Abstract
Introduction
The universal increase in obesity and diabetes has increased the chronic kidney disease (CKD) rate. In 2017, almost 800 million individuals suffered from CKD worldwide. Kidney dialysis becomes necessary as the disease progresses. Dialysis negatively impacts CKD patients' quality of life (QoL). It causes several complications that affect patients' physical, social, psychological, and spiritual aspects of life. This systematic review aims to identify condition-specific tools used to assess CKD patients' quality of life on dialysis.
Material and Methods
A systematic literature search was conducted to investigate studies using QoL tools among patients on dialysis from February 2000 to June 2023. The search was conducted in several databases and followed the PRISMA guidelines. The focus was to identify tools that capture intrinsic factors, such as spiritual subdomains, rather than extrinsic factors, such as environmental subdomains.
Results
The review identified five studies and seven dialysis-specific tools for assessing the QoL of CKD patients on dialysis. The physical domain was the most assessed, followed by the psychological and social domains. Fatigue, muscle weakness, sleep disorders, and pain were identified as the most common concerns in the physical domain.
Conclusion
Dialysis negatively impacts all aspects of QoL in CKD patients. This review can guide clinicians in understanding the disease and treatment burden by identifying the most appropriate tools for assessing the QoL of adult CKD patients undergoing dialysis. There is a need for further studies to explore the detrimental effects of CKD treatment and better understand its impact on patients' QoL.
Keywords: Dialysis, Quality of life, QoL, Tools, Chronic Kidney disease, CKD
1. Introduction
The universal increase in obesity and diabetes has translated into more patients suffering from chronic kidney disease (CKD) (Kovesdy, 2011). In 2017, the number of patients with CKD was estimated at 843.6 million individuals worldwide (Kovesdy, 2011). As the disease progresses and kidneys lose their ability to function, patients enter another phase known as End Stage Renal Failure (ESRD), where dialysis is necessary (Zazzeroni et al., 2017). There are two types of dialysis indicated for ESRD therapy, which are hemodialysis (HD) and peritoneal dialysis (PD) (Zazzeroni et al., 2017).
With recent advances in Medicine, the mortality of ESRD has declined, but the Global Burden of Disease (GBD) studies have identified CKD as a new leading cause of death globally (Kovesdy, 2011). A recent meta-analysis showed that the median five-year survival rate for patients starting dialysis was only 45 % (Bonenkamp and van Eck van der Sluijs A, Hoekstra T, Verhaar MC, van Ittersum FJ, Abrahams AC, 2020). Nonetheless, patients on kidney dialysis are concerned about their quality of life and survival (Bonenkamp and van Eck van der Sluijs A, Hoekstra T, Verhaar MC, van Ittersum FJ, Abrahams AC, 2020). The patients perceive the dialysis process as a heavy burden that affects their health-related quality of life (HRQoL) (Zazzeroni et al., 2017). Patients on hemodialysis should visit the hospital or a dialysis center 2–3 times a week for a dialysis session that lasts around 3–4 h, which can be very limiting and affects the social as well as the professional aspects of their lives (Zazzeroni et al., 2017) Psychiatric disorders are also common among kidney dialysis patients, with depression ranking as the primary disorder (de Alencar et al., 2020).
Several studies have reported kidney dialysis complications and how they affect all aspects of life (Cox et al., 2017, Dąbrowska-Bender et al., 2018, Davey et al., 2019, de Alencar et al., 2020). Patients suffer from increased fatigue, sleep disorders, decreased appetite, malnutrition, physical performance deterioration, sexual dysfunction, cognitive difficulties, pain, and depression (Mollaoğlu, 2013, Dąbrowska-Bender et al., 2018, Caplin et al., 2011, Cox et al., 2017).
Peritoneal dialysis, which can be done from home or work, offers patients flexibility, independence, and normalcy as they perform their daily activities. Peritoneal dialysis is done every 4–5 h for about 30 min (Zazzeroni et al., 2017). Despite the advantages of PD mentioned above, there is no conclusive evidence that patients on PD have higher HRQoL than patients performing hemodialysis (Zazzeroni et al., 2017). Both peritoneal dialysis and hemodialysis increased survival rates for patients with CKD, directing the focus to improving their quality of life.
The objective of this study is to systematically review the tools used to assess the QoL of CKD patients on dialysis or hemodialysis. The study will shed light on the tools the researchers can use to assess QoL among patients with CKD who need dialysis, determine the areas affected, and identify interventions needed to improve the QoL of CKD patients on dialysis.
2. Material and methods
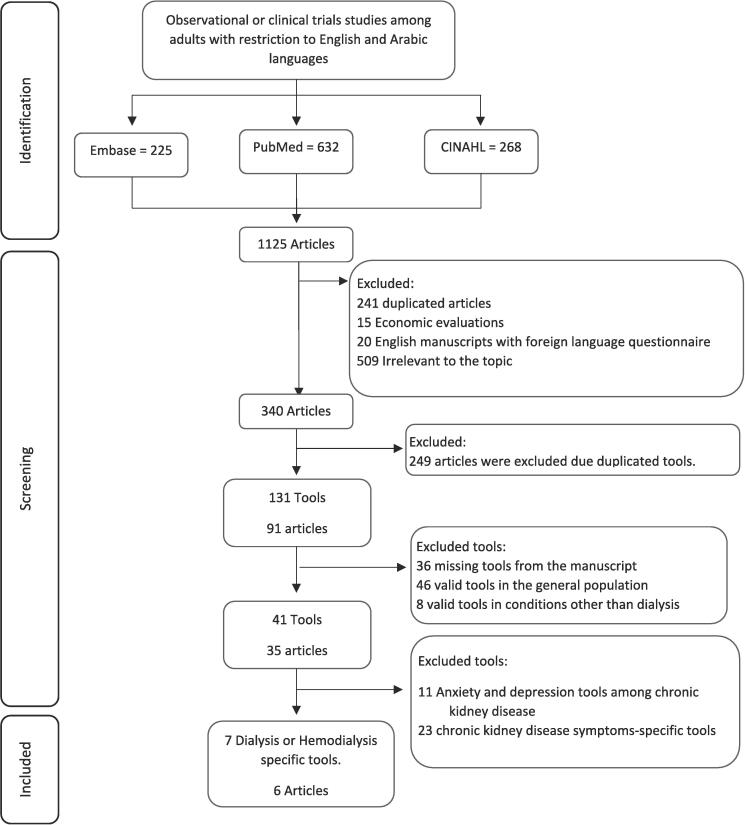
A systematic literature search was performed to investigate studies that reported QoL tools among patients on dialysis and the tools they included. The search included articles from February 2000 to February 2023. This review focused on dialysis-related tools and the studies in which they were reported. Following PRISMA guidelines (PRISMA), database searches were for PubMed, Embase, and CINAHL.
The search used the following keywords or their combinations: quality of life, QoL, health-related quality of life, satisfaction, tools, instruments, measures, questionnaires, scales, surveys, hemodialysis, dialysis, renal replacement therapy, renal dialysis, peritoneal dialysis, and adults. Word variations were also searched.
2.1. Inclusion and exclusion criteria
All observational and clinical studies on adults were included in the first phase of the search. Studies were included if they contained at least one QoL tool used among adult patients on kidney dialysis. The search included studies in both the English and Arabic languages. Economic evaluation studies, studies with English manuscripts but foreign (other than Arabic) language QoL questionnaires, studies with missing tools, and studies on the general population or conditions other than dialysis were excluded.
2.2. Data extraction
The extracted articles were examined for any duplications. Then, a first filtration was done separately by two teams of two investigators. Each team looked at the title and the abstract to determine eligibility. Then, the agreed-upon articles were included. Those articles with differences between the two groups were referred to the third team of two, who looked at the title and the abstract to determine eligibility.
The eligible studies were reviewed, and the following fields were extracted, including the tool's name, study objectives, inclusion and exclusion criteria, sample size, study outcomes, the number of domains and items, the domain description, and the scoring system. Data was also extracted regarding the tools that involved pharmacological interventions and their effect on QoL. The classified QoL domains depended on Ferrell's QoL conceptual framework that included physical well-being, psychological well-being, social well-being, and spiritual well-being. (Ferrell et al., 1995, Ferrell et al., 1996) Although the subdomains under the environment domain are crucial to patients with kidney failure and dialysis, the focus here was to identify tools that capture the intrinsic factors, such as spiritual subdomains, rather than the extrinsic factors, such as the environment's subdomains. The search strategy followed is illustrated in Fig. 1.
Fig. 1.
The search strategy for identifying Physical well-being related Quality of life tools among dialysis patients.
3. Results
3.1. Validated QoL tools in chronic kidney dialysis
From the database search, seven QoL tools were identified using the search criteria. All seven tools were classified as condition-specific and were included in this systematic review for analysis. These tools included the International Physical Activity Questionnaire (IPAQ), Chalder Fatigue Questionnaire (CFQ 11), International Physical Activity Questionnaire short form (IPAQ-SF), Work and Social Adjustment Scale (WSAS), Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F), Functional Independence Measure (FIM), and Piper Fatigue Scale (PFS).
3.2. Description of QoL tools
The systematic review yielded seven different tools varying in the number of items and domains. Each tool has its scoring/scaling system and a different set of domains. The description of these tools is represented in Table 1. The number of items for our tools ranged from 5 to 40, whereas the number of domains ranged from 1 to 6. Each tool evaluated a specific domain, with four tools covering three domains each. No tool assessed all four domains (physical, psychological, social, and spiritual) suggested by Ferrell et al. (Ferrell et al., 1996).
Table 1.
Characteristics of quality-of-life tools in kidney dialysis patients.
| Tools | QoL Domains | Sub-domain number/description | Items | Scaling/Scoring |
|---|---|---|---|---|
| The International Physical Activity Questionnaires (IPAQ) (Suhardjono et al., 2019) | Physical | 5 Job-related; Transportation; Housework, house maintenance, caring for the family; Recreation, sport, leisure time; Time spent sitting. |
27 | The overall score is calculated using all responses. Patients are categorized into 1 of 3 categories based on physical activity: low/inactive, moderate, or high. Self-reported |
| Chalder Fatigue Scale (CFQ 11) (Picariello et al., 2018, Picariello et al., 2021) | Physical, Psychological | 2 Physical fatigue; Psychological fatigue |
11 | The questionnaire measures fatigue severity through 11 items scored against a 4-point Likert-type response scale. Scores range from 0 to 3. Higher scores indicate severe fatigue. Self-administered. |
| Work and Social Adjustment Scale (WSAS) (Picariello et al., 2018, Picariello et al., 2021) | Social, Psychological | 5 Impairment in work, home management, social activities, private leisure activities, close relationships |
5 | A 0–40 scoring system with lower scores indicating better results. Scores exceeding 20 indicate moderately severe or worse psychopathology. Scores between 10 and 20 are associated with significant functional impairment but less severe symptoms Scores below 10 are associated with subclinical populations. Self-administered |
| The International Physical Activity Questionnaires (IPAQ-SF) (Picariello et al., 2018, Picariello et al., 2021) | Physical | 1 General Physical activity |
7 | Divided into 3 categories based on the patient's physical activity: Low, moderate, and high activity. Total scores can provide an indication of a patient's physical activity. Self-reported |
| Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) (J.S. et al., 2017) | Physical; Social; Phycological; | 5 Physical Well-Being; Social/Family Well-Being; Emotional Well-Being; Functional Well-Being; Fatigue |
40 | A 40-item scale that assesses self-reported fatigue and its impact on daily activities and function. Scores range from 0 to 160 Higher scores indicate better QoL. |
| Functional Independence Measure (FIM) (Matsufuji et al., 2015) |
Physical; Psychological; Social | 6 Self-Care; Sphincter control; mobility; locomotion; communication; social cognition |
18 | Used to assess and grade a person's functional status based on the level of assistance he or she requires. FIM scores range from: 1–7, where 1 = Total dependence, and 7 = complete independence with no helper |
| Piper Fatigue Scale (PFS) (Zhang et al., 2020, Reeve et al., 2012) | Phycological; Physical; Social | 4 Behavior; affect; sensory; cognitive/mood |
27 | 22 items, each scaled using 0–10 to measure four dimensions of subjective fatigue. In addition to 5 writing questions that help to describe reasons and states. |
3.3. Review of studies using QoL tools in dialysis patients
Our literature search methodology found five studies reporting using QoL tools in kidney dialysis patients. A summary of these studies is provided in Table 2, including the study objective, population, criteria for inclusion/exclusion, tools used, method of administration, study results, and QoL domains covered. Only some studies examined more than one QoL tool to investigate different QoL aspects in kidney dialysis patients, while others focused only on one tool. Only three out of four QoL aspects, as proposed by Ferrell et al., were used in the five studies. The QoL aspects covered were the physical, social, and psychological domains, whereas the spiritual aspect was not mentioned.
Table 2.
Quality of life studies in chronic kidney disease patients on hemodialysis.
| Study (Type) | Study objective | Patient population | Inclusion/ Exclusion criteria |
Tools used/ Administration |
Results | QoL domains |
|---|---|---|---|---|---|---|
| Picariello et al. (2020) (Picariello et al., 2018, Picariello et al., 2021) RCT |
Evaluation of the applicability of cognitive behavioral therapy (CBT)-based intervention efficacy trial. |
Adult patients undergoing In-center hemodialysis who meet clinical levels of fatigue. (n = 40) |
Inclusion: Age > 18 years; confirmed ESKD diagnosis; fatigue levels exceed 18 on the CFQ; receiving in-center HD; length of time on dialysis > 90 days Exclusion: The presence of any known cognitive impairments ; have a severe mental health disorder; currently receiving psychotherapy; failing on dialysis and approaching the end of life; have a CFQ score below the cut-off at the Pre-randomization assessment |
CFQ, WSAS, IPAQ-SF | Results showed that a cognitive-behavioral therapy (CBT) based intervention for fatigue in hemodialysis appeared necessary and beneficial. The study found moderate to significant treatment effects in favor of the intervention, specifically on fatigue severity, fatigue-related functional impairment, depression, and anxiety. However, there was no significant improvement in sleep quality. | Physical Social Psychological |
| Suhardjono et al. (2019) (Suhardjono et al., 2019) RCT |
Evaluation of the role of intradialytic exercise on physical capacity, inflammation, and nutritional status of dialysis patients. |
N = 120, 18 age or older; on maintenance dialysis for at least 3 months |
Inclusion: dialysis patients aged over 18 years who had undergone routine dialysis for over three months/ Exclusion: traveling on dialysis, being hospitalized for any reason within the past three months, having arrhythmias, being on dialysis for less than 2‐week intervals, having a limited range of motion of extremities, being immobilized |
IPAQ Self-administered |
The study found a notable improvement in the lower extremity strength and physical component score (PCS) of the KDQOL-SF™ tool among patients who underwent aerobic training and combination exercise, as compared to those in the control group. | Physical |
| Matsufuji et al.(2014) (Matsufuji et al., 2015) RCT |
Evaluation of the effect of chair stand exercise on ADL of hemodialysis patients. | N = 27; outpatients on hemodialysis; age ˃ 60 years |
Inclusion: on hemodialysis; age ˃ 60 years; ambulatory Exclusion: symptomatic ischemic heart disease; symptomatic peripheral artery disease; arthritis; history of stroke with severe paralysis; chronic obstructive pulmonary disease; pregnancy. |
(FIM) HCP-administered |
Significant improvement in the FIM subscales related to mobility and locomotion. |
Physical; psychological; social |
| Sihombing et al. (2016) (J.S. et al., 2017) Multicenter prospective study |
Evaluation of the effect of erythropoietin on the QoL of CKD patients. | CKD patients with routine hemodialysis |
Inclusion Criteria: CKD patients with routine hemodialysis for at least three months, aged 20–80 years, use EPO to treat their anemia. Exclusion: Kidney transplant; diagnosed with cancer. |
FACIT-F | Erythropoietin could positively impact the quality of life (QOL) of chronic kidney disease (CKD) patients undergoing routine hemodialysis. | Physical |
| Zhang et al. (2020) (Zhang et al., 2020) Systematic Review and meta-analysis |
Evaluation of the therapeutic efficacy of exercise for patients undergoing hemodialysis on fatigue and HRQoL. |
Patients with ESRD undergoing hemodialysis |
Inclusion Criteria: Patients with ESRD undergoing hemodialysis who were diagnosed using the Kidney Disease Improving Global Outcomes. Exclusion: None. |
PFS, FACIT-F, CFQ) |
Results are not reported yet. | Physical |
Picariello et al. (2020) evaluated the effect of cognitive behavioral therapy (CBT)-based intervention on fatigue in CKD patients. Three tools were utilized in this study (CFQ, WSAS, IPAQ-SF). The study results were published in 2020. Cognitive behavioral therapy (CBT)-based intervention was moderate to largely effective in reducing fatigue severity, fatigue-related functional impairment, depression, and anxiety. However, it did not have any effect on sleep quality (Picariello et al., 2018, Picariello et al., 2021).
Suhardjono et al. (2019) aimed to determine the role of intradialytic exercise on physical capacity, inflammation, and nutritional status in dialysis patients. The IPAQ questionnaire was used to assess the physical performance levels. After 12 weeks of intradialytic exercise, patients significantly improved the lower limbs' muscle strength (Suhardjono et al., 2019).
Using the FIM tool, Matsufuji et al. (2014) evaluated 3 QoL aspects (physical, psychological, and social). The study objective was to assess the effect of chair stand exercises performed three times per week for 12 weeks on the activity of daily living (ADL). Significant improvements were observed in mobility and locomotion (Matsufuji et al., 2015).
Using the FACIT-F scale, Sihombing et al. (2016) evaluated erythropoietin administration's effect on kidney dialysis patients' QoL. Results showed that erythropoietin administration could improve the QoL of CKD patients on dialysis (J.S. et al., 2017).
3.4. Non-pharmacological intervention and QoL
Five studies (Picariello et al., 2018, Picariello et al., 2021, Suhardjono et al., 2019, Matsufuji et al., 2015, J.S. et al., 2017, Zhang et al., 2020) examined the effect of non-pharmacological approaches on QoL among kidney dialysis patients. Suhardjono et al. (2019) reported improved lower exterminates muscle strength following an exercise regimen (Picariello et al., 2018). Matsufuji et al. (2014) confirmed improvement in the ADL of kidney dialysis patients when using the chair stand exercise intervention (Matsufuji et al., 2015).
A study by Picariello et al. (2020) highlighted the benefits of CBT intervention on fatigue severity, fatigue-related functional impairment, depression, and anxiety (Picariello et al., 2018, Picariello et al., 2021).
3.5. Pharmacological intervention and QoL
Only one study, Sihombing et al. (2016), evaluated the impact of erythropoietin as a pharmacologic intervention on the QoL of kidney dialysis patients, where it showed potential benefits (J.S. et al., 2017).
4. Discussion
Patients with dialysis are identified as a vulnerable group of patients owing to their frail and delicate physical and psychological condition. Unfortunately, patients with dialysis have to cope with their lifelong disease and the hardships and limitations it imposes on them, which eventually translates to a diminished QoL. Quality of life is not a luxury. Its assessment has become mandatory to measure the outcomes of adverse events assessment and treatment effectiveness in several disease conditions, including end-stage renal disease (ESRD), among others (Tsai et al., 2010).
For this review, we adopted the QoL model proposed by Ferrell et al. for breast cancer survivors, which involved four QoL dimensions: psychological, social, physical, and spiritual domains (Ferrell et al., 1996).
In our review, dialysis negatively impacted three out of four life domains. These three domains are the physical, psychological, and social domains. We could not find any tools that assessed the spiritual domain of CKD patients on dialysis. Future studies should include the spiritual domain, as many patients with CKD spend hours in dialysis sessions, which may impact their spiritual well-being (Dąbrowska-Bender et al., 2018). Previous studies have shown that patients with spiritual beliefs experience a better quality of life (Dąbrowska-Bender et al., 2018). Religion and faith help patients cope and even fight disease. Some studies have even hypothesized that spirituality prolongs one's life (Puchalski, 2001).
Seven tools were identified as condition-specific tools. Six tools (IPAQ, CFQ11, FACIT-F, IPAQ-SF, FIM, PFS) assessed the physical aspect, five tools (WSAS, CFQ11, FACIT-F, FIM, PFS) assessed the psychological aspect, while four tools assessed the social aspect (WSAS, FACIT-F, FIM, PFS). The widely used SF36 tool was excluded because it was not specific to CKD patients on dialysis. One of the limitations of the identified tools is that none was able to assess all four QoL domains.
In addition to the physical pain of the disease itself, dialysis therapy is exhausting and requires frequent visits to the hospital or dialysis center multiple times per week. Dialysis therapy can also cause various negative symptoms such as pain, fatigue, sleep disorders, nausea, stomachache, and hypotension that further impact the QoL (Hanspal et al., 2021). Patients in our review often complained of fatigue, depression, anxiety, muscle weakness, and sleep disorders. Consequently, more studies are needed to examine the possible pharmacological and non-pharmacological therapies to improve their QoL. Dialysis patients are also forced to make lifestyle changes and occupational arrangements to meet their routine dialysis schedules, which affect their mental and emotional health (Gerogianni et al., 2016); (Davey et al., 2019). This also represents an extra burden as they become threatened with losing their source of income (Hanspal et al., 2021, Gerogianni et al., 2016).
This review also aimed to report the studies that assessed QoL in CKD dialysis patients using validated tools. Quality of life domains are inter-connected and mainly affect one another. Fatigue, for example, is a physical aspect that affects the social domain because the patient cannot go out, work, or perform routine daily activities. Sometimes, fatigue restricts patient mobility, adversely affecting their psychological state as well. Therefore, more studies are needed to explore how these symptoms affect patients' productivity, overall earnings, health services utilization, and cause-specific mortality.
The domain of focus was the physical domain, followed by the psychological and then the social domain. The physical domain's most common concerns included fatigue, muscle weakness, sleep disorders, and pain. Fatigue is a massive contributor to impaired functioning and diminished quality of life; however, it is often under-recognized and even normalized by physicians due to the illness and treatment burden (Davey et al., 2019, Jhamb et al., 2008). The psychological QoL domain came second and had anxiety and depression as the main issues. The minor domain reported in the studies was the social domain, including social interaction, problem-solving, and memory.
It is worth noting that not all complications that dialysis patients go through are related to the disease itself. Unfortunately, long-term dialysis therapy and the chronic use of medications also contribute to this dilemma. Data reports that patients on dialysis are prescribed many medications, increasing their chances of experiencing adverse events, drug interactions, and medication errors. The high number of medications could indicate a more severe disease that requires multiple therapeutic agents (Cardone et al., 2010). This represents a psychological, physical, and economic burden for CKD patients. A report on hemodialysis on 850 patients who were followed for over a year demonstrated that the risk of mortality increases as the number of medications increases (Cardone et al., 2010).
There is a clear need for further studies to explore and evaluate the possible detrimental effects of CKD treatment to understand its actual impact on CKD patients' QoL.
5. Limitations
It is important to note that our study has a few limitations. Firstly, we only included studies that were in the English language, which may have left out relevant studies in other languages. Secondly, some manuscripts we reviewed had missing tools that were not captured in our results. Unfortunately, we cannot evaluate how these missing tools could have affected our findings. Thirdly, we were unable to find any tools that addressed the spiritual domain. Lastly, our research did not assess special cohorts like CKD patients with other comorbidities and the overlapping role they might play in affecting patients’ overall QoL. One of the limitations of the identified tools is that none was able to assess all four QoL domains.
6. Conclusion
This review reported the available tools used to assess QoL in CKD dialysis patients and highlighted disease and treatment effects on all aspects of QoL.
Tools used to assess the QoL in CKD dialysis patients are valuable tools that assist clinicians in understanding the disease and treatment burden and its impact on dialysis patients' QoL. Understanding the factors that could be associated with decreased QoL in dialysis patients, using validated tools to assess their QoL, and finding solutions that enhance different aspects of their lives are essential to the CKD dialysis patients' existence and well-being.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bonenkamp, A.A., van Eck van der Sluijs, A., Hoekstra, T., Verhaar, M.C., van Ittersum, F.J., Abrahams, A.C., et al., 2020. Health-related quality of life in home dialysis patients compared to in-center hemodialysis patients: a systematic review and meta-analysis. Kidney Med., 2:139–154. [DOI] [PMC free article] [PubMed]
- Caplin B., Kumar S., Davenport A. Patients’ perspective of haemodialysis-associated symptoms. Nephrol. Dialysis Transplant. 2011;26:2656–2663. doi: 10.1093/ndt/gfq763. [DOI] [PubMed] [Google Scholar]
- Cardone K.E., Bacchus S., Assimon M.M., Pai A.B., Manley H.J. Medication-related problems in CKD. Adv. Chronic Kidney Dis. 2010;17:404–412. doi: 10.1053/j.ackd.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Cox K.J., Parshall M.B., Hernandez S.H.A., Parvez S.Z., Unruh M.L. Symptoms among patients receiving in-center hemodialysis: A qualitative study. Hemodial. Int. 2017;21:524–533. doi: 10.1111/hdi.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dąbrowska-Bender M., Dykowska G., Żuk W., Milewska M., Staniszewska A. The impact on quality of life of dialysis patients with renal insufficiency. Patient Prefer Adher. 2018;12:577–583. doi: 10.2147/PPA.S156356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.H., Webel A.R., Sehgal A.R., Voss J.G., Huml A. Fatigue in individuals with end stage renal disease. Nephrol. Nurs. J. 2019;46:497–508. [PMC free article] [PubMed] [Google Scholar]
- Alencar SBV de, de Lima, F.M., Dias L do, A., Dias V do, A., Lessa, A.C., Bezerra, J.M., et al., 2020. Depression and quality of life in older adults on hemodialysis. Brazil. J. Psychiatry 42, 195–200. [DOI] [PMC free article] [PubMed]
- Ferrell B.R., Hassey Dow K., Grant M. Measurement of the quality of life in cancer survivors. Qual. Life Res. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- Ferrell B.R., Grant M., Funk B., Garcia N., Otis-Green S., Schaffner M.L. Quality of life in breast cancer. Cancer Pract. 1996;4:331–340. [PubMed] [Google Scholar]
- Gerogianni S., Babatsikou F., Gerogianni G., Koutis C., Panagiotou M., Psimenou E. Social life of patients undergoing haemodialysis. Int. J. Caring Sci. 2016;9:122–134. [Google Scholar]
- Hanspal I., Fathima F., Kedlaya P. Social impact of end-stage renal disease requiring hemodialysis among patients with type-2 diabetes and their caregivers in Bengaluru, Karnataka. Indian J. Community Med. 2021;46:626. doi: 10.4103/ijcm.IJCM_995_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J. S, L. H, M. AT, F. I., 2017. Quality of life of chronic kidney disease patients with routine hemodialysis in general hospitals in SLEMAN YOGYAKARTA. Int. J. Pharm. Pharm. Sci., 9:213.
- Jhamb M., Weisbord S.D., Steel J.L., Unruh M. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am. J. Kidney Diseases. 2008;52:353–365. doi: 10.1053/j.ajkd.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdy C.P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int. Suppl. 2011;2022(12):7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsufuji S., Shoji T., Yano Y., Tsujimoto Y., Kishimoto H., Tabata T., et al. Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. J. Renal Nutrition. 2015;25:17–24. doi: 10.1053/j.jrn.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Mollaoğlu M. Quality of Life in Patients Undergoing Hemodialysis. Suzuki H, editor. InTech. 2013 [Google Scholar]
- Picariello F., Moss-Morris R., Macdougall I.C., Norton S., Da Silva-Gane M., Farrington K., et al. Cognitive-behavioural therapy (CBT) for renal fatigue (BReF): a feasibility randomised-controlled trial of CBT for the management of fatigue in haemodialysis (HD) patients. BMJ Open. 2018;8:e020842. doi: 10.1136/bmjopen-2017-020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picariello F., Moss-Morris R., Norton S., Macdougall I.C., Da Silva-Gane M., Farrington K., et al. Feasibility trial of cognitive behavioral therapy for fatigue in hemodialysis (BReF Intervention) J. Pain Symptom Manage. 2021;61:1234–1246.e5. doi: 10.1016/j.jpainsymman.2020.10.005. [DOI] [PubMed] [Google Scholar]
- PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement criteria. [http://www.prisma-statement.org/].
- Puchalski C.M. The role of spirituality in health care. Baylor University Medical Center Proceedings. 2001;14:352–357. doi: 10.1080/08998280.2001.11927788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve B.B., Stover A.M., Alfano C.M., Smith A.W., Ballard-Barbash R., Bernstein L., et al. The Piper Fatigue Scale-12 (PFS-12): psychometric findings and item reduction in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2012;136:9–20. doi: 10.1007/s10549-012-2212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhardjono U.V., Tedjasukmana D., Setiati S. The effect of intradialytic exercise twice a week on the physical capacity, inflammation, and nutritional status of dialysis patients: A randomized controlled trial. Hemodial. Int. 2019;23:486–493. doi: 10.1111/hdi.12764. [DOI] [PubMed] [Google Scholar]
- Tsai Y-C, Hung C-C, Hwang S-J, Wang S-L, Hsiao S-M, Lin M-Y, et al. Quality of life predicts risks of end-stage renal disease and mortality in patients with chronic kidney disease. Nephrology Dialysis Transplantation [Internet]. 2010;25:1621–6. Available from: https://doi.org/10.1093/ndt/gfp671. [DOI] [PubMed]
- Zazzeroni L., Pasquinelli G., Nanni E., Cremonini V., Rubbi I. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta-analysis. Kidney Blood Press Res. 2017;42:717–727. doi: 10.1159/000484115. [DOI] [PubMed] [Google Scholar]
- Zhang F., Bai Y., Zhao X., Huang L., Zhang Y., Zhang H. The impact of exercise intervention for patients undergoing hemodialysis on fatigue and quality of life. Medicine. 2020;99:e21394. doi: 10.1097/MD.0000000000021394. [DOI] [PMC free article] [PubMed] [Google Scholar]