The rate of Donation after Circulatory Death (DCD) Heart Transplant (HT) increased threefold in the United States from 2019 to 2022.1 While early analyses of DCD HT have suggested similar clinical outcomes compared to donation after brain death (DBD) HT, this may be the result of a restrictive DCD donor and recipient selection process, intended to minimize risk in the first DCD HT cohorts.2 We hypothesized that risk profiles for DCD HT donors and recipients have increased over time, as the use of DCD HT has proliferated beyond the earliest adopting centers and familiarity with DCD HT has increased. We therefore sought to identify longitudinal changes in risk profiles for DCD HT donors and recipients from 2019–2023, and to determine if such changes have impacted post-DCD HT clinical outcomes.
The study was approved by the Institutional Review Board at Duke University. Informed consent was waived. We analyzed the United Network for Organ Sharing (UNOS) database from December 1st, 2019 to June 30th, 2023. The corresponding data can be publicly requested from UNOS. Adult DCD HT recipient (≥18 years old) and donor risk characteristics were evaluated over time for significant trends. Dual organ recipients were excluded. Thirty-day, 180-day, and one-year survival free from death or repeat HT were compared over time (as grouped by year) via Kaplan-Meier methods with logrank testing for significance. Recipients from 2019 (n=7) and 2020 (n=103) were combined for sake of analysis. Recipients who underwent HT in 2023 were excluded from the survival analysis, given insufficient follow up.
During the study period, 858 DCD HT were performed, including 253 (29.4%) from January 1-June 30th, 2023. Recipients were mostly male (79.6%), Caucasian (68.1%), and had a median age of 57 (Q1, Q3: 46, 64) years old. Over time, several recipient and donor characteristics were significantly different as determined by generalized regression models for time trend. Recipients were older (2020: 54 years (42, 61) vs 2023: 57 years (46, 64), p=0.044), more likely to be Status 1 or 2 (2020: Status 1=0% (0/110), Status 2=17.3% (19/110) vs 2023: Status 1=4.0% (10/253), Status 2=50.2% (127/253), p<0.001), more likely to on ECMO at time of HT (2020: 0% (0/110) vs 2023: 2.4% (6/253), p=0.043), and had lower total waitlist time (2020: 43 days (12, 163) vs 2023: 24 days (7, 134), p=0.009). Recipient BMI (2020: 29.6±5.1 kg/m2 vs 2023: 27.9±4.9 kg/m2, p<0.001), and total ischemic time (2020: 6.1 hours (5.0, 6.8) vs 2023: 5.3 hours (3.2, 6.7), p=0.005), decreased. Donors were older (2020: 29.0±7.8 years vs 2023 32.0±8.5 years, p<0.001), and had increased BMI (2020: 26.2 kg/m2 (23.1, 30.5) vs 2023: 26.9 kg/m2 (23.5, 32.0), p=0.039). Recipient diabetes status, creatinine, and use of pre-HT intra-aortic balloon pump did not differ. Post-DCD HT survival, stratified by year, did not differ. (Figure)
Figure:
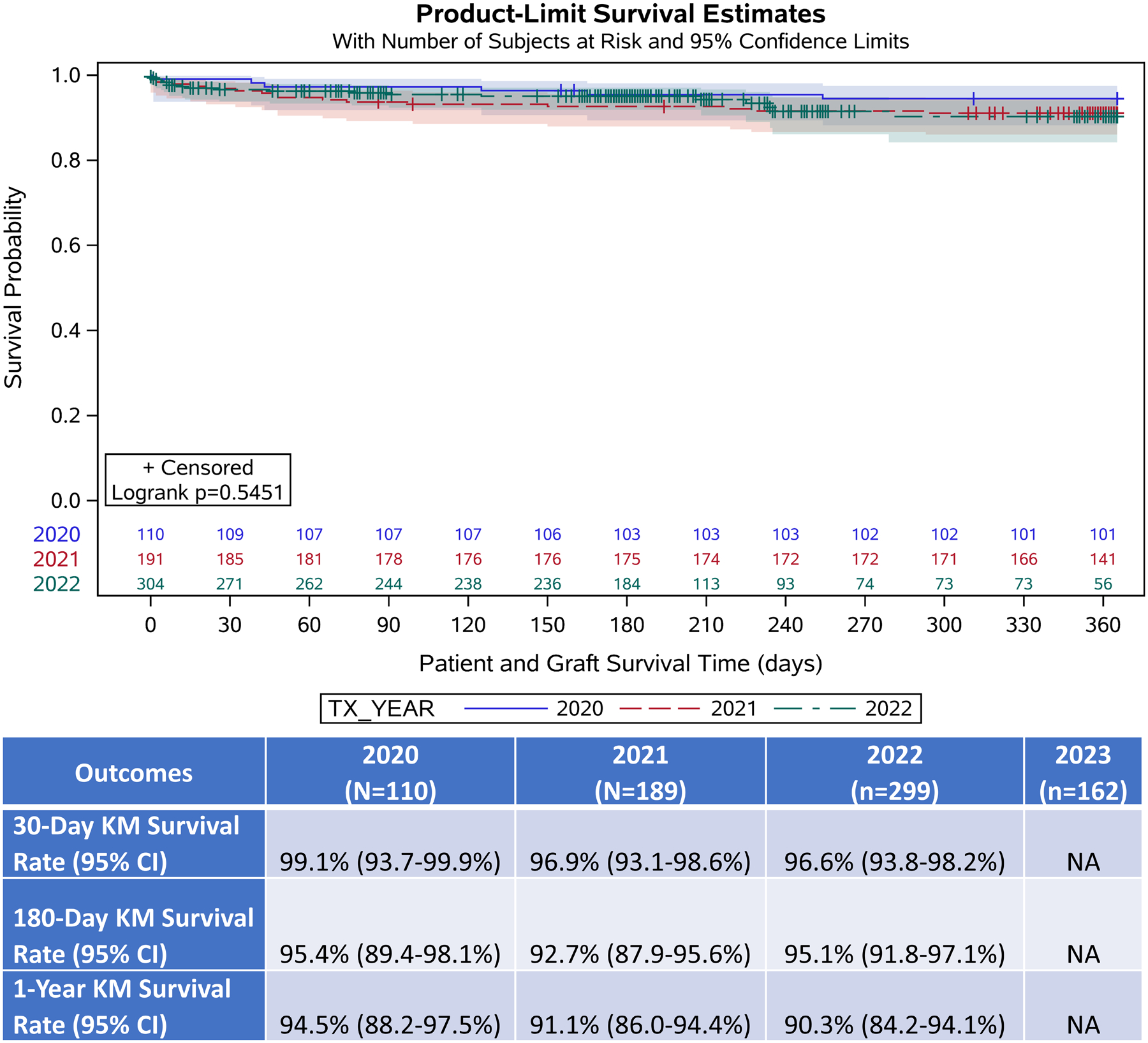
Post-DCD Heart Transplant Kaplan-Meier Survival Free from Death or Repeat HT Estimate by year
Tx-Year: Year of transplant. KM: Kaplan-Meier. CI: Confidence Interval.
To our knowledge, this is the first investigation into longitudinal changes in adult DCD HT recipient and donor risk profiles, as well as concomitant changes in post-DCD HT clinical outcomes. We identified two major findings: 1) Several recipient and donor risk-factors have increased over time, most strikingly the percentage of recipients whom are transplanted at UNOS status 1 or 2 (2020: 17%, 2023: 54.2%), as well as recipient and donor age. 2) This increase in recipient and donor risk factors did not translate into changes in post-DCD HT survival.
HT waitlist mortality remains high.3 As such, safely expanding access to DCD HT to higher-risk recipients, and from a broader proportion of potential donors, is critical. In the recently published DCD Heart Trial, adjusted 6-month survival amongst patients randomized to DCD HT was non-inferior to that of patients randomized to DBD HT.2 The trial, however, had a low rate of UNOS Status 1 (1%) and Status 2 (20%) recipients in the DCD arm.4 Our analysis identifies that in clinical practice, DCD HT has rapidly expanded into these higher-risk recipient populations. DCD donor risk, though more difficult to quantify, has also increased in certain ways, including older donor age, though this is perhaps balanced by a reduction in reported total ischemic time (a variable which is difficult to interpret, given the 64.2% utilization rate of ex vivo perfusion devices in this cohort). Clinical studies in higher risk donor populations will be needed to understand the limits of donor risk which can be acceptably used in DCD HT.
To date, this expansion of DCD HT has not associated with worsened post-HT survival, which remains similar to survival after DBD HT.5 This finding must be interpreted with caution, in the context of our limited sample size, and inability to analyze post-HT outcomes from 2023 (where a further increase in Status 2 recipients was noted). Further analysis, particularly in larger cohorts of UNOS Status 1 and 2 recipients, is required. However, our results suggest that in real-world clinical practice, DCD HT has safely expanded into higher risk recipient and donor groups, and as it grows further, may continue to meaningfully expand the donor pool and improve waitlist outcomes.
Sources of Funding:
Joseph Lerman is supported by National Heart, Lung, and Blood Institute (NHLBI) 5T32HL069749-20. Daniel Guidot is supported by NHLBI 5T32HL160494-02. Additional support from the Duke Clinical Research Institute Executive Director’s Pathway Grant 8588.
Disclosure Statement:
JBL: none. DMG: none. CLG: none. CBP: none. RA: none. NKS: none. JEK: none. CAM: Research Funding- TransMedics. JNS: Research Funding- TransMedics. ADD: Grants/contracts- Amgen, American Heart Association, Biofourmis, Bodyport, Cytokinetics, NHLBI, Novartis, Story Health, Vifor Pharma. Consulting Fees- AbioMed, AstraZeneca, CareDx. Honoraria- AbioMed, Zoll. Travel/Meeting Support- Abbott. DSMB/ Advisory Board- Cardionomic, LivaNova. Leadership or fiduciary role in other board, society, committee or advocacy group- Natera.
Abbreviations:
- DCD
Donation after Circulatory Death
- HT
Heart Transplant
- DBD
Donation after Brain Death
- UNOS
United Network for Organ Sharing
- BMI
body mass index
- ECMO
extra-corporeal membrane oxygenation
References
- 1.Kwon JH, Ghannam AD, Shorbaji K, et al. Early Outcomes of Heart Transplantation Using Donation After Circulatory Death Donors in the United States. Circ Heart Fail. Dec 2022;15(12):e009844. doi: 10.1161/circheartfailure.122.009844 [DOI] [PubMed] [Google Scholar]
- 2.Schroder JN, Patel CB, DeVore AD, et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N Engl J Med. Jun 8 2023;388(23):2121–2131. doi: 10.1056/NEJMoa2212438 [DOI] [PubMed] [Google Scholar]
- 3.Maitra NS, Dugger SJ, Balachandran IC, Civitello AB, Khazanie P, Rogers JG. Impact of the 2018 UNOS Heart Transplant Policy Changes on Patient Outcomes. JACC Heart Fail. May 2023;11(5):491–503. doi: 10.1016/j.jchf.2023.01.009 [DOI] [PubMed] [Google Scholar]
- 4.Sweitzer NK. Safely Increasing Heart Transplantation with Donation after Cardiac Death. N Engl J Med. Jun 8 2023;388(23):2191–2192. doi: 10.1056/NEJMe2303928 [DOI] [PubMed] [Google Scholar]
- 5.Kwon JH, Blanding WM, Shorbaji K, et al. Waitlist and Transplant Outcomes in Organ Donation After Circulatory Death: Trends in the United States. Ann Surg. Oct 1 2023;278(4):609–620. doi: 10.1097/sla.0000000000005947 [DOI] [PubMed] [Google Scholar]


