Abstract
We have produced monoclonal antibodies against Plasmodium yoelii merozoite surface protein 1 (MSP-1) and have assessed their ability to suppress blood stage parasitemia by passive immunization. Six immunoglobulin G antibodies were characterized in detail: three (B6, D3, and F5) were effective in suppressing a lethal blood stage challenge infection, two (B10 and G3) were partially effective, and one (B4) was ineffective. MSP-1 is the precursor to a complex of polypeptides on the merozoite surface; all of the antibodies bound to this precursor and to an ∼42-kDa fragment (MSP-142) that is derived from the C terminus of MSP-1. MSP-142 is further cleaved to an N-terminal ∼33-kDa polypeptide (MSP-133) and a C-terminal ∼19-kDa polypeptide (MSP-119) comprised of two epidermal growth factor (EGF)-like modules. D3 reacted with MSP-142 but not with either of the constituents MSP-133 and MSP-119, B4 recognized an epitope within the N terminus of MSP-133, and B6, B10, F5, and G3 bound to MSP-119. B10 and G3 bound to epitopes that required both C-terminal EGF-like modules for their formation, whereas B6 and F5 bound to epitopes in the first EGF-like module. These results indicate that at least three distinct epitopes on P. yoelii MSP-1 are recognized by antibodies that suppress parasitemia in vivo.
Malaria is a disease caused by parasites of the genus Plasmodium, which have a complex life cycle with several distinct stages in vertebrates. A number of antigens that may be targets of protective host immune responses have been identified. The asexual stage of parasite multiplication within erythrocytes leads to the release of merozoites that invade further erythrocytes. Merozoite surface protein 1 (MSP-1) has been identified as a target of protective immune responses and a potential vaccine candidate. In Plasmodium falciparum this protein is synthesized as a precursor during schizogony and then is processed to a complex of polypeptides on the merozoite surface (19, 27). A similar processing may occur in other species of Plasmodium (5, 12, 20, 28). At or just before invasion, proteolytic cleavage of the C-terminal ∼42-kDa polypeptide (MSP-142) produces a new membrane-bound, ∼19-kDa fragment (MSP-119), which is carried into the newly invaded erythrocyte (2), and a soluble ∼33-kDa fragment (MSP-133), which is shed from the surface with the rest of the complex. MSP-119 is a cysteine-rich domain composed of two epidermal growth factor (EGF)-like modules containing disulfide bonds that maintain the three-dimensional structure (3). Mice immunized with recombinant Plasmodium yoelii MSP-119 are protected against challenge infection with this parasite (9, 23, 31), and this protection appears to be antibody mediated (10, 23, 25). One monoclonal antibody (MAb), MAb 302, that reacts with the C-terminal cysteine-rich region of P. yoelii MSP-1 (6) and on passive immunization is effective against a blood stage challenge infection (26) has been described. Here we describe additional MAbs that bind to P. yoelii MSP-1 and suppress blood stage parasitemia. These inhibitory antibodies define at least three distinct epitopes, two in MSP-119 and a third that is detected only on intact MSP-1 and the C-terminal MSP-142.
MATERIALS AND METHODS
Expression and purification of recombinant proteins.
Both the C-terminal fragment of MSP-1 containing both EGF-like modules (MSP-119, residues 1649 to 1754 in the amino acid sequence [22]) and the two individual modules were expressed in Escherichia coli as glutathione S-transferase (GST) fusion proteins (23, 24) and purified by binding to glutathione-agarose (30). Recombinant MSP-119 (rMSP-119) was produced by cleavage of GST–MSP-119 with the protease factor Xa (Boehringer) and separated from GST by gel filtration (24). Three recombinant GST fusion proteins, corresponding to most of the sequence of MSP-133 and to the N- and C-terminal parts, were produced. The oligonucleotides 5′-CTAGGATCCACACAAAAAGGTTGGGTAG (primer 1), 5′-CCAGAATTCATTTAATTCCGTCAGTAGTTG (primer 2), 5′-CTAGGATCCAAGCTGCAGTAAAGGAACA (primer 3), and 5′-CCAGAATTCAGTGTTTATTATTTTCTGCATG (primer 4) were used to amplify DNA by PCR from the clone PyM4.3 (kindly provided by Alan Lewis), corresponding to amino acid residues Thr1415 to Lys1513 (primers 1 and 2; PyMSP133B), Ala1527 to His1619 (primers 3 and 4; PyMSP133C), and Thr1415 to His1619 (primers 1 and 4; PyMSP133A). The DNA was restricted with BamHI and EcoRI (sites in the primers above are underlined) and then cloned into the corresponding sites of the plasmid pGEX3X. The fusion proteins were purified as described above.
Production and characterization of MAbs.
Female BALB/c mice were immunized intraperitoneally with the recombinant protein GST–MSP-119 by using Freund’s adjuvant (23). Alternatively, the mice were immunized by infection following passive immunization with an inhibitory MAb derived from the first fusion: mice that had been challenged with 5 × 103 parasites after passive immunization with MAb B10 (see below) were inoculated with a further 5 × 108 parasitized erythrocytes on three occasions. Spleen cells were fused with SP2/0-Ag14 mouse myeloma cells in the presence of 50% polyethylene glycol, using 8 × 107 spleen cells and 2 × 107 myeloma cells. The cells were dispensed into 96-well culture plates in 100 μl of hypoxanthine-aminopterin-thymidine selective medium. After 10 days, the supernatants were tested for specific antibody by indirect immunofluorescence on acetone-fixed preparations of erythrocytes infected with P. yoelii YM and by enzyme-linked immunosorbent assay (ELISA) with rMSP-119. The hybridomas producing antibody considered positive in one or both of the assays were cloned by three rounds of limiting dilution in hypoxanthine-thymidine medium, and selected hybridomas were expanded by growth in RPMI 1640 medium containing 10% (vol/vol) fetal calf serum.
The antibodies secreted by the hybridomas B4, B6, B10, D3, F5, and G3 were affinity purified from culture supernatant on columns of protein G-Sepharose 4 Fast Flow (Pharmacia) (1) according to the manufacturer’s recommendations, and purity was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Antibody isotypes were determined by double diffusion and capture ELISA with a kit (Sigma) according to the manufacturer’s recommendations. Antibody titers were determined by indirect immunofluorescence and by ELISA with rMSP-119, using twofold serial dilutions of antibody, essentially as described previously (24).
Passive immunization and parasite challenge.
Female BALB/c mice from 8 weeks of age and bred under specific-pathogen-free conditions were used in groups of 10 animals. The purified MAbs (a total of 1.5 mg/mouse) were administered by intraperitoneal injection on three occasions, i.e., 1 day before, 1 day after and on the day of challenge infection. The parasite used for the challenge was the lethal YM strain of P. yoelii; the parasite stock was stored at −195°C and passaged once before use in the experiment. The challenge was administered by intravenous injection into the lateral tail vein with 5 × 103 parasitized erythrocytes per mouse, at least 1 h after administration of the MAb. Parasitemia was assessed daily on smears made from tail blood and stained with Giemsa’s reagent.
Immunoprecipitation, immunoblotting, and immunofluorescence.
Blood was harvested from P. yoelii-infected mice, passed through a cellulose powder (CF-11) column to eliminate the leukocytes, and eluted with Krebs glucose saline. The parasites were then washed with RPMI 1640 medium (methionine and cysteine free) containing 2 mM glutamine. Trophozoites and schizonts were isolated by centrifugation through a Percoll gradient (60 to 80%) and metabolically radiolabelled with 5 MBq of Tran35S-label (70% l-[35S]methionine, 15% l-[35S]cysteine) (ICN) per ml at 37°C for 2 to 3 h. The preparation of detergent extract and the immunoprecipitation and analysis of labelled polypeptides by SDS-PAGE and fluorography were carried out essentially as described previously (24). For immunoblotting, recombinant proteins or parasite extract was denatured in SDS sample buffer, fractionated by SDS-PAGE, and electrophoretically transferred to nitrocellulose (Schleicher and Schuell; 0.2-μm pore size). The detection of proteins on the blot with specific antibody was carried out as described previously (24). MAb 25.1 (18), normal mouse serum, and a polyclonal antiserum raised by immunization with GST–MSP-119 (24) were used as controls.
For immunofluorescence studies, erythrocytes infected with P. yoelii YM were washed, aliquoted onto multiwell slides, and fixed in methanol-acetone (1:1, vol/vol) for 10 min. MAbs were diluted 1:100 and incubated on the slide for 30 min at room temperature. After washing, the slides were incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (IgG) (Sigma) for 30 min, washed, and dipped in 0.05% Evans blue–1 μg of DAPI (4′,6-diamidino-2-phenylindole) per ml. They were then examined by fluorescence microscopy.
Competition ELISA.
The wells of 96-well microplates (Dynatech Immulon-4) were coated with 1 μg of rMSP-119 per ml and then blocked with bovine serum albumin, as described previously (24). After incubation for 1 h at 37°C with the first MAb, the wells were washed three times with washing buffer and then incubated with the second biotinylated MAb (prepared by using sulfo-NHS-biotin [Pierce] according to the manufacturer’s recommendation) for 1 h at 37°C, using a concentration that had been determined previously by titration. The wells were washed three times, and then 100 μl of horseradish peroxidase-conjugated streptavidin (10 μg/ml) was added and left for 1 h at 37°C. After a final three washes, 100 μl of o-phenylenediamine substrate was added. The color reaction was stopped by addition of 50 μl of 1 M sulfuric acid, and the absorbance was read at 492 nm as described previously (24). The ability of a MAb to inhibit the binding of the biotinylated second MAb to the antigen was analyzed by comparing the mean absorbance values in the presence of different competitor MAbs by the Kruskal-Wallis test.
RESULTS
Passive immunization suppresses parasitemia.
Of 12 hybridoma cell lines that were cloned, 6 (B4, B6, B10, D3, F5, and G3) that secrete antibody specific for P. yoelii MSP-1 were characterized further, and the MAbs were analyzed in detail. Hybridomas B10 and G3 were produced after immunization with the recombinant protein GST–MSP-119, and the others were produced after passive immunization with B10 and infection with the parasite. The subclass of each antibody is shown in Table 1.
TABLE 1.
Properties of MSP-1-specific MAbs
| MAb | Subclass | Reaction with MSP-1a | Binding to recombinant GST fusion proteinb:
|
|||
|---|---|---|---|---|---|---|
| MSP1EGF1 | MSP1EGF2 | MSP-119 | MSP-133 | |||
| B4 | IgG1 | + | − | − | − | + |
| B6 | IgG3 | + | + | − | + | − |
| B10 | IgG2b | + | − | − | + | − |
| D3 | IgG2a | + | − | − | − | − |
| F5 | IgG3 | + | + | − | + | − |
| G3 | IgG1 | + | − | − | + | − |
The reaction with MSP-1 was determined by immunofluorescence, immunoprecipitation, and Western blotting.
The binding of the MAbs to the different recombinant proteins was determined by Western blotting and ELISA. MSP1EGF1 is the first EGF-like module of P. yoelii MSP-119, MSP1EGF2 is the second EGF-like module of MSP-119, MSP-119 is the two combined EGF-like modules, and MSP-133 is the N-terminal part of MSP-142; all of the recombinant proteins were expressed as GST fusion proteins in E. coli.
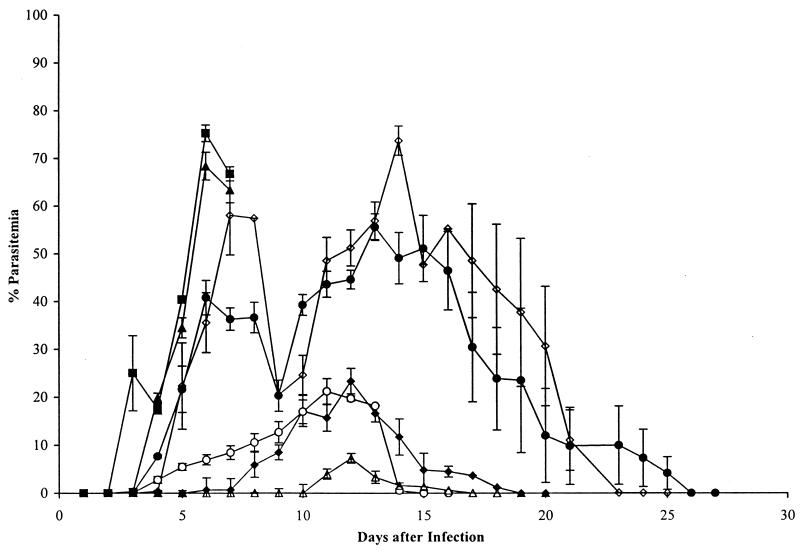
The ability of the MAbs to protect mice against a blood stage challenge infection with P. yoelii YM by passive immunization was evaluated. Purified IgG was inoculated into groups of mice at the time of parasite challenge, and the development of parasitemia was monitored (Fig. 1). One antibody (B4) had no effect on the course of parasitemia; all mice developed a fulminating infection similar to that in the group inoculated with phosphate-buffered saline (PBS) alone, and none survived beyond day 7. Two of the antibodies (B10 and G3) produced a partial suppression of parasite growth. However, the mean parasitemia in the group that received G3 exceeded 70% on day 14, and only 60% of the mice cleared the infection; in the group that received B10, the parasitemia exceeded 50%, although all of the mice cleared the infection and survived. Three of the antibodies (B6, D3, and F5) mediated a substantial reduction in parasitemia, and all of the mice in these groups cleared the infection. In the animals that received MAb D3, the parasites were largely restricted to reticulocytes.
FIG. 1.
Course of P. yoelii YM infection in groups of 10 BALB/c mice injected intraperitoneally with solutions of MAbs, followed by intravenous challenge with 5 × 103 parasitized erythrocytes per mouse. Mice in each group received 0.5 mg of IgG in PBS intraperitoneally on days −1, 0, and +1 relative to the day of parasite challenge (day 0). B6 (▵), F5 (⧫), and D3 (○) substantially modified the course of the infection; B10 (•) and G3 (◊) were less effective. The parasitemias in mice treated with B4 (■) and in mice treated with an irrelevant MAb (data not shown) were indistinguishable from that in the group that received PBS (▴). All of the mice cleared the infection, except for those that received B4 and PBS, which died on day 7, and 40% of the mice treated with G3, which died on days 8 and 9. Each point represents the geometric mean parasitemia of mice in each group at the time after parasite challenge, and the vertical bars indicate the standard errors.
The MAbs react with MSP-1 and its fragments in parasite extracts.
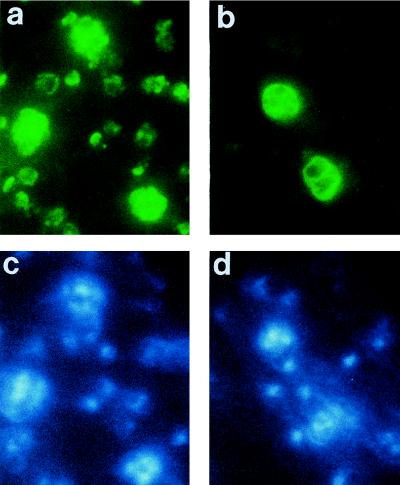
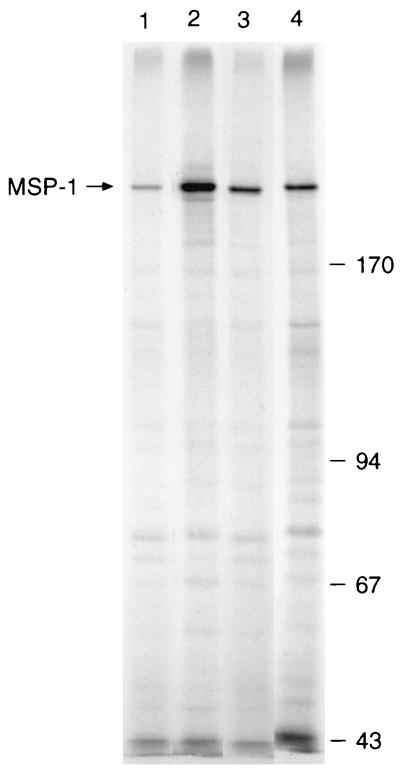
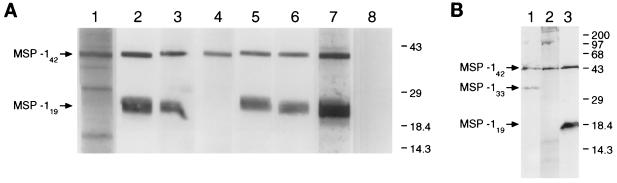
As determined by indirect immunofluorescence, all of the MAbs produced a distinctive pattern with schizonts and merozoites, characteristic of an antigen present on the parasite surface (Fig. 2). In addition, B6 (Fig. 2a), B10, F5, and G3 also reacted with ring stage parasites. All MAbs immunoprecipitated the 230-kDa MSP-1 precursor from detergent lysates of schizonts (Fig. 3); this protein was also recognized by MAb 25.1 (18) and a polyclonal serum specific for MSP-1 (23) but not by antibodies in normal mouse serum. As determined by Western blotting, all of the MAbs recognized MSP-142 in parasite extract enriched for merozoites (Fig. 4A); this protein was also detected by polyclonal antibodies raised by immunization with GST–MSP-119. In addition, MAbs B6 (Fig. 4A, lane 2), B10 (lane 3), F5 (lane 5), and G3 (lane 6) detected MSP-119. In merozoite extracts incubated for 1 h to allow further MSP-1 processing and the formation of MSP-133, MAb B4 detected an additional species of ∼33 kDa (Fig. 4B, lane 1) that was not detected by D3 (lane 2) or F5 (lane 3).
FIG. 2.
Immunofluorescence patterns of MAbs reactive with MSP-1 on methanol–acetone-fixed smears of erythrocytes infected with P. yoelii YM. The smears were incubated with MAb B6 (a) or MAb B4 (b), and antibody binding was detected with a second fluorescein isothiocyanate-labelled antibody. (c and d) Corresponding smears stained with DAPI to locate DNA. Note the reaction with schizonts and ring stages in panel a and with schizonts alone in panel b, despite the presence of young parasites detected in panel d by nuclear staining. No specific fluorescence was detected with an irrelevant first antibody or with the second labelled antibody alone (data not shown).
FIG. 3.
The MAbs immunoprecipitate MSP-1 from extracts of 35S-labelled P. yoelii YM-infected erythrocytes solubilized with buffer containing detergent. In this experiment the following MAbs were used: B6 (lane 1), D3 (lane 2), F5 (lane 3), and G3 (lane 4). The immunoprecipitates were analyzed by SDS-PAGE on a 7.5% polyacrylamide gel and detected by fluorography. The position of MSP-1 on the left and the mobilities of standard molecular mass markers (Pharmacia) (in kilodaltons) on the right are indicated. MSP-1 was also precipitated by B4, B10, 25.1, and a polyclonal antiserum to GST–MSP-119 but not by antibodies in normal mouse serum (not shown).
FIG. 4.
The MAbs react with fragments of MSP-1 in an extract of P. yoelii YM merozoites by Western blotting. (A) The samples were subjected to SDS-PAGE (without prior reduction) on a 5 to 15% polyacrylamide gradient gel, blotted onto nitrocellulose and then probed with MAb B4 (lane 1), B6 (lane 2), B10 (lane 3), D3 (lane 4), F5 (lane 5), or G3 (lane 6) or with polyclonal anti-GST–MSP-119 (lane 7) and normal mouse serum (lane 8). The positions of MSP-142 and MSP-119 on the left and the mobilities of standard molecular mass markers (in kilodaltons) on the right are indicated. (B) The merozoites were incubated in vitro to allow processing to continue before analysis. The samples were fractionated on a 12.5% polyacrylamide gel, blotted, and probed with MAbs B4 (lane 1), D3 (lane 2), and F5 (lane 3). The positions of MSP-142, MSP-133, and MSP-119 on the left and the mobilities of standard molecular mass markers (in kilodaltons) on the right are indicated.
Some of the MAbs react with recombinant proteins.
To locate more precisely the epitopes recognized by the MAbs, the ability of the antibodies to bind to recombinant proteins expressed from parts of the MSP-1 gene was investigated by Western blotting. The results of this analysis are summarized in Table 1. MAbs B6, B10, F5, and G3 bound to GST–MSP-119, indicating that they recognize epitopes in the C-terminal cysteine-rich region of MSP-1. None of the MAbs bound to the second EGF-like module fused to GST (GST-MSP1EGF2), but both B6 and F5 bound to the first EGF-like module fused to GST (GST-MSP1EGF1). This reactivity was abolished when the recombinant proteins were reduced and alkylated with iodoacetic acid before SDS-PAGE, a treatment that destroys epitopes that require disulfide bonds for their integrity (23, 24). MAbs B4 and D3 did not bind to GST–MSP-119. However, B4 reacted with the recombinant proteins GST–MSP-133A (Fig. 5, lane 1) and GST–MSP-133B (Fig. 5, lane 2) but not with GST–MSP-133C (Fig. 5, lane 3), indicating that it bound an epitope in the N-terminal half of MSP-133.
FIG. 5.
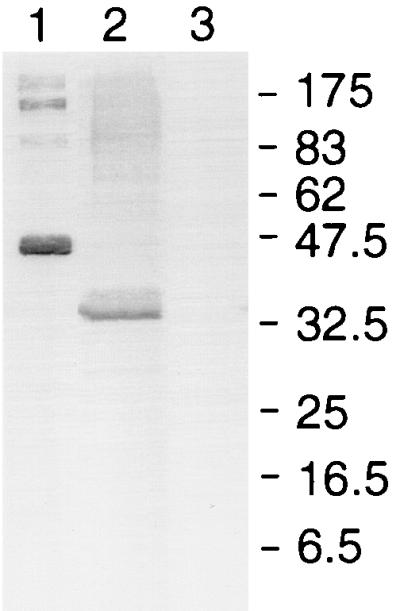
MAb B4 binds to an epitope in the N-terminal end of MSP-133. Recombinant proteins consisting of MSP-133 sequences fused to GST were fractionated by SDS-PAGE on a 12.5% polyacrylamide gel under reducing conditions, transferred to nitrocellulose, and then probed with the MAb. Lane 1, PyMSP133A (Thr1415 to His1619); lane 2, PyMSP133B (Thr1415 to Lys1513); lane 3, PyMSP133C (Ala1527 to His1619). The mobilities of molecular mass standards (New England Biolabs) (in kilodaltons) are shown. The antibody did not react with GST alone, none of the other MAbs reacted with GST–MSP-133A, and each of the fusion proteins was detected with antibody specific for GST (data not shown).
Analysis of the antibodies by competition ELISA.
To study the epitopes on rMSP-119 recognized by the different MAbs, they were analyzed by competition ELISA (Table 2). MAbs G3 and B10 competed with each other for binding to GST–MSP-119 but not with any of the other antibodies. F5 competed with itself but not with B6, although B6 was able to compete effectively with F5. As expected from the Western blot data, D3 and B4 did not bind to rMSP-119 (not shown) and did not compete with the other antibodies.
TABLE 2.
Comparison of the different MAbs by competition ELISA
| Competitor MAb | Competition with labelled MAba:
|
|||
|---|---|---|---|---|
| B6 | B10 | F5 | G3 | |
| B6 | + | − | + | − |
| B10 | − | + | − | +/− |
| F5 | − | − | + | − |
| G3 | − | + | − | + |
| B4 | − | − | − | − |
| D3 | − | − | − | − |
| PBS | − | − | − | − |
+, inhibition of binding of biotinylated MAb to MSP-119 by nonlabelled MAb; −, no inhibition of binding of biotinylated MAb to MSP-119 by nonlabelled MAb.
DISCUSSION
Previous studies have shown that immunization of mice with P. yoelii MSP-1 provides some protection against challenge infection with lethal strains of the parasite (15, 18). Immunization with recombinant protein corresponding to the C-terminal cysteine-rich region of P. yoelii MSP-1 has been found to be highly effective (9, 17, 23), and it has been suggested that antibody is important in mediating the protection observed (10, 17, 23, 25). In previous studies polyclonal antibodies raised by immunization with affinity-purified MSP-1 (15) or a MAb specific for an epitope at the N terminus of the protein (14) was not effective by passive immunization. In contrast, a MAb specific for the C-terminal cysteine-rich region of MSP-1 (26) and polyclonal antibodies raised by immunization with recombinant MSP-119 (10, 25) were at least partly effective in suppressing parasitemia. In this study we report six additional MSP-1-specific MAbs and define additional specificities for antibodies that are at least partially protective after passive immunization. One of these (D3) defines an epitope on the larger C-terminal fragment MSP-142.
Of the three MAbs that were most effective at suppressing the parasitemia after passive immunization, two of them (F5 and B6) are of the IgG3 subclass, and the other (D3) is an IgG2a. The subclass and specificity (see below) of F5 and B6 suggest that they are antibodies similar to MAb 302, the IgG3 reported by Majarian et al. (26). Two other MAbs, B10 and G3 (IgG2b and IgG1, respectively), were also able to modify the course of parasitemia, and some mice survived the challenge infection. MAb B4 was unable to modify the course of infection after passive immunization, and all mice developed a fulminating infection, a pattern similar to that obtained with a control MAb (data not shown). Determination of whether there is a correlation between subclass, as well as epitope specificity, and the ability of IgG to protect mice requires further investigation. The specific contributions of different IgG subclasses to protection mediated by immunization with recombinant proteins corresponding to the C terminus of MSP-1 have not been evaluated in detail, although correlations of protection with subclass have suggested that IgG1, IgG2a, and IgG2b are important (11, 13, 25, 31). Passive immunization with IgG fractions isolated from sera of mice immune to P. yoelii infection suggested that IgG2a was of particular importance (33). The fact that MAbs against P. falciparum MSP-119 that inhibit erythrocyte invasion in vitro appear to do so by inhibiting secondary processing of MSP-142 suggests that their activity is based on a steric effect rather than one requiring a specific Fc-mediated function (4). It will be of interest to determine whether the P. yoelii MSP-1-specific MAbs that are effective after passive immunization also inhibit P. yoelii MSP-1 processing in vitro.
All of the antibodies react with the intact MSP-1 precursor as assessed by immunoprecipitation from schizont extracts and with the C-terminal MSP-142 in merozoite extracts. The Western blotting results with the recombinant proteins indicate that B10 and G3 recognize epitopes in MSP-119 that require both EGF-like modules to be present in the protein, whereas B6 and F5 recognize epitopes in the first EGF-like module. The specificity of B6 and F5 appears to be similar to that of MAb 302, described previously (6). The epitopes for all of these antibodies are constrained by disulfide bonds, since they do not recognize the reduced and alkylated GST–MSP-119. These results are consistent with the results of the competition ELISA, which suggest that the epitopes for B10 and G3 overlap, may be identical, and are different from the epitopes for B6 and F5, which also appear to overlap each other but clearly are distinct epitopes. All of these antibodies recognize schizont and ring stage parasites as determined by indirect immunofluorescence, indicating that they react with the small C-terminal fragment of MSP-1 that results from secondary processing of MSP-1 at the time of invasion and is carried into newly invaded erythrocytes (2, 5, 28).
MAbs D3 and B4 did not recognize ring stage parasites as determined by immunofluorescence, suggesting that they do not bind directly to MSP-119. The epitope for B4 was mapped to the N-terminal region of MSP-142 by reaction with a recombinant protein containing amino acid sequences from this region. This antibody also reacted with an ∼33-kDa polypeptide present in merozoite preparations that had been incubated for 1 h to allow processing to proceed. This polypeptide is probably analogous to the soluble 33-kDa fragment (PfMSP-133) resulting from secondary processing of P. falciparum MSP-142 and shed from the merozoite surface at the time of erythrocyte invasion (4). This is the first description of a MAb to this part of P. yoelii MSP-1, and it will be of use in studying the proteolytic processing of this molecule. MAb D3 reacts with MSP-142 but not with either the N-terminal (MSP-133) or C-terminal (MSP-119) part alone, suggesting that it binds to the site of secondary processing or to an epitope that requires intact MSP-142.
The results are consistent with the demonstration that immunization with the C terminus of P. yoelii MSP-1 can protect mice against challenge infection with blood stage parasites and that this protection is mediated, at least in part, by antibody. Although two of the protective antibodies are directed against the first EGF-like module, immunization with GST-MSP1EGF1 alone does not protect mice against blood stage challenge (7, 24). Immunization with a recombinant protein containing the two EGF-like modules (MSP-119) does protect against a blood stage parasite or sporozoite challenge (9, 17, 23, 29, 31). However, the identification of a MAb that is effective on passive immunization and does not recognize just the C terminus of MSP-1 alone suggests that the optimal immunogen may contain sequence in addition to MSP-119. Successful immunization with a GST–MSP-142 recombinant protein was reported recently, but the protection observed was no better than that mediated by MSP-119 alone (32). For development of a vaccine against P. falciparum, both MSP-142 and MSP-119 are being considered (8, 21), but comparative studies of the two have not been performed so far. The development of effective vaccines based on MSP-1 may also be compromised by the presence of epitopes that induce blocking antibodies that abrogate the action of neutralizing antibodies (4, 16), so it will be important to define in detail the fine specificity of MSP-1-specific IgG with biological activity and to modify the antigen to remove epitopes that induce blocking antibodies without affecting those that induce protective antibodies.
ACKNOWLEDGMENTS
Lilian Spencer was in receipt of a studentship from CONICIT, Venezuela. This work was supported in part by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), EC contract TS3*93 0228, and the Medical Research Council.
We thank M. Goggin for cell culture.
REFERENCES
- 1.Akerstrom B, Brodin T, Reis K, Bjorck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985;135:2589–2592. [PubMed] [Google Scholar]
- 2.Blackman M J, Heidrich H-G, Donachie S, McBride J S, Holder A A. A single fragment of a malaria merozoite surface protein remains on the parasite surface during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman M J, Ling I T, Nicholls S C, Holder A A. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;49:29–34. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 4.Blackman M J, Scott-Finnigan T J, Shai S, Holder A A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackman M J, Dennis E D, Hirst E M A, Kocken C H, Scott-Finnigan T J, Thomas A W. Plasmodium knowlesi: secondary processing of the malaria merozoite surface protein-1. Exp Parasitol. 1996;83:229–239. doi: 10.1006/expr.1996.0069. [DOI] [PubMed] [Google Scholar]
- 6.Burns J M, Majarian W R, Young J F, Daly T M, Long C A. A protective monoclonal antibody recognizes an epitope in the carboxyl-terminal cysteine-rich domain in the precursor of the major merozoite surface antigen of the rodent malarial parasite, Plasmodium yoelii. J Immunol. 1989;143:2670–2676. [PubMed] [Google Scholar]
- 7.Calvo P A, Daly T M, Long C A. Plasmodium yoelii: the role of the individual epidermal growth factor-like domains of the merozoite surface protein-1 in protection from malaria. Exp Parasitol. 1996;82:54–64. doi: 10.1006/expr.1996.0007. [DOI] [PubMed] [Google Scholar]
- 8.Chang S P, Case S E, Gosnell W L, Hashimoto A, Kramer K J, Tam L Q, Hashiro C Q, Nikaido C M, Gibson H L, Lee-Ng C T, Barr P J, Yokota B T, Hui G S N. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly T M, Long C A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly T M, Long C A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- 11.Daly T M, Long C A. Influence of adjuvants on protection induced by a recombinant fusion protein against malaria infection. Infect Immun. 1996;64:2602–2608. doi: 10.1128/iai.64.7.2602-2608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David P H, Hadley T J, Aikawa M, Miller L H. Processing of a major parasite surface glycoprotein during the ultimate stages of differentiation in Plasmodium knowlesi. Mol Biochem Parasitol. 1984;11:267–282. doi: 10.1016/0166-6851(84)90071-9. [DOI] [PubMed] [Google Scholar]
- 13.de Souza J B, Ling I T, Ogun S A, Holder A A, Playfair J H L. Cytokines and antibody subclass associated with protective immunity against blood stage malaria in mice vaccinated with the C terminus of merozoite surface protein 1 plus a novel adjuvant. Infect Immun. 1996;64:3532–3536. doi: 10.1128/iai.64.9.3532-3536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman R R, Trejdosiewicz A J, Cross G A M. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980;284:366–368. doi: 10.1038/284366a0. [DOI] [PubMed] [Google Scholar]
- 15.Freeman R R, Holder A A. Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoelii schizont antigen. Clin Exp Immunol. 1983;54:609–616. [PMC free article] [PubMed] [Google Scholar]
- 16.Guevara Patiño J A, Holder A A, McBride J S, Blackman M J. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J Exp Med. 1997;186:1–11. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirunpetcharat C, Tian J-H, Kaslow D C, van Rooijen N, Kumar S, Berzofsky J A, Miller L H, Good M F. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Sacchromyces cerevisiae. J Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- 18.Holder A A, Freeman R R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981;294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 19.Holder A A, Freeman R R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982;156:1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holder A A, Freeman R R. Characterization of a high molecular weight protective antigen of Plasmodium yoelii. Parasitology. 1984;88:211–219. [PubMed] [Google Scholar]
- 21.Kumar S, Yadava A, Keister D B, Tian J-H, Ohl M, Perdue-Greenfield K A, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis A P. Cloning and analysis of the gene encoding the 230-kDa merozoite surface antigen of Plasmodium yoelii. Mol Biochem Parasitol. 1989;36:271–282. doi: 10.1016/0166-6851(89)90175-8. [DOI] [PubMed] [Google Scholar]
- 23.Ling I T, Ogun S A, Holder A A. Immunisation against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 24.Ling I T, Ogun S A, Holder A A. The two epidermal growth factor-like modules of Plasmodium yoelii Merozoite Surface Protein-1 are required for an effective immune response to the parasite. Parasite Immunol. 1995;17:425–433. doi: 10.1111/j.1365-3024.1995.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 25.Ling I T, Ogun S A, Momin P, Richards R L, Garçon N, Cohen J, Ballou W R, Holder A A. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine. 1997;15:1562–1567. doi: 10.1016/s0264-410x(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 26.Majarian W R, Daly T M, Weidanz W P, Long C A. Passive immunization against murine malaria with IgG3 monoclonal antibody. J Immunol. 1984;132:3131–3137. [PubMed] [Google Scholar]
- 27.McBride J S, Heidrich H-G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987;23:71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- 28.O’Dea K P, McKean P G, Harris A, Brown K N. Processing of the Plasmodium chabaudi chabaudi AS merozoite surface protein 1 in vivo and in vitro. Mol Biochem Parasitol. 1995;72:111–119. doi: 10.1016/0166-6851(95)00090-n. [DOI] [PubMed] [Google Scholar]
- 29.Rénia L, Ling I T, Marussig M, Miltgen F, Holder A A, Mazier D. Immunization with recombinant C terminus of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect Immun. 1997;65:4419–4423. doi: 10.1128/iai.65.11.4419-4423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith D B, Johnson K S. Single step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 31.Tian J-H, Miller L H, Kaslow D C, Ahlers J, Good M F, Alling D W, Berzofsky J A, Kumar S. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;157:1176–1183. [PubMed] [Google Scholar]
- 32.Tian J-H, Kumar S, Kaslow D C, Miller L H. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun. 1997;65:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White W I, Evans C B, Taylor D W. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun. 1991;59:3547–3554. doi: 10.1128/iai.59.10.3547-3554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]