behav 13, e2838. 10.1002/brb3.2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Pablo-Fernandez E et al. (2018) Association between diabetes and subsequent Parkinson disease: A record-linkage cohort study. Neurology 91, e139–e142. 10.1212/WNL.0000000000005771 [DOI] [PubMed] [Google Scholar]
- 45.Deischinger C et al. (2021) Diabetes Mellitus is Associated with a Higher Relative Risk for Parkinson’s Disease in Women than in Men. J Parkinsons Dis 11, 793–800. 10.3233/JPD-202486 [DOI] [PubMed] [Google Scholar]
- 46.Rao SC et al. (2023) Association of women-specific health factors in the severity of Parkinson’s disease. NPJ Parkinsons Dis 9, 86. 10.1038/s41531-023-00524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YH et al. (2019) Beneficial effect of estrogen on nigrostriatal dopaminergic neurons in drug-naive postmenopausal Parkinson’s disease. Sci Rep 9, 10531. 10.1038/s41598-019-47026-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pagano G et al. (2018) Diabetes mellitus and Parkinson disease. Neurology 90, e1654–e1662. 10.1212/WNL.0000000000005475 [DOI] [PubMed] [Google Scholar]
- 49.Zhong Q and Wang S (2023) Association between diabetes mellitus, prediabetes and risk, disease progression of Parkinson’s disease: A systematic review and meta-analysis. Front Aging Neurosci 15, 1109914. 10.3389/fnagi.2023.1109914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chohan H et al. (2021) Type 2 Diabetes as a Determinant of Parkinson’s Disease Risk and Progression. Mov Disord 36, 1420–1429. 10.1002/mds.28551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cereda E et al. (2012) Clinical features of Parkinson disease when onset of diabetes came first: A case-control study. Neurology 78, 1507–1511. 10.1212/WNL.0b013e3182553cc9 [DOI] [PubMed] [Google Scholar]
- 52.Athauda D et al. (2022) The Impact of Type 2 Diabetes in Parkinson’s Disease. Mov Disord 37, 1612–1623. 10.1002/mds.29122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung SJ et al. (2019) Detrimental effect of type 2 diabetes mellitus in a large case series of Parkinson’s disease. Parkinsonism Relat Disord 64, 54–59. 10.1016/j.parkreldis.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 54.Pezzoli G et al. (2023) Onset and mortality of Parkinson’s disease in relation to type II diabetes. J Neurol 270, 1564–1572. 10.1007/s00415-022-11496-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jawaid A et al. (2010) ALS disease onset may occur later in patients with premorbid diabetes mellitus. Eur J Neurol 17, 733–739. 10.1111/j.14681331.2009.02923.x [DOI] [PubMed] [Google Scholar]
- 56.Mitchell CS et al. (2015) Antecedent Disease is Less Prevalent in Amyotrophic Lateral Sclerosis. Neurodegener Dis 15, 109–113. 10.1159/000369812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paganoni S et al. (2015) Pre-morbid type 2 diabetes mellitus is not a prognostic factor in amyotrophic lateral sclerosis. Muscle Nerve 52, 339–343. 10.1002/mus.24688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollinger SK et al. (2016) Antecedent Disease and Amyotrophic Lateral Sclerosis: What Is Protecting Whom? Front Neurol 7, 47. 10.3389/fneur.2016.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y et al. (2015) Risk of Amyotrophic Lateral Sclerosis in Patients With Diabetes: A Nationwide Population-Based Cohort Study. J Epidemiol 25, 445–451. 10.2188/jea.JE20140176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei QQ et al. (2017) Blood hemoglobin A1c levels and amyotrophic lateral sclerosis survival. Mol Neurodegener 12, 69. 10.1186/s13024-017-0211-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mariosa D et al. (2015) Association between diabetes and amyotrophic lateral sclerosis in Sweden. Eur J Neurol 22, 1436–1442. 10.1111/ene.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L et al. (2020) The protective role of pre-morbid type 2 diabetes in patients with amyotrophic lateral sclerosis: a center-based survey in China. Amyotroph Lateral Scler Frontotemporal Degener 21, 209–215. 10.1080/21678421.2019.1704010 [DOI] [PubMed] [Google Scholar]
- 63.Tsai CP et al. (2019) Type II diabetes mellitus and the incidence of amyotrophic lateral sclerosis. J Neurol 266, 2233–2243. 10.1007/s00415-019-09405-x [DOI] [PubMed] [Google Scholar]
- 64.Palmer AK and Jensen MD (2022) Metabolic changes in aging humans: current evidence and therapeutic strategies. J Clin Invest 132. 10.1172/JCI158451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dupuis L et al. (2008) Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70, 1004–1009. 10.1212/01.wnl.0000285080.70324.27 [DOI] [PubMed] [Google Scholar]
- 66.Dardiotis E et al. (2018) Body mass index and survival from amyotrophic lateral sclerosis: A meta-analysis. Neurol Clin Pract 8, 437–444. 10.1212/CPJ.0000000000000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson AG et al. (2022) Higher blood high density lipoprotein and apolipoprotein A1 levels are associated with reduced risk of developing amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 93, 75–81. 10.1136/jnnp-2021-327133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jesus P et al. (2018) Hypermetabolism is a deleterious prognostic factor in patients with amyotrophic lateral sclerosis. Eur J Neurol 25, 97–104. 10.1111/ene.13468 [DOI] [PubMed] [Google Scholar]
- 69.Steyn FJ et al. (2018) Hypermetabolism in ALS is associated with greater functional decline and shorter survival. J Neurol Neurosurg Psychiatry 89, 1016–1023. 10.1136/jnnp-2017-317887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dupuis L et al. (2004) Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci U S A 101, 11159–11164. 10.1073/pnas.0402026101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ludolph AC et al. (2020) Effect of High-Caloric Nutrition on Survival in Amyotrophic Lateral Sclerosis. Ann Neurol 87, 206–216. 10.1002/ana.25661 [DOI] [PubMed] [Google Scholar]
- 72.Apostolatos A et al. (2012) Insulin promotes neuronal survival via the alternatively spliced protein kinase CdeltaII isoform. J Biol Chem 287, 9299–9310. 10.1074/jbc.M111.313080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pardini AW et al. (2006) Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res 1112, 169–178. 10.1016/j.brainres.2006.06.109 [DOI] [PubMed] [Google Scholar]
- 74.Schulingkamp RJ et al. (2000) Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev 24, 855–872. 10.1016/s0149-7634(00)00040-3 [DOI] [PubMed] [Google Scholar]
- 75.Steen E et al. (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis 7, 63–80. 10.3233/jad-2005-7107 [DOI] [PubMed] [Google Scholar]
- 76.Moloney AM et al. (2010) Defects in IGF-1 receptor, insulin receptor and IRS1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 31, 224–243. 10.1016/j.neurobiolaging.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 77.Talbot K et al. (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122, 1316–1338. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de la Monte SM (2012) Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease. Drugs 72, 49–66. 10.2165/11597760-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi M et al. (1996) Insulin receptor mRNA in the substantia nigra in Parkinson’s disease. Neurosci Lett 204, 201–204. 10.1016/0304-3940(96)12357-0 [DOI] [PubMed] [Google Scholar]
- 80.Bassil F et al. (2022) Impaired brain insulin signalling in Parkinson’s disease. Neuropathol Appl Neurobiol 48, e12760. 10.1111/nan.12760 [DOI] [PubMed] [Google Scholar]
- 81.Morris JK et al. (2014) Insulin resistance and gray matter volume in neurodegenerative disease. Neuroscience 270, 139–147. 10.1016/j.neuroscience.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hogg E et al. (2018) High Prevalence of Undiagnosed Insulin Resistance in Non-Diabetic Subjects with Parkinson’s Disease. J Parkinsons Dis 8, 259–265. 10.3233/JPD-181305 [DOI] [PubMed] [Google Scholar]
- 83.Sanchez-Gomez A et al. (2020) Peripheral insulin and amylin levels in Parkinson’s disease. Parkinsonism Relat Disord 79, 91–96. 10.1016/j.parkreldis.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 84.Bosco D et al. (2012) Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci 315, 39–43. 10.1016/j.jns.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 85.Santiago JA and Potashkin JA (2015) Blood Biomarkers Associated with Cognitive Decline in Early Stage and Drug-Naive Parkinson’s Disease Patients. PLoS One 10, e0142582. 10.1371/journal.pone.0142582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leclerc M et al. (2023) Cerebrovascular insulin receptors are defective in Alzheimer’s disease. Brain 146, 75–90. 10.1093/brain/awac309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyazaki K et al. (2012) Early and progressive impairment of spinal blood flow-glucose metabolism coupling in motor neuron degeneration of ALS model mice. J Cereb Blood Flow Metab 32, 456–467. 10.1038/jcbfm.2011.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ludolph AC et al. (1992) Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand 85, 81–89. 10.1111/j.1600-0404.1992.tb04003.x [DOI] [PubMed] [Google Scholar]
- 89.Tauffenberger A et al. (2012) Glucose delays age-dependent proteotoxicity. Aging Cell 11, 856–866. 10.1111/j.1474-9726.2012.00855.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manzo E et al. (2019) Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS. Elife 8. 10.7554/eLife.45114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malpartida AB et al. (2021) Mitochondrial Dysfunction and Mitophagy in Parkinson’s Disease: From Mechanism to Therapy. Trends Biochem Sci 46, 329–343. 10.1016/j.tibs.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 92.Requejo-Aguilar R et al. (2014) PINK1 deficiency sustains cell proliferation by reprogramming glucose metabolism through HIF1. Nat Commun 5, 4514. 10.1038/ncomms5514 [DOI] [PubMed] [Google Scholar]
- 93.Lee SH et al. (2016) Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol Med 8, 779–795. 10.15252/emmm.201506046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SH et al. (2019) Mitochondrial MsrB2 serves as a switch and transducer for mitophagy. EMBO Mol Med 11, e10409. 10.15252/emmm.201910409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heremans IP et al. (2022) Parkinson’s disease protein PARK7 prevents metabolite and protein damage caused by a glycolytic metabolite. Proc Natl Acad Sci U S A 119. 10.1073/pnas.2111338119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jain D et al. (2015) DJ-1 Protects Pancreatic Beta Cells from Cytokine- and Streptozotocin-Mediated Cell Death. PLoS One 10, e0138535. 10.1371/journal.pone.0138535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jain D et al. (2012) Age- and diet-dependent requirement of DJ-1 for glucose homeostasis in mice with implications for human type 2 diabetes. J Mol Cell Biol 4, 221–230. 10.1093/jmcb/mjs025 [DOI] [PubMed] [Google Scholar]
- 98.Geng X et al. (2011) alpha-Synuclein binds the K(ATP) channel at insulin-secretory granules and inhibits insulin secretion. Am J Physiol Endocrinol Metab 300, E276–286. 10.1152/ajpendo.00262.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Araki K et al. (2019) TDP-43 regulates early-phase insulin secretion via CaV1.2-mediated exocytosis in islets. J Clin Invest 129, 3578–3593. 10.1172/JCI124481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shinohara M et al. (2020) Interaction between APOE genotype and diabetes in cognitive decline. Alzheimers Dement (Amst) 12, e12006. 10.1002/dad2.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhen J et al. (2018) Association of ApoE Genetic Polymorphism and Type 2 Diabetes with Cognition in Non-Demented Aging Chinese Adults: A Community Based Cross-Sectional Study. Aging Dis 9, 346–357. 10.14336/AD.2017.0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santiago JA et al. (2019) Transcriptomic and Network Analysis Highlight the Association of Diabetes at Different Stages of Alzheimer’s Disease. Front Neurosci 13, 1273. 10.3389/fnins.2019.01273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hardy J et al. (2022) Diabetes and Alzheimer’s disease: shared genetic susceptibility? Lancet Neurol 21, 962–964. 10.1016/S1474-4422(22)00395-7 [DOI] [PubMed] [Google Scholar]
- 104.Zeng P et al. (2019) Causal association of type 2 diabetes with amyotrophic lateral sclerosis: new evidence from Mendelian randomization using GWAS summary statistics. BMC Med 17, 225. 10.1186/s12916-019-1448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen H et al. (2021) Type 2 Diabetes Mellitus and Amyotrophic Lateral Sclerosis: Genetic Overlap, Causality, and Mediation. J Clin Endocrinol Metab 106, e4497–e4508. 10.1210/clinem/dgab465 [DOI] [PubMed] [Google Scholar]
- 106.Shi Q et al. (2019) Effect of metformin on neurodegenerative disease among elderly adult US veterans with type 2 diabetes mellitus. BMJ Open 9, e024954. 10.1136/bmjopen-2018-024954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Imfeld P et al. (2012) Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc 60, 916–921. 10.1111/j.1532-5415.2012.03916.x [DOI] [PubMed] [Google Scholar]
- 108.Qin X et al. (2021) Association Between Diabetes Medications and the Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Neurol 12, 678649. 10.3389/fneur.2021.678649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moore EM et al. (2013) Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 36, 2981–2987. 10.2337/dc13-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koenig AM et al. (2017) Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis Assoc Disord 31, 107–113. 10.1097/WAD.0000000000000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luchsinger JA et al. (2016) Metformin in Amnestic Mild Cognitive Impairment: Results of a Pilot Randomized Placebo Controlled Clinical Trial. J Alzheimers Dis 51, 501–514. 10.3233/JAD-150493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y et al. (2019) Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep 29, 1511–1523 e1515. 10.1016/j.celrep.2019.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou G et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108, 1167–1174. 10.1172/JCI13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wen Z et al. (2018) Overexpression of miR-185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Mol Med Rep 17, 131–137. 10.3892/mmr.2017.7897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tayara K et al. (2018) Divergent Effects of Metformin on an Inflammatory Model of Parkinson’s Disease. Front Cell Neurosci 12, 440. 10.3389/fncel.2018.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Craft S et al. (2017) Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J Alzheimers Dis 57, 1325–1334. 10.3233/JAD-161256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Claxton A et al. (2015) Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 44, 897–906. 10.3233/JAD-141791 [DOI] [PubMed] [Google Scholar]
- 118.Claxton A et al. (2013) Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis 35, 789–797. 10.3233/JAD-122308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Athauda D et al. (2017) Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390, 1664–1675. 10.1016/S0140-6736(17)31585-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vijiaratnam N et al. (2021) Exenatide once weekly over 2 years as a potential disease-modifying treatment for Parkinson’s disease: protocol for a multicentre, randomised, double blind, parallel group, placebo controlled, phase 3 trial: The ‘Exenatide-PD3’ study. BMJ Open 11, e047993. 10.1136/bmjopen-2020-047993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu W et al. (2015) Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience 303, 42–50. 10.1016/j.neuroscience.2015.06.054 [DOI] [PubMed] [Google Scholar]
- 122.Hunter K and Holscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13, 33. 10.1186/1471-2202-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Holscher C (2014) The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer’s disease. Alzheimers Dement 10, S47–54. 10.1016/j.jalz.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 124.Femminella GD et al. (2019) Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study). Trials 20, 191. 10.1186/s13063-019-3259-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Donahue EK et al. (2022) Physical activity intensity is associated with cognition and functional connectivity in Parkinson’s disease. Parkinsonism Relat Disord 104, 7–14. 10.1016/j.parkreldis.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 126.Schenkman M et al. (2018) Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol 75, 219–226. 10.1001/jamaneurol.2017.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ngandu T et al. (2015) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263. 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 128.Santiago JA and Potashkin JA (2023) Physical activity and lifestyle modifications in the treatment of neurodegenerative diseases. Front Aging Neurosci 15, 1185671. 10.3389/fnagi.2023.1185671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilson DM 3rd et al. (2023) Hallmarks of neurodegenerative diseases. Cell 186, 693–714. 10.1016/j.cell.2022.12.032 [DOI] [PubMed] [Google Scholar]
- 130.De Strooper B and Karran E (2016) The Cellular Phase of Alzheimer’s Disease. Cell 164, 603–615. 10.1016/j.cell.2015.12.056 [DOI] [PubMed] [Google Scholar]
- 131.Sims R et al. (2020) The multiplex model of the genetics of Alzheimer’s disease. Nat Neurosci 23, 311–322. 10.1038/s41593-020-0599-5 [DOI] [PubMed] [Google Scholar]
- 132.Bandres-Ciga S et al. (2020) Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol Dis 137, 104782. 10.1016/j.nbd.2020.104782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grad LI et al. (2017) Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb Perspect Med 7. 10.1101/cshperspect.a024117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Renton AE et al. (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17, 17–23. 10.1038/nn.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Athauda D et al. (2019) Utility of Neuronal-Derived Exosomes to Examine Molecular Mechanisms That Affect Motor Function in Patients With Parkinson Disease: A Secondary Analysis of the Exenatide-PD Trial. JAMA Neurol 76, 420429. 10.1001/jamaneurol.2018.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Santiago JA and Potashkin JA (2015) Network-based metaanalysis identifies HNF4A and PTBP1 as longitudinally dynamic biomarkers for Parkinson’s disease. Proc Natl Acad Sci U S A 112, 2257–2262. 10.1073/pnas.1423573112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hadisurya M et al. (2023) Quantitative proteomics and phosphoproteomics of urinary extracellular vesicles define putative diagnostic biosignatures for Parkinson’s disease. Commun Med (Lond) 3, 64. 10.1038/s43856-023-00294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zheng F et al. (2018) HbA(1c), diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia 61, 839–848. 10.1007/s00125-017-4541-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu ZB et al. (2020) Association between visit-to-visit variability of HbA(1c) and cognitive decline: a pooled analysis of two prospective population-based cohorts. Diabetologia 63, 85–94. 10.1007/s00125-019-04986-8 [DOI] [PubMed] [Google Scholar]
- 140.Satoh-Asahara N et al. (2022) Soluble TREM2 and Alzheimer-related biomarker trajectories in the blood of patients with diabetes based on their cognitive status. Diabetes Res Clin Pract 193, 110121. 10.1016/j.diabres.2022.110121 [DOI] [PubMed] [Google Scholar]
- 141.Ciardullo S et al. (2023) Diabetes Mellitus is Associated With Higher Serum Neurofilament Light Chain Levels in the General US Population. J Clin Endocrinol Metab 108, 361–367. 10.1210/clinem/dgac580 [DOI] [PubMed] [Google Scholar]
- 142.Qu H et al. (2013) Plasma progranulin concentrations are increased in patients with type 2 diabetes and obesity and correlated with insulin resistance. Mediators Inflamm 2013, 360190. 10.1155/2013/360190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rhinn H et al. (2022) Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol Sci 43, 641–652. 10.1016/j.tips.2021.11.015 [DOI] [PubMed] [Google Scholar]
- 144.Chitramuthu BP et al. (2017) Neurotrophic effects of progranulin in vivo in reversing motor neuron defects caused by over or under expression of TDP-43 or FUS. PLoS One 12, e0174784. 10.1371/journal.pone.0174784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chatterjee S et al. (2016) Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 39, 300–307. 10.2337/dc15-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kivimaki M et al. (2019) Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ 365, l1495. 10.1136/bmj.l1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang J et al. (2017) An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract 124, 41–47. 10.1016/j.diabres.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 148.Palacios N et al. (2011) Obesity, diabetes, and risk of Parkinson’s disease. Mov Disord 26, 2253–2259. 10.1002/mds.23855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu W and Tang J (2021) Association between diabetes mellitus and risk of Parkinson’s disease: A prisma-compliant meta-analysis. Brain Behav 11, e02082. 10.1002/brb3.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang L et al. (2022) Association between type 2 diabetes and amyotrophic lateral sclerosis. Sci Rep 12, 2544. 10.1038/s41598-022-06463-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mariosa D et al. (2020) Antidiabetics, statins and the risk of amyotrophic lateral sclerosis. Eur J Neurol 27, 1010–1016. 10.1111/ene.14190 [DOI] [PMC free article] [PubMed] [Google Scholar]


