Abstract
Introduction
Low-dose computed tomography screening (LDCT) and lung nodule programs (LNP) promote early lung cancer detection, improve survival; Multidisciplinary Care Programs (MDC) promote guideline-concordant care. The impact of such program-based care on “real-world” lung cancer survival is unquantified. We evaluated outcomes of lung cancer care delivered through structured programs in a community health care system.
Methods
We conducted a cohort study linking institutional prospective observational LDCT, LNP and MDC databases with Tumor Registry of Baptist Cancer Center facilities. We categorized all patients diagnosed with lung cancer between 2011 and 2021 into program-based care versus non-program-based care cohorts. We compared patient characteristics, stage distribution, treatment modalities, survival and mortality in each pathway of care.
Results
Of 12,148 patients, 237, 1,165, 1,140 and 9,606 were diagnosed through the LDCT, LNP, MDC or no program, respectively; non-program-based care sequentially diminished from 96.3% to 66.5%, diagnosis through LDCT increased from 0.5% to 7.1%, LNP from 3.5% to 20.8%; and MDC alone decreased from a high of 12.8% in 2014 to 5.6% in 2021. Program-based care was associated with earlier stage (p < 0.001), higher surgical resection rates (p < 0.001), greater use of adjuvant therapy (p < 0.001), better aggregate and stage-stratified survival (p < 0.001), and lower all-cause and lung cancer-specific mortality (p < 0.001). Recipients of non-program-based care were considerably less likely to receive lung cancer treatment; results remained consistent when patients receiving no treatment were excluded.
Conclusions
Program-based care was associated with substantially better survival. Increasing access to program-based care should be explored as a matter of urgent public policy.
Keywords: Multidisciplinary Care, Early detection, Lung cancer screening, Lung nodule programs, Quality of care, Public health
Introduction
The aggregate 5-year survival rate of persons diagnosed with lung cancer in the United States (US) is approximately 23%.1 Most patients have regional or metastatic disease, for which treatment is more toxic, less effective and more expensive.1 The National Lung Screening Trial revealed that low-dose computer tomography (LDCT) scanning of healthy volunteers at high risk for lung cancer shifted lung cancer to earlier stage, improved survival, and reduced mortality.2 US lung cancer statistics already reveal an evolving redistribution to earlier stage since 2015, when LDCT lung cancer screening became a covered healthcare benefit.1, 2, 3 However, implementation barriers limit access to screening, especially for marginalized populations.4, 5, 6, 7, 8, 9, 10
Programs to promote guideline-concordant management of incidentally-detected lung nodules provide a complementary approach to early lung cancer detection.11 Such Lung Nodule Programs (LNP) are also associated with improved lung cancer survival.11,12 Multidisciplinary Care Programs (MDC) promote guideline-concordant lung cancer care and potentially improve survival.13,14 We previously reported that patients with lung cancer diagnosed through the early detection programs had considerably better survival than patients diagnosed through MDC.11 Accurate assessment of the value of early lung cancer detection programs in heterogeneous, ‘real-world’ populations requires comparison to those receiving non-program-based care.
We compared the outcomes and selected trends of outcomes of “program-based” lung cancer care through the early detection programs- LDCT, LNP- and MDC versus nonprogram-based care in a large community health care system serving a population with some of the heaviest US lung cancer burden. We sought to quantify the relative benefits of program-based care.
Materials and Methods
Population
The Baptist Memorial Health Care Corporation (BMHCC), a not-for-profit community health care system, serves a diverse population across 111-counties in Eastern Arkansas, Mississippi, Western Tennessee, Southwestern Kentucky, Southeastern Missouri and Northwestern Alabama. Forty-four percent of BMHCC’s service area are Delta Regional Authority counties, identified by the US congress as the most socio-economically disadvantaged, with the highest US per-capita lung cancer incidence and mortality rates.15,16
Data Sources
With BMHCC Institutional Review Board approval, including a waiver of the informed consent requirement for this low-risk observational study, we cross-linked the prospectively collected institutional LDCT, LNP and MDC databases with data from all BMHCC tumor registries.11,14,17 Implementation of the MDC began in 2011. Structured implementation the LDCT and LNP began in 2015 as part of the ongoing ‘Detecting Early Lung Cancer in the Mississippi Delta’ project. For this analysis, we used tumor registry data from 2011, when the MDC program commenced (and the initial National Lung Screening Trial results were published), to 2021 (the most recent year with complete vital statistics).
Program-Based Patient Categories
We categorized patients present in any of the BMHCC program databases as having received program-based care. We followed a mutual exclusivity hierarchy of LDCT > LNP > MDC for those present in multiple program databases.
Patients in the tumor registry who were absent from all three program databases were identified as non-program based care recipients. We defined the MDC cohort strictly as patients whose care had been prospectively reviewed in a structured Multidisciplinary Thoracic Oncology Conference which we implemented with rigorous Team Science principles.14,18, 19, 20, 21, 22 Only such patients were captured in the MDC database. Patients outside this structured program were categorized as non-program-based care recipients, irrespective of discussion in other forums within or outside BMHCC.
Variables and End Points
Data on recipients of non-program-based care were not prospectively collected. For consistency, we extracted all analysis variables from the tumor registry, using the database linkages solely to categorize patients according to program. Variables included patient demographics; co-morbid conditions; cancer characteristics including histologic type, tumor size and stage; treatment including surgical, non-surgical treatments, and non-treatment. We used the Rural-Urban Commuting Area of patients’ residential and facility zip-codes at the time of cancer diagnosis to assign patient- and facility-level rurality.17,23
Statistical Analysis
We summarized patient demographics, cancer and treatment characteristics with means, medians, frequencies, and percentages among each program pathway. To determine differences across programs, we employed Kruskal-Wallis and chi-squared tests (Fisher’s exact tests were used for small sample sizes). We examined survival and mortality differences across pathway groups using several approaches. We first summarized follow-up time since diagnosis with means and median for each pathway and compared with Kruskal-Wallis tests. Furthermore, we calculated overall, 1-, 3-, and 5- year survival estimates using the life table method, present Kaplan-Meier plots, and compared differences with log-rank tests. We quantified the hazards of death with Cox proportional hazards models and compared each program to non-program participants (reference level). We present crude hazard ratios (HRs), with 95% confidence intervals (CIs) and adjusted HRs for age, sex, race, patient-level rurality and histology. Lastly, we examined crude mortality and rates of lung-cancer specific death across the programs with chi-squared tests.
We examined statistical trends over time (2011–2021) for program utilization, stage distribution at time of diagnosis, and treatments. Specifically, we examined trends in the proportion of patients using a program, the proportion of early-staged patients (clinical stage I and II), and proportions of patients receiving surgery, systemic treatment, no treatment, radiation, neoadjuvant, and adjuvant treatment. We first assessed if any monotonic trend was present over the years using a two-sided Mann-Kendall (MK) trend test, then employed a one-sided MK test to evaluate whether the proportion of the outcome increases or decreases annually. To minimize the effect of secular changes in lung cancer care and outcomes, we repeated our survival analyses among patients diagnosed from 2015 to 2021, when all programs had been established.24
Sensitivity Analyses
In an effort to combat missing data, we repeated the survival analysis excluding patients receiving no treatment for both the full (2011–2021) and recent (2015–2021) cohorts. Furthermore, we adjusted for facility- instead of patient-level rurality in the adjusted HR models for this sensitivity analysis. All analyses were conducted in R (R Core Team, 2021) with α level of 0.05.
Results
Cohort Characteristics
From January 2011 to December 2021, 12,148 patients with lung cancer were in the BMHCC tumor registry: 2,542 received program-based care, 237 LDCT, 1,165 LNP, and 1,140 MDC; and 9,606 received non-program-based care (Table 1). The median age of patients ranged from 68 years in the MDC to 70 years in LNP. Women, 46.3% of the whole cohort, were more likely to receive program-based care, including 49.8% of patients in the LDCT, 50.3% in the MDC and 52.5% in the LNP, compared with 45.0% of recipients of non-program-based care (p < 0.001). Black persons, 27.7% of the whole cohort, varied significantly across programs –13.5% of LDCT, 25.3% of LNP, 30.9% of MDC, and 27.9% of non-program-based care recipients (p < 0.001).
Table 1.
Characteristics of Patients Diagnosed With Lung Cancer Through Four Different Program Pathways
| Patient Characteristicsa | LDCT |
LNP |
MDC |
Non-Program |
p-Value |
|---|---|---|---|---|---|
| N = 237 | N = 1165 | N = 1140 | N = 9606 | ||
| Age | <0.001 | ||||
| Mean (SD) | 68.7 (5.9) | 69.5 (9.5) | 67.4 (10.4) | 68.9 (10.4) | |
| Median (IQR) | 69 (65–73) | 70 (63–76) | 68 (60–75) | 6 9(62–76) | |
| (Min–Max) | (55–87) | (37–98) | (24–95) | (19–99) | |
| Sex | <0.001 | ||||
| Male | 119 (50.2) | 553 (47.5) | 567 (49.7) | 5286 (55.0) | |
| Female | 118 (49.8) | 612 (52.5) | 573 (50.3) | 4318 (45.0) | |
| Missing | 0 | 0 | 0 | 2 (0.02) | |
| Race | <0.001 | ||||
| White | 205 (86.5) | 865 (74.3) | 774 (67.9) | 6846 (71.3) | |
| Black | 32 (13.5) | 295 (25.3) | 352 (30.9) | 2681 (27.9) | |
| Asian | 0 | 4 (0.34) | 9 (0.8) | 33 (0.3) | |
| Other | 0 | 1 (0.09) | 3 (0.3) | 19 (0.2) | |
| Missing | 0 | 0 | 2 (0.2) | 27 (0.3) | |
| Insurance | <0.001 | ||||
| Medicare | 190 (80.2) | 860 (73.8) | 771 (67.6) | 6826 (71.1) | |
| Medicaid | 14 (5.9) | 71 (6.1) | 62 (5.4) | 514 (5.3) | |
| Commercial | 33 (13.9) | 199 (17.1) | 256 (22.5) | 1794 (18.7) | |
| Self-insured/not reported | 0 | 35 (3.0) | 51 (4.5) | 472 (4.9) | |
| Rurality | <0.001 | ||||
| Metropolitan | 168 (70.9) | 892 (76.5) | 867 (76.1) | 5269 (54.8) | |
| Rural | 69 (29.1) | 270 (23.2) | 273 (23.9) | 4328 (45.1) | |
| Missing | 0 | 3 (0.3) | 0 | 9 (0.1) | |
| Smoking status | <0.001 | ||||
| Active | 127 (53.6) | 454 (39.0) | 386 (33.9) | 2860 (29.8) | |
| Successfully quit | 90 (38.0) | 518 (44.5) | 488 (42.8) | 2522 (26.2) | |
| Never | 2 (0.8) | 116 (10.0) | 105 (9.2) | 557 (5.8) | |
| Unk/NR | 18 (7.6) | 77 (6.5) | 161 (14.1) | 3667 (38.2) | |
| Smoking typeb | nb = 127 | nb = 454 | nb = 386 | nb = 2860 | 0.305 |
| Cigarettes | 125 (98.4) | 433 (95.4) | 370 (95.8) | 2747 (96.1) | |
| Cigar/pipe | 1 (0.8) | 2 (0.4) | 5 (1.3) | 29 (1.0) | |
| Snuff/chew/smokeless | 1 (0.8) | 17 (3.8) | 6 (1.6) | 60 (2.1) | |
| Combination | 0 | 2 (0.4) | 5 (1.3) | 24 (0.8) | |
| Unk/NR | 0 | 0 | 0 | 0 | |
| Comorbidity Distribution | <0.001 | ||||
| Mean (SD) | 0.5 (0.8) | 0.5 (0.7) | 0.2 (0.6) | 0.2 (0.6) | |
| Median (Q1–Q3) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–0) | |
| (Min–Max) | (0–4) | (0–3) | (0–4) | (0–5) | |
| Charlson comorbidity score | <0.001 | ||||
| 0 | 145 (61.2) | 762 (65.4) | 944 (82.8) | 7947 (82.7) | |
| 1 | 82 (34.6) | 371 (31.8) | 184 (16.1) | 1510 (15.7) | |
| 2 | 10 (4.2) | 32 (2.8) | 12 (1.1) | 149 (1.6) |
IQR, interquartile range; LDCT, lung cancer screening program; LNP, Lung Nodule Program; Max, maximum; MDC, multidisciplinary care program; Min, minimum; NR, not reported; Q, quartile; Unk, unknown.
Percentage is computed by the column head N unless it specified.
Smoking type uses the number of people who actively smoke and those who have successfully quit.
Forty-one percent of patients resided in rural areas, including 29.1% of LDCT, 23.2% of LNP, 24.0% of MDC and 45.1% of non-program-based care recipients (p < 0.001); of which 5.0% received program-based care while 35.6% received non-program-based care (ratio 1:7.1). By contrast, among the metropolitan residents 15.9% received program-based care and 43.4% received non-program-based care (ratio 1:2.7). Patients’ smoking history differed between programs: 53.6% of patients in the LDCT program actively smoked at the time of diagnosis, compared with 39.0% in the LNP, 33.9% in the MDC, and 29.8% of nonprogram-based care recipients; 0.8%, 10.0%, 9.2% and 5.8% of patients in the respective pathways never smoked, p < 0.001. However, smoking history was unknown in 38.2% of the nonprogram-based cohort compared with 6.6% in the LNP to 14.1% in MDC (Table 1).
Adoption of Program-Based Care
A sequentially higher proportion of the cohort, from 3.7% in 2011, to 33.5% in 2021, received program-based care (increasing MK trend, p < 0.001; Supplementary Table 1A and B and Supplementary Fig. 1). The components of program-based care evolved: MDC, the predominant program early on, ranged from 100% from 2011 to 2014, to 76.3% in 2015. From 2016 to 2021 the LNP became the predominant pathway of program-based care increasing to 62.1%, while MDC dropped to 16.7% in 2021. The LDCT pathway expanded from 0.5% of all patients in 2015 to 7.1% by 2021, while LNP evolved from 3.5% to 20.8%. The ratio of lung cancer diagnosis through the LDCT and LNP changed from 1:6.8 in 2015 to 1:2.9 in 2021. Nonprogram-based care sequentially decreased from 96.3% in 2011 to 66.5% in 2021 (Supplementary Fig. 1).
Lung Cancer Characteristics
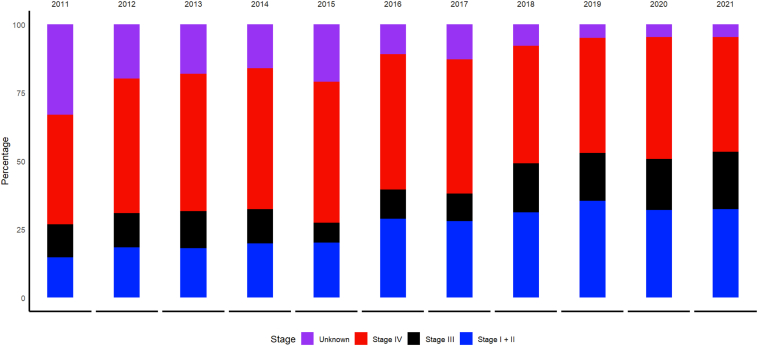
A plurality of patients across all pathways had adenocarcinoma, ranging from 41.1% in the non-program-based cohort to 47.7% in MDC (Supplementary Table 2). Radiologic tumor size was a median of 20 mm (interquartile range [IQR]: 13–34), 25 mm (17–44), 35mm (20–55) and 35 mm (20–55) in the LDCT, LNP, MDC and non-program-based cohorts, respectively (p < 0.001). Clinical stage was early (clinical stage I or II) in 55.7%, 44.8%, 31.0% and 21.9% and stage IV in 17.3%, 31.2%, 34.0% and 50.9%, respectively (p < 0.001). Clinical stage was not reported in 10.1%, 8.6%, 15.9% and 14.2%, in the respective pathways (Supplementary Table 2). The proportion with early-stage disease significantly increased over time (MK trend p < 0.001, Fig. 1), with early aggregate clinical stage evolving from 14.8% in 2011 to 32.5% in 2021 (Supplementary Table 1A and B).
Figure 1.
Stage distribution of lung cancers diagnosed across all pathways of care, 2011 to 2021, indicating an increasing trend in early-stage diagnoses (p < 0.001).
Treatment
Treatment varied significantly between programs – 2,627 patients (21.6%) had surgery, including 46.8%, 35.2%, 39.2% and 17.3%, in the LDCT, LNP, MDC and non-program-based pathways (p < 0.0001), respectively (Table 2). Among recipients of surgery, aggregate pathologic stage was I in 69.4%, 63.9%, 41.0% and 44.2% and II - IV (typically candidates for adjuvant therapy) in 18.9%, 22.4%, 30.3% and 22.7%, respectively (p < 0.001). Surgery was the sole treatment modality in 29.1%, 24.1%, 17.9% and 11.4%, respectively (p < 0.001); although rates differed between programs, the overall rate of surgery was consistent over time (MK trend two-sided p = 0.213; Supplementary Table 1A).
Table 2.
Treatment Received by Patients With Lung Cancer Diagnosed Through Four Different Pathways
| Treatment Characteristics | LDCT |
LNP |
MDC |
Non-Program |
p-Value |
|---|---|---|---|---|---|
| N = 237 | N = 1165 | N = 1140 | N = 9606 | ||
| Modality | <0.0001 | ||||
| Surgery only | 69 (29.1) | 281 (24.1) | 204 (17.9) | 1092 (11.4) | |
| Any surgery | 111 (46.8) | 410 (35.2) | 446 (39.1) | 1660 (17.3) | |
| Any curative-intent treatment: surgery or definitive radiation | 158 (66.7) | 702 (60.3) | 643 (56.4) | 4831 (50.3) | |
| Systemic treatment | 23 (9.7) | 146 (12.5) | 189 (16.6) | 1886(19.6) | |
| Radiation only | 34 (14.4) | 167 (14.3) | 97 (8.5) | 1202 (12.5) | |
| Radiation with other | 55 (23.2) | 251 (21.6) | 334 (29.3) | 2519 (26.2) | |
| No treatment | 14 (5.9) | 190 (16.3) | 74 (6.5) | 2338 (24.3) |
| Adjuvant therapy for recipients of surgery | |||||
|---|---|---|---|---|---|
| Neoadjuvant | na = 111 | na = 410 | na = 446 | na = 1660 | <0.001 |
| No | 109 (98.2) | 394 (96.1) | 391 (87.7) | 1558 (93.9) | |
| Yes | 2 (1.8) | 16 (3.9) | 55 (12.3) | 102 (6.1) | |
| Adjuvant | <0.001 | ||||
| No | 75 (67.6) | 307 (74.9) | 268 (60.1) | 1219 (73.4) | |
| Yes | 36 (32.4) | 103 (25.1) | 178 (39.9) | 441 (26.6) | |
| Both | 0 | 4 (1.0) | 9 (2.0) | 14 (0.84) | 0.038 |
| Pathologic tumor size (mm) | 0.004 | ||||
| Mean (SD) | 24.1 (14.5) | 24.8 (13.9) | 31.26 (18.3) | 26.8 (16.4) | |
| Median (Q1–Q3) | 20 (15–27) | 22 (15–30) | 27 (18–40.8) | 22 (15–33) | |
| (Min–Max) | (8–77) | (2–80) | (0–90) | (0–91) | |
| Aggregate pathologic stage | <0.001 | ||||
| Stage I | 77 (69.4) | 262 (63.9) | 183 (41.0) | 733 (44.2) | |
| Stage II | 10 (9.0) | 49 (11.9) | 63 (14.1) | 184 (11.1) | |
| Stage III | 10 (9.0) | 31 (7.6) | 34 (7.6) | 125 (7.5) | |
| Stage IV | 1 (0.9) | 12 (2.9) | 38 (8.6) | 67 (4.0) | |
| Not Reported | 13 (11.7) | 56 (13.7) | 128 (28.7) | 551 (33.2) | |
| Combined pathologic stage | <0.001 | ||||
| Early stage | 87 (78.4) | 311 (75.8) | 246 (55.2) | 917 (55.2) | |
| Advanced stage | 11 (9.9) | 43 (10.5) | 72 (16.1) | 192 (11.6) | |
| Not reported | 13 (11.7) | 56 (13.7) | 128 (28.7) | 551 (33.2) | |
Modality percentages are used the column head as the denominator; adjuvant therapy percentage used the number of patients underwent surgery; p-value of the modality is computed with four exclusive groups: surgery only, systemic therapy, radiation only and no treatment.
LDCT, lung cancer screening program; LNP, Lung Nodule Program; Max, maximum; MDC, multidisciplinary care program; Min, minimum; Q, quartile.
Denominator for all column percentages (numbers in parentheses) is patients within column category who had surgery.
The proportion that received systemic treatment was lowest among LDCT (9.7%) and highest among non-program patients, while radiation therapy was least used among MDC (8.5%) and most among LDCT and LNP (14.3% and 14.4%, respectively) (p < 0.001; Table 2). The overall rate of systemic treatment decreased over time (MK decreasing trend p = 0.015) while rates of radiation increased over time (MK increasing trend p = 0.002; Supplementary Table 1A and B).
Only 26.6% of recipients of non-program-based care received adjuvant therapy, compared with 32.8% in program-based care, including 32.4%, 25.1% and 39.9% in LDCT, LNP and MDC, respectively (p < 0.001). In the respective programs, 5.9%, 16.3%, and 6.5% of patients received no treatment for their lung cancer. In aggregate, 278 of 2542 patients (10.9%) in any of the three programs received no treatment, compared with 24.3% of patients who received non-program-based care (Table 2). However, there was no change in overall rates of no treatment, neoadjuvant, or adjuvant treatment over time (MK two-sided trend p > 0.05, Supplementary Table 1A).
Survival and Mortality
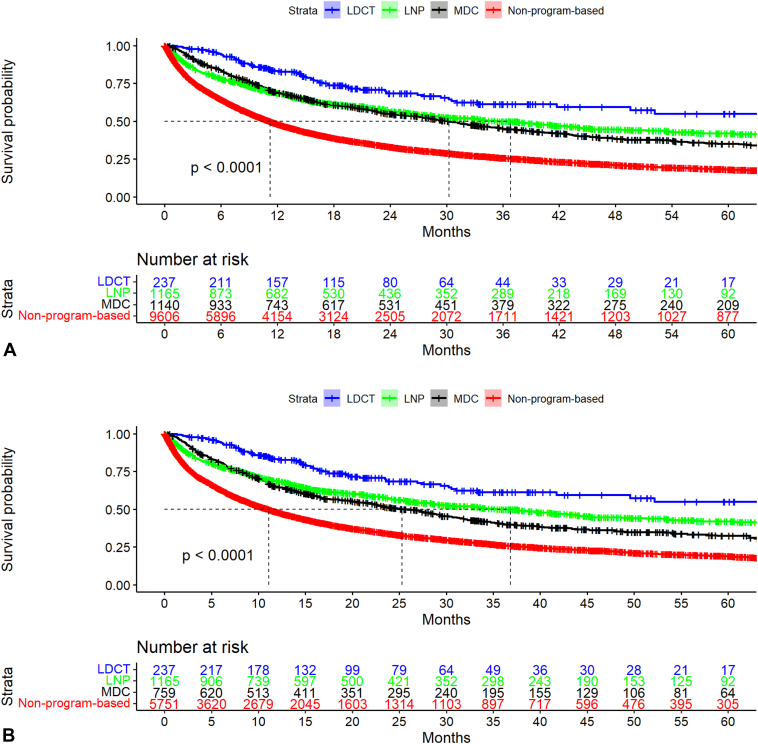
In the whole cohort, the median duration of follow-up from the date of lung cancer diagnosis was 11.0 months (IQR: 3.4–29.0), a combination of 17.4 months (IQR: 10.1–31.7), 15.5 months (IQR: 6.0–35.9), 21.8 months (IQR: 8.7–46.2), and 9.5 months (IQR: 2.8–25.0) in the respective pathways. Median survival was not reached (95% CI: 49.5–not reached) for LDCT versus 36.8 months (29.0–43.8) for LNP versus 30.3 months (27.3–33.7) for MDC versus 11.2 months (10.8–11.7) for non-program, p < 0.001 (Fig. 2A and Supplementary Fig. 2A). Aggregate 3-year survival was 61.0% (95% CI: 54.0%–70.0%) versus 50.0% (47.0%–53.0%) versus 45.0% (42.0%–48.0%) versus 26.0% (25.0%–27.0%), respectively (p < 0.001). Aggregate 5-year survival rates were: 55.0% (46.0%–66.0%), 42.0% (38.0%–46.0%), 35.0% (32.0%–39.0%), and 18.0% (17.0%–9.0%), respectively (p < 0.001). Stage-stratified 3- and 5-year survival also differed in the same pattern (Table 3). With non-program-based care for reference, the crude HR was 0.3 (95% CI: 0.24–0.39), 0.5 (0.48–0.57) and 0.6 (0.53–0.62) for recipients of care through LDCT, LNP and MDC, respectively. Adjusting for age, sex, race, patient-level rurality and histologic type, the adjusted HR (aHR) were 0.3(95% CI: 0.26–0.41), 0.5(0.50–0.59), and 0.6(0.56–0.66).
Figure 2.
(A) Kaplan-Meier plots for survival distribution stratified by program pathway, 2011 to 2021. (B) Kaplan-Meier plots for survival distribution stratified by program pathway, 2015 to 2021.
Table 3.
Survival Rates, Crude and Adjusted Hazards of Patients Diagnosed With Lung Cancer Through Four Different Pathways of Care: 2011 to 2021
| Survival | LDCT |
LNP |
MDC |
Non-Program |
p-Value |
|---|---|---|---|---|---|
| N = 237 | N = 1165 | N = 1140 | N = 9606 | ||
| Duration of follow-up from diagnosis (mo) | <0.001 | ||||
| Mean (SD) | 23.1 (18.5) | 22.8(20.1) | 32.1 (30.4) | 19.9 (26.0) | |
| Median (IQR) | 17.4 (10.1–31.7) | 15.5(6.0–35.9) | 21.8(8.7–46.2) | 9.5 (2.8–25.0) | |
| (Min–Max) | (0–85.8) | (0–91.1) | (0.1–135.5) | (0–139.0) | |
| Crude Overall Survival (95% CI) | |||||
| 1-y | |||||
| Aggregate | 83 (79–89) | 69 (66–71) | 69 (67–72) | 48 (47–49) | <0.001 |
| Stage I | 94 (89–98) | 90 (87–93) | 93 (90–96) | 84 (82–86) | <0.001 |
| Stage II | 100 (100–100) | 75 (66–86) | 79 (71–88) | 70 (65–74) | 0.047 |
| Stage III | 73 (59–89) | 63 (56–71) | 69 (63–75) | 57 (54–59) | <0.001 |
| Stage IV | 52 (38–72) | 40 (35–45) | 45 (41–51) | 28 (26–29) | <0.001 |
| 3-y | |||||
| Aggregate | 61 (54–70) | 50 (47–53) | 45 (42–48) | 26 (25–27) | <0.001 |
| Stage I | 82 (73–91) | 73 (68–78) | 71 (65–77) | 59 (57–62) | <0.001 |
| Stage II | 56 (29–100) | 43 (31–59) | 51 (41–63) | 39 (34–45) | 0.047 |
| Stage III | 39 (22–68) | 44 (36–54) | 43 (37–51) | 27 (24–30) | <0.001 |
| Stage IV | 20 (10–42) | 21 (17–27) | 21 (17–26) | 9 (8–10) | <0.001 |
| 5-y | |||||
| Aggregate | 55 (46–66) | 42 (38–46) | 35 (32–39) | 18 (17–19) | <0.001 |
| Stage I | 78 (68–90) | 61 (55–68) | 61 (54–68) | 44 (42–47) | <0.001 |
| Stage II | 0 (NA–NA) | 38 (26–57) | 39 (29–53) | 27 (22–33) | 0.047 |
| Stage III | 39 (22–68) | 34 (23–50) | 32 (25–40) | 18 (16–21) | <0.001 |
| Stage IV | 20 (10–42) | 17 (12–23) | 15 (11–20) | 5 (5–6) | <0.001 |
| Unadjusted hazard ratio (95% CI) | |||||
| Aggregate | 0.3 (0.24–0.39) | 0.5 (0.48–0.57) | 0.6 (0.53–0.62) | Ref | |
| p-Value | <0.001 | <0.001 | <0.001 | ||
| Stage I | 0.4 (0.23–0.62) | 0.6 (0.53–0.77) | 0.7 (0.54–0.81) | Ref | |
| p-Value | <0.001 | <0.001 | <0.001 | ||
| Stage II | 0.5 (0.21–1.22) | 0.8 (0.57–1.13) | 0.7 (0.52–0.96) | Ref | |
| p-Value | 0.126 | 0.201 | 0.028 | ||
| Stage III | 0.6 (0.38–0.99) | 0.7 (0.57–0.88) | 0.6 (0.53–0.76) | Ref | |
| p-Value | 0.044 | 0.002 | <0.001 | ||
| Stage IV | 0.5 (0.35–0.75) | 0.7 (0.62–0.80) | 0.6 (0.53–0.66) | Ref | |
| p-Value | <0.001 | <0.001 | <0.001 | ||
| Adjusted hazard ratio (age, sex, race, insurance, patient-level rurality, histology) | |||||
| Aggregate | 0.3 (0.26–0.41) | 0.5 (0.50–0.59) | 0.6 (0.56–0.66) | Ref | |
| p-Value | <0.001 | <0.001 | <0.001 | ||
| Stage I | 0.4 (0.24–0.66) | 0.7 (0.54–0.79) | 0.7 (0.59–0.88) | Ref | |
| p-Value | <0.001 | <0.001 | 0.002 | ||
| Stage II | 0.5 (0.20–1.17) | 0.8 (0.57–1.15) | 0.7 (0.51–0.95) | Ref | |
| p-Value | 0.107 | 0.242 | 0.022 | ||
| Stage III | 0.7 (0.42–1.10) | 0.8 (0.61–0.94) | 0.7 (0.56–0.81) | Ref | |
| p-Value | 0.114 | 0.013 | <0.001 | ||
| Stage IV | 0.5 (0.34–0.74) | 0.7 (0.63–0.81) | 0.6 (0.55–0.70) | Ref | |
| p-Value | <0.001 | <0.001 | <0.001 | ||
| Vital statusa | |||||
| Final vital status | <0.001 | ||||
| Alive | 167 (70.5) | 622 (53.4) | 431 (37.8) | 2372 (24.7) | |
| Dead | 70 (29.5) | 543 (46.6) | 709 (62.2) | 7234 (75.3) | |
| Cause of death | na = 70 | na = 543 | na = 709 | na = 7234 | <0.001 |
| Lung cancer-specific | 29 (41.4) | 256 (47.2) | 320 (45.1) | 3021 (41.8) | |
| Other cancer-specific | 1 (1.4) | 5 (0.9) | 7 (1.0) | 95 (1.3) | |
| Other (noncancer) | 6 (8.6) | 42 (7.7) | 50 (7.1) | 1160 (16.0) | |
CI, confidence interval; IQR, interquartile range; LDCT, lung cancer screening program; LNP, Lung Nodule Program; Max, maximum; MDC, multidisciplinary care program; Min, minimum; Ref, reference.
Vital status percentage used the column head as the denominator; Cause of death used the number of deaths as the denominator.
Over the course of follow-up, 8,556 patients (70.4%) died, including 70 (29.5%) in LDCT, 543 (46.6%) in LNP, 709 (62.2%) in MDC and 7,234 (75.3%) in the non-program-based pathway of care (Table 3). The cause of death was not reported in 34, 240, 332 and 2,958 patients in the respective programs. With the caveat that cause of death was unspecified in a high proportion of cases, the LDCT, LNP, MDC and non-program pathways accounted for 0.8%, 7.1%, 8.8% and 83.3% of the known lung cancer-specific deaths, while representing 2%, 9.6%, 9.4% and 79.1% of the whole cohort.
Survival and Mortality in the Subset From 2015 to 2021
When limited to those diagnosed from 2015 to 2021, the median duration of follow-up was, 17.4 (IQR: 10.1–31.7), 15.5 (IQR: 6.0–35.9), 16.7 (IQR: 7.4–35.7), 8.8 (IQR: 2.5–23.0) months, respectively; Median survival was not reached (95% CI: 49.5–not reached) versus 36.8 months (95% CI: 29.0–43.8) versus 25.3 months (95% CI: 22.9–29.5) versus 11.1 months (95% CI: 10.4–11.7), p value less than 0.001 (Fig. 2B, Supplementary Table 3, and Supplementary Fig. 2B). Among the 7,912 patients from 2015 to 2021, 5071 of 7912 (64.1%) had died, including 70 of 237 (29.5%) in LDCT, 543 of 1165 (46.6%) in LNP, 444 of 759 (58.5%) in MDC and 4014 of 5,751 (69.8%) nonprogram-based care patients.
With non-program-based care for reference, the crude HR was 0.3 (95% CI: 0.24–0.39), 0.5 (95% CI: 0.48–0.57) and 0.6 (95% CI: 0.56–0.68) for recipients of care through LDCT, LNP and MDC, respectively. Adjusting for age, sex, race, patient-level rurality and histologic type, the aHR was 0.3 (95% CI: 0.26–0.41), 0.5 (95% CI: 0.50–0.60), and 0.6 (95% CI: 0.58–0.71), respectively. Stage-stratified analysis revealed a similar pattern to the aggregated cohort comparison (Supplementary Table 3).
Sensitivity Analyses
Excluding patients who received no treatment, the aHR was 0.4 (95% CI: 0.27–0.45), 0.5 (0.46–0.57) and 0.7 (0.61–0.71), p value less than 0.001 (Supplementary Table 4). Adjusting for age, sex, race, insurance, facility-level rurality (rather than patient-level rurality) and histologic type, the aHR was 0.4 (0.28–0.46), 0.5 (0.49–0.60) and 0.7 (0.64–0.75), p value less than 0.001. Results were similar in the cohort from 2015 to 2021 (Supplementary Tables 5 and 6).
Discussion
Care within three specific programs to promote lung cancer screening, guideline-concordant management of incidentally-detected lung nodules and structured multidisciplinary decision-making was associated with considerable survival benefit among patients diagnosed with lung cancer in this large community health care system. Program-based care was associated with earlier stage at diagnosis, with 56% of LDCT, 45% of LNP, 31% of MDC and 22% of the non-program-based care cohorts diagnosed at stage I or II. The program-based care cohort was considerably more likely to undergo surgical resection, including surgery without adjuvant therapy.
Despite distribution to lower pathologic stage, the program-based cohort was also more likely to receive adjuvant therapy; non-program-based care was associated higher non-treatment rates. Nevertheless, the survival differences remained consistent when patients who received no treatment were excluded. Non-program-based care remained associated with considerably greater hazard. All-cause mortality and lung cancer-specific mortality were substantially higher in the non-program-based cohort.
Program-based care sequentially increased over time, from 3.7% in 2011 to 33.5% by 2021. Initially dominated by the MDC, from 2016 onward program-based care occurred predominantly through the LNP. The LDCT program sequentially increased as the source of lung cancer diagnoses as compared with LNP. The gap between lung cancer detection through the LDCT versus LNP reduced from a ratio of 1:6.8 in 2015 to 1:2.9 in 2021, suggesting wider reach of screening over time. Women were more likely, Black persons and rural dwellers less likely, to have their lung cancer diagnosed through program-based care.
We previously reported the improved survival of lung cancer patients in the two early detection programs, compared with the MDC.11 However, given that care within the MDC was more likely to be guideline-concordant and associated with better survival than care outside the MDC, that analysis was probably biased to the null, under-estimating the value of implementing these early detection programs.14 In the current analysis, we report that aggregate and stage-stratified survival were considerably better with program-based care, with the hierarchy LDCT > LNP > MDC > nonprogram-based care. Furthermore, mortality was substantially less likely with program-based care.
Program-based care was successfully implemented in this large, diverse community health care system, structurally similar to systems where it is estimated that up to 85% of patients receive lung cancer care in the US. The two approaches to early detection- LDCT screening of ostensibly healthy individuals at high risk for lung cancer, and guideline-concordant management of patients with incidentally detected lung nodules- profoundly redistributed lung cancer to earlier stage. Implementation within the context of structured multidisciplinary decision-making, as recommended, probably increases the likelihood of safe and effective management, including optimal use of curative-intent treatment.25,26
Limitations
The main limitations of this study are the retrospective design and use of tumor registry data to gain access to the non-program-based care data. Inherent weaknesses of this approach include missing data (such as stage), the possibility of mis-classification bias, and inability to attribute causality. The stage distribution of our non-program-based cohort mirrors the US statistics, including the proportion with unknown stage.1 Furthermore, we had no access to certain important details such as patients’ performance status, patient-clinician interactions and decision-making.
Although our findings are from a single regional community-based health care system, multiple prior reports from this health care system have been corroborated by analyses of national and international datasets.11,12,27, 28, 29, 30 For example, a recent analysis of the Surveillance, Epidemiology, and End Results-Medicare database reported aggregate 3-year survival of 59.7% in lung cancer patients who received an LDCT versus 58.2% in those diagnosed after an incidental lung nodule versus 31.7% in a referent population, which is very similar to our report of 61.0%, 50.0% and 26.0%, respectively.12 The care delivery experience of our non-program-based care cohort is probably consonant with the experience of most US (and indeed, worldwide) lung cancer patients, whose diagnosis is not made through structured early detection programs and whose care is often delivered without structured multidisciplinary interaction.
Summary
Program-based lung cancer care was associated with redistribution of lung cancer to earlier stage, greater likelihood of treatment, use of curative-intent surgery, adjuvant therapy, improved survival, reduced mortality, and possibly, reduced lung cancer-specific mortality. Furthermore exploration of this program-based approach as a key strategy to decrease population-level lung cancer mortality should be encouraged as a matter of public policy.
CRediT Authorship Contribution Statement
Wei Liao: Formal analysis, methodology, validation, writing - review and editing.
Meredith A. Ray: Methodology, Validation, Writing – review and editing.
Carrie Fehnel: Project administration, resources, writing –review and editing.
Jordan Goss: Data curation, Writing – review and editing.
Catherine J. Shepherd: Data curation, Writing – review and editing.
Anita Patel: Data curation, Writing – review and editing.
Talat Qureshi: Data curation, Writing – review and editing.
Federico Caro: Data curation, Writing – review and editing.
Jessica Roma: Data curation, Writing – review and editing.
Anna Derrick: Data curation, Writing – review and editing.
Anberitha Matthews: Writing – review and editing.
Nick Faris: Project administration, Resources, Writing – review and editing, Data curation.
Matthew P. Smeltzer: Formal analysis, Methodology, Original draft, Writing – review and editing, Validation.
Raymond Osarogiagbon: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – review and editing, Supervision.
Disclosure
Dr. Osarogiagbon reports as patent, specimen collection kit; stocks: Bridge BIO, Eli Lilly, Gilead Sciences, Pfizer; consultant: AstraZeneca, GE Healthcare, Genentech/Roche, Median Technologies, National Cancer Institute, Tryptych Healthcare Partners. Dr. Matthews reports as Stocks, Pfizer and Boston Scientific. Dr. Smeltzer reports as Research consultant, Association of Community Cancer Centers. Dr. Faris reports as personal fees from Biodesix. The remaining authors declare no conflict of interest.
Acknowledgments
This work was partially supported by research grants from the Baptist Memorial Health Care Foundation (15BD03), the National Institutes of Health (2R01CA172253; 2UG1CA189873) and a Patient-Centered Outcomes Research Institute Award (IH-1304-6147) to Dr. Osarogiagbon. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors or Methodology Committee.
Footnotes
Cite this article as: Liao W, Ray M, Fehnel C, Goss J, et al. Program-based lung cancer care: a prospective observational tumor registry linkage study. JTO Clin Res Rep. 2024;5:100629.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100629.
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCD. Lung Cancer Screening With Low Dose Computed Tomography (LDCT) (210.14) cms.gov. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=364&ncdver=1& Accessed March 10, 2023.
- 4.Tanner N.T., Gebregziabher M., Hughes Halbert C., Payne E., Egede L.E., Silvestri G.A. Racial differences in outcomes within the national lung screening trial. Implications for widespread implementation. Am J Respir Crit Care Med. 2015;192:200–208. doi: 10.1164/rccm.201502-0259OC. [DOI] [PubMed] [Google Scholar]
- 5.Aldrich M.C., Mercaldo S.F., Sandler K.L., Blot W.J., Grogan E.L., Blume J.D. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol. 2019;5:1318–1324. doi: 10.1001/jamaoncol.2019.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera M.P., Katki H.A., Tanner N.T., et al. Addressing disparities in lung cancer screening eligibility and healthcare access. An official American Thoracic Society statement. Am J Respir Crit Care Med. 2020;202:e95–e112. doi: 10.1164/rccm.202008-3053ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S.S., Chow E., Ten Haaf K., et al. Disparities of national lung cancer screening guidelines in the US population. J Natl Cancer Inst. 2020;112:1136–1142. doi: 10.1093/jnci/djaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedewa S.A., Kazerooni E.A., Studts J.L., et al. State variation in low-dose computed tomography scanning for lung cancer screening in the United States. J Natl Cancer Inst. 2021;113:1044–1052. doi: 10.1093/jnci/djaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahar L., Douangchai Wills V.L., Liu K.K., Kazerooni E.A., Dyer D.S., Smith R.A. Using geospatial analysis to evaluate access to lung cancer screening in the United States. Chest. 2021;159:833–844. doi: 10.1016/j.chest.2020.08.2081. [DOI] [PubMed] [Google Scholar]
- 10.Pinsky P.F., Lau Y.K., Doubeni C.A. Potential disparities by sex and race or ethnicity in lung cancer screening eligibility rates. Chest. 2021;160:341–350. doi: 10.1016/j.chest.2021.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osarogiagbon R.U., Liao W., Faris N.R., et al. Lung cancer diagnosed through screening, lung nodule, and neither program: A prospective observational study of the detecting early lung cancer (DELUGE) in the Mississippi Delta cohort. J Clin Oncol. 2022;40:2094–2105. doi: 10.1200/JCO.21.02496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinsky P., Miller E., Faris N., Osarogiagbon R. Pulmonary nodules, lung cancer screening and lung cancer in the Medicare population. Chest. 2023;163:1304–1313. doi: 10.1016/j.chest.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prabhu Das I., Baker M., Altice C., Castro K.M., Brandys B., Mitchell S.A. Outcomes of multidisciplinary treatment planning in US cancer care settings. Cancer. 2018;124:3656–3667. doi: 10.1002/cncr.31394. [DOI] [PubMed] [Google Scholar]
- 14.Ray M.A., Faris N.R., Fehnel C., et al. Survival impact of an enhanced multidisciplinary thoracic oncology conference in a regional community health care system. JTO Clin Res Rep. 2021;2 doi: 10.1016/j.jtocrr.2021.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.About, Delta Regional Authority. https://dra.gov/about/ Accessed July 12, 2023.
- 16.Mokdad A.H., Dwyer-Lindgren L., Fitzmaurice C., et al. Trends and patterns of disparities in cancer mortality among US counties, 1980–2014. JAMA. 2017;317:388–406. doi: 10.1001/jama.2016.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray M.A., Faris N.R., Derrick A., Smeltzer M.P., Osarogiagbon R.U. Rurality, stage-stratified use of treatment modalities, and survival of non-small cell lung cancer. Chest. 2020;158:787–796. doi: 10.1016/j.chest.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osarogiagbon R.U., Phelps G., McFarlane J., Bankole O. Causes and consequences of deviation from multidisciplinary care in thoracic oncology. J Thorac Oncol. 2011;6:510–516. doi: 10.1097/JTO.0b013e31820b88a7. [DOI] [PubMed] [Google Scholar]
- 19.Osarogiagbon R.U., Rodriguez H.P., Hicks D., et al. Deploying team science principles to optimize interdisciplinary lung cancer care delivery: avoiding the long and winding road to optimal care. J Oncol Pract. 2016;12:983–991. doi: 10.1200/JOP.2016.013813. [DOI] [PubMed] [Google Scholar]
- 20.Smeltzer M.P., Rugless F.E., Jackson B.M., et al. Pragmatic trial of a multidisciplinary lung cancer care model in a community healthcare setting: study design, implementation evaluation, and baseline clinical results. Transl Lung Cancer Res. 2018;7:88–102. doi: 10.21037/tlcr.2018.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhoeven D.C., Chollette V., Lazzara E.H., Shuffler M.L., Osarogiagbon R.U., Weaver S.J. The anatomy and physiology of teaming in cancer care delivery: a conceptual framework. J Natl Cancer Inst. 2021;113:360–370. doi: 10.1093/jnci/djaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smeltzer M.P., Ray M.A., Faris N.R., et al. Prospective comparative effectiveness trial of multidisciplinary lung cancer care within a community-based health care system. JCO Oncol Pract. 2023;19:e15–e24. doi: 10.1200/OP.21.00815. [DOI] [PubMed] [Google Scholar]
- 23.USDA Economic Research Service, U.S. Department of Agriculture. Rural-Urban Commuting Area Codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx Accessed September 25, 2023.
- 24.Howlader N., Forjaz G., Mooradian M.J., et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener R.S., Gould M.K., Arenberg D.A., et al. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192:881–891. doi: 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts T.J., Lennes I.T., Hawari S., et al. Integrated, multidisciplinary management of pulmonary nodules can streamline care and improve adherence to recommendations. Oncologist. 2020;25:431–437. doi: 10.1634/theoncologist.2019-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osarogiagbon R.U., Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol. 2012;7:1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 28.Osarogiagbon R.U., Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96:1178–1189. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Edwards J.G., Chansky K., Van Schil P., et al. International Association for the Study of Lung Cancer staging and prognostic factors committee, advisory board members, and participating institutions. The IASLC lung cancer staging project: analysis of resection margin status and proposals for residual tumor descriptors for non-small cell lung cancer. J Thorac Oncol. 2020;15:344–359. doi: 10.1016/j.jtho.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Osarogiagbon R.U., Faris N.R., Stevens W., et al. Beyond margin status: population-based validation of the proposed International Association for the Study of Lung Cancer residual tumor classification recategorization. J Thorac Oncol. 2020;15:371–382. doi: 10.1016/j.jtho.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.