Abstract
Following rapid growth of pediatric antipsychotic prescribing in the early 2000s, especially in the Medicaid population, concerns regarding safety and appropriateness of such prescribing increased. Many states implemented policy and educational initiatives aimed at safer and more judicious antipsychotic use. Antipsychotic use leveled off in the late 2000s, but there have not been recent national estimates of trends in antipsychotic use in children enrolled in Medicaid, and it is unclear how use varied by race and ethnicity. This study observed a sizeable decline in antipsychotic use among children aged 2–17 between 2008–2016. While the magnitude of change varied, declines were observed across foster care status and age, sex, and racial and ethnic groups studied. The proportion of children with an antipsychotic prescription who received any diagnosis associated with an FDA-approved pediatric indication increased from 38%(2008) to 45%(2016), which may indicate a trend toward more-judicious prescribing.
Keywords: Antipsychotics, children, adolescents, trend, Medicaid, racial disparities
Introduction
Selected antipsychotic medications are approved by the US Food and Drug Administration (FDA) for treatment of schizophrenia, irritability associated with autism, Tourette’s disorder, and bipolar disorder in children. Indications for use vary by age(1) and antipsychotics are also used off-label for unapproved indications and ages. Antipsychotics should be prescribed with caution as antipsychotic treatment is associated with potentially serious side effects, including type 2 diabetes, cardiometabolic effects, and unexpected death.(2, 3, 4, 5)
Antipsychotic use in US children increased sharply in the early 2000s, corresponding with rising off-label use in children with ADHD, conduct disorders, and mood disorders(6, 7, 8). Several states enacted policies to address potential inappropriate use including prior authorization restrictions, secondary review by peers, and population-level monitoring of antipsychotic prescription utilization.(9)
Subsequently, a 20-state analysis found growth in pediatric antipsychotic use in Medicaid to level off from 2008 through 2010.(6) Declines in antipsychotic use past 2010 have been observed in privately insured children(10, 11) and samples of Medicaid-enrolled children(12), including children in foster care.(13) However, we are unaware of published national analyses of antipsychotic trends among Medicaid-enrolled children extending into the 2010s. Further, while prior research found US antipsychotic use to be more prevalent in White children compared to Black, Hispanic, and other racial/ethnic groups,(14) there is limited research on whether trends are similar across racial/ethnic groups.
We provide updated estimates of trends in antipsychotic prescribing from 2008 to 2016 among US children enrolled in Medicaid and evaluate trends by race/ethnicity and foster care status.
Methods
Our analysis used 2008–2016 Medicaid administrative claims data from 45 states drawn from the Medicaid Analytic eXtract (MAX) and Transformed Medicaid Statistical Information System (T-MSIS) Analytic Files (TAF), inclusive of the most recently available data at the time of analysis. We used information on eligibility and enrollment, demographics, inpatient and outpatient services, and dispensed pharmacy prescriptions. For each study year, we included children aged 2–17 with full calendar year enrollment in Medicaid. We excluded children with dual Medicaid-Medicare eligibility or in long-term care facilities during the calendar year.
Antipsychotic use was defined as at least one prescription during the calendar year for a first or second-generation antipsychotic. Race/ethnicity, from enrollment files, was classified into mutually exclusive groups: White, non-Hispanic; Black, non-Hispanic; Asian, non-Hispanic (hereafter White, Black, and Asian, respectively); Hispanic, all races; other or multiple races, non-Hispanic; unknown. Foster care status was based on Medicaid eligibility in December of that year.
Among youth with an antipsychotic prescription fill, we examined psychiatric diagnoses during that calendar year. An indicator for a diagnosis with an FDA approved indication (regardless of antipsychotic agent and age) was included. Diagnoses were classified into hierarchical, mutually exclusive categories, ordered by estimated level of evidence for antipsychotic treatment (Appendix exhibit A).(6, 11, 15) Children were assigned to the highest-listed diagnostic group in which they received a diagnostic (ICD-9-CM or ICD-10-CM) code during that year.
Analysis.
The annual proportion of children with antipsychotic use was calculated by dividing the number of eligible enrollees filling an antipsychotic prescription during the year by all eligible enrollees during that year. When calculating annual proportions, we pooled data from all 45 states. Statistical significance trend tests were not performed, given size of full Medicaid population.
Annual antipsychotic use proportions were stratified by foster care status, age, sex, and race/ethnicity. The primary stratification by race/ethnicity was limited to 26 states identified by the Centers for Medicare and Medicaid Services’ DQ Atlas as having low or medium concern for quality of race/ethnicity data in 2016.(16) Two sensitivity analyses on race/ethnicity stratification included: 1) More-restrictive analysis excluding 8 states missing >10% race/ethnicity data any year from 2008–2016; 2) all 45 states (Appendix exhibit B)(15). In post-hoc analyses, we examined trends by sex and race and ethnicity (White, Black, Hispanic) in children ages 13–17 years, given that the largest overall absolute declines were observed in this age group.
We analyzed: 1) diagnoses among antipsychotic users and 2) antipsychotic use within each diagnostic category. 1) We described psychiatric diagnoses among antipsychotic-treated children at the beginning (2008) and end (2016) of our timeframe (2016) and at an intermediary point prior to Medicaid expansion (2013). For a sensitivity analysis, all mental health diagnoses in the calendar year were described (Appendix Exhibit F)(15). 2) We estimated trends in antipsychotic use by mental health diagnosis. For example, among children enrolled in Medicaid with an attention-deficit/hyperactivity disorder (ADHD) diagnosis during that calendar year (denominator), we estimated the proportion with antipsychotic use that calendar year (numerator).
Limitations.
Observed trends in antipsychotic use may be influenced by shifts in the underlying enrolled population such as those following Medicaid expansion. Data completeness and quality may vary across states by managed care organizations and fee-for-service Medicaid.(17) Our study population is limited to children with 12 months of continuous enrollment in Medicaid and reflects filled prescriptions, not ingested medication. Foster care status was determined based on Medicaid eligibility in December; however, status may have differed at the time of antipsychotic prescription. We examine five racial/ethnic groups and do not capture the diversity within each of these groups. For the category pervasive developmental disorder (PDD) and intellectual disabilities, we did not distinguish between these diagnoses. The hierarchical classification of diagnoses captures the highest-listed diagnosis in that calendar year and is a crude approximation of the indication for antipsychotic treatment. We do not have access to the clinical indication motivating the antipsychotic prescription. Additionally, the transitions from MAX to TAF data and from ICD-9-CM to ICD-10-CM codes may have influenced results. We evaluated trends through 2016, which covers the time the majority of initiatives were implemented; as more national data becomes available, future research can determine whether and when trends began to stabilize.
Results
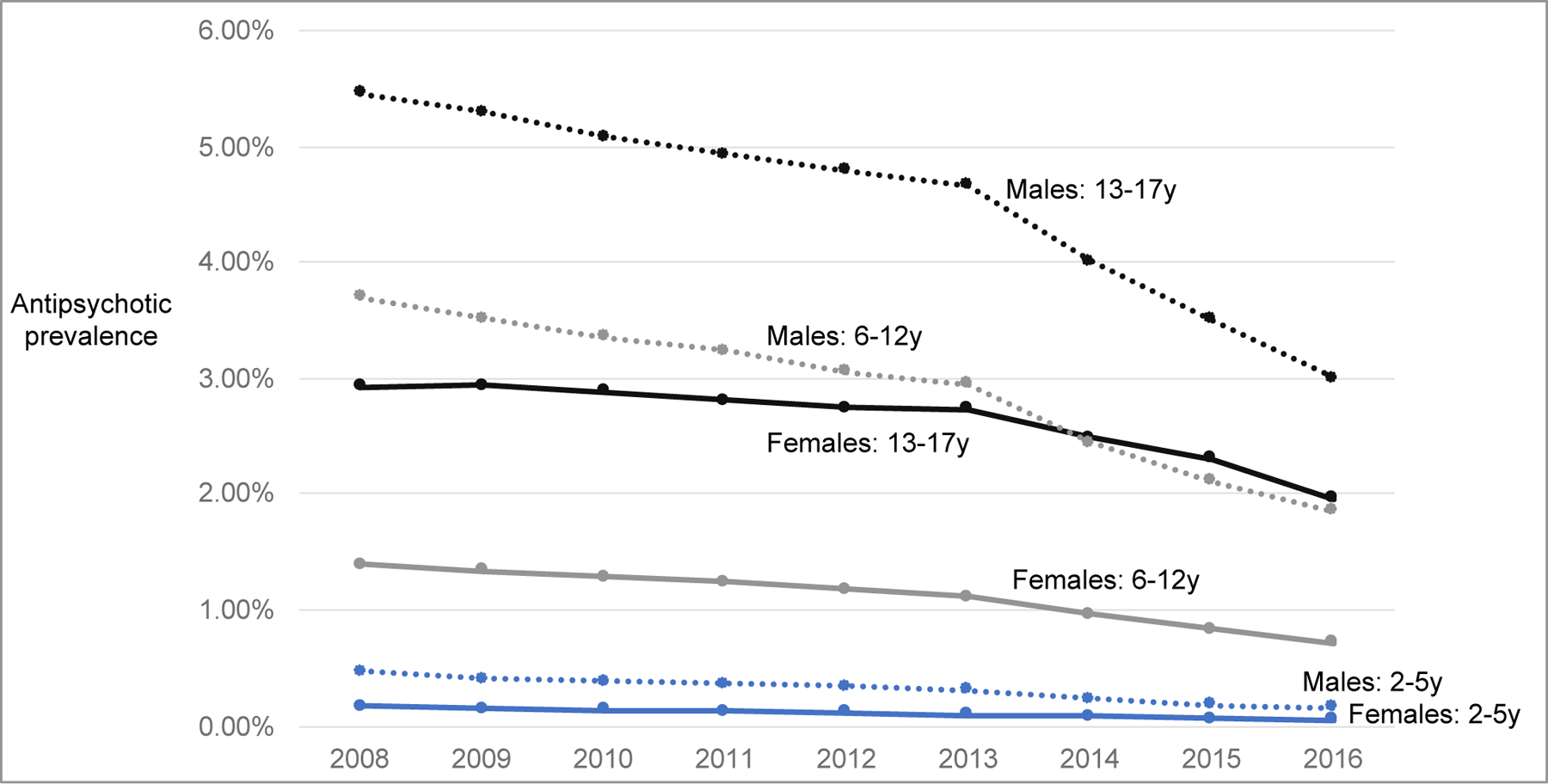
The annual antipsychotic use in Medicaid-enrolled children was 2.31% in 2008 and declined by 43%, to 1.32%, by 2016 (Exhibits 1 and 2). Antipsychotic fills declined in children in foster care and not in foster care, with use remaining substantially higher in foster care children (2016=7.09%) than others (2016=1.19%).
Exhibit 1. Antipsychotic use in children (2–17 years) enrolled in Medicaid from 2008 to 2016 by age and sex.
SOURCE [Authors’ analysis of data from CMS Medicaid data from 2008–2016]
Exhibit 2.
| Year | Children with antipsychotic use, Full sample | Children in foster carec | Children not in foster care | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| 2008 | 348,253 | 2.31 | 68,657 | 10.89 | 279,596 | 1.93 |
| 2009 | 366,072 | 2.20 | 66,578 | 10.86 | 299,494 | 1.87 |
| 2010 | 374,715 | 2.10 | 62,332 | 10.40 | 312,383 | 1.81 |
| 2011 | 385,357 | 2.04 | 58,972 | 10.32 | 326,385 | 1.78 |
| 2012 | 392,168 | 1.98 | 61,582 | 10.05 | 330,586 | 1.72 |
| 2013 | 395,424 | 1.95 | 61,382 | 9.81 | 334,042 | 1.70 |
| 2014 | 372,037 | 1.70 | 50,535 | 9.15 | 321,502 | 1.51 |
| 2015 | 358,904 | 1.52 | 35,389 | 8.88 | 323,515 | 1.39 |
| 2016 | 323,861 | 1.32 | 37,369 | 7.09 | 286,492 | 1.19 |
SOURCE [Authors’ analysis of data from CMS Medicaid data from 2008–2016]
Antipsychotics included first-generation antipsychotic medications: chlorpromazine, fluphenazine, haloperidol, loxapine, mesoridazine, molindone, perphenazine, pimozide, promazine, thioridazine, thiothixene, trifluoperazine, triflupromazine; and second-generation antipsychotic medications: aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, ziprasidone; Combination products olanzapine/fluoxetine and perphenazine/amitriptyline were included.
Medicaid data from 45 states (excluded states include Arizona, Delaware, Nevada, Oregon, Rhode Island, along with the District of Columbia); For 2015 Maryland data, we required only 11 months of enrollment given missing March 2015 monthly enrollment indicator
Foster care status was based on Medicaid eligibility in December of that calendar year
In 2016, antipsychotic use ranged from 3.00% in males aged 13–17 years to 0.05% for females aged 2–5 years (Exhibit 1). The largest relative decline from 2008 to 2016 was in children aged 2–5 years (females: −70%, 0.17% to 0.05%; males: −67%, 0.47% to 0.16%). The largest absolute decline was in males aged 13–17 years (−2.46%, 5.46% to 3.00%).
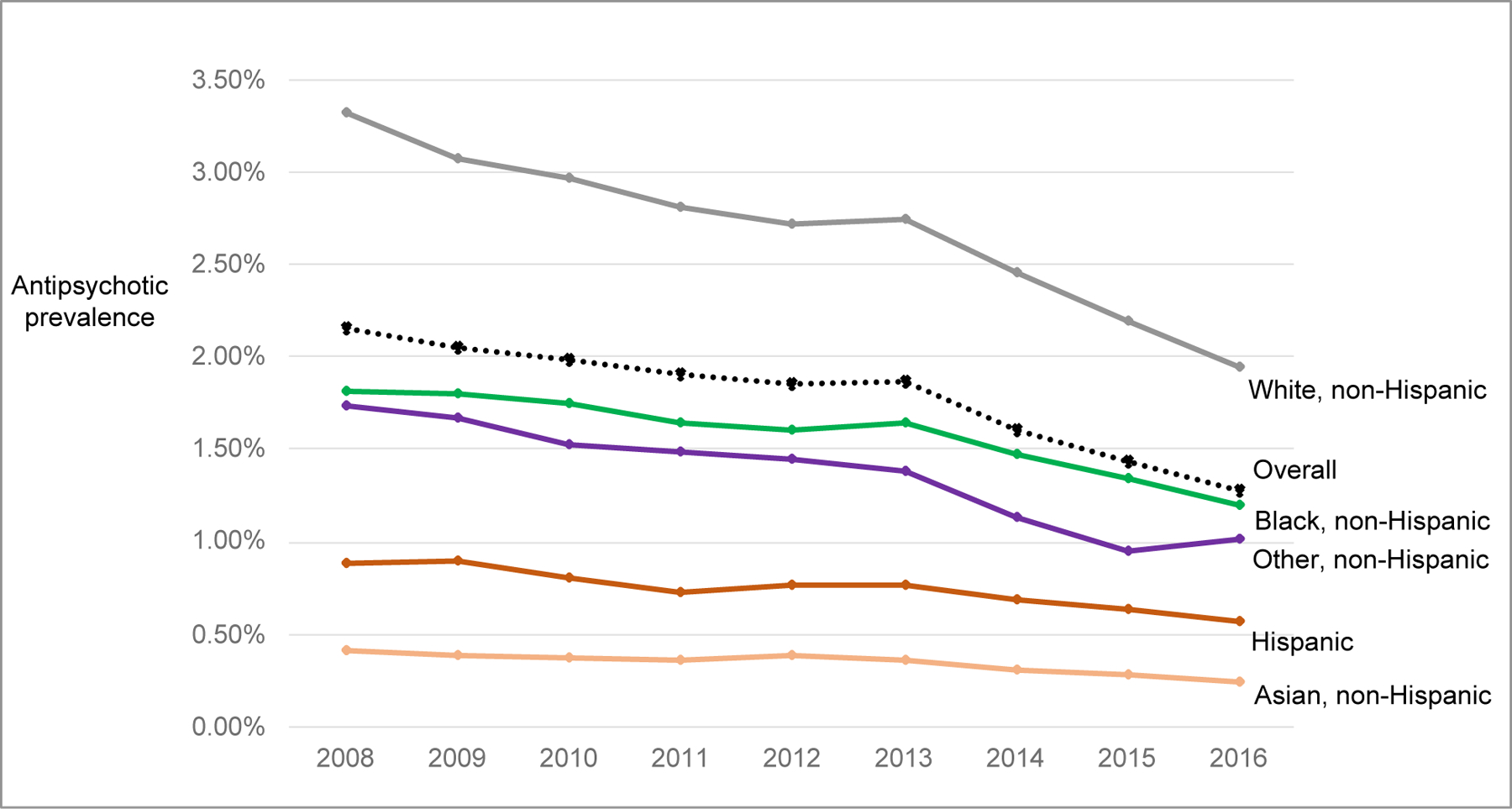
By race/ethnicity, antipsychotic use in 2016 ranged from 1.94% for White individuals to 0.24% in Asian individuals (Exhibit 3). There was a decline in antipsychotic use across all racial/ethnic groups from 2008–2016. Relative changes ranged from −34% (Black children) to −41% (White children). The largest absolute decline was observed in White children (−1.37%) vs. Black (−0.62%), Hispanic (−0.31%), and Asian (−0.17%) children. Overall trends by race and ethnicity were consistent in sensitivity analyses (Appendix exhibit B)(15). The decline in antipsychotic use among children aged 13–17 years was observed across sex and race/ethnicity strata; however, the magnitude of decline varied (Appendix Exhibit C)(15).
Exhibit 3. Antipsychotic use in children enrolled in Medicaid from 2008 to 2016 by race and ethnicitya,b.
SOURCE [Authors’ analysis of data from CMS Medicaid data from 2008–2016]
aRestricted to 26 states identified by the Centers for Medicare and Medicaid Services’ DQ Atlas as having low or medium concern for the quality of race/ethnicity data in 2016 data (AK, CA, FL, GA, ID, IN, IL, ME, MD, MI, MN, MT, NH, NJ, NM, NC, ND, OH, OK, PA, SD, TX, VT, VA, WA, WI); Appendix exhibit B displays results with 45 states and 18 states.
bThe proportion of children with unknown race and ethnicity ranged from 5–8% annually.
When examining diagnoses among antipsychotic users, the proportion of children with antipsychotic use who were diagnosed with an FDA-approved antipsychotic indication (for any age), increased from 38% in 2008 to 45% in 2016 (Exhibit 4). The proportion of antipsychotic-treated children without a psychiatric diagnosis recorded in claims declined (2008=10%, 2016=4%). There was an increase in the proportion of antipsychotic-treated children with PDD or intellectual disability diagnosis (2008=14%, 2016=23%) and slight decline in the proportion with ADHD diagnosis (2008=23%, 2016=20%), under the hierarchical diagnostic classification. Considering all mental health diagnoses in the calendar year, ADHD (2008=54.7%, 2016=61.8%) and conduct or disruptive behavior disorder (2008=32.0%, 2016=35.7%) remained the most common diagnoses in antipsychotic users (Appendix Exhibit D)(15).
Exhibit 4.
Characteristics and mental health diagnoses (hierarchical classification) in children enrolled in Medicaid with antipsychotic use in 2008, 2013, 2016
| Children with antipsychotic use |
||||||
|---|---|---|---|---|---|---|
| Patient characteristic | 2008 N=348,253 |
2013 N=395,424 |
2016 N=323,861 |
|||
| No. | % | No. | % | No. | % | |
| Female | 104,142 | 29.9 | 122,830 | 31.1 | 106,435 | 32.9 |
| Age, median (IQR) | 12 (9–15) | 12 (9–15) | 13 (10–15) | |||
| 2–5 years | 15,176 | 4.4 | 12,229 | 3.1 | 6,748 | 2.1 |
| 6–12 years | 165,879 | 47.6 | 187,766 | 47.5 | 147,651 | 45.6 |
| 13–17 years | 167,198 | 48.0 | 195,429 | 49.4 | 169,462 | 52.3 |
| Race, ethnicity | ||||||
| White, non-Hispanic | 192,587 | 55.3 | 200,169 | 50.6 | 144,839 | 44.7 |
| Black, non-Hispanic | 73,991 | 21.2 | 77,490 | 19.6 | 53,498 | 16.5 |
| Hispanic | 37,563 | 10.8 | 44,939 | 11.4 | 42,985 | 13.3 |
| Asian, non-Hispanic | 1,370 | 0.4 | 1,851 | 0.5 | 2,328 | 0.7 |
| Other non-Hispanic | 5,827 | 1.7 | 9,839 | 2.5 | 4,628 | 1.4 |
| Unknown | 36,915 | 10.6 | 61,136 | 15.5 | 75,583 | 23.3 |
| Any diagnosis associated with an FDA approved indication (1+ drug in class, any age <18) | 133,378 | 38.3 | 160,237 | 40.5 | 145,569 | 44.9 |
| Hierarchical classification (mutually exclusive groups)a | ||||||
| Schizophrenia, psychotic-related disorders | 18,995 | 5.5 | 29,711 | 7.5 | 26,663 | 8.2 |
| Pervasive developmental disorder; Intellectual disabilities | 49,880 | 14.3 | 73,327 | 18.5 | 74,419 | 23.0 |
| Bipolar disorder | 62,086 | 17.8 | 54,123 | 13.7 | 41,808 | 12.9 |
| Tic disorder | 2,417 | 0.7 | 3,076 | 0.8 | 2,679 | 0.8 |
| Conduct, disruptive behavior disorder, without ADHD | 23,448 | 6.7 | 24,480 | 6.2 | 15,856 | 4.9 |
| Conduct, disruptive behavior disorder, with comorbid ADHD | 43,011 | 12.4 | 61,800 | 15.6 | 50,312 | 15.5 |
| ADHD | 79,733 | 22.9 | 88,593 | 22.4 | 65,706 | 20.3 |
| Depression | 11,923 | 3.4 | 14,668 | 3.7 | 15,848 | 4.9 |
| PSTD | 3,318 | 1.0 | 3,432 | 0.9 | 2,980 | 0.9 |
| Anxiety disorder or obsessive-compulsive disorder | 2,956 | 0.8 | 4,078 | 1.0 | 3,993 | 1.2 |
| Adjustment-related disorders | 3,674 | 1.1 | 2,900 | 0.7 | 1,856 | 0.6 |
| Other/unspecified mood disorder | 4,638 | 1.3 | 7,251 | 1.8 | 3,963 | 1.2 |
| Sleep disorder | 887 | 0.3 | 886 | 0.2 | 729 | 0.2 |
| Other mental health diagnosis | 5,865 | 1.7 | 4,985 | 1.3 | 4,146 | 1.3 |
| No mental health diagnosis | 35,422 | 10.2 | 22,114 | 5.6 | 12,903 | 4.0 |
SOURCE [Authors’ analysis of data from CMS Medicaid data from 2008–2016]
Hierarchical classification results presented from 45 states; hierarchical classification results restricted to the 20 states matching Stephen Crystal et al (2016)(6) are available on request.
Antipsychotic use within each diagnostic category are displayed in Appendix Exhibit E(15). Use declined across all mental health diagnoses, remaining highest among children with a diagnosis of schizophrenia or bipolar disorder.
Discussion
Between 2008 and 2016, antipsychotic use declined among children enrolled in Medicaid across all age, sex, and racial and ethnic groups studied along with within diagnostic group. These declines occurred in the context of state-level policy changes aimed at promoting safe antipsychotic use in children.
By 2014, 31 states had implemented prior authorization policies related to pediatric antipsychotic use(18). The implementation of state-level policies was associated with declines in antipsychotic prescribing(10, 19). While the percent difference from 2008–2016 in the pooled 45-state Medicaid data was −1.0%, this difference ranged from −4.8% to +0.6% by state. This state-level heterogeneity in prescribing trends, while beyond the scope of the current paper, may be related to state prior authorization and other state-level policies. State-specific investigations are needed to evaluate the specific impact of local policy changes on within state trends.
In addition to prior authorizations, states implemented a number of other initiatives for judicious prescribing, building on the work of the 16-state Medicaid Medical Directors Learning Network/Rutgers CERTs Antipsychotics in Children consortium.(20) This consortium shared promising practices between states and developed and piloted measures of safe and judicious prescribing. Quality metrics for pediatric antipsychotic use were subsequently endorsed nationally(6, 21, 22). Efforts during the early 2010s to improve antipsychotic use included peer-review programs, state specialized interventions, educational initiatives, and multistate consortium(9, 23, 24, 25). Professional guidelines on antipsychotic prescribing(26) and management of maladaptive aggression,(27) often associated with antipsychotic prescribing, were published along with HEDIS quality measures for pediatric antipsychotic use(21). Other ongoing initiatives include programs such as the Safer Use of Antipsychotics in Youth (SUAY) clinical trial.(28)
The proportion of children with an antipsychotic prescription with any diagnosis associated with an FDA-approved pediatric indication increased from 38% (2008) to 45% (2016), potentially indicating prescribing improvements. Still, most antipsychotic use continued to be among children without such diagnoses. Antipsychotic use declined across all mental health diagnoses. The decline across diagnoses may be influenced by changes in diagnostic practices during the study period such as recording of comorbid diagnoses, severity at diagnosis, and time to diagnosis. These factors may alter the population with affected diagnoses over time and thus alter treatment rates. For example, while the proportion of children with a schizophrenia or psychotic-related diagnosis who received an antipsychotic prescription that calendar year declined from 2008 to 2016, more focused research is required before drawing conclusions concerning quality of care. In general, a higher threshold is used to defined schizophrenia disorders in Medicaid claims data;(29, 30) whereas our definition only required one ICD code and included the broad psychotic-related disorder category. There may also have been shifts in antipsychotic use in children with first-episode psychotic symptoms during the study period.(31, 32)
We observed higher antipsychotic use in White children compared to the other racial/ethnic groups. Similar differences have been previously described.(14, 33, 34) Factors contributing to lower antipsychotic use among non-white children are likely complex and structural, and may include differential access to mental healthcare, diagnostic and treatment biases, views on psychotropic treatment, and trust in the healthcare system(14, 35, 36). The role of under-treatment for children with indications for antipsychotic medications and over-prescription for White children remain key questions. Future research is needed to shed light on disparities by considering appropriateness of prescribing and quality metrics such as psychotherapy and metabolic monitoring.(21)
As described in prior research,(6, 13, 37, 38, 39) antipsychotic use is more prevalent in children in foster care. In California, despite declines in prescribing, lack of adherence to metabolic screening remained for children in foster care.(13) However, shortfalls in management of antipsychotic use are seen across all groups of children insured by Medicaid.(40) One concern is is polypharmacy among children receiving antipsychotics.(41, 42) While antipsychotic prevalence did decline during the study period, safety concerns with antipsychotic use remain.
While there was an annual decline in antipsychotic use, the decline was most pronounced from 2013–2014. Medicaid expansion may have contributed to the post-2013 decline. Among individuals newly eligible for Medicaid(43, 44) antipsychotic use rates may have been lower. We conducted a post-hoc trend analysis stratified by states with and without Medicaid expansion by 2016 (Appendix Exhibit F). In states with Medicaid expansion, the largest decline in antipsychotic use was from 2013–2014 and from 2015–2016; in states that did not expand, the largest declines were not limited to the time post-2013 (2009–2010 and 2014–2015). In addition to Medicaid expansion, the post-2013 decline may reflect growing concerns on metabolic risks of atypical antipsychotics for children(2) and increasing implementation of the above-noted initiatives(9, 21, 23, 24, 26, 27). For example, as an increasing number of states newly imposed prior authorization requirements, many states with existing requirements broadened the covered age range.(18)
Conclusions.
From 2008–2016, national prevalence of antipsychotic use in Medicaid children underwent a sizeable decline. While varying in magnitude, overall declines in antipsychotic use were observed across sex, age, foster care status, and racial and ethnic groups and antipsychotic prescribing became more focused on children with FDA approved indications. Nationwide trends likely reflect the convergence of multiple factors including evolving state oversight policies, clinical practice standards, professional guidance, and deployment of quality metrics for safe and judicious prescribing.
Supplementary Material
Acknowledgements.
The research was funded by AHRQ 5R01HS026001 (PI: Crystal) with support from NIH 5K01DA050769 (PI: Bushnell) and NCATS UL1TR003017. The founding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author disclosures. No disclosures to report.
ENDNOTES.
- 1.Education Medicaid Integrity Contractor for the CMS Medicaid Program Integrity Education (MPIE). Atypical Antipsychotic Medications: Use in Pediatric Patients Washington DC: 2015. Accessed April 5, 2023. [Available from: https://www.cms.gov/medicare-medicaid-coordination/fraud-prevention/medicaid-integrity-education/pharmacy-education-materials/downloads/atyp-antipsych-pediatric-factsheet11-14.pdf. [Google Scholar]
- 2.Galling B, Roldan A, Nielsen RE, Nielsen J, Gerhard T, Carbon M, et al. Type 2 Diabetes Mellitus in Youth Exposed to Antipsychotics: A Systematic Review and Meta-analysis. JAMA Psychiatry 2016;73(3):247–59. [DOI] [PubMed] [Google Scholar]
- 3.Ray WA, Stein CM, Murray KT, Fuchs DC, Patrick SW, Daugherty J, et al. Association of Antipsychotic Treatment With Risk of Unexpected Death Among Children and Youths. JAMA Psychiatry 2019;76(2):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libowitz MR, Nurmi EL. The Burden of Antipsychotic-Induced Weight Gain and Metabolic Syndrome in Children. Front Psychiatry 2021;12:623681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen KG, Correll CU, Ruda D, Klauber DG, Decara MS, Fagerlund B, et al. Cardiometabolic Adverse Effects and Its Predictors in Children and Adolescents With First-Episode Psychosis During Treatment With Quetiapine-Extended Release Versus Aripiprazole: 12-Week Results From the Tolerance and Effect of Antipsychotics in Children and Adolescents With Psychosis (TEA) Trial. J Am Acad Child Adolesc Psychiatry 2019;58(11):1062–78. [DOI] [PubMed] [Google Scholar]
- 6.Crystal S, Mackie T, Fenton MC, Amin S, Neese-Todd S, Olfson M, et al. Rapid Growth Of Antipsychotic Prescriptions For Children Who Are Publicly Insured Has Ceased, But Concerns Remain. Health Aff (Millwood) 2016;35(6):974–82. [DOI] [PubMed] [Google Scholar]
- 7.Patten SB, Waheed W, Bresee L. A review of pharmacoepidemiologic studies of antipsychotic use in children and adolescents. Can J Psychiatry 2012;57(12):717–21. [DOI] [PubMed] [Google Scholar]
- 8.Crystal S, Olfson M, Huang C, Pincus H, Gerhard T. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood) 2009;28(5):w770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackie TI, Hyde J, Palinkas LA, Niemi E, Leslie LK. Fostering Psychotropic Medication Oversight for Children in Foster Care: A National Examination of States’ Monitoring Mechanisms. Adm Policy Ment Health 2017;44(2):243–57. [DOI] [PubMed] [Google Scholar]
- 10.Spence O, Reeves G, dosReis S. Spillover effects of state medicaid antipsychotic prior authorization policies in US commercially insured youth. Pharmacoepidemiol Drug Saf.2020. [DOI] [PubMed]
- 11.Bushnell GA, Crystal S, Olfson M. Trends in Antipsychotic Medication Use in Young Privately Insured Children. J Am Acad Child Adolesc Psychiatry.2021;60(7):877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelsohn GA, Karpov I, Parthasarathy M, Hutchison SL, Castelnovo K, Ghuman J, et al. Trends in Antipsychotic Prescribing in Medicaid-Eligible Youth. J Am Acad Child Adolesc Psychiatry.2017;56(1):59–66. [DOI] [PubMed] [Google Scholar]
- 13.Nunes JC, Naccarato T, Stafford RS. Antipsychotics in the California Foster Care System: A 10-Year Analysis. J Child Adolesc Psychopharmacol.2022;32(7):400–7. [DOI] [PubMed] [Google Scholar]
- 14.Cataife G, Weinberg DA. Racial and Ethnic Differences in Antipsychotic Medication Use Among Children Enrolled in Medicaid. Psychiatr Serv.2015;66(9):946–51. [DOI] [PubMed] [Google Scholar]
- 15.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 16.U.S. Centers for Medicare and Medicaid Services. DQ Atlas. Accessed April 5, 2023. [Available from: https://www.medicaid.gov/dq-atlas/welcome.
- 17.Nguyen JK, Sanghavi P. A national assessment of legacy versus new generation Medicaid data. Health Serv Res.2022;57(4):944–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid I, Burcu M, Zito JM. Medicaid prior authorization policies for pediatric use of antipsychotic medications. JAMA.2015;313(9):966–8. [DOI] [PubMed] [Google Scholar]
- 19.Zito JM, Burcu M, McKean S, Warnock R, Kelman J. Pediatric Use of Antipsychotic Medications Before and After Medicaid Peer Review Implementation. JAMA Psychiatry.2018;75(1):100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medicaid Medical Directors Learning Network and Rutgers Center for Education and Research on Mental Health Therapeutics. Antipsychotic Medication Use in Medicaid Children and Adolescents: Report and Resource Guide from a 16-State Study. 2010. Accessed April 5, 2023. [Available from: https://ifh.rutgers.edu/wp-content/uploads/2019/09/MMDLN-Rutgers-AP_MED_USE_IN_CHILDREN...en_Report_and_Resource_Guide_Final.pdf]
- 21.National Committee for Quality Assurance (NCQA). HEDIS Measures for the Safe & Judicious Use of Antipsychotic Medications in Children and Adolescents. Accessed November 17, 2022. [Available from: https://www.ncqa.org/hedis/reports-and-research/national-collaborative-for-innovation-in-quality-measurement/hedis-measures-for-the-safe-judicious-use-of-antipsychotic-medications-in-children-and-adolescents/.
- 22.Centers for Medicare and Medicaid Services. Annual Reporting on the Quality of Care for Children in Medicaid and CHIP.2015. Accessed April 5, 2023. [Available from: https://www.medicaid.gov/medicaid/quality-of-care/performance-measurement/adult-and-child-health-care-quality-measures/childrens-health-care-quality-measures/index.html]
- 23.Akincigil A, Mackie TI, Cook S, Hilt RJ, Crystal S. Effectiveness of mandatory peer review to reduce antipsychotic prescriptions for Medicaid-insured children. Health Serv Res.2020;55(4):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackie TI, Cook S, Crystal S, Olfson M, Akincigil A. Antipsychotic Use Among Youth in Foster Care Enrolled in a Specialized Managed Care Organization Intervention. J Am Acad Child Adolesc Psychiatry.2020;59(1):166–76 e3. [DOI] [PubMed] [Google Scholar]
- 25.Finnerty M, Neese-Todd S, Bilder S, Olfson M, Crystal S. Best Practices: MEDNET: a multistate policy maker-researcher collaboration to improve prescribing practices. Psychiatr Serv.2014;65(11):1297–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Findling RL, Drury SS, Jensen PS, Rapoport JL, AACAP Committee on Quality Issues. Practice Parameter for the Use of Atypical Antipsychotic Medications in Children and Adolescents.2011. Accessed April 5, 2023. [Available from: https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf.]
- 27.Scotto Rosato N, Correll CU, Pappadopulos E, Chait A, Crystal S, Jensen PS, et al. Treatment of maladaptive aggression in youth: CERT guidelines II. Treatments and ongoing management. Pediatrics.2012;129(6):e1577–86. [DOI] [PubMed] [Google Scholar]
- 28.Penfold RB, Thompson EE, Hilt RJ, Kelleher KJ, Schwartz N, Beck A, et al. Safer use of antipsychotics in youth (SUAY) pragmatic trial protocol. Contemp Clin Trials.2020;99:106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry.2016;173(2):166–73. [DOI] [PubMed] [Google Scholar]
- 30.Olfson M, Gerhard T, Huang C, Lieberman JA, Bobo WV, Crystal S. Comparative effectiveness of second-generation antipsychotic medications in early-onset schizophrenia. Schizophr Bull.2012;38(4):845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute for Health and Care Excellence. Psychosis and schizophrenia in children and young people: recognition and management.2013. Accessed April 5, 2023. [Available from: https://www.nice.org.uk/guidance/cg155] [PubMed]
- 32.Morrison AP, Pyle M, Maughan D, Johns L, Freeman D, Broome MR, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry.2020;7(9):788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook BL, Carson NJ, Kafali EN, Valentine A, Rueda JD, Coe-Odess S, et al. Examining psychotropic medication use among youth in the U.S. by race/ethnicity and psychological impairment. Gen Hosp Psychiatry.2017;45:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heun-Johnson H, Menchine M, Axeen S, Lung K, Claudius I, Wright T, et al. Association Between Race/Ethnicity and Disparities in Health Care Use Before First-Episode Psychosis Among Privately Insured Young Patients. JAMA Psychiatry.2021;78(3):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med.2002;162(21):2458–63. [DOI] [PubMed] [Google Scholar]
- 36.DeLuca JS, Novacek DM, Adery LH, Herrera SN, Landa Y, Corcoran CM, et al. Equity in Mental Health Services for Youth at Clinical High Risk for Psychosis: Considering Marginalized Identities and Stressors. Evid Based Pract Child Adolesc Ment Health.2022;7(2):176–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanderwerker L, Akincigil A, Olfson M, Gerhard T, Neese-Todd S, Crystal S. Foster care, externalizing disorders, and antipsychotic use among Medicaid-enrolled youths. Psychiatr Serv.2014;65(10):1281–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan C, Greiner MV, Nause K, Shahabuddin Z, Beal SJ. Mental Health Diagnoses, Health Care Utilization, and Placement Stability on Antipsychotic Prescribing Among Foster Care Youth. Acad Pediatr.2022. [DOI] [PubMed]
- 39.Dosreis S, Yoon Y, Rubin DM, Riddle MA, Noll E, Rothbard A. Antipsychotic treatment among youth in foster care. Pediatrics.2011;128(6):e1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leckman-Westin E, Finnerty M, Scholle SH, Pritam R, Layman D, Kealey E, et al. Differences in Medicaid Antipsychotic Medication Measures Among Children with SSI, Foster Care, and Income-Based Aid. J Manag Care Spec Pharm.2018;24(3):238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohr WD, Jawad K, Feygin Y, Le J, Creel L, Pasquenza N, et al. Antipsychotic Medications for Low-Income Preschoolers: Long Duration and Psychotropic Medication Polypharmacy. Psychiatr Serv.2022;73(5):510–7. [DOI] [PubMed] [Google Scholar]
- 42.Olfson M, King M, Schoenbaum M. Treatment of Young People With Antipsychotic Medications in the United States. JAMA Psychiatry.2015;72(9):867–74. [DOI] [PubMed] [Google Scholar]
- 43.Patel H, Barnes J, Osazuwa-Peters N, Bierut LJ. Association of State Medicaid Expansion Status With Rates of Suicide Among US Adults. JAMA Netw Open.2022;5(6):e2217228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sommers BD, Blendon RJ, Orav EJ, Epstein AM. Changes in Utilization and Health Among Low-Income Adults After Medicaid Expansion or Expanded Private Insurance. JAMA Intern Med.2016;176(10):1501–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


