Abstract
Infection of human monocyte-derived macrophages (HMDM) and J774 cells (murine macrophage cell line) with several enteroaggregative and cytodetaching Escherichia coli (EAggEC and CDEC, respectively) strains demonstrated that some strains could induce macrophage cell death accompanied by release of lactate dehydrogenase activity and interleukin 1β (IL-1β) into culture supernatants. The mode of cell death differed in the two types of macrophages. Damage to macrophage plasma membrane integrity without changes in nuclear morphology resulted in cytolysis of HMDM. This mechanism of cell death has been previously described for virulent Shigella infection of HMDM and is termed oncosis. In contrast, infection of J774 cells by EAggEC and CDEC strains resulted in apoptosis. The presence of α-hemolysin (Hly) in EAggEC and CDEC strains appears to be critical for both oncosis in HMDM and apoptosis in J774 cells. Bacteria lacking Hly, including Hly− EAggEC strains as well as enterotoxigenic, enteropathogenic, and enterohemorrhagic E. coli strains, behaved like avirulent Shigella flexneri in that the macrophage monolayers were intact, with no release of lactate dehydrogenase activity or IL-1β into the culture supernatants.
A wide range of pathogenic bacteria such as Shigella species, Salmonella species, and Vibrio cholerae interact with the gastrointestinal mucosal surface through the follicle-associated epithelium (FAE) that is unevenly distributed across the epithelial cell layer. The FAE is composed of specialized endocytic cells, M cells, whose basolateral surface is invaginated to form a pocket containing T and B lymphocytes as well as antigen-presenting cells such as dendritic cells and macrophages. The importance of M cells is becoming increasingly recognized in mucosal immunity for the capacity of these cells to ingest, sample, and transport macromolecules and for the role they play in bacterial pathogenesis (26, 34, 35). Bacteria, such as Shigella, that use M cells as an invasion route can infect and destroy M cells and local macrophages, escaping into the relatively less hostile environment of the epithelial monolayer at its basolateral surface (34, 35). Interaction of Shigella with macrophages causes the release of proinflammatory cytokines such as interleukin-1β (IL-1β [14, 45]). A growing body of evidence indicates that processing and presentation of antigens occur during the interaction of pathogens with the M cells, leading to an immune response (26, 34, 35). Thus, the behavior of individual bacterial pathogens with macrophages has become an important focus of study in pathogenesis.
Previous reports have shown that human monocyte-derived macrophages (HMDM) infected with virulent Shigella flexneri in vitro die by a rapid cytolytic process which involves cell swelling, plasma membrane disintegration, and karyolysis (14). This mode of cell death has been termed oncosis (28). Mature IL-1β is released into the culture supernatants during HMDM infection (14). The Shigella IpaB protein plays a critical role in macrophage cell death (14, 45). Cells of the mouse macrophage cell line J774 infected with virulent Shigella also release IL-1β when they die, but death occurs by apoptosis (45). Thus, bacterial strains may activate two entirely different modes of cell death depending on the type of macrophage infected.
Epidemiological studies have associated enteroaggregative Escherichia coli (EAggEC) strains with diarrheal disease in children, particularly among cases of persistent diarrhea (≥14 days) (6, 10, 12, 17, 20, 27). Several factors normally associated with virulence have been identified for some, but not all, EAggEC strains (7). These factors include cytotoxin, enterotoxin, fimbriae, and Hly (1, 12, 20, 24, 32, 33, 37, 40). Cytodetaching E. coli (CDEC) strains have been associated with diarrhea in children less than 18 months old (12, 17). The cytodetaching activity on epithelial cells is due to the presence of an α-hemolysin which is homologous to α-hemolysin seen in urinary tract isolates of E. coli (1, 2, 5). This report describes the results of a comparative analysis of HMDM and J774 macrophage infection with strains of EAggEC and CDEC. Here too, different modes of cell death occurred in each macrophage cell type, and in each type of macrophage, the features of cell death were similar to those previously described for infection with virulent Shigella strains (14, 45). Like the Shigella IpaB protein, the presence of an α-hemolysin in these EAggEC and CDEC strains appears to be critical for both types of macrophage cell death.
MATERIALS AND METHODS
Bacterial strains.
The EAggEC and CDEC bacterial strains used in this study are listed in Table 1. Antibiotics were added at the following concentrations when appropriate: ampicillin (Sigma, St. Louis, Mo.), 100 μg/ml; kanamycin (Sigma), 50 μg/ml; and streptomycin (Sigma), 300 μg/ml.
TABLE 1.
Strains used in this study
| EAggEC or CDEC strain | Serotype | AA probea | Hly presence or absencec | PCR band (hlyA)d | LDH releasee | IL-1β releasee |
|---|---|---|---|---|---|---|
| O42 | O44:H18 | + | − | − | − | − |
| 17-2 | O3:H2 | + | + | + | + | + |
| JM221 | O92:H33 | + | − | − | − | − |
| 69H77-1 | − | − | − | − | − | |
| 52H72.2 | − | NDb | ND | − | ND | |
| DS244R3 | + | − | ND | − | ND | |
| WC216-1-1 | R:H27 | + | − | ND | − | ND |
| WC201-1-4 | O81:NM | − | + | − | − | ND |
| H32-1 | − | − | ND | − | − | |
| Thai501-1-1 | Rough | − | + | − | − | ND |
| DS65-R3 | − | − | ND | − | ND | |
| 81H194-2 | + | − | ND | − | ND | |
| 54H-191-1 | + | − | ND | − | ND | |
| 697 (WRAIR) | ND | + | + | + | + | |
| 55-3 Hly+ (CDEC) | O6:H− | ND | + (two copies) | + | + | + |
| 55-3 Hly− (CDEC) | ND | − | − | − | − |
AA probe refers to a specific DNA probe present in some but not all EAggEC strains (see references 3 and 7).
ND, not determined.
Hly determined on blood agar plates.
hlyA determined by PCR amplification with hlyA primers.
LDH activity and IL-1β release were determined with both HMDM and J774 cells.
Cell culture and macrophage infections.
Monocytes were isolated by counterflow centrifugal elutriation of leukopacks obtained from healthy volunteers and cultivated for macrophages (HMDM) as previously described (14). The murine macrophage-like cell line J774 was cultured as described elsewhere (14). Twenty-four hours prior to infection, the macrophages were resuspended in fresh medium and added to 24-well culture plates or 100-mm-diameter tissue culture plates at a concentration of 106 cells/ml. On the following day, the plates were washed to remove nonadherent cells before infection, and fresh medium without antibiotics was added. For all macrophage infections, overnight cultures of the bacterial strains were diluted 1:50 in 10 ml of Luria-Bertani broth (Difco, Detroit, Mich.) and were incubated at 37°C for 2 h. The bacteria were harvested and resuspended in 1 ml of Hanks balanced salt solution (HBSS; Gibco BRL, Gaithersburg, Md.).
The macrophages were infected as described elsewhere (14). Briefly, 5 to 20 μl of the bacterial suspension (with a multiplicity of infection [MOI] of approximately 3 to 30) was added to each well in a 24-well plate, and the plate was centrifuged at 500 rpm for 5 min in a Sorvall RT6000B instrument at room temperature. The plates were incubated for different periods of time in a CO2 incubator at 37°C. The wells were then washed four to six times with HBSS and incubated again with RPMI medium containing 50 μg of gentamicin per ml. To determine bacterial survival at selected intervals after infection, the medium was removed, and the macrophages were washed and lysed with 0.1% Triton X-100. The number of viable bacteria was determined by plating serial dilutions of macrophage lysates on tryptic soy agar (TSA) plates.
PCR amplification of hemolysin.
Hemolysin primers hlyA-1 (5′-GCACACTGCAGTCTGCAAAG-3′ [residues 1351 to 1370]) and hlyA-2 (5′-TCACTGGCATTACCGGACA-3′ [residues 4345 to 4327]) were obtained from the E. coli J96 chromosomal hemolysin sequence (accession no. M10133). PCR conditions were 5 min at 95°C, followed by 30 cycles of 1 min at 94°C, 1 min at 48°C, and 1 min at 72°C, and an elongation of 7 min at 72°C.
Light microscopy and TEM analysis of infected macrophages.
Human or murine macrophages were seeded into tissue chamber slides (Lab-Tek Chamber Slide; Nalge Nunc Int., Naperville, Ill.) and incubated at 37°C in a humidified 5% CO2 atmosphere. At selected intervals after infection, the slides were washed and stained as described elsewhere (14). Transmission electron microscopic (TEM) analysis of macrophages infected with bacterial strains has been described elsewhere (14).
LDH and cytokine assays.
Macrophages were infected with different bacterial strains, and lactate dehydrogenase (LDH) activity in the culture supernatants was measured at timed intervals with the colorimetric CytoTox96 kit (Promega Corp., Madison, Wis.) according to the manufacturer’s instructions, with modifications as described elsewhere (14). Enzyme-linked immunosorbent assays for human or mouse IL-1β were performed according to the instructions of the manufacturer (Endogen, Woburn, Mass.).
Gel electrophoresis of DNA fragmentation.
Internucleosomal DNA fragmentation of infected macrophages was measured by a method described elsewhere (14, 30). The samples were electrophoresed on 1.2% agarose gels and stained with ethidium bromide.
Statistical analysis.
Statistical analysis was done by Student’s t test with the INSTAT statistical analysis package (Graph Pad Software, Inc., San Diego, Calif.). Significance was defined as P < 0.05.
RESULTS
Survival of EaggEC and CDEC strains during in vitro infection of HMDM and J774 cells.
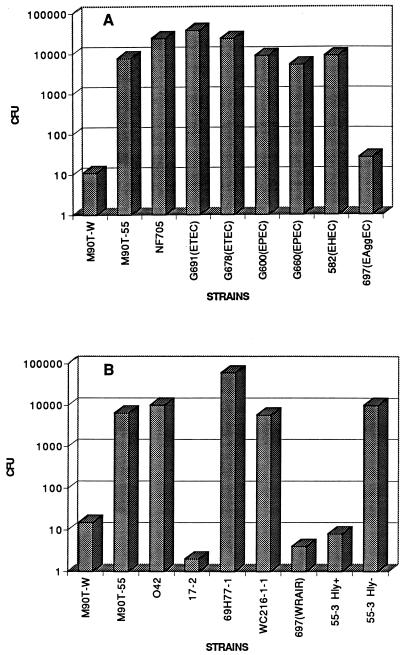
Several pathogenic E. coli strains in both HMDM and J774 macrophages were tested to determine their survival compared to that of Shigella. The results of one representative experiment with HMDM are shown in Fig. 1. Macrophages were infected for 30 min, washed, and further incubated in gentamicin-containing medium for 50 min as previously described (14). The number of CFU was obtained by plating dilutions of the macrophage lysates on tryptic soy agar plates. Invasive S. flexneri 5 strain M9OT-W and the corresponding plasmidless noninvasive strain M9OT-55 were included in each assay for comparative purposes. The numbers of CFU recovered after infection with normal E. coli NF705 or enterotoxigenic E. coli (ETEC) strains were two- to threefold higher than the numbers of CFU recovered after infection with M9OT-55, enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), and noninvasive enteroinvasive E. coli (EIEC) strains (Fig. 1A). In contrast, some EAggEC strains, such as 697 and 17-2 (Fig. 1A and B), gave very few CFU, similarly to what was obtained with M9OT-W infection (Fig. 1A and B), while other EAggEC strains were recovered in numbers either comparable to or higher than those for M9OT-55 (Fig. 1B). M9OT-W is recovered poorly because this strain lyses macrophages, exposing the bacteria to the gentamicin-containing medium (14, 45). Several experiments described below demonstrate that a similar event was also responsible for the lower recovery of 17-2, 697, and Hly+ 55-3 strains.
FIG. 1.
Survival of bacterial strains in HMDM; HMDM infected with various E. coli and Shigella strains (A) and various EAggEC strains (B). CFU represents the total number of bacteria in macrophage cell lysates. M9OT-W, virulent S. flexneri 5; M9OT-55, isogeneic plasmid-cured strain; NF705, normal E. coli strain. The rest of the strains are described in Table 1 and in the text.
Infection with isogenic Hly+ and Hly− strains resulted in poor recovery of Hly+ CDEC strain 55-3, while the corresponding Hly− 55-3 was recovered at values that were 3 log units greater, similarly to the rest of the EAggEC strains and M9OT-55 (Fig. 1B). EAggEC 17-2 and 697 demonstrated hemolytic activity on sheep blood agar plates; the rest of the EAggEC strains were negative in this assay (Table 1). The presence of an α-hemolysin gene in 17-2 and 697 was confirmed by PCR analysis with primers derived from the E. coli hlyA gene (see Materials and Methods).
Macrophage cell death or cytotoxicity was measured by the release of LDH activity in the culture supernatants, which is a reflection of the loss of macrophage plasma membrane integrity. The results of a representative experiment are shown in Table 2. HMDM infected with 17-2, 697, and Hly+ 55-3 released maximal levels of LDH activity within 2 h of infection, comparable to levels reached upon infection with M9OT-W. No LDH activity was detectable in the culture supernatants of macrophages infected with Hly− 55-3, nonhemolytic EAggEC strains, or M9OT-55 (Tables 1 and 2). Similar results were also obtained with J774 macrophages; however, the time taken for obtaining maximal release of LDH was longer (data not shown).
TABLE 2.
Determination of macrophage cytotoxicity
| Strain | Time (h) | Amt of IL-1β (pg/ml) | % of Cytotoxicitya | CFUb |
|---|---|---|---|---|
| M9OT-W | 1 | 44 | 44 | ND |
| 2 | 375 | 90 | 15 | |
| M9OT-55 | 1 | 0 | 1.3 | ND |
| 2 | 0 | 1.5 | 6.4 × 103 | |
| Hly+ CDEC | 1 | 11 | 52 | ND |
| 2 | 25.6 | 98 | 8 | |
| Hly− CDEC | 1 | 0 | 1.4 | ND |
| 2 | 0 | 2 | 10 × 104 |
Percentage of cytotoxicity refers to LDH released from HMDM calculated as a percentage of maximal release from uninfected cultures.
CFU obtained after 30 min of infection followed by 50 min of incubation in gentamicin-containing medium. ND, not determined.
Infection of HMDM and J774 cells with Hly+ 55-3 resulted in the secretion of mature IL-1β into the culture supernatant (Tables 1 and 2), a feature shared with virulent Shigella infection (14, 45). However, significantly less IL-1β was released from macrophages infected with Hly+ CDEC strains than M9OT-W during the same time period (Table 2).
Light microscopic analysis of human and murine macrophages infected with EAggEC and CDEC strains.
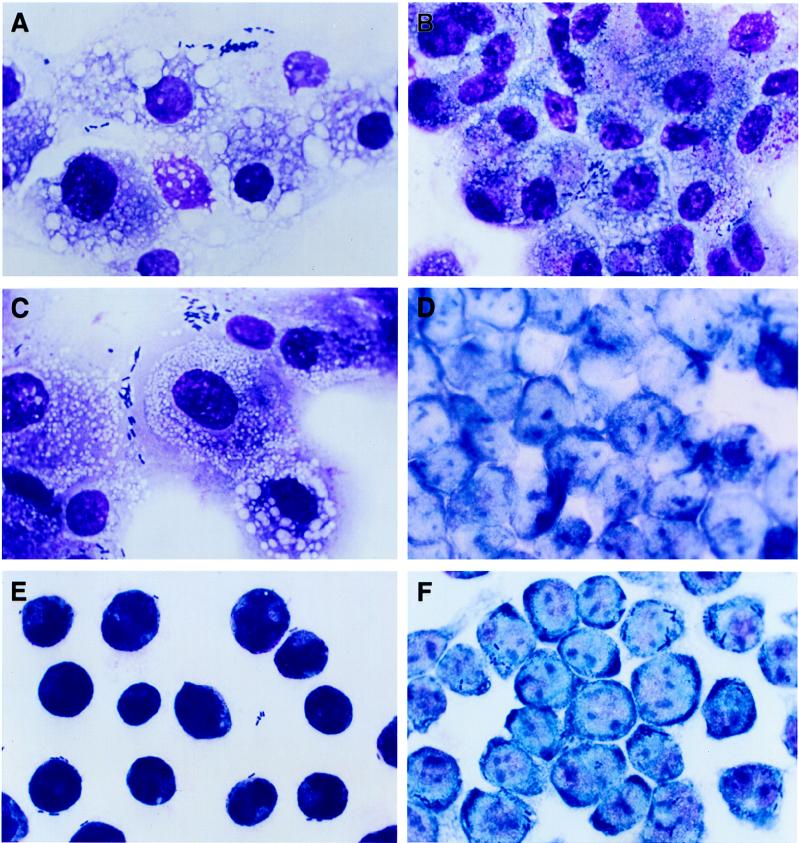
In order to determine the mode of macrophage cell death, infected HMDM and J774 macrophages were studied by light microscopy. HMDM infected with Hly+ 55-3 (Fig. 2A) and EAggEC strains 17-2 and 697 showed cytolysis which disrupted the cell monolayers. The cells appeared swollen and vacuolated, with morphological characteristics of oncosis similar to the changes described for M9OT-W-infected HMDM (Fig. 2C) (14). Internalized bacteria were observed in some cells. The nuclei appeared morphologically similar to uninfected macrophages, with no evidence of the compaction and condensation characteristic of apoptosis. HMDM infected with the Hly− 55-3 strain (Fig. 2B) or with any of the other EAggEC strains had larger numbers of intracellular bacteria. The monolayers appeared relatively intact, and none of the large vacuoles could be observed in these cells (Fig. 2B). The nuclear morphology was similar to that of uninfected or Hly+-infected macrophages. In contrast, J774 cells infected with Hly+ E. coli (Fig. 2E) showed compacted and condensed nuclei which were clearly different from the nuclei of uninfected (Fig. 2D) or Hly−-infected macrophages (Fig. 2F). Some cell shrinkage could be observed in macrophages infected with Hly− cells compared to uninfected macrophages (compare Fig. 2D with F).
FIG. 2.
Light microscopic analysis of HMDM and J774 murine macrophages infected with EAggEC and CDEC strains. Bacteria were left in contact with HMDM (A, B, and C) and J774 macrophages (D, E, and F) for 1 h. Macrophages were stained with a modified Wright’s stain after infection with Hly+ CDEC 55-3 (A and E), Hly− CDEC 55-3 (B and F), S. flexneri 5 M9OT-W (C), and uninfected J774 (D). Magnification, ×910.
Analysis of nuclear DNA by fragmentation assays.
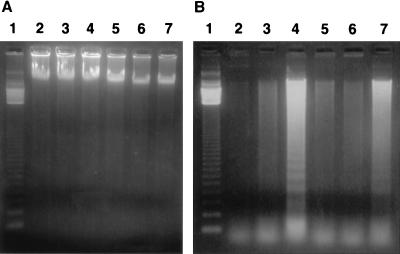
Demonstration of internucleosomal DNA fragmentation is often used to indicate cells undergoing apoptosis. In order to confirm the occurrence of two different modes of cell death induced by the same organism, DNA extracted from macrophages after infection was subjected to agarose gel electrophoresis. DNA extracted from HMDM after infection with Hly+ EAggEC and CDEC strains showed no evidence of chromatin cleavage, confirming that these macrophages are killed by a mechanism distinct from apoptosis (Fig. 3A). On the other hand, extraction of DNA from J774 macrophages infected with Hly+ E. coli indicated a ladder pattern of low-molecular-weight DNA characteristic of apoptosis (Fig. 3B). These results correlate well with nuclear condensation observed with light microscopy (Fig. 2). These characteristic apoptotic responses were also seen in J774 cells infected with M9OT-W (45) but were much less pronounced in cells not infected with Hly+ E. coli.
FIG. 3.
DNA fragmentation assays on agarose gels. DNA was isolated from macrophages infected with different EAggEC and CDEC strains. M9OT-W and M9OT-55 were included for comparative purposes. The DNA was electrophoresed on 1.2% agarose gels for 3 h at 100 V. DNA was isolated from HMDM (A) or J774 (B) murine macrophages infected with M9OT-55 (lanes 3), EAggEC 697 (Hly+) (lanes 4), CDEC 55-3 (Hly+) (lanes 5), CDEC 55-3 (Hly−) (lanes 6), and M9OT-W (lanes 7). Lanes 1, 123-bp DNA ladder molecular-weight-marker (BRL); lanes 2, DNA isolated from noninfected macrophages.
TEM analysis of HMDM infected with EAggEC and CDEC strains.
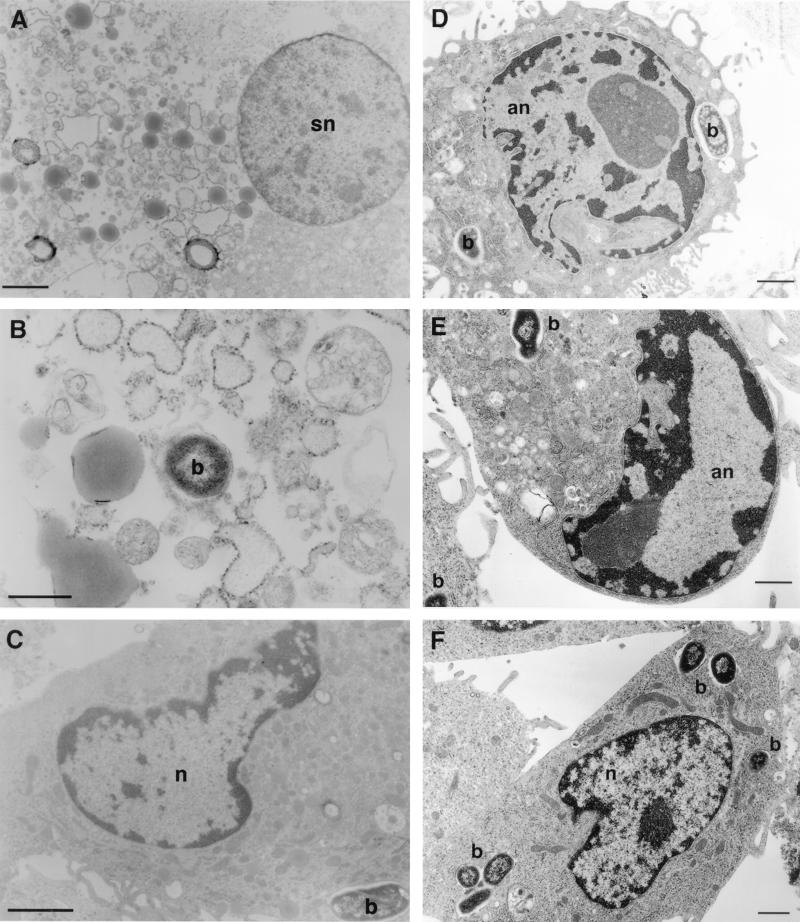
TEM analysis of infected macrophages confirmed observations made by both light microscopy and DNA analysis. Prominent features of cell lysis, i.e., swollen mitochondria and lysed and swollen nuclei, could be demonstrated in HMDM infected with Hly+ 55-3, even at an MOI of 3 bacteria per macrophage (Fig. 4A and B). Similar cell destruction was also observed when HMDM were infected with 17-2, 697, and M9OT-W (data not shown [14]). There was no evidence of apoptosis in HMDM infected with these hemolytic strains. HMDM infected with Hly− 55-3 looked like uninfected macrophages with internalized bacteria within vacuoles (Fig. 4C). In contrast, J774 cells infected with Hly+ 55-3 showed striking condensation and marginalization of chromatin material characteristic of apoptosis (Fig. 4D and E). Nuclei of J774 cells infected with Hly− 55-3 strains looked like uninfected macrophages, with several bacteria seen within enclosed vacuoles (Fig. 4F). The nuclei of Hly+ and Hly− EAggEC strains showed characteristics similar to those of Hly+ and Hly− CDEC strains as described above (data not shown).
FIG. 4.
TEM of HMDM and J774 cells infected with hemolysin-positive and -negative EAggEC and CDEC strains. (A and B) HMDM infected with Hly+ EAggEC shown in a state of necrosis; (C) HMDM infected with Hly− EAggEC or CDEC strains; (D and E) J774 cells infected with Hly+ CDEC strain 55-3; (F) J774 cells infected with Hly− CDEC strain 55-3. Bar markers: 2 μm (A and C) and 1 μm (B, D, E, and F). b, bacteria; n, nucleus; and sn, swollen nucleus; an, apoptotic nucleus.
DISCUSSION
Hemolysin-positive EAggEC and CDEC strains caused oncosis in HMDM and apoptosis in J774 cells. These two forms of cell death can be distinguished morphologically and biochemically (39, 43, 44). Apoptotic cells are characterized by rapid, irreversible condensation of the cytoplasm and compaction and marginalization of chromatin in the nucleus. Eventually, the cells split into a cluster of membrane-bound bodies (43). In vivo, apoptotic cells are phagocytosed and disappear rapidly from the tissue without generating an inflammatory response. This is a hallmark of programmed cell death in many developmental systems (43, 44). In vitro demonstrations of apoptosis rely on microscopy as well as internucleosomal cleavage of DNA as shown in this study with J774 cells. In contrast to J774 macrophages, HMDM infected with Hly+ E. coli showed oncosis, i.e., cell swelling, alteration of cytoplasmic organelles, normal chromatin disposition, rupture of the plasma membrane, and cytolysis. Recently, several reports of apoptosis with no evidence of DNA laddering and DNA ladders in cells without apoptotic morphology have also been described (8, 9, 11, 16, 38). For example, MDCK cells undergoing necrosis showed DNA fragmentation which was abolished by serine protease inhibitors but not by inhibitors of ICE protease (11). Apoptosis in these cells was excluded by phase-contrast and electron microscopy and by Hoechst staining. These and other studies emphasize that techniques based solely on demonstration of DNA fragmentation may not reliably distinguish between apoptosis and necrosis (8, 9, 11, 16, 38) and must be accompanied by evidence of morphological changes in the nuclei.
It is unclear what molecular events determine which pathway of cell death will occur. Whether a toxin induces necrosis or apoptosis appears to be related to the rapidity of cell membrane damage and to the extent of such damage (22, 29). For example, low concentrations of S. aureus alpha toxin and Actinobacillus leukotoxin bind with high affinity to plasma membrane receptors, form small pores in the host cell membrane which allow influx of Na+ but not Ca+ ions, and induce apoptosis (22, 29). At higher concentrations, larger pores are formed, resulting in influx of Ca+, depletion of ATP, and necrosis. It can be speculated that HMDM are more sensitive to membrane damage during bacterial infection and, therefore, more susceptible to oncosis than J774 cells. While this hypothesis cannot be ruled out, a 10-fold greater or lesser MOI did not alter the outcome in either type of macrophage (unpublished observations). Furthermore, similar differences in endpoint outcome between the two types of macrophages was also observed after infection with virulent Shigella (14), indicating that perhaps the differences in response between HMDM and J774 cells may be related, in each macrophage type, to the accessibility of one pathway over another. This, in turn, could be a reflection of the unique surface and microenvironmental properties of these two types of macrophages.
That heterogeneity of response exists between different macrophage types is evidenced in the killing of intracellular pathogens. Murine peritoneal macrophages activated with gamma interferon and tumor necrosis factor alpha can kill several pathogens by a mechanism involving reactive N2 intermediates, whereas similar mechanisms of intracellular killing have not been demonstrated in HMDM (4, 15). Heterogeneity of cellular responses may also be reflective of species specificity, state of maturity, and differentiation. While the majority of human T cells treated with Actinobacillus leukotoxin undergo necrosis, approximately 30% of the cells appear unaffected, and 10% show signs of apoptosis (29). Heterogeneity of response to the same pathogen is seen with listeriolysin O-containing Listeria monocytogenes, which grows within mouse peritoneal macrophages and J774 cells but induces apoptosis in hepatocytes, lymphocytes, and dendritic cells (15, 18, 25, 31, 36). Furthermore, some macrophages are able to kill L. monocytogenes, whereas other macrophages in the same host are unable to do so (15). Intracellular levels of iron and recruitment of receptors that internalize the bacteria, as well as the effect of cytokines, have all been shown to influence listericidal activity. Thus, the induction of a cellular response to infection will be determined by the type of cell infected, its trophic environment, its ability to modulate the expression of cell survival genes, and perhaps other factors related to the restoration of homeostasis (43, 44).
The widespread occurrence of Hly in many enteric and nonenteric strains is attributed to transposition events and may account for its distribution in some EAggEC strains. While a clear role for Hly in E. coli strains that cause urinary tract infections has been established, the role of the Hly gene in enteric infections is less clear. In the rabbit RITARD model, Hly+ CDEC was associated with twice as much inflammation in the small intestine compared to nonhemolytic bacteria and with four times as much inflammation in the large intestine (13). Hly was also associated with greater frequency and severity of diarrhea as well as greater histopathological changes, including edema, necrosis, inflammation, and infiltration of polymorphonuclear leukocytes (13). The ability of Hly+ CDEC and EAggEC strains to kill macrophages and release proinflammatory cytokines may contribute to the manifestation of disease. Although hemolysin-positive EAggEC and CDEC strains were observed within the phagocytic vacuoles of the macrophages, it is likely, based on observations with purified E. coli HlyA and other bacterial toxins, that bacterial uptake by macrophages is not necessary for cell death (21–23, 41–43).
E. coli HlyA is a prototype Ca2+-dependent, pore-forming cytolysin found in gram-negative bacteria (2). HlyA is highly toxic to different types of cells, and cell death occurs primarily due to necrosis accompanied by rapid membrane permeabilization, ionic fluxes, and ATP depletion (5).
Intracellular increases of calcium are responsible for the secondary processes accompanying cell death, which may include DNA fragmentation (5, 21). Yet in J774 macrophages, Hly+ E. coli strains can also cause apoptosis. The physiological relevance of these different endpoint outcomes and mechanisms that select one over the other can only be approximated from in vitro studies, since infection of monocytes and macrophages with these microbes in vivo occurs in substantially different microenvironment.
REFERENCES
- 1.Abe C M, Marques L R M, Gomes T A T. Abstracts of the 97th Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Alpha-hemolysin production and cell-detaching activity in fecal Escherichia coli strains harbouring DNA sequence(s) associated with putative or established enteropathogenic categories of E. coli, abstr. B-154. [Google Scholar]
- 2.Baldwin T J, Knutton S, Sellers L, Manjarrez Hernandez H A, Aitken A, Williams P H. Enteroaggregative Escherichia coli strains secrete a heat-labile toxin antigenically related to E. coli hemolysin. Infect Immun. 1992;60:2092–2095. doi: 10.1128/iai.60.5.2092-2095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudry B, Savarino S J, Vial P, Kaper J B, Levine M M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990;161:1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez L E. Differential mechanism of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activation of human and murine macrophages: the role of nitric oxide. Clin Exp Immunol. 1993;91:277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutin L. The different hemolysins of Escherichia coli. Med Microbiol Immunol. 1991;180:167–182. doi: 10.1007/BF00215246. [DOI] [PubMed] [Google Scholar]
- 6.Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kimar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 7.Chart H, Spencer J, Smith H R, Rowe B. Identification of enteroaggregative Escherichia coli based on surface properties. J Appl Microbiol. 1997;83:712–717. doi: 10.1046/j.1365-2672.1997.00296.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen G M, Sun X M, Snowden R T, Dinsdale D, Skilleter D N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1993;286:331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins R J, Harmon B V, Gobe G C, Kerr J F R. Internucleosomal DNA cleavage should not be the sole criteria for identifying apoptosis. Int J Rad Biol. 1991;61:451–453. doi: 10.1080/09553009214551201. [DOI] [PubMed] [Google Scholar]
- 10.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 11.Dong Z, Saikumar P, Weinberg J M, Venkatachalam M A. Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death: involvement of serine but not cysteine proteases. Am J Pathol. 1997;151:1205–1213. [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott S J, Nataro J P. Enteroaggregative and diffusely adherent Escherichia coli. Rev Med Microbiol. 1995;6:196–206. [Google Scholar]
- 13.Elliott S J, Srinivas S, Albert M J, Alam K, Robins-Browne R M, Gunzburg S T, Mee B J, Chang B J. Characterization of the roles of hemolysin and other toxins in enteropathy caused by alpha-hemolytic Escherichia coli linked to human diarrhea. Infect Immun. 1998;66:2040–2051. doi: 10.1128/iai.66.5.2040-2051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Prada C M, Hoover D L, Tall B D, Venkatesan M M. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infect Immun. 1997;65:1486–1496. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming S D, Campbell P A. Some macrophages kill Listeria monocytogenes while others do not. Immunol Rev. 1997;158:69–77. doi: 10.1111/j.1600-065x.1997.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda K, Kojiro M, Chiu J. Demonstration of extensive chromatin cleavage in transplanted Morris hepatoma 7777 tissue: apoptosis or necrosis? Am J Pathol. 1993;142:935–946. [PMC free article] [PubMed] [Google Scholar]
- 17.Gunzburg S T, Chang B J, Elliott S J, Burke V, Gracey M. Diffuse and enteroaggregative patterns of adherence of enteric Escherichia coli isolated from Aboriginal children from the Kimberley region of Western Australia. J Infect Dis. 1993;167:755–758. doi: 10.1093/infdis/167.3.755. [DOI] [PubMed] [Google Scholar]
- 18.Guzman C A, Domann E, Rohde M, Bruder D, Darji A, Weiss S, Wehland J, Chakrabarti T, Timmis K N. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microb. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 19.Henkart P. ICE family of proteases: mediators of all apoptotic death. Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 20.Hicks S D, Candy C A, Phillips D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonas D, Schultheis B, Klas C, Krammer P H, Bhakdi S. Cytocidal effects of E. coli hemolysin on human T lymphocytes. Infect Immun. 1993;61:1715–1721. doi: 10.1128/iai.61.5.1715-1721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonas D, Walev I, Berger T, Leibtrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alphatoxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages; role of adenylate-cyclase hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutton S, Shaw R K, Bhan M K, Smith H, McConnell M M, Cheasty T, Williams P H, Baldwin T J. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992;60:2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn M, Goebel W. Responses by murine macrophages infected with Listeria monocytogenes crucial for the development of immunity to this pathogen. Immunol Rev. 1997;158:57–67. doi: 10.1111/j.1600-065x.1997.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 26.Lamm M E. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 27.Levine M M, Prado V, Robins-Browne R, Lior H, Kaper J B, Moseley S L, Gicquelais K, Nataro J P, Vial P, Tall B. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J Infect Dis. 1988;158:224–228. doi: 10.1093/infdis/158.1.224. [DOI] [PubMed] [Google Scholar]
- 28.Majno G, Joris I. Apoptosis, oncosis and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 29.Mangan D, Norton F, Taichman S, Lally E T, Wahl S M. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect Immun. 1991;59:3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangan D F, Wahl S M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 31.Merrick J C, Edelson B J, Bharadwaj V, Swanson P E, Unanue E R. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am J Pathol. 1997;151:785–790. [PMC free article] [PubMed] [Google Scholar]
- 32.Nataro J P, Deng Y, Cookson S, Cravioto A, Savarino S J, Guers L D, Levine M M, Tacket C O. Heterogeneity of enteroaggregative Escherichia coli (EaggEC) virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 33.Nataro J P, Kaper J B, Robins-Browne R, Prado V, Vial P, Levine M M. Patterns of adherence of diarrheagenic E. coli to Hep-2 cells. Pediatr Infect Dis. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Neutra M R, Frey A, Kraehenbuhl J P. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 35.Neutra M R, Pringault E, Kraehenbuhl J P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immun. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 36.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1997;156:679–684. [PubMed] [Google Scholar]
- 37.Tzipori S, Montanaro J, Robins-Browne R M, Vial P, Gibson R, Levine M M. Studies with Enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun. 1992;60:5302–5306. doi: 10.1128/iai.60.12.5302-5306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueda N, Walker P D, Hsu S M, Shah S V. Activation of a 15 kDa endonuclease in hypoxia/oxygenation injury without morphologic features of apoptosis. Proc Natl Acad Sci USA. 1995;92:7202–7206. doi: 10.1073/pnas.92.16.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaux D L, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vial P A, Robins-Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 41.Walker K E, Weiss A A. Characterization of the dermonecrotic toxin in members of the genus Bordetella. Infect Immun. 1994;62:3817–3828. doi: 10.1128/iai.62.9.3817-3828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch R A. Pore-forming cytolysins of Gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 43.Wyllie A H. Apoptosis: an overview. Br Med Bull. 1997;53:451–465. doi: 10.1093/oxfordjournals.bmb.a011623. [DOI] [PubMed] [Google Scholar]
- 44.Yuan J Y. Transducing signals of life and death. Curr Opin Cell Biol. 1997;2:247–251. doi: 10.1016/s0955-0674(97)80069-5. [DOI] [PubMed] [Google Scholar]
- 45.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]