Abstract
目的
探究双层软组织缝合封闭技术在单纯应用抗骨吸收药物引起的发生在下颌骨的中早期药物相关性颌骨骨坏死(medication-related osteonecrosis of the jaw, MRONJ)患者手术治疗中的临床应用效果。
方法
选择2021年10月至2022年9月于北京大学口腔医院四病区经手术治疗的中早期下颌骨MRONJ患者的病历资料进行回顾性分析,收集患者术前基线临床资料,包括原发疾病、伴发疾病、用药方案(药物种类、用药时长)、MRONJ分期、临床症状、影像学表现等,所有患者在手术中行下颌骨边缘切除术去除坏死骨,运用双层软组织缝合封闭技术关闭伤口,术后定期复查随访,评价双层软组织缝合封闭技术的治疗效果及并发症,并对患者进行疼痛评分和功能状态评价。
结果
研究共纳入13例患者(女12例,男1例),年龄(66.69±13.14)岁。原发疾病包括骨质疏松7例,肺癌2例,乳腺癌3例,前列腺癌1例;2例伴发糖尿病,2例伴发心血管疾病,1例伴发干燥综合征。9例患者静脉注射唑来膦酸,平均用药时间(37.7±20.0)个月,7例患者同时服用了来曲唑片等其他药物;3例患者应用地舒单抗注射液,平均用药时间(10.3±11.9)个月;5例患者服用阿仑膦酸钠片,平均用药时间(55.20±27.20)个月,2例患者不同程度地服用醋酸泼尼松片或阿卡波糖片。MRONJ 1期4例,2期9例。13例患者均采用双层软组织缝合封闭技术关闭伤口,术后平均随访11.9个月(9~17个月),13例患者皆治愈,无溢脓等并发症发生。患者术前Karnofsky功能状态评分量表(Karnofsky performance status, KPS)评分为(68.46±14.05)分,术后评分为(82.31±15.36)分,差异有统计学意义(P < 0.05)。患者术前疼痛评估视觉模拟评分量表(visual analogue scale,VAS)评分为(5.77±0.73)分,术后评分为(0.38±0.51)分,差异有统计学意义(P < 0.001)。
结论
双层软组织缝合封闭技术在中早期单纯使用抗骨吸收类药物的下颌骨MRONJ患者中可以取得良好的临床治疗效果,可为用药情况更加复杂的MRONJ患者提供临床治疗思路。
Keywords: 药物相关性颌骨骨坏死, 双层软组织缝合封闭技术, 边缘性颌骨切除, 抗骨吸收药物, 外科治疗
Abstract
Objective
To investigate the clinical application effect of double-layer soft tissue (DLST) suture closure technique in patients with mandible medication-related osteonecrosis of the jaw (MRONJ) of early and medium stages resulted in application of anti-bone-resorptive drugs.
Methods
Early to medium stage mandible MRONJ patients who underwent surgical treatment in the fourth ward of Peking University School and Hospital of Stomatology from October 2021 to September 2022 were included. Clinical information of the patients were collected, including primary disease, concomitant disease, medication regimen (drug type, duration of medication), MRONJ stage, clinical symptoms, imaging manifestations, etc. During surgery, after using marginal mandibulae resection to remove the necrotic bone, the wound was closed using DLST closure technique. Regular post-operative follow-up was performed to evaluate the therapeutic effect and complications of the DLST technique, the pain score and functional status of the patiens were evaluated.
Results
This study totally included 13 patients, 12 women and 1 man, aged (66.69±13.14) years. Seven patients had osteoporosis, 2 had lung cancer, 3 had breast cancer and 1 had prostate cancer among their primary diseases; 7 had no concomitant diseases, 2 had diabetes mellitus, 2 had cardiovascular disease and 1 had dry syndrome. Intravenous zoledronic acid were used in 9 patients, the average duration was (37.7±20.0) months, and other drugs, such as letrozole tablets were taken in 7 patients at the same time; Denosumab injection was used in 3 patients for an average of (10.3±11.9) months; Alendronate sodium tablets were taken in 5 patients for an average of (55.20±27.20) months, and prednisone acetate tablets or acarbose tablets were taken to varying degrees in 2 patients. The average post-operative follow-up was 11.9 months (9 to 17 months), and all the 13 patients were cured without complications, such as pus overflow and so forth. The pre-operative score of Karnofsky performance status (KPS) in the patients was 68.46±14.05, and the post-operative score was 82.31±15.36, and the difference was statistically significant (P < 0.05). The pre-operative score of visual analogue scale (VAS) in the patients was 5.77±0.73 and the post-operative score was 0.38±0.51, and the difference had statistical significance (P < 0.001).
Conclusion
The double-layer soft tissue suture closure technique can achieve good clinical results in patients with MRONJ of the mandible using anti-bone-resorptive drugs alone, and can provide clinical treatment ideas for MRONJ patients with more complicated drug use.
Keywords: Medication-related osteonecrosis of the jaw, Double-layer soft tissue suture closure technique, Marginal mandibulectomy, Anti-bone-resorptive drugs, Surgical treatment
药物相关性颌骨骨坏死(medication-related osteonecrosis of the jaw, MRONJ)是目前口腔临床工作中愈发常见的一种疾病。2003年,Marx[1]首次报道静脉注射双膦酸盐类药物后颌骨出现坏死,并指出骨坏死与双膦酸盐类药物的使用相关。当前研究表明,除双膦酸盐等抗骨吸收药物外,与这类疾病相关的还有抗血管生成药物(舒尼替尼、贝伐珠单抗)和免疫调节药物(如英夫利昔单抗、利妥昔单抗)等[2]。在临床上,患者可出现牙齿松动、溢脓、软组织瘘管、红肿、颌骨暴露及骨折等,影响进食,患者的生活质量严重下降[3]。
MRONJ的临床治疗可分为手术治疗和保守治疗,但是对于MRONJ的治疗原则,目前尚未取得统一意见。2014年美国口腔颌面外科医师协会(American Association of Oral and Maxillofacial Surgeons, AAOMS)建议手术治疗仅限于3期病变及2期病变保守治疗效果不佳者[2],但随着手术治疗取得了令人满意的临床治疗效果,2022年AAOMS建议1~3期患者均可进行手术治疗[4]。
下颌骨MRONJ中早期(1、2期)患者病变仍局限在牙槽突范围内,术式多选择下颌骨边缘切除术以清除坏死骨,手术后创口裂开或积液感染容易引起MRONJ疾病复发。对切除坏死骨后如何严密地关闭伤口、维持黏膜闭合、促进愈合是临床上亟需解决的难题之一。本研究设计了针对下颌骨MRONJ中早期手术中的双层软组织封闭技术缝合创面,并对该项技术的临床初步应用情况进行回顾性分析。
1. 资料与方法
1.1. 纳入对象
选择2021年10月至2022年9月于北京大学口腔医院四病区就诊并在手术中运用双层软组织缝合封闭技术的13例下颌骨MRONJ患者进行回顾性分析。本研究开始前已经北京大学口腔医院生物医学伦理委员会审查批准(PKUSSIRB-201949114)。
纳入标准:有抗骨吸收药物应用史,确诊的下颌骨MRONJ 1、2期患者,全身情况能耐受手术且同意采用双层软组织缝合封闭术式的患者。
排除标准:有头颈部放疗史者;有抗血管生成药物应用史者;不能耐受手术者。
MRONJ诊断标准参照2022年AAOMS文献[4]。
1.2. 收集患者基线资料
收集患者基线临床资料,包括原发疾病、伴发疾病、用药方案(药物种类、用药时长)、MRONJ分期、临床症状、影像学表现等。
1.3. 手术方法
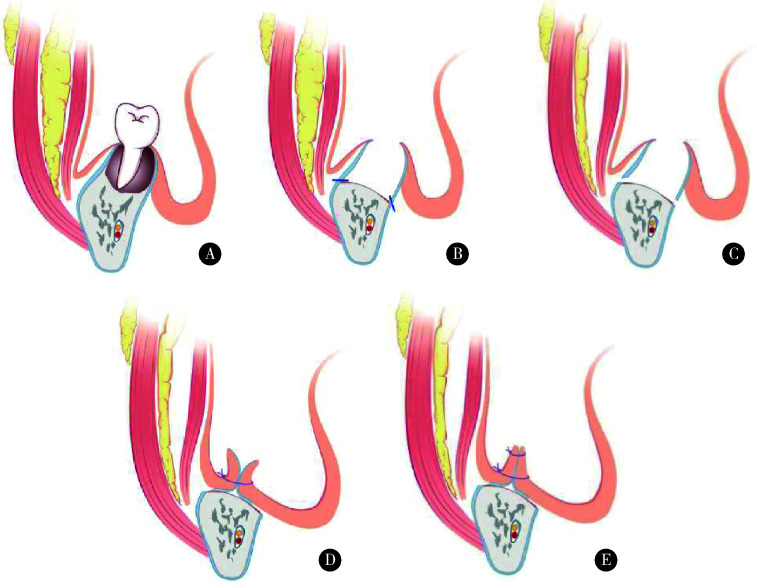
预防性应用抗生素:术前1 d开始应用抗生素(以头孢类抗生素为主,头孢类抗生素过敏者可选用阿奇霉素),术后再继续用5~7 d。手术操作在全身麻醉下进行,口内沿瘘口、死骨外侧黏膜及牙龈缘切开,拔除不能保留的患牙,充分暴露出下颌骨骨病变区及两侧正常骨质。通过行下颌骨边缘性颌骨切除,去除坏死骨,至达到正常骨质边界(有活力的出血骨区域),修整骨创面边缘至光滑、圆钝;分离颊侧、舌侧黏骨膜,于骨切缘水平行横向骨膜切开,制备颊舌侧黏骨膜瓣,将颊舌侧黏骨膜瓣纵向双层缝合,关闭伤口(图 1为示意图,图 2为术中图),手术由本研究同一作者按照统一的手术方案进行。
图 1.
双层软组织缝合封闭设计示意图(下颌骨)
Schematic of double-layer soft tissue suture closure design (mandible)
A, osteonecrosis of mandible in stage Ⅱ; B, marginal mandibulectomy for the removement of the osteonecrosis; C, periosteum membrane cut off to increase the freeness of mucoperiosteal flap; D, the purpose of the first layer of suture is to reduce the tissue dead cavity and make the soft tissue as close to the remaining mandibular bone as possible; E, the purpose of the second layer of suture is to further allow the mucoperiosteal flaps of the cheek side and the lingual side close to increase the tightness.
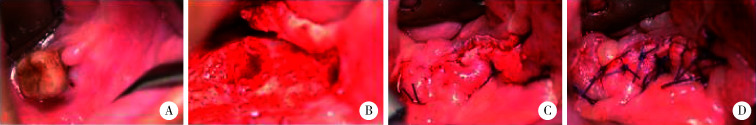
图 2.
患者双层软组织缝合封闭术手术过程
Operative process of double-layer soft tissue suture closure
A, MRONJ stage Ⅱ mandibular osteonecrosis; B, bucco-lingual mucoperiosteal flap was prepared after trimming the bone wound; C, the first layer of suture; D, the second layer of suture.
1.4. 手术治疗效果评价
参照Yarom等[5]2019年发表的临床治疗指南,根据患者术后黏膜覆盖情况、症状/疼痛、感染/炎症及影像学表现,将手术治疗效果分为治愈、改善、稳定和进展四个等级。
1.5. 术后随访及评价
术后2周、1个月、3个月、6个月、1年复诊随访,查看患者口腔内软组织伤口愈合情况,是否存在伤口开裂、脂肪液化、感染等现象并进行影像学检查。
疼痛及功能状态评价:分别于术前1天及术后3个月采用疼痛评估视觉模拟评分量表(visual analogue scale, VAS)和Karnofsky功能状态评分量表(Karnofsky performance status, KPS)对患者进行问卷调查并评分。
1.6. 统计学分析
采用SPSS 24.0统计软件,计量资料以均数±标准差表示,采用配对样本的非参数检验Wilcoxon符号秩检验进行分析,判断患者手术前后的疼痛、功能状态差异。P < 0.05认为差异有统计学意义。
2. 结果
2.1. 患者临床基线资料
研究共纳入13例患者,其中女性12例,男性1例,年龄(66.69±13.14)岁(40~87岁,表 1)。原发疾病为骨质疏松7例,肺癌2例,乳腺癌3例,前列腺癌1例。2例患者伴发糖尿病,1例伴发高血压,1例伴发冠心病,1例伴发干燥综合征。9例静脉注射唑来膦酸,平均用药时间(37.67±20.04)个月,7例患者同时服用了来曲唑片等其他药物;3例患者运用地舒单抗注射液,平均用药时间(10.3±11.9)个月;5例患者服用阿仑膦酸钠片,平均用药时间(55.20±27.20)个月,2例患者不同程度地服用醋酸泼尼松片或阿卡波糖片。MRONJ 1期患者4例,2期患者9例。临床表现为颌骨暴露者1例,牙龈软组织瘘管者12例。13例患者中2例患者影像学上表现为局部的骨质增厚,7例出现分离的死骨形成,8例出现弥漫性骨质硬化(表 1)。
表 1.
患者的基线临床资料
Baseline data of patients
| Case | Gender | Age/years | Primary disease | Concomitant disease | Chemotherapeutics | Stage of MRONJ | Clinical manifestation | Imaging manifestation | |
| Anti-bone resorption drugs/months | Else | ||||||||
| 1 | Female | 63 | Lung cancer | - | Zoledronic acid/26, denosumab/2 | Crizotinib capsule | 2 | Soft tissue fistula | Local thickening of bone |
| 2 | Male | 71 | Prostatic cancer | - | Zoledronic acid/31 | Flutamide tablets, leuprorelin acetate, microspheres for injection | 2 | Soft tissue fistula | Diffuse sclerosis of bone |
| 3 | Female | 40 | Breast cancer | - | Zoledronic acid/30, pamidronate/28 | Palbociclib, fulvestrant, everolimus tablets | 2 | Soft tissue fistula | Local thickening of bone |
| 4 | Female | 64 | Osteoporosis | - | Alendronate sodium/48, zoledronic acid/1 | - | 1 | Soft tissue fistula | Sequestration |
| 5 | Female | 79 | Osteoporosis | - | Zoledronic acid/60 | - | 1 | Soft tissue fistula | Sequestration,diffuse sclerosis of bone |
| 6 | Female | 87 | Osteoporosis | Diabetes | Alendronate sodium/103 | Acarbose tablets | 1 | Soft tissue fistula | Sequestration,diffuse sclerosis of bone |
| 7 | Female | 85 | Osteoporosis | - | Alendronate sodium/41 | - | 1 | Soft tissue fistula | Diffuse sclerosis of bone |
| 8 | Female | 63 | Lung cancer | - | Zoledronic acid/57 | - | 2 | Soft tissue fistula | Sequestration,diffuse sclerosis of bone |
| 9 | Female | 63 | Breast cancer | Diabetes | Zoledronic acid/64, denosumab/5 | Trastuzumab, pertuzumab, paclitaxel, fulvestrant, capecitabine tablets | 2 | Soft tissue fistula | Diffuse sclerosis of bone |
| 10 | Female | 70 | Osteoporosis | Hypertension | Zoledronic acid/40, alendronate sodium/48 | - | 1 | Soft tissue fistula | Sequestration |
| 11 | Female | 74 | Osteoporosis | Coronary heart disease | Denosumab/24 | Leiflunomide tablets, tripterygium glycosides tablets, | 1 | Bone exposure | Sequestration,diffuse sclerosis of bone |
| 12 | Female | 52 | Breast cancer | - | Zoledronic acid/30 | Letrozole tablets, fulvestran, abemaciclib | 2 | Soft tissue fistula | Uneven alveolar bone density |
| 13 | Female | 56 | Osteoporosis | Sicca syndrome | Alendronate sodium/36 | Prednisone acetate tablets | 1 | Soft tissue fistula | Sequestration,diffuse sclerosis of bone |
2.2. 患者手术治疗效果及随访
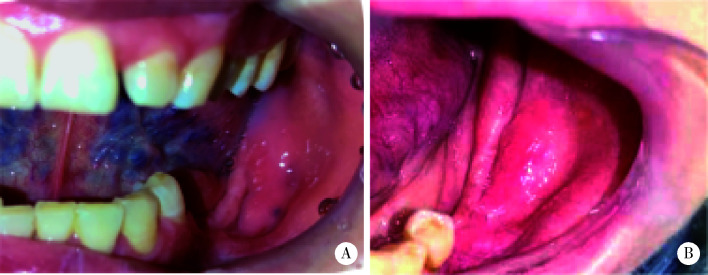
13例患者均密切随访,平均随访时间11.9个月(9~17个月)。13例患者手术治疗效果皆为治愈,黏膜完全覆盖,局部黏膜稍高起,术后无疼痛、感染和炎症,影像学骨小梁形态正常或改善,无死骨形成(图 3、图 4)。
图 3.
病例4(A)和病例9(B)术后6个月复查口腔内软组织伤口愈合
Cases 4 (A) and 9 (B) 6-month post-operative wound healing of intra-oral soft tissue
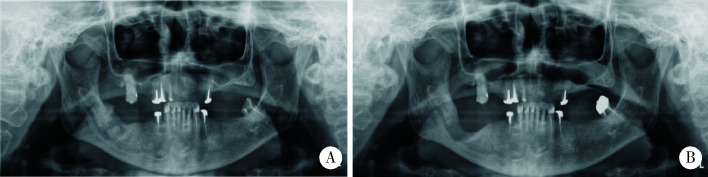
图 4.
病例6术前(A)和术后(B)6个月曲面断层X线片
Panoramic radiograph of pre-operation (A) and 6-month post-operation (B) of case 6
2.3. 患者疼痛及功能状态评价
患者术前KPS平均评分为(68.46±14.05) 分,术后平均评分为(82.31±15.36)分,差异有统计学意义(P < 0.05)。患者术前VAS平均评分为(5.77± 0.73)分,术后平均评分为(0.38±0.51)分,差异有统计学意义(P < 0.001)。
3. 讨论
药物相关性颌骨骨坏死已成为口腔临床工作中越来越常见的一类疾病,症状上可表现为颌骨暴露、软组织瘘管、口腔异味等,严重影响患者的生活质量和精神状态,其治疗仍非常具有挑战性,目前尚未见确定的金标准治疗方法。MRONJ的临床治疗可分为保守治疗和手术治疗,虽然保守治疗仍然是MRONJ的一种治疗选择,但越来越多的学者认为手术治疗在疾病的各个阶段都有很高的成功率[6-11]。
MRONJ手术治疗去除坏死骨的方式主要包括下颌骨边缘性切除术和下颌骨区段截骨术, 在去除病变骨质的同时,刮除部分健康骨组织,直达新鲜渗血的骨面[12]。下颌骨边缘性切除术是指切除牙槽骨,保留下颌骨的连续性,下颌骨区段截骨术是指下颌骨的连续性丧失并可通过钛板置入等其他手术方式进行重建。MRONJ 1、2期患者因病变仍局限在牙槽突范围内,可行下颌骨边缘切除术去除坏死骨。Altay等[13]对12例病变局限于下颌骨牙槽突的患者行边缘切除术,最终12例(100%)患者在初次手术后均达到黏膜的初期愈合。Carlson[6]对48例下颌骨MRONJ 1、2期患者行下颌骨边缘切除术,最终治愈率可达87.5%(42/48)。MRONJ 3期患者因颌骨病变范围超过牙槽突,达下颌骨下缘,多采用下颌骨区段截骨术并进行重建。安金刚等[14]报道40例下颌骨MRONJ 3期患者,术中分别采用下颌骨区段截骨术后重建钛板固定联合下颌下腺转位, 或联合颏下岛状瓣嵌合下颌下腺,90%患者黏膜实现初期愈合,10%患者术后出现钛板折断。Caldroney等[15]对11例MRONJ 3期患者行手术切除和同期血管化骨重建治疗,包括7例腓骨皮瓣和4例肩胛骨骨皮瓣,所有患者均报告症状消退,X线片显示骨完全愈合(100%)。
对MRONJ手术而言,实现严密的伤口关闭,维持黏膜的长期愈合对疾病的治愈十分重要。Klingelhöffer等[16]的一项前瞻性研究,对40例患者进行了76次手术干预,通过边缘切除术去除坏死骨后使用黏骨膜瓣行伤口关闭,发现仅27.6%的病例实现了黏膜闭合的长期维持,但术后81%的2期患者降至1期(P < 0.01)。Mücke等[17]报道26例下颌骨MRONJ患者,术中采用下颌舌骨肌瓣关闭创口,发现其术后复发率(15.4%)显著低于采用局部黏骨膜瓣者(29.6%)。Lemound等[18]报道32例MRONJ患者,分别采用鼻唇沟瓣和黏骨膜瓣行伤口关闭,发现16例鼻唇沟瓣患者68.8%实现了一期伤口闭合,16例黏骨膜瓣患者有81.2%出现复发,相较于邻位瓣辅助伤口关闭,黏骨膜直接拉拢关闭的临床治疗效果不尽如人意。
研究认为MRONJ术后无张力的黏膜闭合对于促进软组织愈合至关重要[6, 10, 12]。由于黏膜组织量不足,切除死骨后黏骨膜直接拉拢关闭伤口时存在一定的张力,黏膜下方留有死腔,积液的存积会直接影响伤口的愈合。本研究针对下颌骨MRONJ中早期患者设计的双层软组织缝合封闭技术,在关闭伤口时可有效地消灭死腔,加强伤口的严密程度,使骨缺损得到机械稳定、血管化良好的覆盖,并降低软组织和骨组织在口腔中的机会感染率。手术操作过程相对简单快捷,手术时间较短,术者约40 min即可完成,手术效果较好,并发症少,具有一定的普适性。
本研究存在一定的局限性,首先患者样本量少,不足以全面地反映双层软组织缝合封闭技术临床应用的真实情况,且患者的随访时间较短;其次,本研究纳入的患者为仅使用抗骨吸收药物的下颌骨MRONJ患者,对于复杂用药患者此种术式的临床效果还需要进一步研究。
综上所述,双层软组织缝合封闭技术在中早期下颌骨MRONJ患者手术治疗的临床应用中取得了较为满意的效果。
Funding Statement
北京大学口腔医院国家临床重点专科建设项目(PKUSSNKP-202114)、中国博士后自然基金(2022M720289)、中华口腔医学会临床研究基金(CSA-SIS2022-0)、北京市自然科学基金(7212137)和国家口腔医学中心适宜技术推广项目(2023NCSHTP03)
Supported by the National Clinical Key Discipline Construction Project (PKUSSNKP-202114), China Postdoctoral Science Foundation (2022M720289), Chinese Stomatological Association Clinical Research Fund(CSA-SIS2022-01), Beijing Municipal Natural Science Foundation (7212137), and the National Stomatology Center Suitable Technology Promotion Project(2023NCSHTP03)
Footnotes
利益冲突 所有作者均声明不存在利益冲突。
作者贡献声明 周颖:收集数据和撰写论文;赵宁、黄竑远、李庆祥:分析、整理数据;郭传瑸:总体把关;郭玉兴:手术操作、提出研究思路和审定论文。
References
- 1.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–1117. doi: 10.1016/S0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero SL, Dodson TB. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw: 2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 3.郭 玉兴, 王 佃灿, 王 洋, et al. 二膦酸盐药物治疗乳腺癌骨转移发生颌骨坏死的临床特点. 北京大学学报(医学版) 2016;48(1):80–83. doi: 10.3969/j.issn.1671-167X.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero SL, Dodson TB, Aghaloo T, et al. American association of oral and maxillofacial surgeons' position paper on medication-related osteonecrosis of the jaws: 2022 update. J Oral Maxillofac Surg. 2022;80(5):920–943. doi: 10.1016/j.joms.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Yarom N, Shapiro CL, Peterson DE, et al. Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO clinical practice guideline. J Clin Oncol. 2019;37(25):2270–2290. doi: 10.1200/JCO.19.01186. [DOI] [PubMed] [Google Scholar]
- 6.Carlson ER. Management of antiresorptive osteonecrosis of the jaws with primary surgical resection. J Oral Maxillofac Surg. 2014;72(4):655–657. doi: 10.1016/j.joms.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Abu-ld MH, Warnke PH, Gottschalk J, et al. "Bis-phossy jaws"-high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008;36(2):95–103. doi: 10.1016/j.jcms.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Wilde F, Heufelder M, Winter K, et al. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):153–163. doi: 10.1016/j.tripleo.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Hayashida S, Soutome S, Yanamoto S, et al. Evaluation of the treatment strategies for medication-related osteonecrosis of the jaws (MRONJ) and the factors affecting treatment outcome: A multicenter retrospective study with propensity score matching analysis. J Bone Miner Res. 2017;32(10):2022–2029. doi: 10.1002/jbmr.3191. [DOI] [PubMed] [Google Scholar]
- 10.Williamson RA. Surgical management of bisphosphonate induced osteonecrosis of the jaws. Int J Oral Maxillofac Surg. 2010;39(3):251–255. doi: 10.1016/j.ijom.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Lopes RN, Rabelo GD, Rocha AC, et al. Surgical therapy for bisphosphonate-related osteonecrosis of the jaw: Six-year experience of a single institution. J Oral Maxillofac Surg. 2015;73(7):1288–1295. doi: 10.1016/j.joms.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 12.何 悦, 陈 珩, 安 金刚, et al. 药物相关性颌骨坏死临床诊疗专家共识. 中国口腔颌面外科杂志. 2023;21(4):313–325. [Google Scholar]
- 13.Altay MA, Radu A, Pack SE, et al. Medication-related osteonecrosis of the jaw: An institution' s experience. Cranio. 2020;38(5):333–341. doi: 10.1080/08869634.2018.1528711. [DOI] [PubMed] [Google Scholar]
- 14.安 金刚, 吕 晓鸣, 贾 宽宽, et al. 下颌下腺转位在下颌骨3期药物相关性颌骨坏死手术中的应用. 中华口腔医学杂志. 2021;56(5):441–446. doi: 10.3760/cma.j.cn112144-20210203-00058. [DOI] [Google Scholar]
- 15.Caldroney S, Ghazali N, Dyalram D, et al. Surgical resection and vascularized bone reconstruction in advanced stage medication-related osteonecrosis of the jaw. Int J Oral Maxillofac Surg. 2017;46(7):871–876. doi: 10.1016/j.ijom.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Klingelhöffer C, Zeman F, Meier J, et al. Evaluation of surgical outcome and influencing risk factors in patients with medication-related osteonecrosis of the jaws. J Craniomaxillofac Surg. 2016;44(10):1694–1699. doi: 10.1016/j.jcms.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Mücke T, Koerdt S, Jung M, et al. The role of mylohyoid flap in the treatment of bisphosphonate-related osteonecrosis of the jaws. J Craniomaxillofac Surg. 2016;44(4):369–373. doi: 10.1016/j.jcms.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Lemound J, Muecke T, Zeller AN, et al. Nasolabial flap improves healing in medication-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2018;76(4):877–885. doi: 10.1016/j.joms.2017.09.021. [DOI] [PubMed] [Google Scholar]