Key Points
Question
Is alirocumab an efficacious lipid-lowering therapy (LLT) for pediatric patients (8-17 years) with heterozygous familial hypercholesterolemia (HeFH) inadequately controlled with statins?
Findings
In this randomized clinical trial of 153 patients, alirocumab treatment showed statistically significant reductions in low-density lipoprotein cholesterol (LDL-C) vs placebo in 2 dosing cohorts.
Meaning
The findings indicate that alirocumab may be an effective adjunct LLT for pediatric patients with HeFH inadequately controlled with statins.
This randomized clinical trial evaluates the efficacy of alirocumab in pediatric patients with heterozygous familial hypercholesterolemia.
Abstract
Importance
Many pediatric patients with heterozygous familial hypercholesterolemia (HeFH) cannot reach recommended low-density lipoprotein cholesterol (LDL-C) concentrations on statins alone and require adjunct lipid-lowering therapy (LLT); the use of alirocumab in pediatric patients requires evaluation.
Objective
To assess the efficacy of alirocumab in pediatric patients with inadequately controlled HeFH.
Design, Setting, and Participants
This was a phase 3, randomized clinical trial conducted between May 2018 and August 2022 at 43 centers in 24 countries. Pediatric patients aged 8 to 17 years with HeFH, LDL-C 130 mg/dL or greater, and receiving statins or other LLTs were included. Following consecutive enrollment into dosing cohorts, 25 of 99 patients screened for dosing every 2 weeks (Q2W) failed screening; 25 of 104 patients screened for dosing every 4 weeks (Q4W) failed screening. A total of 70 of 74 Q2W patients (95%) and 75 of 79 Q4W patients (95%) completed the double-blind period.
Interventions
Patients were randomized 2:1 to subcutaneous alirocumab or placebo and Q2W or Q4W. Dosage was based on weight (40 mg for Q2W or 150 mg for Q4W if <50 kg; 75 mg for Q2W or 300 mg for Q4W if ≥50 kg) and adjusted at week 12 if LDL-C was 110 mg/dL or greater at week 8. After the 24-week double-blind period, patients could receive alirocumab in an 80-week open-label period.
Main Outcomes and Measures
The primary end point was percent change in LDL-C from baseline to week 24 in each cohort.
Results
Among 153 patients randomized to receive alirocumab or placebo (mean [range] age, 12.9 [8-17] years; 87 [56.9%] female), alirocumab showed statistically significant reductions in LDL-C vs placebo in both cohorts at week 24. Least squares mean difference in percentage change from baseline was −43.3% (97.5% CI, −56.0 to −30.7; P < .001) Q2W and −33.8% (97.5% CI, −46.4 to −21.2; P < .001) Q4W. Hierarchical analysis of secondary efficacy end points demonstrated significant improvements in other lipid parameters at weeks 12 and 24 with alirocumab. Two patients receiving alirocumab Q4W experienced adverse events leading to discontinuation. No significant difference in adverse event incidence was observed between treatment groups. Open-label period findings were consistent with the double-blind period.
Conclusions and Relevance
The findings in this study indicate that alirocumab Q2W or Q4W significantly may be useful for reducing LDL-C and other lipid parameters and be well tolerated in pediatric patients with HeFH inadequately controlled with statins.
Trial Registration
ClinicalTrials.gov Identifier: NCT03510884
Introduction
Heterozygous familial hypercholesterolemia (HeFH) is an autosomal dominant disorder affecting approximately 1 in 313 individuals characterized by severely elevated low-density lipoprotein cholesterol (LDL-C) concentrations.1,2 If left untreated, patients may develop early-onset atherosclerotic cardiovascular disease. Detection and intervention to control LDL-C concentrations in pediatric patients can prevent atherosclerosis progression and premature cardiovascular events in adulthood.3,4 American College of Cardiology/American Heart Association guidelines define acceptable LDL-C concentrations in pediatric and adolescent patients as less than 110 mg/dL (to convert to mmol/L, multiply by 0.0259) and recommend initiation of statins (or nonstatins if statin intolerant) from as young as 8 years.5,6 Current European Atherosclerosis Society pediatric guidelines recommend LDL-C reduction to less than 130 mg/dL for patients over 10 years, or by at least 50% for patients from 8 years.7 Other lipid-lowering therapies (LLTs), including ezetimibe and bile-acid sequestrants, are available as adjuncts to statins or as alternative therapy for those who are statin intolerant.5
However, some pediatric patients cannot achieve LDL-C targets despite high-dose statins or multiagent LLTs. Additional LLTs are needed for these patients to help control LDL-C concentrations and reduce risk of atherosclerotic disease in adulthood. Recent therapeutic developments include inhibitors of proprotein convertase subtilisin kexin type 9 (PCSK9), a protease that promotes degradation of the low-density lipoprotein receptor responsible for clearing excess LDL-C from the blood.8,9
Alirocumab is a fully human monoclonal antibody that binds and inhibits circulating PCSK9. Alirocumab has been shown to reduce LDL-C and other atherogenic lipoproteins in adults with hyperlipidemia—including patients with HeFH—either alone or in combination with other LLTs. Alirocumab has shown to reduce cardiovascular event risk in adult patients with established cardiovascular disease.10,11 In adults in the US, the starting dose for alirocumab is 75 mg once every 2 weeks (Q2W) or 300 mg once every 4 weeks (Q4W); in the European Union, the starting dose is 75 mg Q2W, and for patients requiring LDL-C reduction of greater than 60% the starting dose is 150 mg Q2W or 300 mg Q4W.12,13,14
A phase 2 dose-finding study, the 8-Week Open-Label, Sequential, Repeated Dose-Finding Study to Evaluate the Efficacy and Safety of Alirocumab in Children and Adolescents With Heterozygous Familial Hypercholesterolemia Followed by an Extension Phase (ODYSSEY KIDS),8 evaluated alirocumab in pediatric patients with HeFH inadequately controlled with statins. Alirocumab was well tolerated and reduced LDL-C with results at higher doses (−46.1% at week 8 for 40 mg/75 mg Q2W if <50 kg/≥50 kg), consistent with reductions reported in adults, supporting further investigation in pediatric HeFH. This phase 3 trial assessed the efficacy of alirocumab in pediatric patients with HeFH inadequately controlled with statins.
Methods
Study Design
This was a phase 3, randomized, double-blind, placebo-controlled, multicenter clinical trial. Patients were screened at 43 centers in 24 countries (Argentina, Austria, Brazil, Bulgaria, Canada, Czech Republic, Denmark, Finland, France, Hungary, Italy, Lebanon, Mexico, the Netherlands, Norway, Poland, Russia, Slovenia, South Africa, Spain, Sweden, Taiwan, Turkey, and the US), with 40 centers randomizing patients. The study was conducted in accordance with the protocol (Supplement 1) and international guidelines, including the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice, and all applicable local laws and regulations. The study protocol was approved by the independent review boards or ethics committees of participating centers. Signed parental consent, with or without patient consent, was obtained for all patients. The study is registered at ClinicalTrials.gov (NCT03510884). The study protocol and the statistical analysis plan are available in Supplement 1.
Patient Eligibility Criteria
Patients aged 8 to 17 years were eligible if they had inadequately controlled HeFH (LDL-C ≥130 mg/dL) despite treatment with optimal statin dose (or nonstatin LLT, if statin intolerant), with or without other LLT, for at least 4 weeks. HeFH diagnosis was confirmed by genotyping or Simon Broome criteria.15 Optimal statin dose was defined as a stable daily dose prescribed based on regional or local guidelines or the maximally tolerated dose. Statin intolerance was defined as the inability to tolerate at least 2 statins (1 at the lowest daily dose, the others at any dose) or patients not receiving a daily statin. Patients who had participated in ODYSSEY KIDS8 were eligible and not excluded based on LDL-C less than 130 mg/dL, as LDL-C 130 mg/dL or greater was required at screening for ODYSSEY KIDS.
Patients were excluded if they were less than 25 kg in body weight, aged 8 to 9 years and not at Tanner Stage 1, or aged 10 to 17 years and not at Tanner Stage 2 or higher. Other exclusion criteria included secondary hyperlipidemia; homozygous FH; prior lipid apheresis within 2 months of screening or planned apheresis during the study; uncontrolled type 1 or 2 diabetes (per local guidelines), thyroid disease or hypertension; severe kidney impairment; abnormal liver function; or creatine phosphokinase greater than 3 times the upper limit of normal.
Study Interventions
The study included a 4-week run-in period and a 2-week screening period; ODYSSEY KIDS8 participants were required to have a washout of at least 10 weeks between their last alirocumab dose and screening lipid assessment. Patients were randomized 2:1 in 2 consecutive dosing cohorts (Q2W or Q4W) to receive alirocumab or matching placebo for 24 weeks in addition to their stable background LLT. Randomization was stratified by previous participation in ODYSSEY KIDS8 and baseline weight. In the Q2W cohort, alirocumab dose was 40 mg for patients weighing less than 50 kg and 75 mg for those weighing 50 kg or more. In the Q4W cohort, the alirocumab dose was 150 mg for patients weighing less than 50 kg and 300 mg for those weighing 50 kg or more. If LDL-C at week 8 was 110 mg/dL or higher, the alirocumab dose was adjusted at week 12 to 75 mg Q2W for patients weighing less than 50 kg and 150 mg Q2W for those weighing 50 kg or more, regardless of cohort. Lipid values during the 24-week double-blind period were not communicated to the investigator; continuation of dose or modification at week 12 was a blinded and automated process. Dosing by weight was matched in the placebo arms (Supplement 1).
After completing 24 weeks of double-blind treatment, all patients could enter an open-label period to receive alirocumab in addition to their LLT for up to 80 weeks. The dose was adjusted based on investigator judgment (taking LDL-C and weight into consideration) (Supplement 1).
Assessments
Blood samples monitoring LDL-C concentrations, other lipids, and clinical laboratory parameters were collected at baseline and weeks 8, 12, and 24 of the double-blind period, and at weeks 32, 44, 68, and 104 (study completion) of the open-label period. Assessment of efficacy end points were handled by a central laboratory. Safety was assessed throughout the study and included adverse event (AE) reports, vital signs, weight, and height measurements, Cogstate battery test score, Tanner stage, laboratory data, and antialirocumab antibodies.
Study End Points
The primary end point was the percent change in LDL-C from baseline to week 24 in each cohort. Key secondary efficacy end points were percent change in LDL-C at week 12 and other lipid parameters at weeks 12 and 24, including: apolipoprotein B (apo B), non–high-density lipoprotein cholesterol (non–HDL-C), total cholesterol, high-density lipoprotein cholesterol (HDL-C), lipoprotein (a) (Lp[a]), triglycerides, and apolipoprotein A-1. Safety, tolerability, and development of antialirocumab antibodies after 24 weeks were also secondary end points.
Statistical Analysis
The trial planned for a sample size of 90 patients to provide 92% power in identifying a difference in mean percent change in LDL-C of 30% between alirocumab and placebo. The size was increased to 150 patients to properly assess safety and tolerability.
The primary efficacy end point was analyzed in the intention-to-treat population using a mixed-effects model with repeated-measures approach; the model included treatment group, randomization strata, time points, treatment-by–time point interaction, strata-by–time point interaction, continuous fixed covariates of baseline value, and baseline value-by–time point interaction. Parameters were estimated using restricted maximum likelihood method with the Newton-Raphson algorithm. To address multiple testing, a Bonferroni adjustment was applied, using a 2-sided α level of .025 within each cohort. Due to the sequential enrollment of participants in 2 different cohorts, comparisons were made between treatment groups within each dosing cohort separately.
Continuous secondary efficacy end points anticipated to have a normal distribution were analyzed using the same approach as the primary end point; those anticipated to have a nonnormal distribution were analyzed using a multiple imputation approach followed by a robust regression model using M-estimation with treatment group and randomization strata as main effects and corresponding baseline value as covariate. Categorical secondary efficacy end points were analyzed using a multiple imputation approach followed by logistic regression model adjusting for treatment group as main effect and corresponding baseline value as covariate, stratified by randomization factors. To address multiple testing, key secondary efficacy end points were analyzed in each cohort if the primary end point was met and using a hierarchical testing procedure with a 2-sided α of .05.
Efficacy analyses were performed on the intention-to-treat population, defined as all randomized patients. For analyses of the Q4W cohort, participation in ODYSSEY KIDS was not included in randomization strata because there were too few patients. Efficacy data for the open-label period were summarized descriptively.
Safety data were summarized with descriptive statistics for the safety population of the double-blind period and the open-label population. The safety population consisted of all randomized patients who received at least 1 dose or partial dose of study treatment, analyzed for the actual treatment received.
Results
Patients
A total of 153 patients (mean [range] age, 12.9 [8-17] years; 87 [56.9%] female) were randomized 2:1 to alirocumab or placebo, following Q2W (n = 74; 49 alirocumab and 25 placebo) or Q4W (n = 79; 52 alirocumab and 27 placebo) dosing. At week 12, alirocumab doses were up-titrated in 22 patients (44.9%) in Q2W and 15 (28.8%) in Q4W. The first patient visit was on May 31, 2018, and the last patient visit on August 5, 2022. Seventy patients (95%) in the Q2W cohort and 75 (95%) in Q4W completed the double-blind treatment period. Subsequently, 145 patients entered and 138 (95%) completed the open-label period (Figure 1).
Figure 1. Participant Flow Diagram.
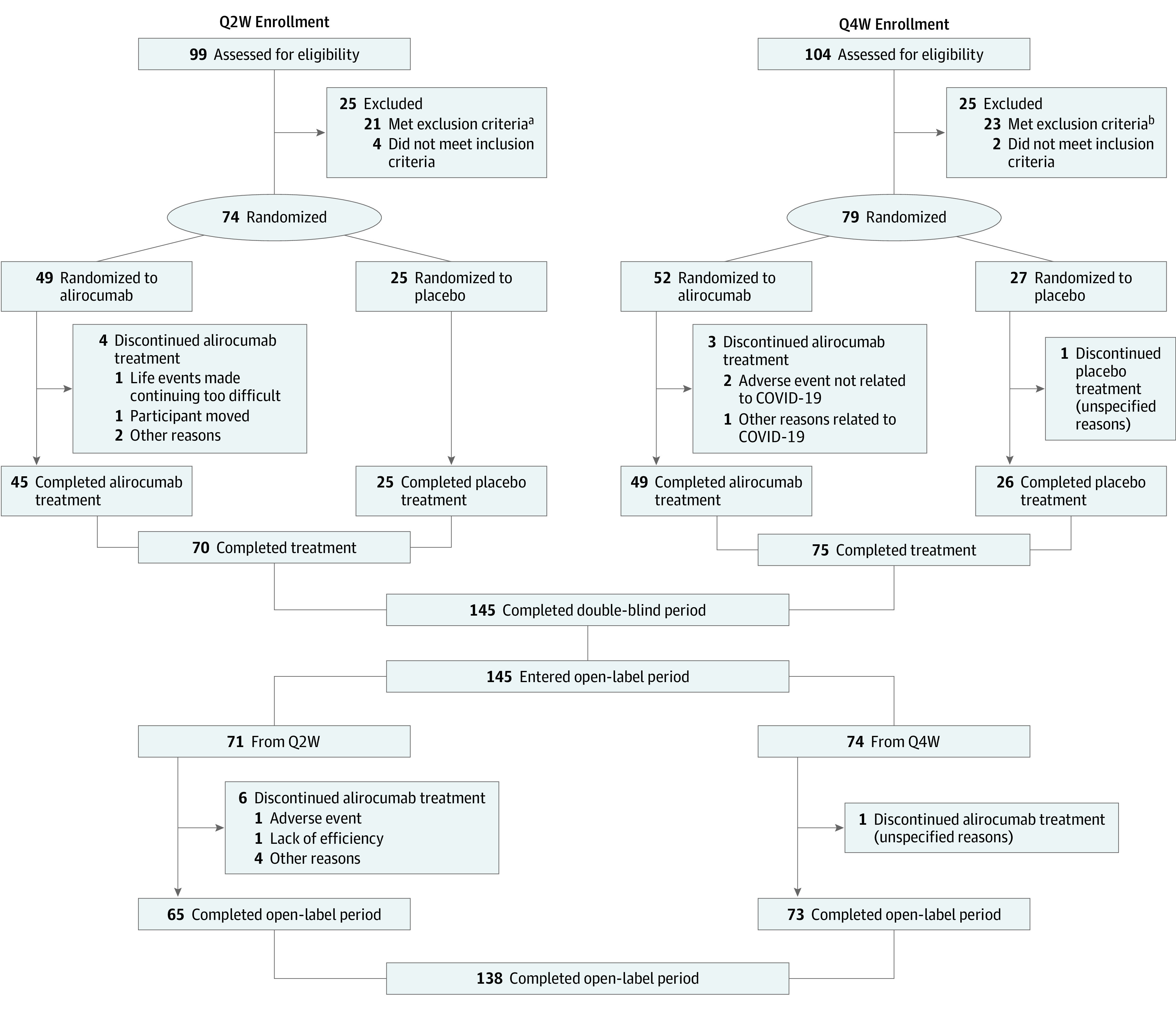
Q2W indicates dosing once every 2 weeks; Q4W, dosing once every 4 weeks.
aIncludes 12 patients (12.1%) with low-density lipoprotein cholesterol less than 130 mg/dL (to convert to mmol/L, multiply by 0.0259) after the patient had been receiving stable lipid-modifying treatment for at least 4 weeks; 5 patients (5.1%) whose parents withdrew consent during the screening period; 3 patients (3.0%) aged 8 to 9 years not at Tanner stage 1 or aged 10 to 17 years not at Tanner stage 2 or higher; 1 patient (1.0%) with uncontrolled type 1 or 2 diabetes (ie, hemoglobin A1C levels above local guidelines or equivalent); and 1 patient (1.0%) with creatine phosphokinase 3 times the upper limit of normal or higher.
bIncludes 14 patients (13.5%) with low-density lipoprotein cholesterol less than 130 mg/dL after the patient had been receiving stable lipid-modifying treatment for at least 4 weeks; 4 patients (3.8%) with any abnormal clinical condition or disease during screening that would hinder study completion or any other condition in the investigator’s opinion as making the patient inappropriate for the study; 3 patients (2.9%) whose parents withdrew consent during the screening period; and 2 patients (1.9%) aged 8 to 9 years not at Tanner stage 1 or aged 10 to 17 years not at Tanner stage 2 or higher.
Baseline patient demographic characteristics, disease characteristics, and lipid parameters were broadly similar between treatment groups and dosing cohorts, with some exceptions (Table 1). In the Q2W cohort, there were more patients weighing less than 50 kg, more ODYSSEY KIDS participants, and fewer female participants than in the Q4W cohort. At baseline, LDL-C concentrations were similar between treatment groups in the Q2W and Q4W cohorts.
Table 1. Baseline Characteristics of the Intention-to-Treat Population.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Q2W cohort (n = 74) | Q4W cohort (n = 79) | Overall (N = 153) | ||||
| Alirocumab (n = 49) | Placebo (n = 25) | Alirocumab (n = 52) | Placebo (n = 27) | Q2W (n = 74) | Q4W (n = 79) | |
| Age, mean (SD), y | 12.5 (2.7) | 13.2 (2.4) | 13.1 (3.0) | 12.8 (3.0) | 12.8 (2.6) | 13.0 (3.0) |
| <12 y | 19 (38.8) | 6 (24.0) | 20 (38.5) | 10 (37.0) | 25 (33.8) | 30 (38.0) |
| ≥12 y | 30 (61.2) | 19 (76.0) | 32 (61.5) | 17 (63.0) | 49 (66.2) | 49 (62.0) |
| Sex | ||||||
| Female | 30 (61.2) | 8 (32.0) | 34 (65.4) | 15 (55.6) | 38 (51.4) | 49 (62.0) |
| Male | 19 (38.8) | 17 (68.0) | 18 (34.6) | 12 (44.4) | 36 (48.6) | 30 (38.0) |
| Racea | ||||||
| White | 42 (85.7) | 23 (92.0) | 38 (73.1) | 22 (81.5) | 65 (87.8) | 60 (75.9) |
| Otherb | 7 (14.3) | 2 (8.0) | 14 (26.9) | 5 (18.5) | 9 (12.2) | 19 (24.1) |
| Weight, mean (SD), kg | 53.9 (22.2) | 50.4 (15.1) | 54.7 (20.2) | 50.9 (14.0) | 52.7 (20.1) | 53.4 (18.3) |
| <50 kg | 25 (51.0) | 13 (52.0) | 20 (38.5) | 10 (37.0) | 38 (51.4) | 30 (38.0) |
| ≥50 kg | 24 (49.0) | 12 (48.0) | 32 (61.5) | 17 (63.0) | 36 (48.6) | 49 (62.0) |
| Height, mean (SD), cm | 156.2 (16.5) | 156.6 (14.0) | 155.6 (16.5) | 154.2 (13.0) | 156.3 (15.6) | 155.1 (15.3) |
| ODYSSEY KIDS study participation | 19 (38.8) | 10 (40.0) | 2 (3.8) | 1 (3.7) | 29 (39.2) | 3 (3.8) |
| Time to diagnosis, mean (SD), y | 4.21 (3.11) | 4.35 (3.76) | 2.45 (2.66) | 3.71 (3.80) | 4.26 (3.32) | 2.88 (3.13) |
| Diagnosis of HeFH | ||||||
| SB criteria | 29 (59.2) | 14 (56.0) | 22 (42.3) | 11 (40.7) | 43 (100) | 33 (100) |
| Genotyping prior to or during the study | 43 (87.8) | 19 (76.0) | 50 (96.2) | 26 (96.3) | 62 (83.8) | 76 (96.2) |
| Cardiovascular disease history in the family/cardiovascular risk factors | 48 (98.0) | 25 (100) | 51 (98.1) | 26 (96.3) | 73 (98.6) | 77 (97.5) |
| Never smoked | 49 (100) | 25 (100) | 51 (98.1) | 26 (96.3) | 74 (100) | 77 (97.5) |
| Baseline lipid parameters | ||||||
| LDL-C, mean (SD), mg/dL | 169.69 (46.74) | 175.29 (50.23) | 176.79 (53.93) | 176.57 (49.01) | 171.58 (47.68) | 176.71 (51.99) |
| Apolipoprotein B, mean (SD), mg/dL | 115.7 (24.5) | 115.2 (25.8) | 119.7 (29.3) | 118.4 (31.2) | 115.5 (24.8) | 119.2 (29.8) |
| Non–HDL-C, mean (SD), mg/dL | 186.75 (48.29) | 191.61 (50.64) | 197.16 (55.14) | 195.37 (53.98) | 188.39 (48.81) | 196.55 (54.41) |
| Total cholesterol, mean (SD), mg/dL | 234.69 (49.65) | 242.88 (59.06) | 246.98 (57.66) | 249.74 (53.49) | 237.46 (52.75) | 247.92 (55.94) |
| HDL-C, mean (SD), mg/dL | 48.24 (10.61) | 51.16 (13.07) | 49.62 (11.06) | 54.74 (14.20) | 49.23 (11.50) | 51.37 (12.38) |
| HDL-C ≥40 mg/dL | 38 (77.6) | 22 (88.0) | 42 (80.8) | 23 (85.2) | 60 (81.1) | 65 (82.3) |
| Fasting triglyceride, median (IQR), mg/dL | 65.00 (54.00-89.38) | 68.14 (51.33-85.84) | 91.00 (72.57-110.62) | 82.00 (52.98-125.66) | 66.57 (51.33-89.38) | 87.17 (61.95-116.81) |
| Lipoprotein(a), median (IQR), mg/dL | 13.2 (2.0-44.1) | 22.0 (6.3-69.4) | 12.7 (5.6-35.0) | 17.8 (5.5-43.4) | 16.2 (5.2; 54.7) | 13.9 (5.5-40.9) |
| Free PCSK9, mean (SD), ng/mL | 188.4 (89.2) | 212.1 (79.9) | 168.0 (87.2) | 158.8 (76.5) | NA | NA |
| Baseline treatment | ||||||
| Statin intolerant | 2 (4.1) | 1 (4.0) | 5 (9.6) | 3 (11.1) | 3 (4.1) | 8 (10.1) |
| Ezetimibe | 2 (4.1) | 3 (12.0) | 12 (23.1) | 4 (14.8) | 5 (6.8) | 16 (20.3) |
| Any statin | 49 (100) | 24 (96.0) | 48 (92.3) | 24 (88.9) | 73 (98.6) | 72 (91.1) |
| Atorvastatin, mg | ||||||
| <10 | 2 (4.1) | 2 (8.0) | 3 (5.8) | 3 (11.1) | 4 (5.4) | 6 (7.6) |
| 10 | 22 (44.9) | 6 (24.0) | 6 (11.5) | 5 (18.5) | 28 (37.8) | 11 (13.9) |
| 20 | 6 (12.2) | 8 (32.0) | 8 (15.4) | 5 (18.5) | 14 (18.9) | 13 (16.5) |
| 40 | 0 | 0 | 13 (25.0) | 4 (14.8) | 0 | 17 (21.5) |
| 80 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other dose | 1 (2.0) | 0 | 1 (1.9) | 0 | 1 (1.4) | 1 (1.3) |
| Rosuvastatin, mg | ||||||
| <5 | 1 (2.0) | 0 | 0 | 0 | 1 (1.4) | 0 |
| 5 | 3 (6.1) | 0 | 2 (3.8) | 2 (7.4) | 3 (4.1) | 4 (5.1) |
| 10 | 6 (12.2) | 1 (4.0) | 4 (7.7) | 1 (3.7) | 7 (9.5) | 5 (6.3) |
| 20 | 0 | 2 (8.0) | 1 (1.9) | 1 (3.7) | 2 (2.7) | 2 (2.5) |
| 40 | 0 | 0 | 2 (3.8) | 0 | 0 | 2 (2.5) |
| Other dose | 0 | 1 (4.0) | 2 (3.8) | 1 (3.7) | 1 (1.4) | 4 (3.8) |
| Pravastatin, mg | ||||||
| <10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 1 (2.0) | 2 (8.0) | 1 (1.9) | 0 | 3 (4.1) | 1 (1.3) |
| 20 | 3 (6.1) | 1 (4.0) | 3 (5.8) | 1 (3.7) | 4 (5.4) | 4 (5.1) |
| 40 | 1 (2.0) | 0 | 0 | 0 | 1 (1.4) | 0 |
| >40 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other dose | 1 (2.0) | 0 | 0 | 0 | 1 (1.4) | 0 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; HeFH, familial heterozygous hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; Q2W, dosing every 2 weeks; Q4W, dosing every 4 weeks; SB, Simon Broome.
Race and ethnicity data were collected via questionnaire and reported because these data are required by the US Food and Drug Administration and the European Medicines Agency.
To preserve anonymity, racial categories with small numbers of patients have been grouped together: American Indian or Alaska Native, Asian, Black, Black and multiple races, Native Hawaiian or Other Pacific Islander, Native Hawaiian or Other Pacific Islander and White, and not reported.
Efficacy
Alirocumab was associated with LDL-C reductions from baseline at first assessment (week 8) of the double-blind period in both cohorts, with reductions maintained through week 24 (Figure 2). For the primary end point, alirocumab significantly reduced LDL-C compared with placebo in both dosing cohorts at week 24, with a least squares (LS) mean difference of −43.3% (97.5% CI, −56.0 to −30.7; P < .001) in Q2W and −33.8% (97.5% CI, −46.4 to −21.2; P < .001) in Q4W. The LDL-C reduction associated with alirocumab at week 24 was maintained across all subgroups of interest, including those defined by baseline weight, sex, age, and LDL-C concentration (eFigure 1 in Supplement 2).
Figure 2. Change in Low-Density Lipoprotein Cholesterol (LDL-C) From Baseline in the Intention-to-Treat Population.
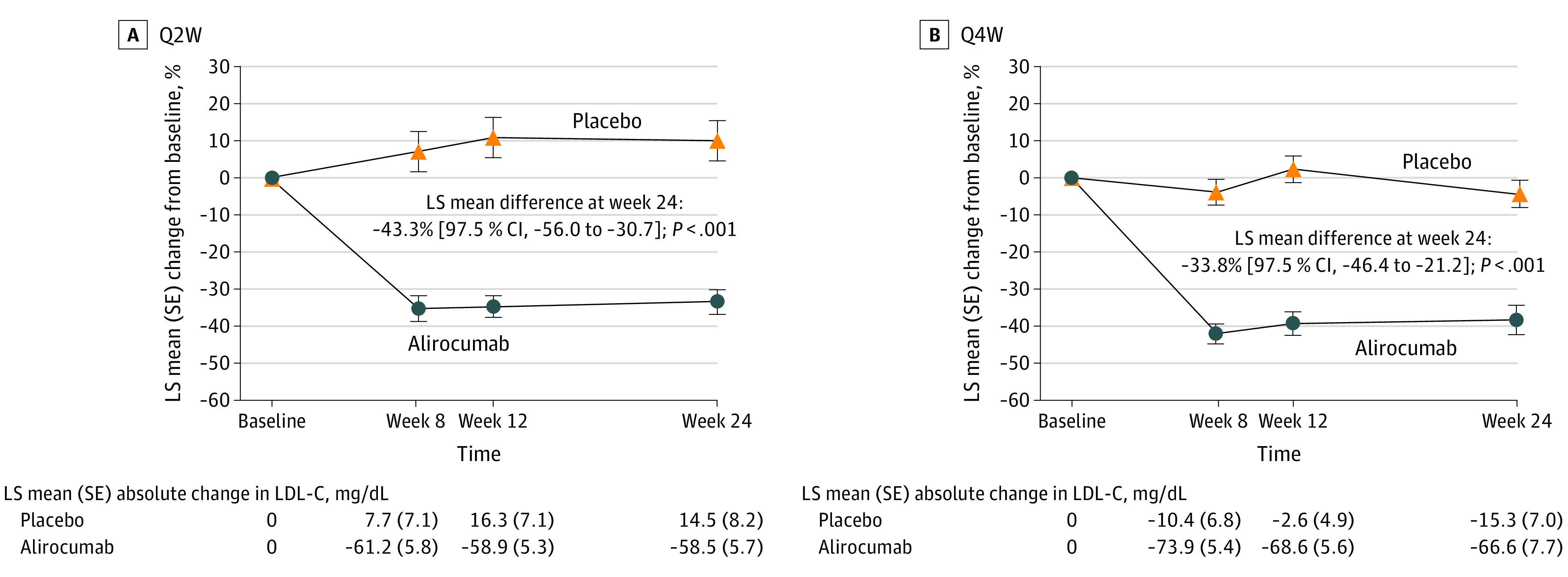
LS means and SEs taken from mixed-effect model with repeated-measures analysis. The model includes the fixed categorical effects of treatment group, stratum previous participation (yes or no) in the ODYSSEY KIDS study (except for Q4W cohort), baseline body weight (<50 or ≥50 kg), time point (week 8, week 12, and week 24), treatment-by–time point interaction, and strata-by–time point interaction, as well as the continuous fixed covariates of baseline LDL-C value and baseline LDL-C value-by–time point interaction. LS indicates least squares; Q2W, dosing once every 2 weeks; Q4W, dosing once every 4 weeks.
At baseline, free PCSK9 concentrations were similar across treatment groups (Table 1). At week 24, mean (SD; IQR) free PCSK9 was 17.4 (54.0; 0.0-0.0) ng/mL (−86.0% from baseline) for alirocumab Q2W and 19.0 (42.4; 0.0-0.0) ng/mL (−88.8%) for alirocumab Q4W, compared with 180.4 (66.5; 121.0-240.0) ng/mL (−5.5%) for placebo Q2W and 104.8 (52.4; 57.0-136.0) ng/mL (−17.1%) for placebo Q4W.
Alirocumab was associated with significant reductions vs placebo through hierarchical testing of the first 12 key secondary efficacy end points, including apo B, non-HDL-C, and total cholesterol. At week 24, there was a −15.2% (97.5% CI, −30.3 to −0.1) reduction in the adjusted mean difference of Lp(a) in the Q2W cohort and a −24.9% (97.5% CI, −44.4 to −5.4) reduction in Q4W (Table 2). At week 24, 38 patients (77.3%) receiving alirocumab in the Q2W cohort and 39 (76.3%) in Q4W achieved LDL-C less than 130 mg/dL, and 10 (21.6%) in Q2W and 17 (32.4%) in Q4W achieved LDL-C reduction of 50% or greater.
Table 2. Secondary Efficacy End Points in the Intention-to-Treat Population.
| Least squares mean difference in change from baseline: alirocumab vs placebo, % (97.5% CI) | ||||
|---|---|---|---|---|
| Q2W cohort (n = 74) | P value | Q4W cohort (n = 79) | P value | |
| Key secondary end points a | ||||
| LDL-C to week 12 | −45.5 (−56.3 to −34.7) | <.001 | −41.5 (−52.7 to −30.2) | <.001 |
| Apolipoprotein B to week 24 | −37.8 (−47.5 to −28.2) | <.001 | −30.7 (−42.0 to −19.4) | <.001 |
| Non–HDL-C to week 24 | −40.7 (−52.2 to −29.1) | <.001 | −31.9 (−44.1 to −19.7) | <.001 |
| Total cholesterol to week 24 | −30.8 (−39.8 to −21.9) | <.001 | −23.3 (−33.5 to −13.1) | <.001 |
| Apolipoprotein B to week 12 | −38.9 (−48.2 to −29.6) | <.001 | −32.8 (−42.8 to −22.7) | <.001 |
| Non–HDL-C to week 12 | −42.8 (−53.8 to −31.8) | <.001 | −37.5 (−47.9 to −27.0) | <.001 |
| Total cholesterol to week 12 | −32.7 (−41.3 to −24.2) | <.001 | −27.9 (−35.6 to −20.2) | <.001 |
| Combined estimates for odds ratio (97.5% CI): alirocumab vs placebo | ||||
| LDL-C <130 mg/dL at week 24b | 77.6 (6.3 to 960.0) | .001 | 14.9 (3.2 to 69.8) | <.001 |
| LDL-C <130 mg/dL at week 12b | 26.5 (4.0 to 174.8) | <.001 | 40.9 (5.7 to 290.9) | <.001 |
| LDL-C <110 mg/dL at week 24 | 52.7 (3.5 to 804.3) | .001 | 43.1 (3.7 to 498.6) | <.001 |
| LDL-C <110 mg/dL at week 12–LOCF | 41.3 (6.6 to I)c | <.001 | 104.8 (5.2 to 2095.9) | <.001 |
| Adjusted mean difference (97.5% CI) in change from baseline: alirocumab vs placebo, % | ||||
| Lipoprotein(a) to week 24 | −15.2 (−30.3 to −0.1) | .02 | −24.9 (−44.4 to −5.4) | .004 |
| Lipoprotein(a) to week 12b | −5.6 (−21.7 to 10.4) | .43 | −13.5 (−32.7 to 5.7) | .11 |
| HDL-C to week 24d | 6.4 (0.5 to 12.3) | NA | 4.4 (−3.6 to 12.5) | NA |
| Fasting triglyceride to week 24e | 4.3 (−19.1 to 27.6) | NA | −19.0 (−41.5 to 3.5) | NA |
| Apolipoprotein A1 to week 24e | 1.1 (−5.9 to 8.2) | NA | 8.9 (1.3 to 16.5) | NA |
| HDL-C to week 12d | 5.6 (−3.1 to 14.3) | NA | 7.5 (−1.7 to 16.6) | NA |
| Fasting triglyceride to week 12e | −8.7 (−28.8 to 11.4) | NA | −8.1 (−31.3 to 15.1) | NA |
| Apolipoprotein A1 to week 12d | −1.6 (−7.4 to 4.1) | NA | 5.7 (−2.7 to 14.1) | NA |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Q2W, dosing every 2 weeks; Q4W, dosing every 4 weeks; NA, not applicable.
Regarding secondary end points, the proportion of patients reaching 50% or greater reduction in LDL-C at week 24 was 21.6% in the alirocumab Q2W cohort (vs 0% in placebo) and 32.4% in the alirocumab Q4W cohort (vs 9.1% in placebo). At week 12, the proportion of patients reaching 50% or greater reduction in LDL-C was 25.2% in the alirocumab Q2W cohort (vs 0% in placebo) and 31.9% in the alirocumab Q4W cohort (vs 0.1% in placebo).
At the time this study was initiated, the pediatric LDL-C recommendation was <130 mg/dL. Continuous secondary efficacy end points anticipated to have a normal distribution were analyzed using the same approach as the primary end point; those anticipated to have a nonnormal distribution were analyzed using a multiple imputation approach followed by a robust regression model using M-estimation with treatment group and randomization strata as main effects and corresponding baseline value as covariate. Categorical secondary efficacy end points were analyzed using a multiple imputation approach followed by logistic regression model adjusting for treatment group as main effect and corresponding baseline value as covariate, stratified by randomization factors.
Exacts odds ratio vs placebo; hierarchical testing procedure did not establish significance.
Least squares mean difference.
Combined estimate for adjusted mean difference.
In the open-label period, LDL-C reductions were well maintained in patients who received alirocumab during the double-blind period, while those who switched from placebo to alirocumab had reductions in LDL-C at week 32 that were maintained through week 104. In the Q2W cohort, the LS mean (SD) change in LDL-C from baseline to week 104 was −26.3% (28.0) and −23.9% (33.5) in the Q4W cohort. At week 104, 45 (63.7%) in the Q2W cohort and 44 (59.6%) in the Q4W cohort achieved LDL-C less than 130 mg/dL, while 10 (13.8%) in Q2W and 15 (21.1%) in Q4W achieved ≥50% LDL-C reduction.
Safety
During the double-blind period, AEs occurred in 26 patients (53.1%) receiving alirocumab and 13 (52.0%) receiving placebo in the Q2W cohort and 26 (50.0%) and 16 (59.3%), respectively, in Q4W (Table 3). Most AEs were of mild or moderate intensity, with no relevant difference in distribution across the treatment groups. Treatment-related AEs were infrequent (11 [10.9%] for alirocumab vs 1 [1.9%] for placebo), and most were injection site reactions (3 [6.1%] for alirocumab Q2W and 2 [3.8%] for alirocumab Q4W). There were no clinically meaningful differences between alirocumab and placebo treatment in hematologic or liver parameters.
Table 3. Safety End Points in the Safety Population.
| Adverse event | No. (%) | |||
|---|---|---|---|---|
| Q2W cohort (n = 74) | Q4W cohort (n = 79) | |||
| Alirocumab (n = 49) | Placebo (n = 25) | Alirocumab (n = 52) | Placebo (n = 27) | |
| Any adverse eventa | 26.0 (53.1) | 13.0 (52.0) | 26.0 (50.0) | 16.0 (59.3) |
| Most common adverse eventsa | ||||
| Laryngitis | 0 | 0 | 1.0 (1.9) | 0 |
| Nasopharyngitis | 7.0 (14.3) | 2.0 (8.0) | 1.0 (1.9) | 2.0 (7.4) |
| Pharyngitis | 1.0 (2.0) | 0 | 0 | 1.0 (3.7) |
| Rhinitis | 1.0 (2.0) | 1.0 (4.0) | 1.0 (1.9) | 0 |
| Sinusitis | 1.0 (2.0) | 0 | 0 | 0 |
| Tonsillitis | 3.0 (6.1) | 1.0 (4.0) | 0 | 1.0 (3.7) |
| Upper respiratory tract infection | 3.0 (6.1) | 3.0 (12.0) | 3.0 (5.8) | 3.0 (11.1) |
| Headache | 3.0 (6.1) | 2.0 (8.0) | 4.0 (7.0) | 1.0 (3.7) |
| Migraine | 0 | 2.0 (8.0) | 0 | 0 |
| Adverse events of special interest | ||||
| Injection site reaction | 3.0 (6.1) | 0 | 2.0 (3.8) | 0 |
| General allergic reaction | 0 | 0 | 2.0 (3.8) | 0 |
| Disturbance in attention and memory | 0 | 0 | 1.0 (1.9) | 0 |
| Hypoesthesia | 0 | 0 | 1.0 (1.9) | 0 |
| Adverse events leading to discontinuation | ||||
| Disturbance in attention and memory | 0 | 0 | 1.0 (1.9) | 0 |
| Syncope | 0 | 0 | 1.0 (1.9) | 0 |
| Serious adverse event | ||||
| Appendicitis | 0 | 1.0 (4.0) | 0 | 0 |
| Depression | 1.0 (2.0) | 0 | 0 | 0 |
| Abdominal hernia | 1.0 (2.0) | 0 | 0 | 0 |
| Abdominal pain | 1.0 (2.0) | 0 | 0 | 0 |
| Sympathetic posterior cervical syndrome | 1.0 (2.0) | 0 | 0 | 0 |
| Syncope | 0 | 0 | 2.0 (3.8) | 0 |
| Noncardiac chest pain | 0 | 0 | 0 | 1.0 (3.7) |
| Treatment-related adverse event | 4.0 (8.2) | 1.0 (4.0) | 7.0 (13.5) | 0 |
| Developmental parameters | ||||
| Height change from baseline at week 24, mean (SD), cm | 2.73 (4.15) | 2.13 (2.28) | 1.68 (2.11) | 1.75 (1.27) |
| Weight change from baseline at week 24, mean (SD), kg | 1.71 (2.47) | 1.89 (2.91) | 1.66 (3.24) | 2.15 (2.86) |
| Cogstate Battery test change from baseline at week 24, mean (SD) | ||||
| Detection test | −0.05 (0.07) | −0.04 (0.13) | −0.04 (0.08) | −0.04 (0.06) |
| Identification test | −0.05 (0.07) | −0.04 (0.08) | −0.05 (0.08) | −0.02 (0.13) |
| One Card Learning test | 0.03 (0.12) | −0.02 (0.24) | −0.06 (0.11) | 0.04 (0.11) |
| Groton Maze Learning test | 4.04 (14.57) | −13.13 (29.83) | −0.31 (24.75) | 5.19 (16.37) |
| Composite score | −0.313 (0.444) | −0.403 (1.008) | −0.136 (0.637) | −0.218 (0.501) |
| Tanner stage, boys | ||||
| Prepubescent | ||||
| Baseline | 4 (21.1) | 1 (5.9) | 0 | 5 (41.7) |
| Week 24 | 3 (17.6) | 0 | 0 | 1 (9.1) |
| Pubescent | ||||
| Baseline | 13 (68.4) | 13 (76.5) | 14 (77.8) | 4 (33.3) |
| Week 24 | 11 (64.7) | 13 (76.5) | 12 (70.6) | 7 (63.6) |
| Postpubescent | ||||
| Baseline | 2 (10.5) | 3 (17.6) | 4 (22.2) | 3 (25.0) |
| Week 24 | 3 (17.6) | 4 (23.5) | 5 (29.4) | 3 (27.3) |
| Tanner stage, girls | ||||
| Prepubescent | ||||
| Baseline | 4 (13.3) | 1 (12.5) | 7 (20.6) | 1 (6.7) |
| Week 24 | 4 (13.3) | 1 (12.5) | 2 (7.4) | 1 (8.3) |
| Pubescent | ||||
| Baseline | 16 (53.3) | 6 (75.0) | 13 (38.2) | 8 (53.3) |
| Week 24 | 15 (53.6) | 5 (62.5) | 16 (59.3) | 6 (50.0) |
| Postpubescent | ||||
| Baseline | 10 (33.3) | 1 (12.5) | 14 (41.2) | 6 (40.0) |
| Week 24 | 9 (32.1) | 2 (25.0) | 9 (33.3) | 5 (41.7) |
| hs-CRP, mean (SD), mg/L | ||||
| Baseline | 2.4 (7.9) | 0.6 (0.8) | 1.7 (3.4) | 0.4 (0.3) |
| Week 24 | 1.1 (1.6) | 1.2 (2.1) | 1.8 (4.6) | 0.6 (0.6) |
Abbreviations: hs-CRP, high-sensitivity C-reactive protein; Q2W, dosing every 2 weeks; Q4W, dosing every 4 weeks.
Treatment-emergent adverse events with high-level term ≥5% in any treatment group in the safety population.
Serious AEs (SAEs) were reported by 4 patients (8.2%) receiving alirocumab vs 1 (4.0%) receiving placebo in the Q2W cohort and 2 patients (3.8%) receiving alirocumab vs 1 (3.7%) receiving placebo in Q4W. Most SAEs were reported as resolved or recovered at the end of the double-blind period. The 2 SAEs in patients receiving alirocumab Q4W (syncope for both) were considered treatment related by the investigator. In one of these patients, syncope led to treatment discontinuation, and an additional patient receiving alirocumab Q4W discontinued due to a nonserious disturbance in attention and memory. No patients discontinued alirocumab Q2W due to AEs.
Developmental parameters, including height, weight, Cogstate score, and Tanner stage, were similar between the alirocumab and placebo groups in both cohorts (Table 3). During the open-label period, the types and rates of AEs were consistent with those reported during the double-blind period. No patients developed antialirocumab antibodies with neutralizing status, and no deaths were reported.
Discussion
In this study of pediatric patients aged 8 to 17 years with HeFH, 2 dosing regimens of alirocumab were associated with significantly reduced LDL-C and other proatherogenic lipid parameters compared with placebo after 24 weeks of treatment. Reduction in LDL-C was achieved by first assessment after 8 weeks of alirocumab treatment for both dosing regimens, and improvements were maintained at week 24 and week 104. Alirocumab was well tolerated with no safety concerns.
As HeFH is an inherited disorder, early intervention to control LDL-C is crucial to mitigate disease burden later in life. Statins are the preferred treatment for pediatric HeFH management; statins effectively reduce LDL-C concentrations, cardiovascular disease risk, and cardiovascular events.3,4,5,16 In the short term, statins are effective and safe in pediatric patients, and there is some evidence that the risk of longer-term AEs is low.4 However, a proportion of patients remain unable to achieve LDL-C targets with statins alone.17,18,19 Additional LLTs are needed for pediatric patients who cannot meet LDL-C targets with statins alone. Recent therapeutic developments include anti-PCSK9 compounds, such as the monoclonal antibodies alirocumab8 and evolocumab9 and the small interfering ribonucleic acid, inclisiran.20
The efficacy of alirocumab reported here in pediatric patients with HeFH is generally consistent with that in adult patients, although the LDL-C reduction in the current study appeared to be lower than reductions previously reported in adults.10 In the Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy and Safety of SAR236553/REGN727 in Patients With Heterozygous Familial Hypercholesterolemia Not Adequately Controlled With Their Lipid-Modifying Therapy (ODYSSEY FH I) and Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of REGN727/SAR236553 in Patients With Heterozygous Familial Hypercholesterolemia Not Adequately Controlled With Their Lipid-Modifying Therapy (FH II),10 adults with inadequately controlled HeFH receiving alirocumab had −57.9% and −51.4% placebo-adjusted reductions in LDL-C from baseline to week 24. In the current study, the placebo-adjusted reduction from baseline to week 24 was −43.3% in the Q2W cohort and −33.8% in Q4W. Similar lipid-lowering disparities between adults and pediatric patients with HeFH have been observed with evolocumab, indicated in adult21,22 and pediatric HeFH.9 In the phase 3 Double-blind, Randomized, Placebo-controlled, Multicenter Study to Evaluate Safety, Tolerability and Efficacy of AMG 145 on LDL-C in Subjects With Heterozygous Familial Hypercholesterolemia (RUTHERFORD-2)22 of adults with HeFH, absolute LDL-C change at week 12 was −59.2% and −61.3% for Q2W and Q4W dosing, whereas in the phase 3 Double-blind, Randomized, Multicenter, Placebo-Controlled Study to Characterize the Efficacy, Safety, and Tolerability of 24 Weeks of Evolocumab for LDL-C Reduction in Pediatric Subjects 10 to 17 Years of Age With HeFH (HAUSER)9 of pediatric patients the placebo-adjusted LDL-C change was −38.3% at week 24 with evolocumab Q4W (absolute reduction, −44.5%). During the open-label extension of HAUSER, this reduction was maintained, at week 80 the mean percentage change in LDL-C was −35.3%.23 It is unclear if there are clinical factors that might explain this apparent difference in response to PCSK9 inhibitors between pediatric and adult patients with HeFH. Concentrations of free PCSK9 can differ between pediatric and adult patients.24 However, it has been shown that free PCSK9 concentrations do not appear to predict alirocumab response8; therefore, it is unlikely that this explains the lipid-lowering disparity observed between adult and pediatric populations. Others have suggested that differences in patient populations related to ezetimibe intolerance may be associated with alirocumab response12; however, there was relatively low use of ezetimibe in this study compared with FH I and FH II (56.0% and 67.1%, respectively).10 Regardless, comparisons across clinical trials should be considered cautiously given differences in study design and patient populations, particularly given the differences in treatment targets and up-titration thresholds between adult and pediatric patients.9,10,21,22
Pediatric guidelines recommend an LDL-C goal of less than 130 mg/dL or a reduction of 50% or more.7,25 In this study, more than75% of participants achieved LDL-C less than 130 mg/dL and nearly one-third achieved 50% or greater reduction with alirocumab across both dosing cohorts. Additionally, significant improvements were observed with alirocumab in other lipid parameters, including a significant reduction in Lp(a) at week 24. The HAUSER study showed a modest, nonstatistically significant reduction in Lp(a) with evolocumab.9 Additionally, significant reductions were seen at weeks 12 and 24 in both non–HDL-C and apo B, established biomarkers of cardiovascular risk.26,27
Alirocumab was generally well tolerated, with a safety profile consistent with previous studies in pediatric and adult patients with HeFH.8,10 There were 2 incidences of syncope, 1 of which led to discontinuation. Both cognitive and physical development were consistent with those expected for this age group, in line with previous developmental progression of pediatric patients in HAUSER,9,23 and neurocognitive AEs were rare and did not appear to differ from placebo.
Limitations
The strengths of this study include the double-blind, randomized trial design with minor eligibility restrictions. While the sample size was relatively small, adequate power for statistical analysis was provided and was similar to other studies investigating lipid-lowering therapies in pediatric populations with familial hypercholesterolemia.4,9,21 Most patients were White, potentially limiting generalizability. However, race was not a significant covariate in an analysis of alirocumab efficacy in adult patients,28 so it is unlikely that race would impact efficacy in pediatric patients. Safety is a key factor for adherence to statin therapy in pediatric patients, and it is also known that increasing statin dose has limited additional efficacy. Therefore, although most patients were not receiving maximal approved statin doses nor using ezetimibe, the study population reflects that of specialized centers treating pediatric patients with HeFH.9,18,21 It may be noted that there was a difference between LDL-C lowering with alirocumab relative to placebo in the 2 cohorts, likely driven by the LDL-C increase for placebo in Q2W vs a decrease in Q4W at week 24. However, no direct comparisons can be made between dosing cohorts due to sequential enrollment of the Q2W and Q4W cohorts. Although reduction in LDL-C with alirocumab appeared to be moderate during the open-label period, this was likely due to dose adjustments being directed by investigators rather than required per study protocol. Furthermore, potential variability in protocol adherence or deviations is inherent to performing a study using different sites.
Conclusions
In this trial, 2 dosing regimens of alirocumab significantly reduced LDL-C in pediatric patients with HeFH inadequately controlled with statins, with continued efficacy over 2 years of treatment. Both regimens were generally well tolerated and consistent with previous studies in pediatric and adult patients with HeFH. Alirocumab presents a suitable adjunct option to stable LLT in pediatric patients as young as 8 years old with HeFH.
Amended study protocol and statistical analysis plan
eFigure. Percent change from baseline in LDL-C at Week 24 for subgroup analyses in the a) Q2W and b) Q4W cohorts (ITT population)
eTable 1. Patient disposition per country
eTable 2. Safety end points during the open-label period
Data sharing statement
References
- 1.Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol. 2020;75(20):2553-2566. doi: 10.1016/j.jacc.2020.03.057 [DOI] [PubMed] [Google Scholar]
- 2.Sharifi M, Rakhit RD, Humphries SE, Nair D. Cardiovascular risk stratification in familial hypercholesterolaemia. Heart. 2016;102(13):1003-1008. doi: 10.1136/heartjnl-2015-308845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturm AC, Knowles JW, Gidding SS, et al. ; Convened by the Familial Hypercholesterolemia Foundation . Clinical genetic testing for familial hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2018;72(6):662-680. doi: 10.1016/j.jacc.2018.05.044 [DOI] [PubMed] [Google Scholar]
- 4.Luirink IK, Wiegman A, Kusters DM, et al. 20-Year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019;381(16):1547-1556. doi: 10.1056/NEJMoa1816454 [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209. doi: 10.1016/j.jacc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5(Suppl 5):S213-256. doi: 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiegman A, Gidding SS, Watts GF, et al. ; European Atherosclerosis Society Consensus Panel . Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36(36):2425-2437. doi: 10.1093/eurheartj/ehv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels S, Caprio S, Chaudhari U, et al. PCSK9 inhibition with alirocumab in pediatric patients with heterozygous familial hypercholesterolemia: the ODYSSEY KIDS study. J Clin Lipidol. 2020;14(3):322-330.e5. doi: 10.1016/j.jacl.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 9.Santos RD, Ruzza A, Hovingh GK, et al. ; HAUSER-RCT Investigators . Evolocumab in pediatric heterozygous familial hypercholesterolemia. N Engl J Med. 2020;383(14):1317-1327. doi: 10.1056/NEJMoa2019910 [DOI] [PubMed] [Google Scholar]
- 10.Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36(43):2996-3003. doi: 10.1093/eurheartj/ehv370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth EM, Kastelein JJP, Cannon CP, et al. Pharmacodynamic relationship between PCSK9, alirocumab, and LDL-C lowering in the ODYSSEY CHOICE I trial. J Clin Lipidol. 2020;14(5):707-719. doi: 10.1016/j.jacl.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg HN, Rader DJ, Raal FJ, et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc Drugs Ther. 2016;30(5):473-483. doi: 10.1007/s10557-016-6685-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regeneron. Praluent (alirocumab) injection (prescribing information). Accessed July 25, 2023. https://www.regeneron.com/downloads/praluent_pi.pdf
- 14.Sanofi. Praluent, SmPC. Accessed July 25, 2023. https://products.sanofi.us/praluent/praluent.pdf
- 15.Al-Rasadi K, Al-Waili K, Al-Sabti HA, et al. Criteria for diagnosis of familial hypercholesterolemia: a comprehensive analysis of the different guidelines, appraising their suitability in the Omani Arab Population. Oman Med J. 2014;29(2):85-91. doi: 10.5001/omj.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masson W, Corral P, Barbagelata L, et al. Reduction of cardiovascular events with the use of lipid-lowering medication in patients with familial hypercholesterolemia or severe primary hypercholesterolemia: a systematic review. J Clin Lipidol. 2022;16(5):562-573. doi: 10.1016/j.jacl.2022.07.004 [DOI] [PubMed] [Google Scholar]
- 17.de Ferranti SD, Shrader P, Linton MF, et al. Children with heterozygous familial hypercholesterolemia in the United States: data from the Cascade Screening for Awareness and Detection-FH Registry. J Pediatr. 2021;229:70-77. doi: 10.1016/j.jpeds.2020.09.042 [DOI] [PubMed] [Google Scholar]
- 18.Ramaswami U, Futema M, Bogsrud MP, et al. Comparison of the characteristics at diagnosis and treatment of children with heterozygous familial hypercholesterolaemia (FH) from eight European countries. Atherosclerosis. 2020;292:178-187. doi: 10.1016/j.atherosclerosis.2019.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogsrud MP, Langslet G, Wium C, Johansen D, Svilaas A, Holven KB. Treatment goal attainment in children with familial hypercholesterolemia: a cohort study of 302 children in Norway. J Clin Lipidol. 2018;12(2):375-382. doi: 10.1016/j.jacl.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 20.Reijman MD, Schweizer A, Peterson ALH, et al. Rationale and design of two trials assessing the efficacy, safety, and tolerability of inclisiran in adolescents with homozygous and heterozygous familial hypercholesterolaemia. Eur J Prev Cardiol. 2022;29(9):1361-1368. doi: 10.1093/eurjpc/zwac025 [DOI] [PubMed] [Google Scholar]
- 21.Santos RD, Stein EA, Hovingh GK, et al. Long-term evolocumab in patients with familial hypercholesterolemia. J Am Coll Cardiol. 2020;75(6):565-574. doi: 10.1016/j.jacc.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 22.Raal FJ, Stein EA, Dufour R, et al. ; RUTHERFORD-2 Investigators . PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331-340. doi: 10.1016/S0140-6736(14)61399-4 [DOI] [PubMed] [Google Scholar]
- 23.Santos RD, Ruzza A, Hovingh GK, et al. Paediatric patients with heterozygous familial hypercholesterolaemia treated with evolocumab for 80 weeks (HAUSER-OLE): a single-arm, multicentre, open-label extension of HAUSER-RCT. Lancet Diabetes Endocrinol. 2022;10(10):732-740. doi: 10.1016/S2213-8587(22)00221-2 [DOI] [PubMed] [Google Scholar]
- 24.Burke AC, Dron JS, Hegele RA, Huff MW. PCSK9: regulation and target for drug development for dyslipidemia. Annu Rev Pharmacol Toxicol. 2017;57(1):223-244. doi: 10.1146/annurev-pharmtox-010716-104944 [DOI] [PubMed] [Google Scholar]
- 25.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 26.Glavinovic T, Thanassoulis G, de Graaf J, Couture P, Hegele RA, Sniderman AD. Physiological bases for the superiority of apolipoprotein B over low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol as a marker of cardiovascular risk. J Am Heart Assoc. 2022;11(20):e025858. doi: 10.1161/JAHA.122.025858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novartis Pharmaceuticals . Study to evaluate efficacy and safety of inclisiran in adolescents with heterozygous familial hypercholesterolemia (ORION-16). Updated November 27, 2023. Accessed December 21, 2024. https://clinicaltrials.gov/study/NCT04652726
- 28.Robinson JG, Farnier M, Krempf M, et al. ; ODYSSEY LONG TERM Investigators . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489-1499. doi: 10.1056/NEJMoa1501031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amended study protocol and statistical analysis plan
eFigure. Percent change from baseline in LDL-C at Week 24 for subgroup analyses in the a) Q2W and b) Q4W cohorts (ITT population)
eTable 1. Patient disposition per country
eTable 2. Safety end points during the open-label period
Data sharing statement


