Abstract
Background:
The RENAL Nephrometry score (RNS) is widely used to describe renal mass complexity and inform patient counseling for partial nephrectomy (PN). However, in cases with multiple tumors, it is unknown which features drive perioperative outcomes.
Objective:
To employ a novel scoring equation (Multiplex Score, MS) derived from RNS to assess outcomes of multiplex PN at our institution.
Design, Setting, and Participants:
A total of 62 consecutive multiplex PN (median (range) # tumors = 4(2–11), 65% robotic) were performed by a single surgeon. The MS was defined a priori as a weighted score derived from RNS (# low risk (LR) lesions) + 2*(# intermediate risk (IR)) + 4*(# high risk (HR)) based on published complication rates.
Outcome Measurements and Statistical Analysis:
MS was dichotomized into favorable/unfavorable based on median score. Patient outcomes were maintained prospectively. MS was compared with other potential RNS derived scoring systems.
Results and Limitation:
A total of 249 tumors were scored. Median (range) MS was 6(range 2–20, IQR 3–8). Complications occurred in 10 patients (16.1%). Only 1 complication occurred in the favorable MS(<6) group, and MS was associated with perioperative complication (p=0.02) and blood loss (p<0.001). When compared to other potential scoring systems, MS had the best area under the curve (AUC) to predict operative complications (0.75).
Conclusions:
The novel Multiplex Score was associated with complications and blood loss. This tool may facilitate standardized reporting of complexity for multiplex series, with special relevance for hereditary cancer syndromes.
Keywords: Partial nephrectomy, multiplex, imaging score, nephrometry, hereditary kidney cancer
Patient Summary:
For patients who have one kidney tumor, there are established scoring systems to help patients and surgeons decide on the surgical plan. However currently, for patients with more than one renal tumor, there is no such scoring system. Here, we present the “Multiplex Score” to aid shared-decision-making in cases with more than one renal tumor.
Introduction
The RENAL Nephrometry score (RNS) is widely used to describe renal mass complexity and inform patient counseling for partial nephrectomy (PN). First described in 2009 by Kutikov and Uzzo, its creation was motivated by the increasing use of nephron-sparing surgery for renal masses.1 Since its introduction, the score has been validated as a useful measure for riskstratification for postoperative complications, and a high score indicates more than triple the operative risk of a favorable score lesion.2 Indicative of its impact on patient counseling, the RNS has been shown to identify patients more likely to choose radical nephrectomy (RN) versus PN, and also those who elect for active surveillance of their renal masses.3,4
Importantly, the RNS has also allowed for standardized reporting and direct comparison of outcomes after various surgical approaches. For example, Deng et al were able to show in a detailed fashion that robotic PN was superior to laparoscopic PN only in the setting of RNS greater than or equal to 7, as measured by operative time, warm ischemia time (WIT), and length of stay.5 A 2019 meta-analysis further confirmed that RNS is an independent predictor of WIT, complication rate, and glomerular filtration rate increase after PN.6 RNS has also been useful in describing patient appropriateness for advanced maneuvers such as segmental artery clamping, or off-clamp procedure.7
One of the more challenging scenarios in the care of patients with kidney cancer is multiplex partial nephrectomy, where multiple masses in the same renal unit are resected during the same surgical procedure. Multiplex PN is defined as 3 or more masses in the same renal unit, however we also studied cases with 2 masses in the same renal unit, and analyzed these separately.8 These procedures can be associated with high potential morbidity, with reported complication rates approaching 50%.9 Here, each tumor may be separately assigned a RENAL Nephrometry score, but it is not clear which features drive perioperative complications. It is unknown whether the total number of tumors carries more weight than the RNS of the most unfavorable lesion. This clinical question has special relevance for hereditary kidney cancer syndromes, where patients often have bilateral multifocal tumors and require several PN during their lifetime.10 For these patients, preservation of renal function is paramount, and this population is especially vulnerable to perioperative complications and conversion to RN. In the present report, we evaluated the performance of a Multiplex Score (MS) equation for multiple tumors, defined a priori based on published complication rates for low, intermediate, and high RENAL Nephrometry scores. This scoring system was applied in a prospectively collected single surgeon database at our center.
Patients and Methods
Patients:
From 10/2017–8/2019, 62 consecutive multiplex PN (2+ masses in the same renal unit) were performed by a single surgeon (MWB). Patient outcomes were maintained prospectively under National Cancer Institute IRB-approved protocol 97-c-0147. Complications were graded according to the Clavien-Dindo classification and were assessed at the time of discharge and at the three month clinic visit.11 See Table 1 for demographic features of the cohort.
Table 1.
Cohort Demographics
| Median Age (IQR) | 50 (37–58) |
|---|---|
| Race, n (%) | |
| White | 42 (68) |
| Black | 12 (19) |
| Asian | 5 (8) |
| Other | 3 (5) |
| Gender, n (%) | |
| Female | 29 (47) |
| Male | 33 (53) |
| Prior Renal Surgery, n (%) | 26 (42) |
| Robotic Approach, n (%) | 43 (69) |
| EBL (Median, IQR) | 850 (500–1650) |
| Renal Ischemia Type | |
| Off clamp | 32 (52) |
| Warm | 23 (37) |
| Cold | 7 (11) |
| Median Warm Ischemia Time (IQR) | 18 (13–25) |
| Multiplex Score (Median, IQR) | 6 (3–8) |
| Hereditary Cancer Syndrome (%) | 48 (77) |
| Hereditary Syndromes (n=48) | |
| von Hippel-Lindau | 40 (83) |
| Birt Hogg Dube | 4 (8) |
| Hereditary papillary renal carcinoma | 1 (2) |
| Tuberous sclerosis | 3 (6) |
| Predominant Pathology Per Case | |
| Clear cell | 45 (73) |
| Papillary | 10 (16) |
| Hybrid oncocytic/chromophobe | 5 (8) |
| Oncocytoma | 1 (2) |
| Angiomyolipoma | 1 (2) |
The demographic characteristics of the 62 patients are shown. IQR = Interquartile Range
Surgical technique:
Both open and robotic approaches were utilized. The open approach was performed via extraperitoneal flank incisions. The robotic approach was performed with the DaVinci Xi robot using a four-arm technique. In both approaches, minimizing unnecessary dissection was prioritized given that patients had already or were expected to require future surgery on the ipsilateral renal unit or adrenal gland. For example, hilar dissection was minimized when possible to avoid scarring for future procedures. When performed, hilar clamping was accomplished with bulldog clamps on the main renal artery. In a minority of cases, selective clamping and/or a robotic retroperitoneal approach was used. Retroperitoneal access was established using a space maker balloon (Coviden, Dublin, Ireland), and a four-arm approach was utilized.
Tumor enucleation was preferentially used except in patients with hereditary leiomyomatosis and renal cell cancer (HLRCC) where partial nephrectomy wide resection was utilized. For tumor enucleation, the capsule is incised sharply circumferentially and the pseudocapsule of the tumor is identified.12 Using blunt dissection, tumors are rolled away from the normal renal parenchyma. Large defects are closed with a 2-layer renorrhaphy with an inner layer 3–0 barbed suture and an outer layer 2–0 polyglactin suture, secured with Hem-o-lok clips (Teleflex, Wayne, PA) and Lapra-Tys (Ethicon, Somerville, NJ). Drains were left at the surgeon’s discretion based on perceived risk of entry into the collecting system during the procedure.
Tumor complexity analysis:
To assess the impact of tumor complexity on perioperative outcomes (any complication and estimated blood loss (EBL)), the following grading schemes were considered: 1) number of tumors scored, 2) highest score of any tumor, 3) number of high risk RNS tumors, 4) number of intermediate or high risk RNS tumors, and 5) the novel multiplex score (See Table 2 and below).
Table 2.
Univariate Analysis of Grading Schemes with Complications and Blood Loss
| Grading Scheme | High Grade Complication | EBL | AUC (p-value comparing all 0.048) | ||
|---|---|---|---|---|---|
| OR | p value | Coefficient | p value | p value | |
| # tumors scored | 1.31 | 0.06 | 346 | <0.001 | 0.71 |
| Highest score (RNS) | 1.43 | 0.2 | 265 | 0.04 | 0.62 |
| # HR (RNS) tumors | 1.47 | 0.6 | 730 | 0.05 | 0.52 |
| # IR or HR (RNS) tumors | 1.64 | 0.01 | 566 | <0.001 | 0.73 |
| Multiplex Score | 1.23 | 0.02 | 242 | <0.001 | 0.75 |
OR = Odds Ratio; RNS = RENAL Nephrometry Score; HR = High Risk; IR = Intermediate Risk; EBL = Estimated Blood Loss.
Multiplex Score:
The MS was defined a priori as a weighted score (# low risk (LR) lesions) + 2*(# intermediate risk (IR)) + 4*(# high risk (HR)) based on published Clavien-Dindo III-V complication rates of 6.4%, 11.1%, and 21.9% for LR (RNS 4–6 pts), IR (RNS 7–9 pts), and HR (RNS 10–12 pts) RNS respectively.2 MS was dichotomized into favorable/unfavorable based on median score. Imaging review with RENAL Nephrometry score assignment to each primary tumor visible on preoperative imaging was performed by two urologists (HC and NY) based on the preoperative contrast-enhanced MRI.
Statistical Analysis:
Binary outcomes were evaluated with logistic regression and continuous outcomes were evaluated with univariate linear regression. Statistical analysis was performed with Stata 15 (StataCorp, College Station, TX). Two-sided p values < 0.05 were considered significant.
Results
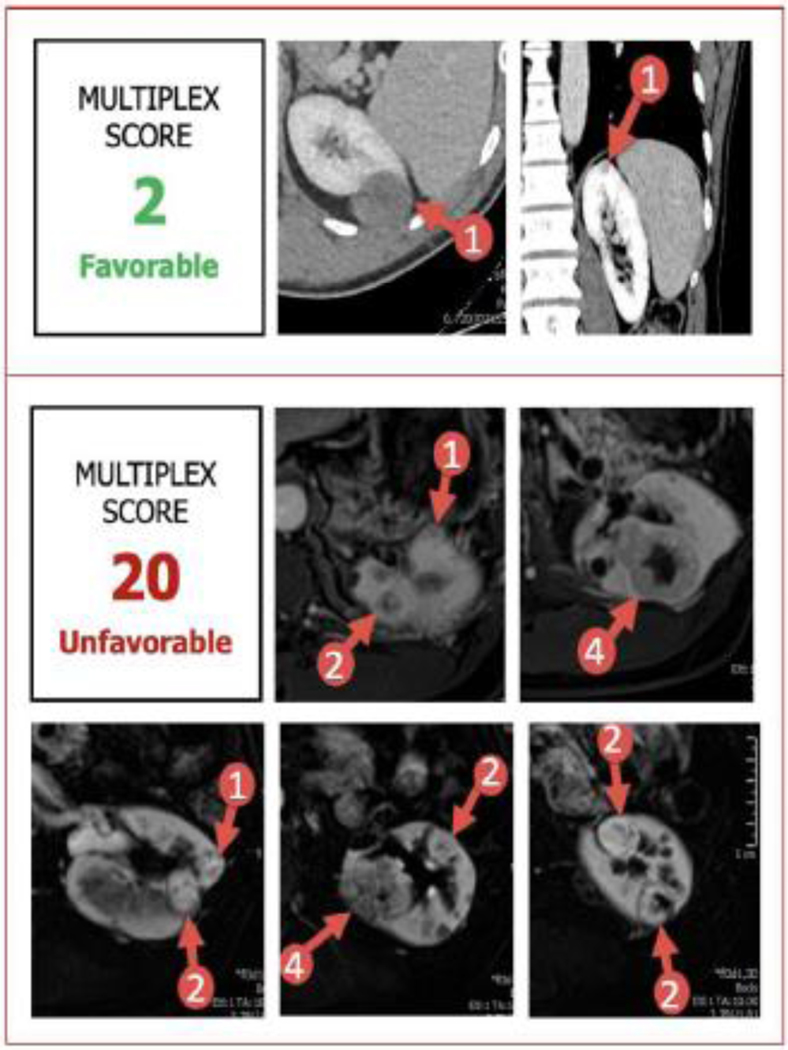
During the study period, 62 consecutive multiplex PN (median (range) # tumors = 4 (2–11), 65% robotic) were performed by a single surgeon. Median (range) number of tumors on preoperative imaging was 4 (2–11). A total of 249 tumors were scored overall. 24 patients (39%) had prior surgery on the ipsilateral kidney. Median (range) MS was 6 (2–20), thus favorable MS was defined as <6 and unfavorable MS was defined as >=6. Figure 1 shows an example of a case with a favorable Multiplex Score of 2 with two tumors. A comparative case with an unfavorable Multiplex Score of 20 is shown with 9 tumors.
Figure 1. Representative Imaging of Sample Patients Assigned Favorable vs. Unfavorable Multiplex Scores.
Postoperative complications occurred in 10 patients (16%). Urine leak (N=5, 8%) and bleed requiring embolization (N=5, 8%) were the most common (See Table 3). Only 1 complication occurred in the favorable score group and MS was significantly associated with perioperative complication (OR 1.23, p=0.02) and blood loss (242 mL, p<0.001). Hilar clamping was omitted in 32 (52%) cases with median warm ischemia time of 18min when used. Every 1 additional HR or IR tumor was associated with a 2-minute increase in clamp time (2.01, p=0.048). Change in renal function was negligible change in our cohort (median ΔCr 0 mg/dL, IQR −0.61 – 0.1). In our cohort, there were no conversions from partial to radical nephrectomy. Open conversion was only done in 3 (7.5%) cases, all of which were assigned unfavorable MS. The scores were 8, 7, 12. Median (IQR) MS for planned robotic approach was 5 (3–7), and for open 7 (5–12), p = 0.005. In this cohort, reoperative surgery had a higher median EBL than initial surgery (1150 vs 625 mL, p < 0.001)
Table 3.
Description of Patients who Experienced Post-Operative Complications
| Patient # | Description of Complication(s) | Multiplex Score | Tumors Removed | Highest Nephrometry Tumor | Prior ipsilateral renal surgery | Approach |
|---|---|---|---|---|---|---|
| 1 | Bleed (embolization) | 5 | 2 | 10 | No | Robotic |
| 2 | Gastric injury requiring repair, bleed (embolization), IR drain placement. | 6 | 4 | 8 | Yes | Open |
| 3 | Bleed (embolization) | 6 | 5 | 9 | No | Robotic |
| 4 | Urine leak | 7 | 3 | 10 | Yes | Open |
| 5 | Urine leak | 7 | 4 | 8 | No | Robotic |
| 6 | Bleed (embolization) | 8 | 5 | 9 | No | Open |
| 7 | Urine leak + bleed (embolization) | 8 | 4 | 9 | Yes | Open |
| 8 | Urine leak, prolonged stent, drain placement | 12 | 6 | 8 | Yes | Open |
| 9 | Urine leak, prolonged stent, drain placement | 13 | 9 | 8 | Yes | Open |
| 10 | Bleed (embolization) | 20 | 9 | 10 | Yes | Open |
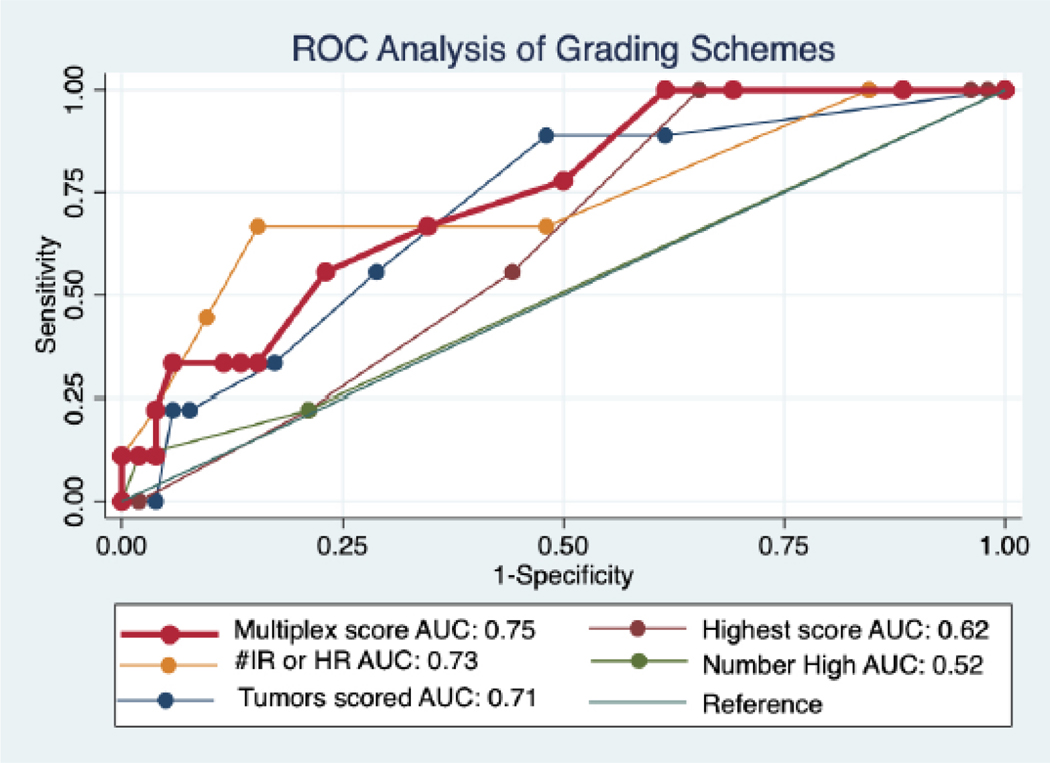
To compare the five grading schemes previously outlined (Table 2), receiver operating characteristic (ROC) curves were generated and an area under the curve analysis for any complication was performed. MS performed the best with an AUC of 0.75 (Figure 2). In comparison, the number of tumors scored had an AUC of 0.73, highest score of any tumor had an AUC of 0.69, number of high risk RNS tumors had an AUC = 0.56, and finally number of intermediate or high risk RNS tumors had an AUC of 0.68.
Figure 2. ROC Analysis of Grading Schemes.
ROC = Receiver operating characteristics; AUC = Area under the curve; IR = intermediate risk; HR = high risk
Discussion
There are at least seventeen genes implicated in hereditary cancer syndromes that increase an individual’s risk for the development of renal cell carcinoma (RCC), often with concomitant renal cysts and bilateral, multifocal tumors.13, 14 These patients require specialized treatment paradigms, and surgical management must consider not only the tumors present at the time of diagnosis but also anticipate the need for future surgery by sparing hilar dissection and omitting clamping when safe and feasible. Yet another group of patients have a sporadic form of bilateral or multifocal renal masses, and these patients often undergo radical nephrectomy due to the perceived difficulty of multiplex PN if they are not referred to centers of excellence. Currently, there is no established scoring system to predict the operative complexity of multifocal RCC. Here, we evaluated a Multiplex Score that integrates the RENAL Nephrometry scores of individual lesions with a weighted equation based on published complication rates for partial nephrectomy of a single tumor. This scoring system predicted both post-operative complications and EBL, and compared favorably to other potential scoring systems. Of note, other scoring systems such as total number of tumors and number of IR or HR tumors had similar AUCs approaching that of the MS, however in addition to the highest AUC the multiplex score has the added benefit of presenting maximal information which can help to standardize literature reporting.
In certain more indolent forms of hereditary RCC, a “three centimeter” threshold is used to predict the optimal time for operative intervention.15 This tumor size cutoff is based on the observed malignant potential of such lesions by size, with minimal risk for metastasis below 3 cm. However, anecdotally urologists understand that it may be prudent to intervene early for certain lesions growing in an unfavorable location.16 The Multiplex Score may help to standardize discussion in these circumstances and assist patients and urologists with decision making. In an instance with two or three tumors in the same renal unit, a urologist may use the MS to help decide if they will perform the surgery locally or consider referral to a specialized center.
In the present study, we found that the MS was a useful tool to understand the risk of perioperative outcomes for patients with multifocal disease. However, there were several limitations. First, this is a single institution, retrospective study with the inherent limitations of that study design. Additionally, we did not include patients undergoing radical nephrectomy; however, surgeons at our institution preferentially perform partial nephrectomy in the overwhelming majority of cases. Of note, only a single early career surgeon’s cases were included, however the learning curve for multiplex PN has previously been evaluated and for the first 100 cases, no difference in surgical duration, EBL or complications have been noted among multiple surgeons.17Also, RNS was not routinely used at our institution before this study period and we felt that the addition of other surgeons would introduce potential confounders to our analysis. Understandably, only lesions visible on preoperative imaging were scored, yet additional small tumors were often found at time of surgery and were resected, which was not included in this analysis. Another limitation is that the RNS for each tumor was assigned by 1 of 2 urologists, although we do know that the RENAL Nephrometry score has been shown to have good interobserver variability.18 Additionally, we did not include the anterior/posterior/hilar portions of the RENAL Nephrometry score in our analysis and assessed performance with a cutoff value as opposed to as a continuous variable. Another unaccounted variable is the manner in which any prior surgery on the same renal unit was conducted; for example, closure of the Gerota’s fascia is a standard practice at our center but is not uniformly performed elsewhere. Similarly, case-by-case decisions such as open versus robotic, trans versus retroperitoneal, and dissection on or off clamp are all additional variables that may impact outcomes. Given the relatively small number of events, we were limited by our inability to model all of these variables. Finally, while the present report was based on only 249 tumors arising from 62 patients, it is comparable to the original 2009 report by Kutikov et al that served as the basis for all subsequent nephrometry work.1
Conclusions
In conclusion, the novel Multiplex Score is associated well with complications, blood loss, and open conversion. This tool may facilitate standardized reporting of complexity for multiplex series, with special relevance for hereditary cancer syndromes.
Highlights.
There is currently no scoring system for patients with multiple renal tumors
We assessed several potential scoring systems.
The Multiplex Score assigns points based on the R.E.N.A.L. Nephrometry Score.
The Multiplex Score is associated with intraoperative blood loss and post-operative complications.
Source of Funding:
“This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References –
- 1).Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009. Sep;182(3):844–53. doi: 10.1016/j.juro.2009.05.035. Epub 2009 Jul 17. [DOI] [PubMed] [Google Scholar]
- 2).Simhan J, Smaldone MC, Tsai KJ, Canter DJ, Li T, Kutikov A, Viterbo R, Chen DY, Greenberg RE, Uzzo RG. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol. 2011 Oct;60(4):724–30. doi: 10.1016/j.eururo.2011.05.030. Epub 2011. May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Canter D, Kutikov A, Manley B, Egleston B, Simhan J, Smaldone M, Teper E, Viterbo R, Chen DY, Greenberg RE, Uzzo RG. Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology. 2011. Nov;78(5):1089–94. doi: 10.1016/j.urology.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Tomaszewski JJ, Uzzo RG, Kocher N, Li T, Manley B, Mehrazin R, Ito T, Abbosh P, Viterbo R, Chen DY, Greenberg RE, Canter D, Smaldone MC, Kutikov A. Patients with anatomically “simple” renal masses are more likely to be placed on active surveillance than those with anatomically “complex” lesions. Urol Oncol. 2014 Nov;32(8):1267–71. doi: 10.1016/j.urolonc.2014.05.003. Epub 2014. Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Deng W, Li J, Liu X, Chen L, Liu W, Zhou X, Zhu J, Fu B, Wang G. Robot-assisted versus laparoscopic partial nephrectomy for anatomically complex T1b renal tumors with a RENAL nephrometry score ≥7: A propensity score-based analysis. Cancer Med. 2020. Jan;9(2):586–594. doi: 10.1002/cam4.2749. Epub 2019 Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Veccia A, Antonelli A, Uzzo RG, Novara G, Kutikov A, Ficarra V, Simeone C, Mirone V, Hampton LJ, Derweesh I, Porpiglia F, Autorino R. Predictive Value of Nephrometry Scores in Nephron-sparing Surgery: A Systematic Review and Meta-analysis. Eur Urol Focus. 2020. May 15;6(3):490–504. doi: 10.1016/j.euf.2019.11.004. Epub 2019 Nov 24. [DOI] [PubMed] [Google Scholar]
- 7).Qian J, Jiang J, Li P, Zhang S, Bao M, Qin C, Meng X, Shao P, Wang Z. Factors Influencing the Feasibility of Segmental Artery Clamping During Retroperitoneal Laparoscopic Partial Nephrectomy. Urology. 2019. Jul;129:92–97. doi: 10.1016/j.urology.2019.03.024. Epub 2019 Apr 5. [DOI] [PubMed] [Google Scholar]
- 8).Baiocco JA, Ball MW, Pappajohn AK, Rayn KN, Bratslavsky G, Boyle SL, Linehan WM, Metwalli AR. A comparison of outcomes for standard and multiplex partial nephrectomy in a solitary kidney: The National Cancer Institute experience. Urol Oncol. 2019. Jun;37(6):356.e1–356.e7. doi: 10.1016/j.urolonc.2019.02.015. Epub 2019 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Fadahunsi AT, Sanford T, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of partial nephrectomy for resection of at least 20 tumors in a single renal unit. J Urol. 2011. Jan;185(1):49–53. doi: 10.1016/j.juro.2010.09.032. Epub 2010 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Baiocco JA, Ball MW, Pappajohn AK, Rayn KN, Bratslavsky G, Boyle SL, Linehan WM, Metwalli AR. A comparison of outcomes for standard and multiplex partial nephrectomy in a solitary kidney: The National Cancer Institute experience. Urol Oncol. 2019. Jun;37(6):356.e1–356.e7. doi: 10.1016/j.urolonc.2019.02.015. Epub 2019 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. Aug;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Lebastchi AH, Haynes B, Gurram S, Bratslavsky G, Metwalli AR, Linehan WM, Ball MW. X-Capsular Incision for Tumor Enucleation (X-CITE)-Technique: A Method to Maximize Renal Parenchymal Preservation for Completely Endophytic Renal Tumors. Urology. 2021 Aug;154:315–319. doi: 10.1016/j.urology.2021.03.032. Epub 2021. Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Linehan WM, Schmidt LS, Crooks DR, Wei D, Srinivasan R, Lang M, Ricketts CJ. The Metabolic Basis of Kidney Cancer. Cancer Discov. 2019. Aug;9(8):1006–1021. doi: 10.1158/2159-8290.CD-18-1354. Epub 2019 May 14. [DOI] [PubMed] [Google Scholar]
- 14).Ball MW, Shuch BM. Inherited kidney cancer syndromes. Curr Opin Urol. 2019. Jul;29(4):334–343. doi: 10.1097/MOU.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 15).Metwalli AR, Linehan WM. Nephron-sparing surgery for multifocal and hereditary renal tumors. Curr Opin Urol. 2014;24(5):466–473. doi: 10.1097/MOU.0000000000000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Pierorazio PM, Hyams ES, Mullins JK, Allaf ME. Active surveillance for small renal masses. Rev Urol. 2012;14(1–2):13–9. [PMC free article] [PubMed] [Google Scholar]
- 17).Telfer S, Gurram S, Gomella PH, et al. Surgical learning curve for robotic multiplex partial nephrectomy: The National Cancer Institute Experience. Journal of Clinical Oncology 38 (6_suppl), 660–660. [Google Scholar]
- 18).Spaliviero M, Poon BY, Aras O, Di Paolo PL, Guglielmetti GB, Coleman CZ, Karlo CA, Bernstein ML, Sjoberg DD, Russo P, Touijer KA, Akin O, Coleman JA. Interobserver variability of R.E.N.A.L., PADUA, and centrality index nephrometry score systems. World J Urol. 2015. Jun;33(6):853–8. doi: 10.1007/s00345-014-1376-4. Epub 2014 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]