Summary
Bradyrhizobium is a genus of nitrogen-fixing bacteria, with some species producing nodules in leguminous plants. Investigations into Bradyrhizobium have recently revealed its substantial genetic resources and agricultural benefits, but a comprehensive survey of its genetic diversity and functional properties is lacking. Using a panel of various strains (N = 278), this study performed a comparative genomics analysis to anticipate genes linked with symbiotic nitrogen fixation. Bradyrhizobium’s pan-genome consisted of 84,078 gene families, containing 824 core genes and 42,409 accessory genes. Core genes were mainly involved in crucial cell processes, while accessory genes served diverse functions, including nitrogen fixation and nodulation. Three distinct genetic profiles were identified based on the presence/absence of gene clusters related to nodulation, nitrogen fixation, and secretion systems. Most Bradyrhizobium strains from soil and non-leguminous plants lacked major nif/nod genes and were evolutionarily more closely related. These findings shed light on Bradyrhizobium’s genetic features for symbiotic nitrogen fixation.
Subject areas: Bacteriology, Interaction of plants with organisms, Genomics
Graphical abstract
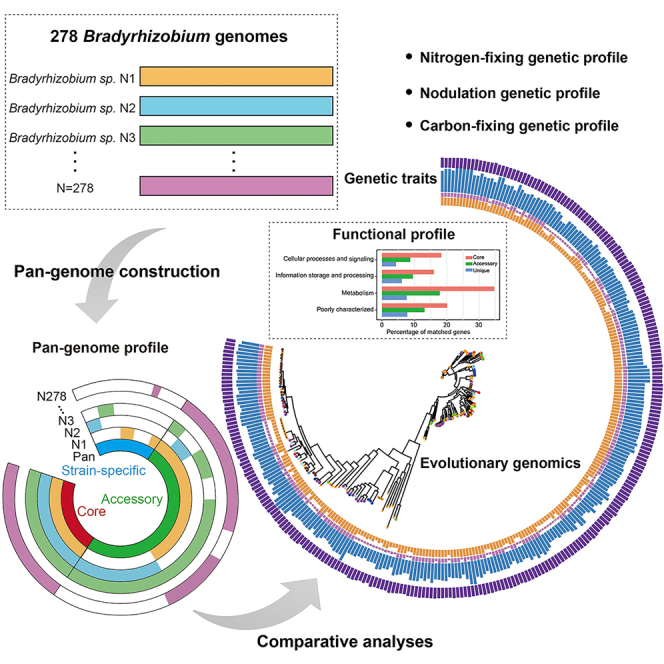
Highlights
-
•
Comparative genomics on a collection of 278 Bradyrhizobium strains was performed
-
•
The genomic diversity of Bradyrhizobium represents an “open” pan-genome model
-
•
Bradyrhizobium strains have dramatically different symbiotic nitrogen fixation genes
-
•
Most Bradyrhizobium strains from soil lack the nif and nod gene clusters
Bacteriology; Interaction of plants with organisms; Genomics
Introduction
As important members of the Rhizobia, bacterial species from the genus Bradyrhizobium are characterized by their diverse biochemical functions, including biological nitrogen fixation and carbon fixation.1,2 They are one of the most prevalent groups in the soil, and many representatives have been identified as being involved in the development and adaptation of host plants.3 This genus belongs to the rhizobia family, which comprises commercially important soil bacteria that interact with the roots of leguminous plants to fix atmospheric nitrogen.4,5 The nitrogen-fixing Bradyrhizobium symbiosis leads to the formation of plant nodules, which boosts nitrogen availability to plants and has agricultural applications.6 The genus Bradyrhizobium is currently encompassing more than 70 species that have been isolated worldwide. Some are symbiotic, able to fix nitrogen and form nodules on legumes, while others are free-living and unable to form nodules.7,8 Variation in the genetic composition of Bradyrhizobium strains has been documented with respect to symbiotic associations and nitrogen fixation.9 The soybean symbionts Bradyrhizoium elkanii, B radyrhizoium Japonicum, and B radyrhizoium diazoefficiens can nodulate soybean and have a remarkable genetic diversity.10 Bradyrhizobium sp. S23321 has been described as free-living and unable to form nodules.5
Bradyrhizobium members have excellent prospects for agricultural research, emphasizing the importance of studying their genomes and identifying the variety embedded in this genus. There is a significant level of diversity knowledge within the Bradyrhizobium genus, as shown by studies on well-adapted Bradyrhizobium species.11,12 Numerous Bradyrhizobium nod and nif factors have recently been characterized, which are responsible for nod-independent nodulation formation and nitrogen fixation, respectively.13 These nodulation or nitrogen fixation genes are also highly variable among different bradyrhizobial species.14 For example, Bradyrhizobium sp. G22 lacked both the nodulation gene nodABC and the nitrogen fixation gene nifDKH that were present in B. diazoefficiens USDA 110.15 According to comparative genomic analysis research, nod genes and nif factors are species and host specific.16,17 Although legumes of the genus Bradyrhizobium are known to be capable of forming symbiotic nodules, the genetic repertoire for nitrogen/carbon fixation is still under investigation. Additionally, studies have shown that nod and nif factors are typically arranged as gene clusters that govern nodulation and nitrogen fixation. Recent studies have shown that while some nonsymbiotic Bradyrhizobium isolates lacking the nod gene cluster are unable to nodulate legumes but are nevertheless common in soils.7,18 These soil isolates have been demonstrated to be deficient in both nodulation and nitrogen fixation, and their genomes lack functional genes related to both processes.7 According to a recent study, some Bradyrhizobium can participate in nod-independent nodulation via the T3SS pathway and do not require nodulation components for nodulation.15 Even though some nonsymbiotic photosynthetic Bradyrhizobium from legumes lack the nod gene, they can fix nitrogen.19 The genetic properties of nod-dependent or nod-independent nitrogen also vary on the host type, which requires the determination of their genomic variation.
The Bradyrhizobium genomes’ accessibility has made it possible to characterize genetic diversity in great detail. Recent research has identified the processes that differ in symbiotic nitrogen fixation in Bradyrhizobium strains with varied genotypes and phenotypes.20 Bradyrhizobium arachidis from Arachis hypogaea and Sophora flavescens have been compared for symbiotic nitrogen fixation, but the genetic diversity within the genus is still under-represented.21 With the availability of a large number of Bradyrhizobium genomes, a pan-genome investigation would certainly be beneficial in uncovering intra-species diversity, such as nitrogen fixation capacity. As the primary determinants of nodulate legumes are frequently encoded by genes that are not shared by all strains, capturing functional gene differences is particularly relevant for Bradyrhizobium.
In this study, we performed a detailed comparative genomics analysis of 278 Bradyrhizobium strains. We constructed the pan-genome of Bradyrhizobium, allowing to characterize different functional gene sets within the genus. We explored the role of core, accessory, and unique genomes to obtain in-depth comparisons of the functional potential of representative strains. We developed a novel phylogenetic understanding of the genus, and also elucidated the genomic variation of symbiosis and nitrogen fixation in Bradyrhizobium, and further explained the genetic diversity of Bradyrhizobium assimilating carbon by fixation. The results of this study will shed new light on the nitrogen and carbon-fixing lifestyle of Bradyrhizobium.
Results and discussion
Bradyrhizobium open pan-genome
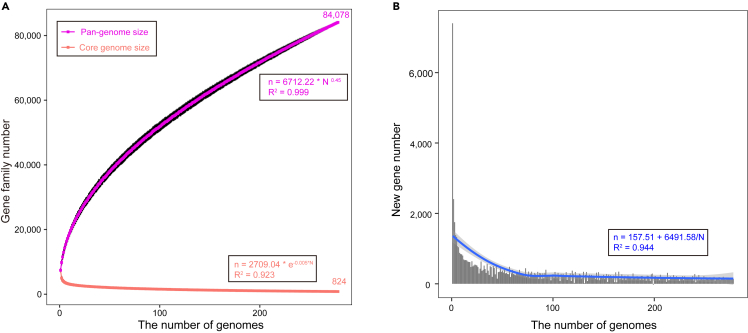
To study the genomic diversity among the members of the genus Bradyrhizobium, 278 strains with complete genomes or draft assemblies (Table S1) from 70 species were downloaded from NCBI for comparative genomics analysis. The OrthoMCL algorithm was applied to protein sequences from all 278 genomes to generate the Bradyrhizobium pan-genome, which contained a total repertoire of 84,078 gene families (non-redundant genes). The pan-genome of Bradyrhizobium consisted of total 824 core gene families (genes shared by all strains), 42,409 accessory gene families (genes shared by two or more strains), and 40,845 unique gene families (genes unique to individual strains). Bradyrhizobium genomes were found to be remarkably diverse, with only a minimal core genome component (0.98% of the pan-genome shared by the genus, and the remaining 99.02% being variable genes, of which up to 48.58% of which were strain-specific genes).
Bradyrhizobium genomes were highly heterogeneous, with an open pan-genome. We constructed accumulation curves for pan- and core genes against the sampled genomes. The power-law regression based on Heap’s law was used to determine the fitted curve of pan-genome accumulation. The developed pan-genome fitting model was represented as n = 6712.22×N0.45, where n is the predicted number of genes (pan-genome size) for a given number of genomes and N is the number of genomes. The Bradyrhizobium pan-genome could be deemed ‘open’ with a power-law coefficient α of 0.45, and demonstrated a progressive expansion by adding new genomes, with no obviously sharp plateau when adding up to 278 strain genomes (Figure 1A). The core genome analysis of 278 genomes converged to a core gene subset of 824 genes, and the number of shared genes decreased when more genomes were included (Figure 1A). The core genome fitting curve followed the exponential regression decay n = 2709.04e−0.005×N. Furthermore, each genome acquired an average of 302 novel genes (Figure 1B). The number of novel genes and core genes gradually decreased as more strains were added, approaching saturation with the addition of 278 strains.
Figure 1.
Prediction of Bradyrhizobium pan- and core genome size
(A) Genome size evolution curves of the pan-genome (purple) and core-genome (red).
(B) Curve (blue) for the number of new genes with an increase in the number of Bradyrhizobium genomes.
Functional characterization of the core, accessory and unique genes
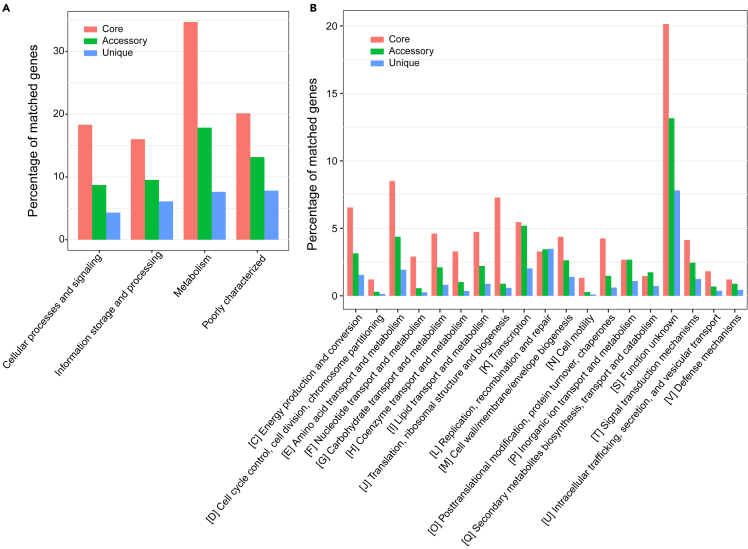
To assess the functional diversity within Bradyrhizobium pan-genome, the COG analysis was employed. The core, accessory, and unique genes were mostly found in the COG functional categories of metabolism (7.63–34.71%), cellular processes and signaling (4.31–18.33%), information storage and processing (6.10–16.02%), and poorly characterized (7.81–20.15%). Approximately 7.8–20.15% of the core, accessory, and unique genes with poorly characterized function indicate that Bradyrhizobium strains have potential pathways or capabilities that have not been determined by the existing COG classification. The percentage of genes related to metabolism was higher in the core, accessory and unique genomes (Figure 2A). The detailed categories of the pan-genome with the highest representation were ‘transcription’ (n = 3,075), ‘replication, recombination and repair’ (n = 2,912), ‘amino acid transport and metabolism’ (n = 2,718) and ‘function unknown’ (n = 8,936). The most common functions in the core genomes are associated with ‘amino acid transport and metabolism’ (8.5%), ‘translation, ribosomal structure and biogenesis’ (7.28%), and ‘energy production and conversion’ (6.55%) (Figure 2B). COG analysis revealed that core genes are functionally diverse and perform basic housekeeping functions regardless of their isolated host. The most abundant categories in the accessory genome include ‘transcription’ (5.19%), ‘amino acid transport and metabolism’ (4.38%) and ‘replication, recombination, and repair’ (3.45%). The unique genes were expected to encode a large number of putative proteins with unknown functions. And ‘replication, recombination, and repair’ accounted for around 3.48% of the unique genome content. Given the open state of the Bradyrhizobium pan-genome, the results of COG enrichment analysis for accessory and unique genomes, in particular ‘replication, recombination, and repair’, support the perspective that the Bradyrhizobium genome was highly plastic. However, a high proportion (10.80%, 50.73%, and 74.14%) of the core, accessory, and unique genome did not have homologs outside the genus. This suggests that deeper gene function analyses would provide more detailed and novel functions for the genus Bradyrhizobium. In addition, the Gene Ontology (GO) enrichment analysis of the pan-genome showed that core genes were enriched in ‘structural constituent of ribosome’, ‘cytosolic large ribosomal subunit’, ‘large ribosomal subunit’, etc. The accessory genes were enriched in ‘cytosol’, ‘cytoplasm’, ‘ATPase activity’, ‘4 iron, 4 sulfur cluster binding’, and ‘integral component of plasma membrane’. Unique genes were enriched in ‘cytosolic large ribosomal subunit’, ‘integral component of plasma membrane’, ‘cytoplasm’, ‘4 iron, 4 sulfur cluster binding’, and ‘large ribosomal subunit’ (Figure S1).
Figure 2.
Classifications of core, accessory and unique genes based on COG categories
(A) Distribution in major COG categories.
(B) Type of gene groups by detailed COG categories. The horizontal coordinate was the content of each COG classification and the vertical coordinate was the percentage of matched genes. Only the proportion of genes that could be annotated to COG was shown.
Phylogenetic relationship of Bradyrhizobium strains
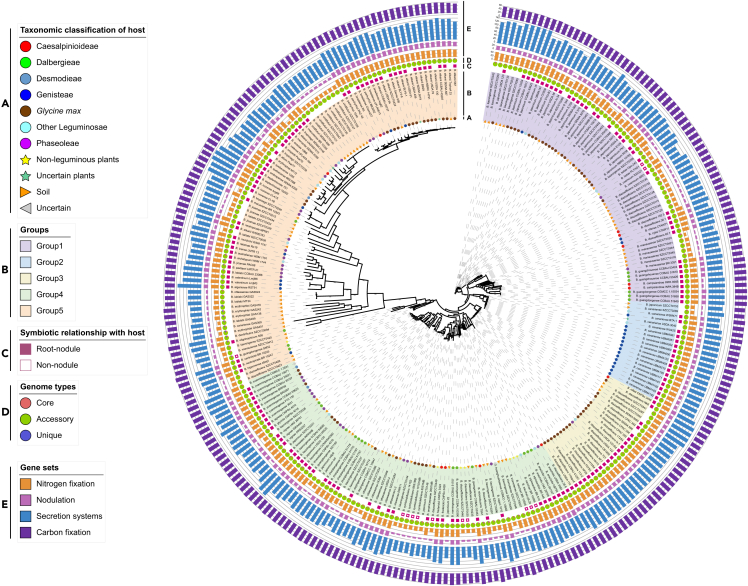
Using concatenated single-copy orthologous genes to resolve phylogenetic relationships could avoid rearrangement-biased phylogenetic tree reconstructions, although it may miss many differences caused by single genes.22,23 The phylogenetic analysis based on the single-copy core genes of the pan-genome provided a complementary perspective on the evolutionary relationships among Bradyrhizobium strains. The phylogenetic tree showed the existence of several clades but revealed a more complex structure within Bradyrhizobium with additional groups (Figure 3). The genetic similarity of most strains varies with different groups of type species of this genus, but not all. Interestingly, the majority of B. japonicum species were assigned to group 1, whereas B. japonicum SZCCT0153 was assigned to group 2 and B. japonicum SZCCT0231, B. japonicum USDA 135, B. japonicum in8p8, B. japonicum is5, B. japonicum SZCCT0148, B. japonicum 22 were assigned to group 4. We found that the strains of B. canariense species clustered in group 2, with the exception of B. canariense GAS369, which was in group 5. In addition, the strain Bradyrhizobium manausense SZCCT0412 also diverged from other B. manausense strains. The genomic profile of the number of accessory genes in these strains was identical, with an exceptionally high proportion of accessory genes in the genome of each strain.
Figure 3.
Phylogenetic tree and genetic profile of the 278 Bradyrhizobium strains
Phylogenetic tree was based on 625 single-copy core genes, and bootstrap support values were calculated from 100 replicates; values above 75 are indicated. Representation of the data (inner to outer layers): (A). Taxonomic classification of isolate host; (B). Phylogenetic clades of strains; (C). Nod-dependent (solid box) and nod-independent (hollow box) relationships with plant hosts, the strains isolated uncertain or from soil were not shown.
(D) Proportion of core, accessory, and strain-specific genes in each strain; (E) The number of functional genes of nitrogen fixation, nodulation, secretion systems, and carbon fixation.
The phylogeny reconstructed using these single-copy genes was largely congruent with previous ones inferred mainly from marker genes by multilocus sequence analysis (MLSA).20 Our results were based on the study of phenotypically variable strains of Bradyrhizobium isolated from a wide range of plants and soils, in which many legume species overlapped and clustered in different subgroups. Despite having partially overlapping ecological niches, there was significant genetic differentiation between B. diazoefficiens, B. japonicum, and B. elkanii. Previous studies have shown that recombination and migration are important evolutionary forces that make species cohesive.24 Several new positions were discovered for some species. For example, B. japonicum, B. diazoefficiens, etc. showed significant intraspecific genetic differentiation. Strains of the same species appeared to be clearly divergent and appeared in different monophyletic clusters, which could be due to a tendency for interspecific recombination or, alternatively, that previous classifications were inaccurate, which requires further investigation and research to confirm. Host-specific clustering was identified in subclades based on the single-copy core genome phylogenetic tree. There appeared to be a relationship between the isolation source of the strains and their evolutionary relationship with other strains. For example, in group 3, the evolutionary relationships of B. diazoefficiens strains isolated from plants tend to cluster more closely together, as do strains from soil, which are also evolutionarily closer to each other. Similar clustering was observed for B. diazoefficiens in group 1.
Repertoire of nitrogen fixation genetic traits
Bradyrhizobium includes both symbiotic nitrogen-fixing bacteria that can form nodules on soybeans and non-symbiotic members that cannot.7,8 The superior symbiotic nitrogen-fixing ability of Bradyrhizobium members is often generated by nif genes and nod genes.13 Characterizing of the genomic features of strains with different symbiotic phenotypes can provide a better understanding of Bradyrhizobium nitrogen fixation and distinguish symbiotic from non-symbiotic strains in Bradyrhizobium. To identify possible genomic signatures associated with nitrogen fixation phenotypes, we examined the distribution of nodulation and nitrogen fixation genes in the pan-genome of Bradyrhizobium, and found 190 genes (Table S3) involved in nodulation and nitrogen fixation, of which 5 were core genes, 141 were accessory genes, and 44 were strain-specific genes. The number of nitrogen fixation and nodulation genes within individual genomes ranged from 10 to 51 and 3 to 29, respectively (Figure 3). The distribution of nitrogen fixation and nodulation genes of these Bradyrhizobium strains appeared to be host specific, with 85.05% of strains from soil harboring fewer nodulation genes (less than 10), whereas strains from legumes had more nodulation genes (Figure 3). For example, B. japonicum SZCCT0280, B. japonicum SZCCT0401, B. japonicum SZCCT0402, B. japonicum SZCCT0403, and B. japonicum SZCCT0395, which were isolated from soil, had fewer nitrogen fixation genes and nodulation genes than those B. japonicum isolated from Glycine max nodules, despite their closer evolutionary relationship in the phylogenetic tree. A similar genetic profile was also observed in species such as B. diazoefficiens and B. canariense. We carried out a clustering analysis and comparisons based on the presence or absence of genes involved in nitrogen fixation and nodulation. It is noteworthy that the clustering presence/absence profiles showed that 278 Bradyrhizobium strains could be classified into two major functionally similar groups (Figure S2), and that the diversity of gene combinations in different nodulation and nitrogen fixation was responsible for the diverse spectrum of host-symbiont interactions.
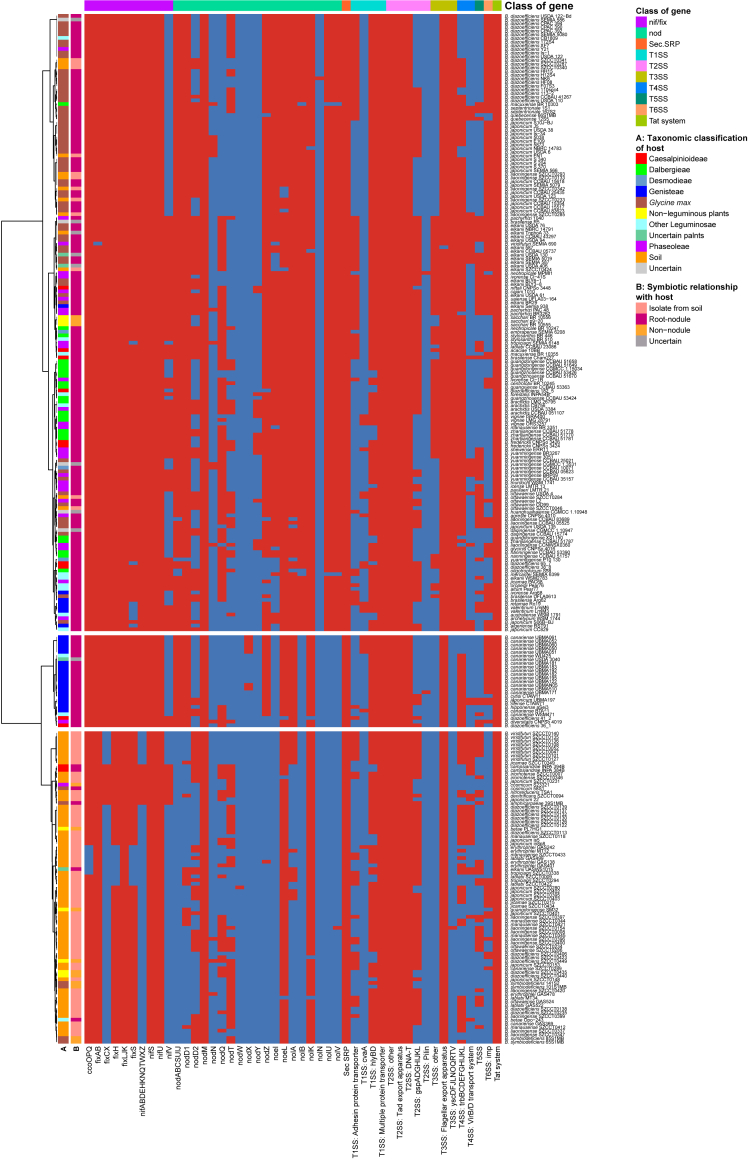
Specific T3SS components in rhizobia have been found to mediate nodule development and have either positive or negative effects in various host nodules.15,25 To further reveal the potential for symbiotic nitrogen fixation, we also detected 401 genes involved in secretion systems, including type I (T1SS), type II (T2SS), type III (T3SS), type IV (T4SS), type V (T5SS) and type VI (T6SS), which have been reported to be important in symbiosis.26 The genes required for nodulation or nitrogen fixation by Bradyrhizobium have been reported typically occur together in a gene cluster.15,20 FixLJK, which responds to hypoxic conditions in soil and nodules and controls the expression of nitrogen fixation and denitrification genes,27 was common among these strains. The critical symbiosis gene clusters for nodulation (nodABCSUIJ) and nitrogen fixation (nifABDEHKNQTWXZ) are functional determinants of symbiotic nitrogen fixation, and Bradyrhizobium lacking these genes is likely to be incapable of nodule formation and nitrogen fixation.20 These gene clusters were found to be associated with host type. We found that the Bradyrhizobium strains isolated from root nodules of Glycine max, Desmodieae, Dalbergieae and other Leguminosae plant hosts had both the nif and nod gene clusters, as well as the ysc gene cluster of the T3SS system (Figure 4), indicating that they could be used as rhizobial inoculants. Although isolated from non-legumes, strains B. sacchari BR 10555, B. sacchari BR 10556, and B. sacchari p9-20 were symbiotic strains carrying nif, nod, and ysc gene clusters. Most of the strains from Genisteae nodules carried the nif and nod gene clusters, but not the ysc gene cluster. The majority of the soil-isolated strains lacked the nif, nod and ysc gene clusters, suggesting that these isolates may not exhibit nod-dependent symbiotic nitrogen fixation, but do contain additional nitrogen-fixing genes such as fixLJK, nifS, nodM (Figures 4 and S2). The strains Bradyrhizobium symbiodeficiens 141S2, B. symbiodeficiens 101S1MB, B. symbiodeficiens 85S1MB, B. symbiodeficiens 65S1MB isolated from Glycine max were free-living members, which could be due to the absence or inactivation of efficient nodulation gene clusters, consistent with their inability to form symbiotic nodules with legumes.28
Figure 4.
Presence (red)/absence (blue) pattern of functional module gene clusters involved in nodules, nitrogen fixation, and secretion system in each Bradyrhizobium strain
Each column represents a cluster of homologous genes. A red box indicates presence of the gene in the corresponding strain while a blue box indicates an absence. The dendrogram was generated based on the ward D method for clustering of Euclidean distances. A strain is considered to contain a gene cluster if 80% of the genes in that cluster are present.
Some strains from the same species, despite their close phylogenetic relationship, they exhibited differences in symbiosis gene cluster, for example, the content of the symbiotic gene content of B. viridifuturi SEMIA 690 differed from that B. viridifuturi species of other strains, which could reflect host characteristics. Previous studies have shown that horizontal gene transfer likely facilitates nif expansion among the free-living Bradyrhizobium.29 Free-living Bradyrhizobium containing nif gene clusters are of particular interest as they may play as yet unidentified roles in the nitrogen cycle in soil.29 Bradyrhizobium members without nitrogen-fixing gene clusters do not fix nitrogen in the soil, but may be adapted to use aromatic soil components.7 The B. cosmicum 58S1 and B. amphicarpaeae 39S1MB strains, which were isolated from Glycine max, did not have the nod and ysc gene clusters, but did have nif. They may use a nod-independent mechanism or they may be nodule-inhabiting strains that have the potential to interact with plants while not nodulating legumes. B. cosmicum S23321 isolated from Phaseoleae is non-nodulating, it contained the nif cluster, and the fixAB, fixCX. The vast majority of the soil group are non-symbiotic lineages. Strains isolated from soil, such as B. japonicum 22, B. nitroreducens TSA1, B. iriomotense SZCCT0007, B. denitrificans SZCCT0094, B. japonicum SZCCT0231, and B. iriomotense SZCCT0346, were free-living nif-carrying Bradyrhizobium. In addition, in combination with the phylogeny, the vast majority of free-living members of the soil B. diazoefficiens formed an independent cluster (Figure 3), and they lacked the nif, nod and ysc gene clusters. We could predict that these strains cannot fix atmospheric nitrogen. Overall, each Bradyrhizobium strain had its own set of genes for nodule formation, nitrogen fixation, and secretion systems, which were expected to collaborate to establish the molecular basis for symbiotic association and nitrogen fixation. We have accessed a previously unrecognized part of the functional diversity of this taxonomic group in soils. However, many gaps remain, including the identification of the correlations between genome size variance and the phylogeny of strains, and symbiotic relationship with the host. This could be overcome by developing a holistic model of symbiosis evolution.20 It is important to continue this research on genes with unknown functions to better estimate mechanisms driving the evolution of mobile genetic elements.
Diversities of genes involved in carbon fixation
The genus Bradyrhizobium is characterized by the presence of a number of genes for carbon fixation. The pan-genome of the 278 strains contained a collection of genes (139, Table S4) for carbon fixation, including 18 core genes, 95 accessory genes, and 26 strain-specific genes (Figure S3). Each strain contained carbon fixation genes ranging from 45 to 67 (Figure 3). Previous studies have shown that not all Bradyrhizobium are capable of photosynthesis,30 and in our study, the genes for photosynthetic carbon fixation, such as ACAT, FBA, GAPDH, glpX-SEBP, maeB, mdh, pgk, rpiA, tktA, and TPI, were present in all strains, suggesting that despite the presence of carbon fixation genes many strains do not possess photosynthetic ability. The strain B. elkanii USDA61 has been noted to be non-photosynthetic,31 but carbon fixation genes were present. This was consistent with the nitrogen-fixing ability of the strain Bradyrhizobium sp. S23321 isolated from paddy soil in Japan, which was found to lack nodulation ability despite the presence of nitrogen fixation (nif) genes.5 These carbon fixation genes did not exhibit host-specific grouping, in contrast to the nodule genes. The accBD, fumAB, and ACAT genes were present in all strains. The succinate dehydrogenase, represented by the sdhABD genes and engaged in carbon oxidation, was detected in all members of the genus. Many Bradyrhizobium strains can fix carbon dioxide via the Calvin cycle, and RuBisCo is the key enzyme at the first step in the Calvin cycle.32 The rbcL gene of RuBisCO was found in 272 strains of the genus Bradyrhizobium, but the remaining six strains all lacked it: B. elkanii 587, B. manausense BR 3351, Bradyrhizobium lablabi SZCCT0009, Bradyrhizobium erythrophlei GAS138, B. lablabi GAS499, and B. erythrophlei GAS401. Our results highlight an unsuspected distribution and diversity of carbon fixation genes within the genus Bradyrhizobium, which may contribute to the genus’s agricultural and environmental applications.
Conclusions
This study presented a comprehensive analysis of Bradyrhizobium, including the largest number (N = 278) of whole Bradyrhizobium genomes retrieved from publicly available repositories. We outlined the genomic variability of this Bradyrhizobium genus, demonstrating that approximately 0.98% (824/84,078 genes) of the pan-genome was found to constitute the core genome and was present in 100% of the strains studied, accomplishing general molecular functions for cell maintenance such as amino acid transport and metabolism, translation, ribosomal structure and biogenesis, energy production and conversion. The accessory genome, on the other hand, demonstrated a wide range of functions, from broad-spectrum nitrogen fixation to nodulation factors potentially establishing effective symbiosis with host cells. However, nitrogen fixation genetic traits were also observed in the core genome.
This study aimed to uncover the evolutionary processes occurring in Bradyrhizobium that may help to understand the molecular mechanisms that drive nitrogen fixation. The phylogenetic relationships of these strains have a tendency to host-specific clustering, with strains from the same host forming many monophyletic clusters, especially those from soil. These strains differed in the gene clusters of functional modules involved in symbiotic nitrogen fixation, such as nodulation, nitrogen fixation and secretion systems, primarily the nod, nif, and ysc gene clusters. Most strains from legumes carried both the nif, nod, and ysc gene clusters, suggesting that they could be used as rhizobial inoculants. Some strains from Genisteae primarily lacked the ysc gene cluster, and most strains from soil were nif-carrying and non-nif-carrying free-living (nod-free) members. This study provided a relatively complete pan-genomic description of the genus Bradyrhizobium as well as a comprehensive understanding of Bradyrhizobium’s nitrogen fixation and carbon fixation genes, which would provide useful understanding in the agricultural applications of Bradyrhizobium.
Limitations of the study
A limitation of this work is that 278 draft genomes were examined, while some genes may be missing from the draft genome assembly but allow for a more comprehensive analysis of Bradyrhizobium. Also, a role for the nif gene cluster in the free-living lifestyle has not yet been recognized, although we have accessed a previously unrecognized part of the functional diversity in symbiotic and nonsymbiotic strains. It remains unclear whether nif-carrying Bradyrhizobium can use a nod-independent strategy to fix nitrogen, and if so, how this mechanism functions needs to be further investigated.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Genome data | NCBI | Table S1 |
| Software and algorithms | ||
| OrthoMCL | Li et al.33 | http://orthomcl.org/orthomcl/ |
| MUSCLE v5.1.0 | Edgar et al.34 | https://www.drive5.com/muscle/ |
| Gblocks v0.91b | Talavera et al.35 | http://molevol.cmima.csic.es/castresana/Gblocks/ |
| RAxML v8.2.12 | Stamatakis et al.36 | https://github.com/stamatak/standard-RAxML |
| eggNOG-mapper | Huerta-Cepas et al.37 | http://eggnog-mapper.embl.de/ |
| KofamKOALA | Aramaki et al.38 | https://www.genome.jp/tools/kofamkoala/ |
| KEGG mapping | Kanehisa et al.39 | https://www.kegg.jp |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Prof. Zhonghua Zhang (gxtczzh@126.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The published article contains all data sets generated or analyzed during this study.
-
•
The codes and scripts used for the phylogenomic and comparative genomic analyses in this study are deposited in the online repository at https://github.com/zhongchaofang/Bradyrhizobium_pangenome.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
This study does not include experimental model or study participant.
Method details
Bradyrhizobium genome sequences
For pan-genome analysis, whole-genome sequences for Bradyrhizobium strains were available from the NCBI Reference Sequence (RefSeq) database. If a strain had multiple genomes, we selected the one with the highest level of genomic integrity for comparison study. In addition, if a strain had multiple replicons (chromosomes and plasmids), a primary chromosome was selected. As a result, 278 Bradyrhizobium genomes from 66 species were carefully included in this study, consisting 52 complete genomes and 226 draft genomes, and 62 genome sequences were from type strains. Their genome information is given in Table S1. The quality and Average Nucleotide Identity (ANI) of the genomes were assessed using CheckM and dRep, and the results were shown in Table S2 and Figure S4.
Pan- and core genome construction
Orthologous pairs were determined for all 278 Bradyrhizobium genomes using OrthoMCL version 2.0.9.33 The BLASTP searches were conducted among mixed protein sequences with an E value of 1.0×10-5, and the minimum score value used in BLASTP results was set to 50%. The MCL with an expansion parameter of 1.5 was used to cluster the filtered BLASTP results, which has been widely utilized in earlier studies of microbial genomes to uncover orthologs between various strains.
Phylogenetic analysis
For phylogenetic analysis, the protein sequences of 625 single-copy core genes were first subjected to the multiple sequence alignment using MUSCLE v5.1.034 with default parameters, and then Gblocks v0.91b35 were used to make the selection of blocks from the multiple sequence alignment results (parameters: -t=p), the Gblocks results were then concatenated sequentially, followed by phylogenetic analysis using RAxML v8.2.1236 to construct a maximum likelihood tree with 100 bootstrap iterations (parameters: -f a -x 12345 -N 100 -m PROTGAMMALGX -n ex -T 30 -p 12345).
Functional analysis
Clusters of orthologous groups (COG) of pan-genome of strains were determined using eggNOG-mapper software.37 Functional annotations of the pan-genome were performed using Kyoto encyclopedia of genes and genomes (KEGG) database. KofamKOALA was used to annotate the functions of pan-genome proteins, which assigns KEGG orthology identifier to proteins via HMMER/HMMSEARCH.38 The results of KofamKOALA were processed by the KEGG Mapper-Reconstruct Pathway utility with manual curation.39 The gene function information associated with nitrogen fixation, nodulation, secretion system, and carbon fixation were then analyzed using R statistical analysis.
The 'pheatmap' function in the 'R' software was used to analyze and visualize the presence/absence pattern of the functional module gene clusters. We clustered the presence (1)/absence (0) matrix using a hierarchical clustering approach, where the Euclidean distance was chosen as the distance metric for the clustering process.
Quantification and statistical analysis
The genome quality assessment was performed in dRep with ANI clustering threshold of 0.95. The statistical analyses of homologous sequences were performed in BLASTP with the E-value < 1e-5. COG, GO and KEGG functions were statistically analyzed with an E-value < 1e-5. Significant enrichment of GO terms was measured by the Fisher's exact test with a p-value < 0.05.
Acknowledgments
This research was supported by the Guangxi Science and Technology Base and Talent Special Project (AD22080023), the Guangxi Natural Science Foundation (2021GXNSFFA196005), and the National Natural Science Foundation of China (42301073).
Author contributions
Z.H.Z., K.N., and C.F.Z. designed and managed the whole project; Z.H.Z. and K.N. led the analyses and manuscript preparation; C.F.Z. performed the analyses and wrote the initial manuscript, and all authors commented on the draft and revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: January 18, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.108948.
Contributor Information
Zhonghua Zhang, Email: gxtczzh@126.com.
Kang Ning, Email: ningkang@hust.edu.cn.
Supplemental information
References
- 1.Rivas R., Martens M., de Lajudie P., Willems A. Multilocus sequence analysis of the genus Bradyrhizobium. Syst. Appl. Microbiol. 2009;32:101–110. doi: 10.1016/j.syapm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R.S., Mok A. Phylogenomics and signature proteins for the alpha proteobacteria and its main groups. BMC Microbiol. 2007;7:106. doi: 10.1186/1471-2180-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uroz S., Buée M., Murat C., Frey-Klett P., Martin F. Pyrosequencing reveals a contrasted bacterial diversity between oak rhizosphere and surrounding soil. Environ. Microbiol. Rep. 2010;2:281–288. doi: 10.1111/j.1758-2229.2009.00117.x. [DOI] [PubMed] [Google Scholar]
- 4.Ormeño-Orrillo E., Martínez-Romero E. A Genomotaxonomy View of the Bradyrhizobium Genus. Front. Microbiol. 2019;10:1334. doi: 10.3389/fmicb.2019.01334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okubo T., Tsukui T., Maita H., Okamoto S., Oshima K., Fujisawa T., Saito A., Futamata H., Hattori R., Shimomura Y., et al. Complete Genome Sequence of Bradyrhizobium sp. S23321: Insights into Symbiosis Evolution in Soil Oligotrophs. Microbes Environ. 2012;27:306–315. doi: 10.1264/jsme2.ME11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker M.A. The spread of Bradyrhizobium lineages across host legume clades: from Abarema to Zygia. Microb. Ecol. 2015;69:630–640. doi: 10.1007/s00248-014-0503-5. [DOI] [PubMed] [Google Scholar]
- 7.VanInsberghe D., Maas K.R., Cardenas E., Strachan C.R., Hallam S.J., Mohn W.W. Non-symbiotic Bradyrhizobium ecotypes dominate North American forest soils. ISME J. 2015;9:2435–2441. doi: 10.1038/ismej.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowan-Nash A.D., Korry B.J., Mylonakis E., Belenky P. Cross-Domain and Viral Interactions in the Microbiome. Microbiol. Mol. Biol. Rev. 2019;83 doi: 10.1128/MMBR.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itakura M., Saeki K., Omori H., Yokoyama T., Kaneko T., Tabata S., Ohwada T., Tajima S., Uchiumi T., Honnma K., et al. Genomic comparison of Bradyrhizobium japonicum strains with different symbiotic nitrogen-fixing capabilities and other Bradyrhizobiaceae members. ISME J. 2009;3:326–339. doi: 10.1038/ismej.2008.88. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko T., Maita H., Hirakawa H., Uchiike N., Minamisawa K., Watanabe A., Sato S. Complete Genome Sequence of the Soybean Symbiont Bradyrhizobium japonicum Strain USDA6T. Genes. 2011;2:763–787. doi: 10.3390/genes2040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal S.K., Dakora F.D. Widespread Distribution of Highly Adapted Bradyrhizobium Species Nodulating Diverse Legumes in Africa. Front. Microbiol. 2019;10:310. doi: 10.3389/fmicb.2019.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siqueira A.F., Ormeño-Orrillo E., Souza R.C., Rodrigues E.P., Almeida L.G.P., Barcellos F.G., Batista J.S.S., Nakatani A.S., Martínez-Romero E., Vasconcelos A.T.R., Hungria M. Comparative genomics of Bradyrhizobium japonicum CPAC 15 and Bradyrhizobium diazoefficiens CPAC 7: elite model strains for understanding symbiotic performance with soybean. BMC Genom. 2014;15:420. doi: 10.1186/1471-2164-15-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepkowski T., Hughes C.E., Law I.J., Markiewicz Ł., Gurda D., Chlebicka A., Moulin L. Diversification of lupine Bradyrhizobium strains: evidence from nodulation gene trees. Appl. Environ. Microbiol. 2007;73:3254–3264. doi: 10.1128/AEM.02125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grönemeyer J.L., Kulkarni A., Berkelmann D., Hurek T., Reinhold-Hurek B. Rhizobia Indigenous to the Okavango Region in Sub-Saharan Africa: Diversity, Adaptations, and Host Specificity. Appl. Environ. Microbiol. 2014;80:7244–7257. doi: 10.1128/AEM.02417-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patra D., Mandal S. Nod–factors are dispensable for nodulation: A twist in bradyrhizobia-legume symbiosis. Symbiosis. 2022;86:1–15. [Google Scholar]
- 16.Lozano M.J., Redondo-Nieto M., Garrido-Sanz D., Mongiardini E., Quelas J.I., Mengucci F., Dardis C., Lodeiro A., Althabegoiti M.J. Comparative Analysis of Three Bradyrhizobium diazoefficiens Genomes Show Specific Mutations Acquired during Selection for a Higher Motility Phenotype and Adaption to Laboratory Conditions. Microbiol. Spectr. 2021;9 doi: 10.1128/Spectrum.00569-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R., Feng Y., Chen H., Zhang C., Huang Y., Chen L., Hao Q., Cao D., Yuan S., Zhou X. Whole-Genome Sequencing of Bradyrhizobium diazoefficiens 113-2 and Comparative Genomic Analysis Provide Molecular Insights Into Species Specificity and Host Specificity. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.576800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollowell A.C., Regus J.U., Gano K.A., Bantay R., Centeno D., Pham J., Lyu J.Y., Moore D., Bernardo A., Lopez G., et al. Epidemic Spread of Symbiotic and Non-Symbiotic Bradyrhizobium Genotypes Across California. Microb. Ecol. 2016;71:700–710. doi: 10.1007/s00248-015-0685-5. [DOI] [PubMed] [Google Scholar]
- 19.Giraud E., Moulin L., Vallenet D., Barbe V., Cytryn E., Avarre J.C., Jaubert M., Simon D., Cartieaux F., Prin Y., et al. Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science. 2007;316:1307–1312. doi: 10.1126/science.1139548. [DOI] [PubMed] [Google Scholar]
- 20.Weisberg A.J., Rahman A., Backus D., Tyavanagimatt P., Chang J.H., Sachs J.L. Pangenome Evolution Reconciles Robustness and Instability of Rhizobial Symbiosis. mBio. 2022;13 doi: 10.1128/mbio.00074-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W.F., Meng X.F., Jiao Y.S., Tian C.F., Sui X.H., Jiao J., Wang E.T., Ma S.J. Bacteroid Development, Transcriptome, and Symbiotic Nitrogen-Fixing Comparison of Bradyrhizobium arachidis in Nodules of Peanut (Arachis hypogaea) and Medicinal Legume Sophora flavescens. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.01079-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salichos L., Rokas A. Inferring ancient divergences requires genes with strong phylogenetic signals. Nature. 2013;497:327–331. doi: 10.1038/nature12130. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N., Zeng L., Shan H., Ma H. Highly conserved low-copy nuclear genes as effective markers for phylogenetic analyses in angiosperms. New Phytol. 2012;195:923–937. doi: 10.1111/j.1469-8137.2012.04212.x. [DOI] [PubMed] [Google Scholar]
- 24.Vinuesa P., Silva C., Werner D., Martínez-Romero E. Population genetics and phylogenetic inference in bacterial molecular systematics: the roles of migration and recombination in Bradyrhizobium species cohesion and delineation. Mol. Phylogenet. Evol. 2005;34:29–54. doi: 10.1016/j.ympev.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki S., Tittabutr P., Teulet A., Thouin J., Fardoux J., Chaintreuil C., Gully D., Arrighi J.-F., Furuta N., Miwa H., et al. Rhizobium–legume symbiosis in the absence of Nod factors: two possible scenarios with or without the T3SS. ISME J. 2016;10:64–74. doi: 10.1038/ismej.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tampakaki A.P. Commonalities and differences of T3SSs in rhizobia and plant pathogenic bacteria. Front. Plant Sci. 2014;5 doi: 10.3389/fpls.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrières L., Francez-Charlot A., Gouzy J., Rouillé S., Kahn D. FixJ-regulated genes evolved through promoter duplication in Sinorhizobium meliloti. Microbiology. 2004;150:2335–2345. doi: 10.1099/mic.0.27081-0. [DOI] [PubMed] [Google Scholar]
- 28.Bromfield E.S.P., Cloutier S., Nguyen H.D.T. Description and complete genome sequences of Bradyrhizobium symbiodeficiens sp. nov., a non-symbiotic bacterium associated with legumes native to Canada. Int. J. Syst. Evol. Microbiol. 2020;70:442–449. doi: 10.1099/ijsem.0.003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao J., Wang S., Liao T., Luo H. Evolutionary origin and ecological implication of a unique nif island in free-living Bradyrhizobium lineages. ISME J. 2021;15:3195–3206. doi: 10.1038/s41396-021-01002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin C.-Z., Wu X.-W., Zhuo Y., Yang Y., Li T., Jin F.-J., Lee H.-G., Jin L. Genomic insights into a free-living, nitrogen-fixing but non nodulating novel species of Bradyrhizobium sediminis from freshwater sediment: Three isolates with the smallest genome within the genus Bradyrhizobium. Syst. Appl. Microbiol. 2022;45 doi: 10.1016/j.syapm.2022.126353. [DOI] [PubMed] [Google Scholar]
- 31.Okazaki S., Kaneko T., Sato S., Saeki K. Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system. Proc. Natl. Acad. Sci. USA. 2013;110:17131–17136. doi: 10.1073/pnas.1302360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hügler M., Sievert S.M. Beyond the Calvin Cycle: Autotrophic Carbon Fixation in the Ocean. Ann. Rev. Mar. Sci. 2011;3:261–289. doi: 10.1146/annurev-marine-120709-142712. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Stoeckert C.J., Jr., Roos D.S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar R.C. MUSCLE v5 enables improved estimates of phylogenetic tree confidence by ensemble bootstrapping. bioRxiv. 2021 doi: 10.1101/2021.06.20.449169. [DOI] [Google Scholar]
- 35.Talavera G., Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 36.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huerta-Cepas J., Forslund K., Coelho L.P., Szklarczyk D., Jensen L.J., von Mering C., Bork P. Fast Genome-Wide Functional Annotation through Orthology Assignment by eggNOG-Mapper. Mol. Biol. Evol. 2017;34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aramaki T., Blanc-Mathieu R., Endo H., Ohkubo K., Kanehisa M., Goto S., Ogata H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2020;36:2251–2252. doi: 10.1093/bioinformatics/btz859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanehisa M., Sato Y., Kawashima M. KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 2022;31:47–53. doi: 10.1002/pro.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The published article contains all data sets generated or analyzed during this study.
-
•
The codes and scripts used for the phylogenomic and comparative genomic analyses in this study are deposited in the online repository at https://github.com/zhongchaofang/Bradyrhizobium_pangenome.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.