Abstract
Identifying patients at higher risk of developing dementia is important. The usefulness of the Mattis Dementia Rating scale-Second Edition (MDRS-2) to detect and differentiate between patients with amnestic mild cognitive impairment (A-MCI), Parkinson’s disease and MCI (PD-MCI), PD with dementia (PDD), and Alzheimer’s disease (AD) was investigated. In all, 22 healthy controls (HC), 22 A-MCI, 22 PD-MCI, 16 PDD, and 22 AD patients were evaluated using an extensive neuropsychological battery, including the MDRS-2. The MDRS-2 total standardized score detected all groups of patients. The dementia groups performed worse than HC on the 5 MDRS-2 subscales. Alzheimer’s disease patients scored higher than PDD on MDRS-2 conceptualization and lower on memory. Healthy controls were better than PD-MCI on MDRS-2 initiation/perseveration and memory and better than A-MCI on memory. No difference was found between the MCI groups. The MDRS-2 is a suitable short scale for MCI and dementia screening but is not specific enough to differentiate between A-MCI and PD-MCI.
Keywords: mild cognitive impairment, Parkinson’s disease, Alzheimer’s disease, Mattis Dementia Rating scale, screening, sensitivity
Introduction
Mild cognitive impairment (MCI) was first described as the preclinical phase of Alzheimer’s disease (AD), and this concept focused on memory impairment. 1 New criteria were elaborated in order to better reflect the possible phenotypes and outcomes of this clinical condition. 2 Mild cognitive impairment is now diagnosed according to the predominance or not of memory impairment, that is amnestic- or nonamnestic-MCI, and also according to the number of cognitive domains impaired (eg, single or multiple domains). For all these subtypes, activities of daily living must remain essentially intact.2,3 The recent development in the conceptualization of MCI has led authors to apply these criteria to other neurodegenerative disorders, such as idiopathic Parkinson’s disease (PD). The prevalence estimates of MCI vary from 22% to 53% in the well-established PD.4,5 Recently, in an incident cohort of newly diagnosed drug-naive PD patients, nearly 20% were found to have MCI, which was twice the risk of cognitive alteration compared with healthy controls. 6 Moreover, longitudinal studies demonstrated that patients with PD having MCI have a 3-fold risk of developing dementia compared to those who are cognitively intact. 5 Thus, MCI is now recognized as a significant risk factor for the development of dementia in PD, along with older age, male gender, higher severity of motor symptoms, and the presence of visual hallucinations. 7
Amnestic MCI (A-MCI) is more likely to predict AD, 8 whereas single- and multiple-domain nonamnestic subtypes are more associated with later development of PD with dementia (PDD). 5 Distinguishing AD and PDD at an earlier clinical stage is of great importance, given that treatment strategies are expected to be different in these disorders. 9 Moreover, pharmacological agents are known to be more efficacious when administered at the beginning of the dementia process.10,11
Neuropsychological assessment is usually the “gold standard” for detecting cognitive impairment and is recommended for the diagnosis of MCI in PD. 6 However, in some clinical or research settings, specialized services and financial resources may be insufficient to administer an extensive neuropsychological battery. In these circumstances, having access to a brief and efficient cognitive screening test becomes important. The Mattis Dementia Rating scale (MDRS)12,13 is a widely used and well-accepted cognitive test in the elderly population. 14 It was designed to provide an overall score of neuropsychological deficits based upon the performances on a variety of cognitive tasks. The MDRS-Second Edition (MDRS-2) contains 5 subscales (eg, attention, initiation/perseveration, construction, conceptualization and memory) within which the items are presented in a hierarchical manner, that is a correct answer to the first items of a subscale allows the examiner to give credits for the subsequent items (max = 144). For this reason, the administration lasts from 10 to 15 minutes in healthy elderly participants and from 30 to 45 minutes in severely impaired individuals. 15
Many studies have documented the ability of the MDRS-2 to detect AD.16–19 The MDRS-2 is also able to discriminate between AD and other types of dementia, such as PDD.20,21 Previous results showed that the MDRS-2 could detect patients with A-MCI, 22 considered to be at high risk of AD. However, little is known about the sensitivity of the MDRS-2 to detect individuals with MCI in PD (PD-MCI) and to differentiate them from A-MCI. Therefore, the goal of this study was to document this important question and to extend it to patients with PDD and AD. We hypothesized that (1) the healthy controls (HC) will score higher than the 4 patient groups on the MDRS-2 total standardized score, (2) the patients with AD will score higher than patients with PDD on the initiation/perseveration subscale and lower than the patients with PDD on the memory subscale, (3) the patients with A-MCI will score lower than HC on the memory subscale, (4) the patients with PD-MCI will score lower than HC on the initiation/perseveration subscale, and (5) the patients with A-MCI will score higher than the patients with PD-MCI on the initiation/perseveration subscale and lower than the PD-MCI on the memory subscale (see Table 1 for the summary of the hypotheses with the underlying biological rationale).
Table 1.
Biological Rationale of the Hypotheses of the Study
| Hypotheses | Biological Rationale | |
|---|---|---|
| (1) | HC > PD-MCI, PDD, A-MCI, AD on the MDRS-2 total standardized score | The HC shall not present with significant cognitive deficit on the MDRS-2, as opposed to the other groups |
| (2) | AD < PDD on the memory subscale of the MDRS-2 | Hippocampal atrophy is extremely severe in the earliest stages of AD, whereas the alteration of the prefrontal-subcortical loops is very severe in the earliest stages of PDD |
| PDD < AD on the initiation/perseveration subscale of the MDRS-2 | ||
| (3) | A-MCI < HC on the memory subscale of the MDRS-2 | A-MCI is supposed to be the prodromal stage of AD and shall therefore present with predominant hippocampal atrophy |
| (4) | PD-MCI < HC on the initiation/perseveration subscale of the MDRS-2 | PD-MCI is supposed to be the prodromal stage of PDD and shall therefore present with a predominant alteration of the prefrontal-subcortical loops |
| (5) | PD-MCI < A-MCI on the initiation/perseveration subscale of the MDRS-2 | The alteration of the prefrontal-subcortical loops shall be more severe in PD-MCI than in A-MCI |
| A-MCI < PD-MCI on the memory subscale of the MDRS-2 | The hippocampal atrophy shall be more severe in A-MCI than in PD-MCI |
Abbreviations: HC, healthy controls; PD-MCI, Parkinson’s disease with mild cognitive impairment; A-MCI, amnestic mild cognitive impairment; PDD, Parkinson’s disease with dementia; AD, Alzheimer’s disease; MDRS-2, Mattis Dementia Rating Scale-Second Edition.
Methods
Participants
The present cross-sectional study was approved by the local Ethics Committee. Informed written consent was obtained from each participant or their legal substitute. All participants had to be aged 50 or older. Healthy controls came from an ongoing research on normal aging conducted at the Laboratoire de Recherche en Neuropsychologie Gériatrique at Laval University in Quebec City, Canada. Patients with A-MCI and AD came from studies on pathological aging and were administered an exhaustive neuropsychological evaluation including the MDRS-2. Patients with PD were recruited at the Département des Sciences Neurologiques of the CHAUQ (Hôital de l'Enfant-Jésus). The diagnosis of PD was made by an experienced neurologist (N.D. or M.L.) according to the consensual criteria.23,24 Data were included in a non-nominative database. Potential participants were not included if they (1) had any neurological or systemic problems other than PD and AD known to impair cognition (eg, traumatic brain injury, tumor, etc), (2) have undergone deep brain stimulation or other brain neurosurgery, (3) had a current or past history of alcohol or drug abuse, or (4) had a chronic psychiatric illness or an acute episode of major depression. This last exclusion criterion was ascertained using the Hamilton Depression Rating scale (Ham-D) 25 in PD-MCI (n = 21), PDD (n = 13), and AD (n = 20) groups. Patients who scored >13 on the Ham-D, indicative of a major depressive disorder,25,26 were excluded. However, because depressive symptoms are a common psychiatric feature in PD and AD,27,28 patients taking an antidepressant medication were accepted only if the dosage was stable for at least 6 months. Of the 22 patients with A-MCI, 3 were assessed with the Ham-D because they were stable on antidepressants. The other patients with A-MCI (not taking any psychotropic medications) were screened for depression using the Neuropsychiatric Inventory (NPI) 29 and according to the clinical interview. Patients with A-MCI who obtained a total NPI score above 1 were not included in the study. Finally, patients had to score ≥15 (mild-to-moderate dementia) on the Mini-Mental State Examination (MMSE) 30 in order to be included in the study.
Diagnosis of MCI
The MCI diagnosis was made according to the most recent Petersen criteria.2,3 The presence of cognitive complaints was questioned by asking the patients whether they had noticed any change in their intellectual functioning. They, and a proxy when available, were also asked to complete the Multifactorial Memory Questionnaire (abilities subtest).31,32 Patients had to score approximately 1.5 standard deviations (SD) below the mean on at least 1 of 5 cognitive domains. Cognitive deficits were required to not affect daily functioning as measured by the Disability Assessment for Dementia (DAD), 33 which was completed by a proxy. In case of doubt, clinical judgment was applied, as suggested by Petersen. 34 Patients were classified as having A-MCI single or multiple domains or nonamnestic MCI single or multiple domains.
Diagnosis of Dementia
Diagnosis of probable AD was performed according to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition [DSM-IV]) 35 and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-DRDA) criteria. 36 Dementia in PD was diagnosed following the last criteria proposed by the Movement Disorder Society Task Force 37 : impairment in more than 1 cognitive domain, with an insidious onset and slow progression, development of dementia within the context of established PD, and cognitive deficits must represent a decline from the premorbid level. Deficits had to be severe enough to impair daily life functions (social, occupational, or personal care) and had to be independent of the impairment due to motor or autonomic symptoms. In order to exclude patients with possible dementia with Lewy bodies, the PD diagnosis had to be made at least a year before the onset of cognitive impairments. 37
Assessments
All participants responded to a structured interview on clinical and demographic information. Pharmacological treatments were gathered through medical charts and directly from patients and HC prescriptions. Patients with PD were encouraged to take their regularly scheduled PD medications during the study visit so that they would be evaluated during the “ON” state. In order to establish the diagnosis of MCI or dementia, several cognitive domains were evaluated. Visuomotor speed was assessed with the Motor speed condition of the Trail Making test from the Delis-Kaplan Executive Function System (D-KEFS). 38 Attention was assessed using Digit span (forward and backward) of the Wechsler Adult Intelligence Scale- Third Edition (WAIS-III), 39 and the Visual scanning condition of the D-KEFS Trail Making Test. 38 Verbal episodic memory and learning were evaluated using the California Verbal Learning Test-Second Edition, 40 while the Clock Drawing Test 41 was used as a visuospatial organization measure. Executive functions were evaluated by tests of letter fluency (T, N, and P), category fluency (animals) and by the Number-letter switching condition of the D-KEFS Trail Making Test. 38 The MMSE was administered to patients in order to assess global cognitive functioning. The severity of motor dysfunction in PD was evaluated using the Hoehn and Yahr (H & Y) stages. 42 Healthy controls received the same cognitive assessments as the other groups of patients, except for the MMSE. All tests were administered and scored according to the standard procedures. Results were compared to the most representative set of published norms and transformed into Z scores. Finally, the MDRS-2 43 was administered to all patients and HC but, it was not used to establish MCI nor dementia diagnoses in order to avoid circularity bias. Moreover, 59.5% (62 of 104) of all MDRS-2 protocols were independently scored by experienced and well-trained graduate students in neuropsychology (L.J. and S.T.). The recent robust and expanded Mayo's Older Americans Normative Studies (MOANS) norms were used to adjust the MDRS-2 raw scores. 44
Data Analysis
The statistical analysis was performed using the SPSS software version 18.0 for Windows. The α level was set at P = .05. Between-group comparisons on demographic and clinical characteristics were made using analyses of variance (ANOVA or multivariate analysis of variance [MANOVA]). Post hoc comparisons were done with the Tukey or Dunnet T3 tests according to the assumption of equality of variances or not, respectively. Categorical variables were compared with the chi-square test. Including all possible data for each participants, associations between the MDRS-2 age- and education-corrected total score (n = 104), the age-corrected score on each of the 5 MDRS-2 subscales (n = 104), age (n = 104), education (n = 103), MMSE total raw score (n = 81), H & Y stage (n = 32), and score on the Ham-D (n = 57) were analyzed with Pearson correlations (bilateral). If confounding factors differed between groups, and if they correlated significantly with the MDRS-2 total standard score, they were included as covariables (multivariate analysis of covariance [MANCOVA]).
Receiver operator characteristics (ROC) curves were generated to test the criterion validity and diagnostic performance of the MDRS-2 in the MCI groups and dementia groups. Area under the curve (AUC) and its 95% confidence intervals (95% CI) were calculated. The optimal screening cutoff point was defined as the lowest value that achieved nearby or more than 80% sensitivity. Likelihood ratios, positive (LR+) and negative (LR-), were also computed as indicators of the clinical utility of the MDRS-2.
In order to determine which variables could better differentiate the MCI groups on one side and the dementia groups on the other side, 2 discriminant function analyses (DFAs), stepwise forward method (F to enter = .05 and F to remove = .10) were computed. Age, education, and age-corrected scores of the 5 MDRS-2 subscales were entered as potential contributory variables, and group membership as predicted variable.
Results
All participants were French-speaking Caucasians. As presented in Table 1, demographic features of the groups were similar, except for age (F(4, 99) = 10.29, P = .000). Dementia groups scored lower than the MCI groups on the MMSE (F(3, 77) = 33.095, P = .000). The severity of mood symptoms, as measured by the Ham-D, did not differ. Duration of PD was not statistically different between PD-MCI (8.27 ± 4.9 years) and PDD (11.13 ± 6.4 years) groups. However, motor symptoms were slightly more severe in patients with PDD (H & Y: 2.92 ± .87) than in patients with PD-MCI (H & Y: 2.07 ± .54; F(1, 30) = 11.341, P = .002). Mean levodopa dosages were 597.6 (363.5) mg in patients with PD-MCI and 700.3 (362.4) mg in patients with PDD, while 43% of patients with PD-MCI were prescribed dopamine agonist versus 20% of patients with PDD. These prescriptions were not statistically different. Regarding other medications, the groups registered different utilization profiles for acetylcholinesterase inhibitors (AChEIs), anticholinergics, and benzodiazepines but not antidepressants (Table 2 ).
Table 2.
Demographic and Clinical Characteristics (Mean and Standard Deviation) of the 5 Groups
| Variables | HC, n = 22 | PD-MCI, n = 22 | A-MCI, n = 22 | PDD, n = 16 | AD, n = 22 |
|---|---|---|---|---|---|
| Age (year)a,b | 64.1 (6.3) | 68.3 (9.3) | 70.0 (6.8) | 73.5 (7.7) | 77.9 (7.3) |
| Education (year) | 13.7 (4.9) | 13.1 (4.5) | 13.7 (4.4) | 10.0 (4.1) | 12.4 (4.5) |
| Men (%) | 45.5 | 54.5 | 45.5 | 75.0 | 36.4 |
| MMSEa,c | N/A | 27.8 (1.4) | 28.8 (1.8) | 23.7 (4.0) | 22.1 (2.3) |
| Ham-D | N/A | 4.0 (3.1) | 4.3 (3.5) | 5.2 (2.8) | 2.3 (3.0) |
| Antidepressants (%) | 0 | 28.6 | 16.7 | 26.7 | 23.8 |
| Anticholinergics (%) d | 0 | 27.3 | 5.6 | 13.3 | 0 |
| Benzodiazepines (%) a | 0 | 33.3 | 22.2 | 46.7 | 19.0 |
| AChEIs (%) a | 0 | 0 | 5.6 | 13.3 | 71.4 |
Abbreviations: HC, healthy controls; PD-MCI, Parkinson’s disease with mild cognitive impairment; A-MCI, Amnestic mild cognitive impairment; PDD, Parkinson’s disease with dementia; AD, Alzheimer’s disease; MMSE, Mini-Mental State Examination, Ham-D, Hamilton Depression Rating Scale, AChEIs, acetylcholinesterase inhibitors.
a P < .001.
b Test post hoc: HC < PDD = AD; A-MCI = PD-MCI < AD.
c Test post hoc: A-MCI = PD-MCI > AD = PDD.
d P < .01.
The composition of the PD-MCI group was different from that of the A-MCI group (P = .000). Almost 41% (40.9%) of patients in the A-MCI group presented memory impairment only (single-domain A-MCI), whereas 59.1% of these patients presented with memory impairment together with deficits in at least 1 other cognitive domain (multiple-domain A-MCI), according to Petersen nomenclature. Fifty percent of patients in the PD-MCI group had a predominant nonmemory deficit combined with deficits in another cognitive domain (nonmemory multiple-domain MCI according to Petersen nomenclature), 27.3% presented with a predominant memory impairment together with deficits in at least 1 other cognitive domain (multiple-domain A-MCI), 13.6% had only cognitive impairment in 1 domain other than memory (nonmemory single-domain MCI), and 9.1% had only an amnestic deficit (single-domain A-MCI).
Results on the MDRS-2
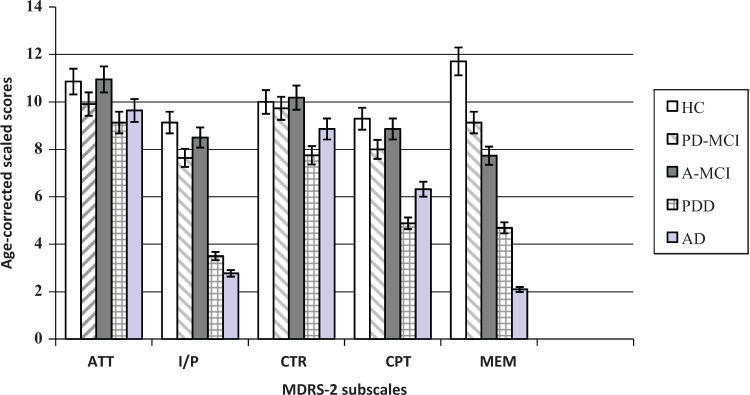
The MDRS-2 age- and education-corrected total score was strongly associated with the MMSE total score (r = .65, P = .000), moderately with age (r = −.44, P = .000) but not related to education and gender. In PD groups, no correlations were observed between the MDRS-2 total standard score and age at diagnosis, PD duration, and the H & Y stages. The Ham-D score was not associated with the MDRS-2 total and the 5 subscales standard scores. Because comparisons showed that the 5 groups were different on age, this variable was included as covariable in between-group comparisons on the MDRS-2. Figure 1 illustrates the performances obtained on the 5 MDRS-2 subscales by the MCI and dementia groups.
Figure 1.
Cognitive profile of the 5 groups on the MDRS-2 subscales.
ATT indicates attention; I/P, initiation/perseveration; CTR, Construction; CPT, Conceptualization; MEM, Memory.
Detailed standardized scores on the MDRS-2 and the effect sizes (Cohen d) for all possible contrasts are also listed in Table 2 (MCI groups) and Table 3 (dementia groups).
Table 3.
Standard Scores (Mean and Standard Deviation) of the MCI and HC Groupsa on the MDRS-2
| HC (1) | PD-MCI (2) | A-MCI (3) | Cohen Effect Size (d) a | |||
|---|---|---|---|---|---|---|
| 1-2 | 1-3 | 2-3 | ||||
| MDRS-2, total | 11.1 ± 2.4 | 7.9 ± 3.2 | 8.01 ± 3.1 | 1.11 | 1.12 | 0.01 |
| MDRS-2, attention | 10.9 ± 1.4 | 9.9 ± 1.9 | 10.9 ± 1.9 | 0.56 | 0.05 | 0.55 |
| MDRS-2, initiation/perseveration b | 9.3 ± 1.4 | 7.8 ± 2.1 | 8.5 ± 2.9 | 0.79 | 0.34 | 0.27 |
| MDRS-2, construction | 9.9 ± 1.2 | 9.7 ± 1.6 | 10.2 ± 1.4 | 0.12 | 0.21 | 0.30 |
| MDRS-2, conceptualization | 9.4 ± 2.0 | 8.0 ± 2.7 | 8.9 ± 2.2 | 0.61 | 0.28 | 0.35 |
| MDRS-2, memory c | 11.9 ± 1.9 | 9.2 ± 3.5 | 7.7 ± 4.2 | 0.95 | 1.26 | 0.37 |
Abbreviations: HC, healthy controls; PD-MCI, Parkinson’s disease with mild cognitive impairment; A-MCI, amnestic mild cognitive impairment; MDRS-2, Mattis Dementia Rating Scale-Second Edition.
a Age-corrected scaled scores for all MDRS-2 subtests and age- and education-scaled scores for MDRS-2 total.
b HC = A-MCI, HC > PD-MCI, A-MCI = PD-MCI; P = .000.
c HC > A-MCI = PD-MCI; P = .000.
A MANCOVA, applied to MDRS-2 standardized scores with age as covariable, showed a significant multivariate group effect (F(6, 93) = 8.98, P = .000). Post hoc pairwise comparisons revealed that HC obtained a higher global score than patients (all Ps < .001). The performances of the 2 MCI groups were similar on the MDRS-2 total score (P = .894) so were the performances of the 2 dementia groups (P = .111). Regarding the performances on the MDRS-2 subscales, the dementia groups scored lower than HC on all subscales, while AD performed better than PDD on conceptualization (P = .043) and lower than PDD on memory (P = .016). On the other hand, HC were better than PD-MCI on initiation/perseveration (P = .035) and better than PD-MCI (P = .007) and A-MCI (P = .000) on memory. The performances of the 2 MCI groups did not differ significantly on the 5 MDRS-2 subscales.
In order to identify which variables best discriminate between HC and dementia groups, a first DFA (stepwise forward method) was computed. The results showed that 2 discriminant functions, including the same 4 variables, provided an optimal discrimination between the groups. The first function (canonical correlation = .940) selected the performances on the MDRS-2 memory subscale (step 1), initiation/perseveration subscale (step 2), age (step 3), and conceptualization subscale (step 4). The second discriminant function (canonical correlation = .399) first entered in the equation the performances on the MDRS-2 conceptualization subscale (step 1), memory subscale (step2), initiation/perseveration subscale (step 3), and age (step 4). Then, a classification analysis based upon these 4 variables demonstrated an overall classification accuracy of 81.7%, with 100% of the HC group, 86.4% of the AD group, and 50% of the PDD group being correctly classified. In order to determine which variables could better differentiate between HC, A-MCI, and PD-MCI, another DFA was conducted. Only 1 discriminant function based on the MDRS-2 memory subscale was significant (canonical correlation = .464). This variable correctly classified 68.2% of the HC group, 50.0% of the A-MCI group, and 31.8% of the PD-MCI group (overall classification accuracy = 50%; Table 4 ).
Table 4.
Standard Scores (Mean and Standard Deviation) of the Dementia and HC Groups a on the MDRS-2
| HC (1) | PDD (4) | AD (5) | Cohen’s Effect Size (d) b | |||
|---|---|---|---|---|---|---|
| 1-4 | 1-5 | 4-5 | ||||
| MDRS-2, total b | 11.1 ± 2.4 | 3.4 ± 2.5 | 1.7 ± 1.3 | 3.10 | 4.85 | 0.86 |
| MDRS-2, attention b | 10.9 ± 1.4 | 9.1 ± 2.7 | 9.6 ± 2.8 | 0.78 | 0.55 | 0.18 |
| MDRS-2, initiation/perseveration b | 9.3 ± 1.4 | 3.5 ± 2.4 | 2.8 ± 1.4 | 2.86 | 4.59 | 0.37 |
| MDRS-2, construction b | 9.9 ± 1.2 | 7.7 ± 3.2 | 8.9 ± 3.0 | 0.89 | 0.46 | 0.36 |
| MDRS-2, conceptualization c | 9.4 ± 2.0 | 4.9 ± 2.9 | 6.3 ± 2.9 | 1.85 | 1.25 | 0.50 |
| MDRS-2, memory d | 11.9 ± 1.9 | 4.7 ± 3.8 | 2.1 ± 0.3 | 2.40 | 7.24 | 0.97 |
Abbreviations: HC, healthy controls; PDD, Parkinson’s disease with dementia; AD: Alzheimer’s disease; MDRS-2, Mattis Dementia Rating Scale-Second Edition.
a Age-corrected scaled scores for all MDRS-2 subscales and age- and education-scaled scores for MDRS-2 total.
b HC > PDD = AD.
c HC > AD > PDD.
d HC > PDD > AD.
Finally, clinical validity of the MDRS-2 for the detection of MCI was examined using an ROC curve analysis after excluding patients with dementia. As defined previously, the optimal cutoff point was 139-140/144 (sensitivity = 0.80; specificity = 0.68; LR+ = 2.5, LR− = 0.4). The AUC (95% CI) was 0.81 (0.71-0.91). Then, including only patients with dementia and HC, a cutoff value of 132-133/144 yielded perfect sensitivity (1.00) and specificity (1.00), with an AUC (95% CI) of 1.0 (1.0-1.0).
Discussion
To our knowledge, this is the first study to assess the clinical validity of the MDRS-2 for screening A-MCI, PD-MCI as well as AD and PDD. The MCI and dementia diagnoses were carefully done following the administration of a broad neuropsychological battery and based on clinical judgment as recommended 34 ; this is a strength of the study. However, this diagnostic neuropsychological battery included fewer tests of attention and visuospatial organization than tests of verbal memory and executive functions. This might have influenced the results, notably regarding the classification of MCI (eg, single vs multiple domains).
Mattis Dementia Rating Scale-Second Edition as a Screening Tool for Dementia
The present study confirms that the MDRS-2 is a good screening test for AD and PDD. Indeed, participants with dementia scored lower than HC on the total score and on each of the MDRS-2 5 subscales. Moreover, a cutoff raw score of ≤132 correctly identified 100% of patients with dementia and 100% of HC. Clinicians can be confident when using this cutoff value for the screening of PDD or AD. Various cutoff scores were proposed over the years to accurately diagnose either unspecified dementia (<132), 18 AD (<129), 16 or PDD (<123) 45 with the MDRS. Demographics, participant’s provenance (eg, community vs specialized clinics), and severity of global cognitive deterioration may explain these discrepancies between the aforementioned studies, emphasizing the importance of using norms representative of the population when choosing a cutoff.
The current results also revealed that the pattern of performances between PDD and AD groups differed on the MDRS-2 subscales. As expected, and according to the results of the previous studies,20,21,46 patients with AD scored lower than patients with PDD on the memory subscale. At a similar level of global cognitive impairment, the memory difficulties of patients with mild AD were still more severe than those of patients with mild PDD. Patients with AD also obtained a higher score than patients with PDD on the conceptualization subscale, which measures a component of executive functions. 15 This is in line with the results from some studies47,48 but not with others. 20 A lower raw score on the conceptualization subscale at baseline has already been found to be predictive of incident dementia in PD, along with memory impairment. 48 However, depressive symptoms seemed to account for the association between this subscale and future PDD in the study of Levy and colleagues. 48 This was not the case in the present study, given the absence of correlation between the performance on the conceptualization subscale and the total score on the Ham-D.
On the other hand, the hypothesis that patients with PDD would perform significantly worse than patients with AD on the initiation/perseveration subscale was not confirmed. This finding did not support the results of the previous research. 21 The discrepancy between Aarsland and the present report may be explained by demographic and methodological differences. The patients in the study of Aarsland were older than the present patients with PDD (77.2 ± 5.6 vs 73.5 ± 7.7 years old) and their disease duration was longer (15.4 ± 5.0 vs 11.13 ± 6.4 years) as well. Furthermore, the age- and education-corrected total scores on the MDRS of their patients with PDD (n = 17) were significantly worse than those of the patients with AD (n = 11). 21 However, the authors did not take this difference into account when they compared the performances on age-adjusted scores of the MDRS subscales. Therefore, the more severe global cognitive impairment in PDD compared to AD might be responsible for the different performance profiles registered between these 2 patient groups on the MDRS in the study of Aarsland, as opposed to the present study.
Mattis Dementia Rating Scale-Second Edition as a Screening Tool for MCI
The current results confirmed that the MDRS-2 is sensitive to the presence of MCI. Indeed, patients with PD-MCI and A-MCI obtained a significantly lower standardized total score than HC on the MDRS-2, thus indicating lower global cognitive functioning. All these patients were considered “normal” on the MMSE, thus showing the superiority of the MDRS-2 to detect individuals at the earliest stage of cognitive impairment. Other studies have demonstrated the poor sensitivity of the MMSE in PD-MCI 49 and A-MCI. 50 Moreover, yielding large effect sizes, the initiation/perseveration and memory subscales were useful in discriminating between PD-MCI and HC. As expected, the A-MCI patients also scored lower than HC on the memory subtest.
However, the A-MCI and PD-MCI groups were similar on all the MDRS-2 subscales, thus infirming the fifth hypothesis (see Table 1). On the basis of the results obtained by Aarsland and colleagues, 21 the authors of the study first anticipated that patients with PD-MCI would have an inferior performance compared to that of patients with A-MCI on the initiation/perseveration subscale, measuring some executive functions. No difference was found between the 2 groups, thus suggesting that patients with A-MCI may also present with subtle executive deficits. 51 This is congruent with prior research, demonstrating that cognitive tests assessing episodic memory and executive functions are among the first to show deterioration long before AD symptoms become clinically apparent. 52 Moreover, the MDRS-2 initiation/perseveration subscale is largely composed by a semantic verbal fluency task (eg, “supermarket”) known to be impaired in both PD-MCI 53 and A-MCI.9,51 Semantic fluency, but not phonemic fluency, was recently revealed as a significant predictor of dementia in PD. 54
The present results also demonstrated that the performance of patients with A-MCI was not different from that of PD-MCI on the MDRS-2 memory subscale, as it was hypothesized. This finding may be explained by some factors. First, after executive dysfunction, memory impairment was the most predominant deficit in 36% of patients with PD-MCI. Some previous studies also reported significant memory decline in early PD,5,55 while others have found relatively spared memory functioning.56,57 Second, the MDRS-2 memory subscale has been related to hippocampal atrophy in AD, 58 a cerebral structure also known to present with atrophy in PD without dementia. 59 Unfortunately, the MDRS-2 does not allow to precise which component of memory processing (eg, encoding, retrieval, or retention) contributes to the memory difficulties. Being able to do so would be of great interest, given that this information may improve the capacity to perform a differential diagnosis between distinct forms of neurodegenerative diseases such as AD and PD and eventually between A-MCI and PD-MCI.
Another interesting finding of the present study concerns the medications prescribed differentially in PD and AD. The results showed that anticholinergics were prescribed in 27% of patients with PD-MCI and, to a lesser extent, in 13% of patients with PDD. Moreover, patients with PD-MCI (33%) and PDD (47%) took benzodiazepines in a greater proportion than patients with A-MCI (22%) and AD (19%). Anticholinergics improve movement problems, 60 while benzodiazepines, such as “clonazepam,” are useful for the treatment of rapid eye movement (REM) sleep behavior in PD. 61 However, these molecules must be prescribed with caution in cognitively impaired patients because they are known to have a deleterious impact on cognition, as they may induce confusion and memory problems.60,62,63 Nearly, 73% of patients with AD received acetylcholinesterase inhibitor (AChEI), whereas only 13% PDD were administered these nootropics. These data are interesting, given that functional positron emission tomography (PET) studies have reported an even greater cholinergic decrement in cortical areas of PDD than in AD of similar severity. 64 In addition, as in AD, the use of AChEI for PDD is nowadays considered a safe and efficacious symptomatic treatment. 65 Thus, it is possible that the cognitive performances on the MDRS-2 were influenced by the different pharmacological treatments received by the 4 patient groups.
The present data support the clinical validity of the MDRS-2 for the screening of dementia of the Alzheimer’s type and dementia secondary to PD. The results also suggest that the MDRS-2 is sensitive to the presence of mild cognitive syndromes. However, it may not be specific enough to discriminate between PD-MCI and A-MCI, as defined here. In order to detect PD-MCI, the MDRS-2 appears to be superior to the MMSE, but as sensitive as the Montreal Cognitive Assessment (MoCA) test, 66 another widespread general cognitive screening test. The MDRS-2 has the advantage to come with a valid alternative version, 67 which allows avoiding practice effects as much as possible. This is clearly a strong asset, given that several follow-up assessments are mandatory for the diagnosis of dementia. Moreover, the MDRS-2 is able to capture mild degrees of functional difficulties, 68 to document cognitive deterioration, 19 and change following pharmacological interventions. 69
Some shortcomings of this study shall be mentioned. First, the current findings were obtained with a small number of patients and thus need to be replicated. The small sample size might have affected the statistical power of the study and might explain that some hypotheses were not verified (see hypotheses 2 and 5 in Table 1). The samples involved patients who were exclusively Caucasians, aged in average 60 years and older and relatively well educated (at least 10 years of schooling). Therefore, generalization of the present results is limited to individuals sharing similar demographics. The fact that the proposed cutoff score for the MCI diagnosis may produce a high rate of false positives must also be mentioned. It is thus recommended to remain cautious regarding the underlying causes of subtle cognitive deficits when they are evidenced by the MDRS-2, given its limited specificity. The potential impact of vascular risk factors on cognition, such as high blood pressure and hypercholesterolemia, cannot be ruled out, as some patients in the present study might have had these symptoms. Finally, the cross-sectional design of this study does not allow confirmation as to whether the MDRS-2 and the criteria used to define MCI are predictive of future PDD and AD. In order to answer these important questions, longitudinal clinicopathological studies including larger cohorts of patients are required.
Acknowledgments
EM is supported by the Canadian Institutes of Health Research (CIHR), the Alzheimer Society of Canada, by the Fonds de la Recherche en Santé du Québec, and by the Canadian Federation of University Women (doctoral awards). MS is engaged in research funded by the Alzheimer Society of Canada and the CIHR (Institute of Aging). These funding sources had no involvement in the present study apart from financial support of EM. The authors would like to extend their thanks to Dr Evelyn Keller for her help with the translation of the manuscript.
Footnotes
The authors declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Peterson RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56(3):303–08. [DOI] [PubMed] [Google Scholar]
- 2. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment- beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. [DOI] [PubMed] [Google Scholar]
- 3. Petersen RC. Mild Cognitive Impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. [DOI] [PubMed] [Google Scholar]
- 4. Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22(9):1272–7. [DOI] [PubMed] [Google Scholar]
- 5. Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: Progression to dementia. Mov Disord. 2006;21(9):1343–9. [DOI] [PubMed] [Google Scholar]
- 6. Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: The norwegian parkwest study. Neurology. 2009;72(13):1121–6. [DOI] [PubMed] [Google Scholar]
- 7. Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289(1-2):18–22. [DOI] [PubMed] [Google Scholar]
- 8. Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63(5):674–81. [DOI] [PubMed] [Google Scholar]
- 9. Saka E, Elibol B. Enhanced cued recall and Clock Drawing Test performances differ in Parkinson's and Alzheimer’s disease-related cognitive dysfunction. Parkinsonism Relat Disord. 2009;15(9):688–91. [DOI] [PubMed] [Google Scholar]
- 10. Simard M, Sampson E. Dementia: Pharmacological and non-pharmacological treatments and guideline review. In: Tyrer P, Silk K. (Eds.), The Cambridge Textbook of Effective Treatment in Psychiatry. Cambridge: Cambridge University Press;2008: 217–43. [Google Scholar]
- 11. Rowan E, McKeith IG, Saxby BK, O'Brien JT, Burn D, Mosimann U, et al. Effects of donepezil on central processing speed and attentional measures in Parkinson’s disease with dementia and dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2007;23(3):161–7. [DOI] [PubMed] [Google Scholar]
- 12. Coblentz JM, Mattis S, Zingesser LH, Kasoff SS, Wisniewski HM, Katzman R. Presenile dementia. Clinical aspects and evaluation of cerebrospinal fluid dynamics. Arch Neurol. 1973;29(5):299–308. [DOI] [PubMed] [Google Scholar]
- 13. Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak T, Karasi TB. (Eds.), Geriatric psychiatry. New York: Crane and Stratten;1976: 77–121. [Google Scholar]
- 14. Schultz SK, Magnotta V, Duff K, Boles Ponto LL, Moser DJ. Evaluation of older persons with mild cognitive deficits: Potential utility of magnetic resonance imaging. Ann Clin Psychiatry. 2008;20(4):204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lezak MD, Howieson DB, Loring WL. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 16. Monsch AU, Bondi MW, Salmon DP, Butters N, Thal LJ, Hansen LA, et al. Clinical validity of the Mattis Dementia Rating Scale in detecting dementia of the Alzheimer type. A double cross-validation and application to a community-dwelling sample. Arch Neurol. 1995;52(9):899–904. [DOI] [PubMed] [Google Scholar]
- 17. Vitaliano PP, Breen AR, Russo J, Albert M, Vitiello MV, Prinz PN. The clinical utility of the Dementia Rating Scale for assessing Alzheimer patients. J Chronic Dis. 1984;37(9-10):743–53. [DOI] [PubMed] [Google Scholar]
- 18. Green RC, Woodard JL, Green J. Validity of the Mattis Dementia Rating Scale for detection of cognitive impairment in the elderly. J Neuropsychiatry Clin Neurosci. 1995;7(3):357–60. [DOI] [PubMed] [Google Scholar]
- 19. Shay KA, Duke LW, Conboy T, Harrell LE, Callaway R, Folks DG. The clinical validity of the Mattis Dementia Rating Scale in staging Alzheimer's dementia. J Geriatr Psychiatry Neurol. 1991;4(1):18–25. [DOI] [PubMed] [Google Scholar]
- 20. Paolo AM, Troster AI, Glatt SL, Hubble JP, Koller WC. Differentiation of the dementias of Alzheimer's and Parkinson’s disease with the Dementia Rating Scale. J Geriatr Psychiatry Neurol. 1995;8(3):184–88. [DOI] [PubMed] [Google Scholar]
- 21. Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the Dementia Rating Scale in Parkinson’s disease with dementia and dementia with Lewy bodies: Comparison with progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psych. 2003;74(9):1215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matteau E, Simard M, Jean L, Turgeon Y. Detection of mild cognitive impairment using cognitive screening tests: A critical review and preliminary data on the Mattis Dementia Rating Scale. In: Tsai JP. (Ed.), Leading-edge cognitive disorders research. Hauppauge (NY): Nova Science Publishers Inc;2008:9–58. [Google Scholar]
- 23. Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: A clinicopathologic study. Neurology. 1992;42(6):1142–6. [DOI] [PubMed] [Google Scholar]
- 24. Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57(8):1497–9. [DOI] [PubMed] [Google Scholar]
- 25. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–96. [DOI] [PubMed] [Google Scholar]
- 26. Leentjens AF, Verhey FR, Lousberg R, Spitsbergen H, Wilmink FW. The validity of the Hamilton and Montgomery-Asberg Depression Rating Scales as screening and diagnostic tools for depression in Parkinson’s disease. Int J Geriatr Psychiatry. 2000;15(7):644–9. [DOI] [PubMed] [Google Scholar]
- 27. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14. [DOI] [PubMed] [Google Scholar]
- 28. Vanderheyden JE, Gonce M, Bourgeois P, Cras P, De Nayer AR, Flamez A, et al. Epidemiology of major depression in belgian Parkinsonian patients. Acta Neurol Belg. 2010;110(2):148–56. [PubMed] [Google Scholar]
- 29. Karttunen K, Karppi P, Hiltunen A, Vanhanen M, Valimaki T, Martikainen J, et al. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’fs disease. Int J Geriatr Psychiatry. 2010; [DOI] [PubMed] [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 31. Troyer AK, Rich JB. Psychometric properties of a new metamemory questionnaire for older adults. J Gerontol A Biol Sci Med Sci. 2002;57(1):P19–27. [DOI] [PubMed] [Google Scholar]
- 32. Fort I, Adoul L, Holl D, Kaddour J, Gana K. Psychometric properties of the french version of the Multifactorial Memory Questionnaire for adults and the elderly. Can J Aging. 2004;23(4):347–57. [DOI] [PubMed] [Google Scholar]
- 33. Gauthier S, Gelinas I, Gauthier L. Functional disability in Alzheimer’ffs disease. Int Psychoger. 1997;9(Suppl 1):163–5. [DOI] [PubMed] [Google Scholar]
- 34. Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild Cognitive Impairment: Ten years later. Arch Neurol. 2009;66(12):1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Psychiatric Association A. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 36. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the nincds-adrda work group under the auspices of Department of Health and Human, Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–44. [DOI] [PubMed] [Google Scholar]
- 37. Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–707. quiz 837. [DOI] [PubMed] [Google Scholar]
- 38. Delis DC, Kaplan E, Kramer JH. In: Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 39. Weschler D. Weschler Adult Intelligence Scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 40. Delis DC, Kramer JH, Kaplan EF, Ober BA. In: California Verbal Learning Test: Adult version manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 41. Cahn DA, Salmon DP, Monsch AU, Butters N, Wiederholt WC, Corey-Bloom J, et al. Screening for dementia of the Alzheimer type in the community: The utility of the Clock Drawing Test. Arch Clin Neuropsychol. 1996;11(6):529–39. [PubMed] [Google Scholar]
- 42. Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17(5):427–42. [DOI] [PubMed] [Google Scholar]
- 43. Mattis S. In: Dementia Rating Scale: Professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 44. Pedraza O, Lucas JA, Smith GE, Petersen RC, Graff-Radford NR, Ivnik RJ. Robust and expanded norms for the Dementia Rating Scale. Arch Clin Neuropsychol. 2010;25(5):347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Llebaria G, Pagonabarraga J, Kulisevsky J, Garcia-Sanchez C, Pascual-Sedano B, Gironell A, et al. Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson’s disease. Mov Disord. 2008;23(11):1546–50. [DOI] [PubMed] [Google Scholar]
- 46. Cahn-Weiner DA, Grace J, Ott BR, Fernandez HH, Friedman JH. Cognitive and behavioral features discriminate between Alzheimer's and Parkinson’s disease. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(2):79–87. [PubMed] [Google Scholar]
- 47. Litvan I, Mohr E, Williams J, Gomez C, Chase TN. Differential memory and executive functions in demented patients with Parkinson's and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1991;54(1):25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levy G, Jacobs DM, Tang MX, Cote LJ, Louis ED, Alfaro B, et al. Memory and executive function impairment predict dementia in Parkinson’s disease. Mov Disord. 2002;17(6):1221–6. [DOI] [PubMed] [Google Scholar]
- 49. Mamikonyan E, Moberg PJ, Siderowf A, Duda JE, Have TT, Hurtig HI, et al. Mild cognitive impairment is common in Parkinson’s disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15(3):226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 51. Kramer JH, Nelson A, Johnson JK, Yaffe K, Glenn S, Rosen HJ, et al. Multiple cognitive deficits in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22(4):306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, et al. Preclinical Alzheimer disease: Neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63(12):2341–7. [DOI] [PubMed] [Google Scholar]
- 53. Song IU, Kim JS, Jeong DS, Song HJ, Lee KS. Early neuropsychological detection and the characteristics of Parkinson’s disease associated with mild dementia. Parkinsonism Relat Disord. 2008;14(7):558–62. [DOI] [PubMed] [Google Scholar]
- 54. Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the campaign cohort. Brain. 2009;132(Pt 11):2958–69. [DOI] [PubMed] [Google Scholar]
- 55. Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology. 2010;75(12):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woods SP, Troster AI. Prodromal frontal/executive dysfunction predicts incident dementia in Parkinson’s disease. J Int Neuropsychol Soc. 2003;9(1):17–24. [DOI] [PubMed] [Google Scholar]
- 57. McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D. Cognitive characteristics associated with mild cognitive impairment in Parkinson’s disease. Dement Geriatr Cogn Disord. 2009;28(2):121–9. [DOI] [PubMed] [Google Scholar]
- 58. Fama R, Sullivan EV, Shear PK, Marsh L, Yesavage JA, Tinklenberg JR, et al. Selective cortical and hippocampal volume correlates of Mattis Dementia Rating Scale in Alzheimer disease. Arch Neurol. 1997;54(6):719–28. [DOI] [PubMed] [Google Scholar]
- 59. Bruck A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75(10):1467–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003(2):CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aurora RN, Zak RS, Auerbach SH, Casey KR, Chowdhuri S, Karippot A, et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389–401. [PMC free article] [PubMed] [Google Scholar]
- 62. Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: A cohort study. J Neurol Neurosurg Psychiatry. 2010;81(2):160–5. [DOI] [PubMed] [Google Scholar]
- 63. Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. 2005;66(Suppl 2):9–13. [PubMed] [Google Scholar]
- 64. Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in Parkinsonian dementia than in Alzheimer disease: An in vivo positron emission tomographic study. Arch Neurol. 2003;60(12):1745–8. [DOI] [PubMed] [Google Scholar]
- 65. van Laar T, De Deyn PP, Aarsland D, Barone P, Galvin JE. Effects of cholinesterase inhibitors in Parkinson’s disease dementia: A review of clinical data. CNS Neurosci Ther. 2010. July 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23(7):1043–6. [DOI] [PubMed] [Google Scholar]
- 67. Schmidt K. In: Dementia rating scale-2: Alternate form supplemental manual. Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- 68. Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, Weintraub D, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010;25(9):1170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dujardin K, Devos D, Duhem S, Destee A, Marie RM, Durif F, et al. Utility of the Mattis Dementia Rating Scale to assess the efficacy of rivastigmine in dementia associated with Parkinson's disease. J Neurol. 2006;253(9):1154–9. [DOI] [PubMed] [Google Scholar]