Abstract
Glomerular filtration rate (GFR) was estimated by plasma clearance of iohexol (PCio) in 52 conscious cats presented for a variety of reasons to Angel Animal Hospital over a 2-year period. Cats were divided into four groups according to their clinical conditions and reasons for measuring PCio. The median PCio (ml/min/kg) was 3.68 in normal cats (NM), 2.39 in cats with suspected renal disease (SP), 1.35 in cats referred to confirm renal dysfunction (RD), and 0.84 in cats with apparent clinical signs of renal failure (RF). There was a significant difference between the results for each group. The respective medians of blood urea nitrogen (BUN) and plasma creatinine concentration (Pcr) (mg/dl) were 15 and 1.40 in NM cats, 21 and 1.71 in SP cats, 30 and 2.20 in RD cats, and 48 and 3.30 in RF cats. The reference values of BUN and Pcr were 21 ± 7 mg/dl and 1.5 ± 0.4 mg/dl (mean ± SD). Diminished renal function could not be detected in SP cats by either BUN or Pcr, while a marked decrease of GFR was demonstrated before BUN and Pcr increased, indicating the insensitivity of BUN and Pcr in detecting renal dysfunction in cats. PCio can be performed non-invasively in conscious cats, which improves the veterinarian's ability to detect early stages of chronic renal disease.
Cats provide a special companionship to humans and the longer companion cats live, the stronger the relationship between cats and their owners. Older cats, however, show clinical signs indicating that skin, heart, kidney, thyroid gland, and brain are undergoing progressive and irreversible changes (Goldston 1995). In an age distribution study, Lulich et al (1992) reported that 62% of the cats with chronic renal failure were 10 years of age or older.
Although chronic renal disease seems to be a major cause of illness and death in older cats (Lund et al 1999), laboratory methods which can accurately evaluate renal function in these animals are limited. The evaluation of glomerular filtration rate (GFR) is pivotal in the diagnosis of patients with renal dysfunction because it is directly related to the functional renal mass. Blood urea nitrogen (BUN) and plasma creatinine concentration (Pcr) are the usual parameters for estimating GFR in patients referred to clinical practice. However, BUN is greatly affected by protein intake and protein metabolism, and Pcr is influenced by lean body mass (Levey at al 1991). The most accepted methods for estimating GFR in cats are urinary clearance of inulin and exogenous creatinine (Ross & Finco 1981), but they are rarely performed in clinical settings due to their labour intensive nature and an associated risk of lower urinary tract infection. Therefore, more precise, non-invasive, and convenient methods for estimating GFR are urgently needed for the early assessment and monitoring of declined renal function in cats, especially in geriatric cats.
Plasma clearance of iohexol (PCio) is one method for estimating GFR that has been postulated as a promising alternative to traditional urinary clearance methods in humans (Levey et al 1990), dogs (Brown et al 1996), and cats (Brown et al 1996, Miyamoto 2000). This method is very attractive because there is no need for timed urine collection nor for a constant infusion of indicator, and iohexol is readily available as a radiographic contrast agent.
In the present study, PCio was employed to evaluate a variety of degrees of renal function in feline patients presented at a rural animal hospital in the southern part of Japan over a 2-year period. The purpose of this study is to elucidate the relation between both BUN and Pcr and GFR estimated by PCio using conscious cats that are normal as well as those with renal dysfunction.
Materials and methods
Fifty two cats presented for a variety of reasons to Angel Animal Hospital in Yatsushiro City Kumamoto, between November 1997 and October 1999 were studied for PCio determination. Cats were divided into four groups according to their clinical conditions and the reasons for PCio measurement: (1) cats presented for annual revaccination and defined as healthy by a routine physical examination, complete blood count, and chemical profile (NM; n=17); (2) cats presented with anorexia and depression, but during routine examination renal disease was suspected (SP; n=5); (3) cats referred to confirm renal dysfunction (RD; n=9); and (4) cats with apparent clinical evidence of chronic renal failure (RF; n=21). Of fifty-two cats, 50 were domestic short-haired cats, one was Persian, and one was Himalayan. Cats were fasted overnight at home prior to the clearance study, but were allowed free access to water.
The reference values for BUN and Pcr were taken from the medical record of 87 clinically healthy cats presented for annual revaccination between January 1997 and December 1999. BUN and Pcr had been determined by enzymatic dry reagent with an Ektachem DT 60 Analyser. The mean values of BUN and Pcr in clinically normal cats was 21 ± 7 mg/dl and 1.5 ± 0.4 mg/dl (mean ± SD), respectively. The 95% confidence interval (mean ± 1.96×SD) for BUN and Pcr was 7–34 mg/dl and 0.7–2.3 mg/dl, respectively.
BUN, Pcr, and PCio were determined once for all 52 patient at the first presentation without any chemical restraint. The dosage of iohexol and sampling times should be determined in each study, because the range of iodine concentration which can be determined was different depending on the analyser used (Brown et al 1996, Miyamoto 2000). The dosage of iohexol in this study was 90 mg of iodine/kg for non-azotemic cats and 45 mg of iodine/kg for azotemic cats. A half millilitre of heparinised blood was collected from the jugular vein immediately before iohexol injection. Iohexol was administered via the cephalic vein (taken as time 0). Then, 0.5 ml of heparinised blood was again sampled at the following times after iohexol injection: 120, 180, and 240 min for non-azotemic cats and 120, 240, and 360 min for azotemic cats (Miyamoto 2000).
To determine plasma iohexol concentration, iohexol was deiodinated by alkaline hydrolysis and the released iodine was subsequently measured according to the ceric arsenite colorimetric method as described elsewhere (Back et al 1988).
PCio was calculated by the one-compartment model corrected with the Broshner-Mortensen formula (Broshner-Mortensen 1972). In brief, AUC was estimated from the slope (β) and intercept (B) of the elimination phase of the curve, as determined by linear regression analysis of the final three plasma samples. A clearance value (Cl) was arrived at, with Cl=dose/AUC; AUC=B/β. The PCio was then calculated as PCio= 0.990778 ×Cl–0.001218 × Cl2.
In the statistical analysis, values are reported as medians except where noted. The difference in median values between groups was evaluated by Mann–Whitney's U-test (Dunn 1977). A P value less than 0.05 was considered significant.
Results
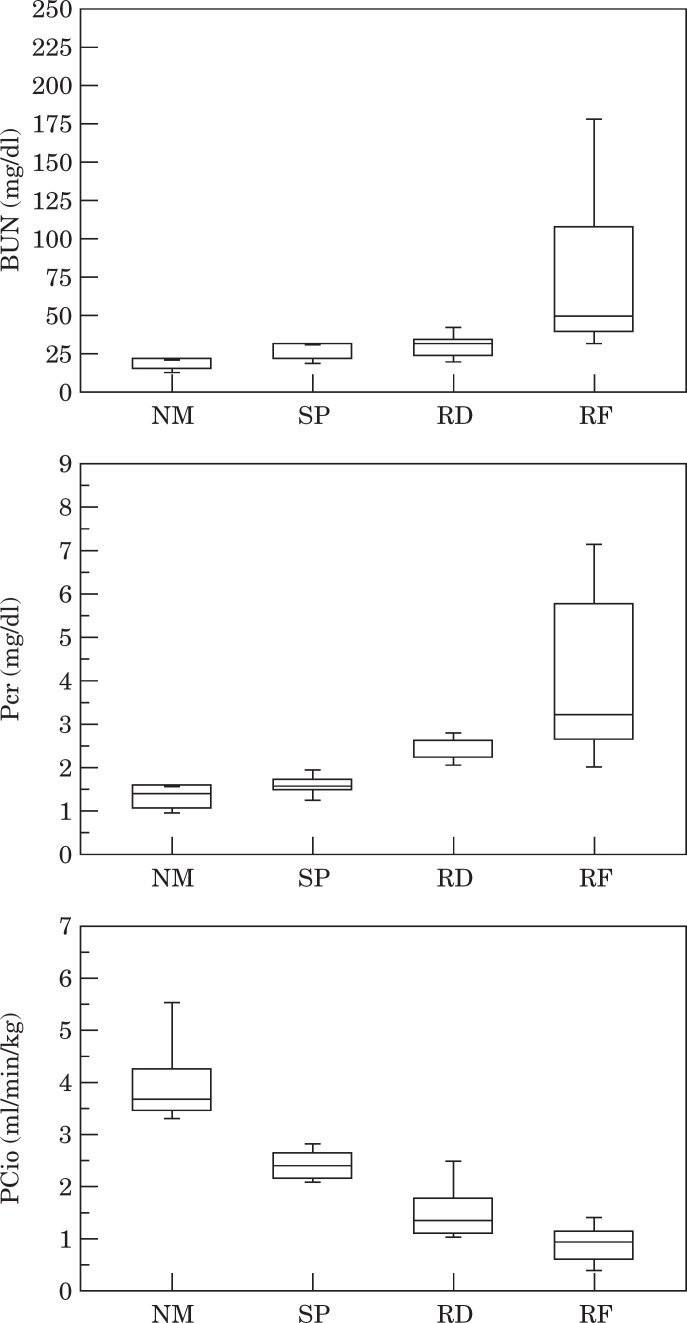
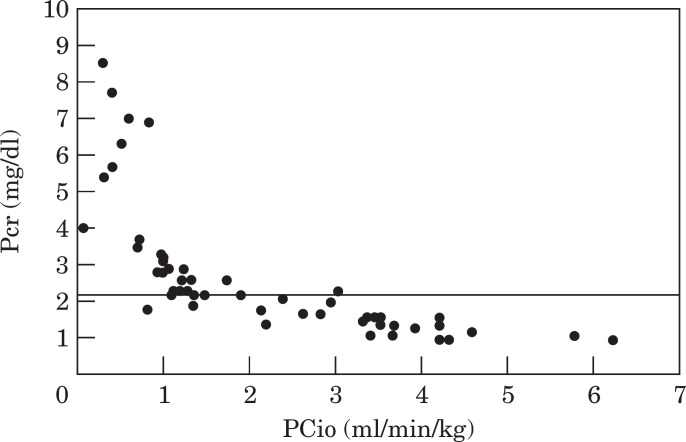
PCio—The median value (range) of bodyweight was 3.54 kg (3.10–4.36) in NM, 3.68 kg (2.9–4.10) in SP, 4.20 kg (3.68–4.6) in RD, and 3.20 kg (2.08–5.32) in RF cats, respectively (Table 1). There was no significant difference between them. Median value (range) of BUN was 15 mg/dl (8–27) in NM, 21 mg/dl (18–30) in SP, 30 mg/dl (17–46) in RD, and 48 mg/dl (28–243) in RF cats, respectively (Table 1; Fig. 1). Median value (range) of Pcr was 1.40 mg/dl (1.0–2.3) in NM, 1.71 mg/dl (1.4–2.1) in SP, 2.20 mg/dl (2.0–2.9) in RD, and 3.3 mg/dl (1.8–8.5) in RF cats, respectively (Table 1; Fig. 1). Median value (range) of PCio was 3.68 ml/min/kg (3.22–6.23) in NM, 2.39 ml/min/kg (2.14–2.83) in SP, 1.35 ml/min/kg (1.07–2.94) in RD, and 0.84 ml/min/kg (0.08–1.35) in RF cats, respectively (Table 1; Fig. 1). There were significant differences between groups in Pcr, BUN, and PCio (Table 1). The relation between BUN and PCio is depicted in Fig. 2, and that between Pcr and PCio is in Fig. 3.
Table 1.
The median of bodyweight (BW), BUN, plasma creatinine concentration (Pcr), and plasma clearance of iohexol (PCio) in normal cats (NM), cats with suspected renal disease (SP), cats referred to confirm renal dysfunction (RD), and cats with apparent clinical signs of chronic renal failure (RF)
| BW (kg) | BUN (mg/dl) | Pcr (mg/dl) | PCio (ml/min/kg) | |
|---|---|---|---|---|
| NM cats | 3.54 (3.10–4.36) | 15 (8–27) | 1.4 (1.0–2.3) | 3.68 (3.22–6.23) |
| SP cats | 3.68 (2.90–4.10) | 21 (18–30)* | 1.7 (1.4–2.1)* | 2.39 (2.14–2.83)* |
| RD cats | 4.20 (3.68–4.60) | 30 (17–46) | 2.2 (2.0–2.9)* | 1.35 (1.07–2.94)* |
| RF cats | 3.20 (2.08–5.32) | 48 (28–243)* | 3.3 (1.8–8.5)* | 0.84 (0.08–1.35)* |
Values are expressed as median (range).
Significantly different from preceding group at P<0.05.
Fig 1.
BUN, plasma creatinine concentration (Pcr), and plasma clearance of iohexol (PCio) in normal cats (NM), cats with suspected renal disease (SP), cats referred to confirm renal dysfunction (RD), and cats with apparent clinical signs of chronic renal failure (RF). The bars depict 90, 75, 50 (median), 25, and 10 percentile, from the top down.
Fig 2.
The relation between BUN and plasma clearance of iohexol (PCio) in cats presented with a variety of renal functions (n=52). Also depicted is a line representing a 95% confidence interval for reference value.
Fig 3.
The relation between plasma creatinine concentration (Pcr) and plasma clearance of iohexol (PCio) in cats presented with a variety of renal functions (n=52). Also depicted is a line representing a 95% confidence interval for reference value.
Discussion
Although several methods have been established as reliable for estimating GFR, they have not prevailed in the clinical setting. The usual methods for estimating renal function are BUN and Pcr in feline practice, but they are relatively insensitive parameters of GFR in that 75% or more total renal functional mass must be lost before increases are observed (Finco 1995). Therefore, practitioners can rarely detect the early stages of chronic renal disease that exhibit no clinical, historical, or laboratory abnormalities.
Recently, the plasma clearance method has been introduced in veterinary medicine and PCio has proved to be a useful alternative to standard urinary clearance methods in cats (Miyamoto 2000). PCio is very attractive for clinical purposes, because it is a more sensitive parameter for GFR than BUN and Pcr. Furthermore, PCio can be determined by using a small volume and number of plasma samples (50 μl and three plasma samples in this study). In fact, these small plasma samples can be collected non-invasively, even in conscious debilitated cats.
The median bodyweight of the cats was not significantly different between groups (Table 1), probably due to the fact that cats were not categorised strictly by clinical histories, clinical signs, and laboratory findings. Information about age and background could not be obtained for some recently adopted normal cats and cats with apparent clinical signs of chronic renal failure, thus analysis of these factors could not be conducted.
PCio was able to clearly distinguish normal cats from others (Table 1), indicating that PCio is a useful screening test for differential diagnosis of renal dysfunction. In addition, PCio could differentiate between groups of cats with declined renal function, indicating it may be used for tracking the process of chronic renal disease more precisely.
Although significant differences were also observed in the medians of both Pcr and BUN between groups, all cats with suspected renal disease and about 50 to 75% of cats referred to confirm renal dysfunction could not be differentiated from normal cats by these parameters. The range of references for BUN (7–34 mg/dl) and Pcr (0.7–2.3 mg/dl) was wide enough to mask the existence of renal dysfunction which was detected by PCio Table 1).
A marked reduction in GFR was observed before BUN and Pcr increased, but the relation between GFR and Pcr was much better than that between GFR and BUN at a low level of GFR (Fig. 3 and 4). These findings are similar to those reported in dogs (Finco 1995) and point out the poor level of reliability of BUN and Pcr in detecting the early decline of GFR in cats. In a recent clinical study of a large number of cats with chronic renal failure, 50% of the uraemic cats had a history of weight loss and cachexia was found in 36% of these cats during physical examination (Elliott & Barber 1998). Creatinine production and its excretion largely depend on lean body mass and GFR, respectively (Finco 1995). Thus, for cats with evidence of renal dysfunction who showed weight loss, Pcr may have lost its reliability as an index of GFR and is likely to lead to an erroneous assessment of the patient's renal function. The author of the clinical study cited above concluded that Pcr remained the most useful screening test for renal dysfunction in cats in the absence of a practical non-invasive method for estimating GFR. Recently, Brown (1999) reported a new, staged approach for chronic renal disease using Pcr and BUN in cats. However, if PCio can be applied to a larger population of cats with chronic renal disease at some time in the future, current claims for the utility of Pcr or categorisations of the disease process are likely to be changed.
At present, there is no proper criterion defining the stage of chronic renal disease in which GFR is between normal and 25% of normal, while chronic renal disease, by definition, takes from several months to several years to conclude in uremia and final death. If renal dysfunction can be detected before GFR declines to 25% of normal, more appropriate medical strategies can be carried out to ameliorate factors contributing to deterioration or to delay the progressive nature of the disease.
In conclusion, this study has demonstrated for the first time the relation between GFR and both BUN and Pcr in conscious cats with a variety of renal functions. Results illustrated that BUN and Pcr are not sensitive indicators of GFR.
Acknowledgements
I would like to acknowledged the invaluable contributions made by my professional colleagues. Thanks are also due to Ms Motoko Miyamoto and Yuko Shiiba (Angel Animal Hospital) for their enthusiastic technical assistants.
References
- Back SE, Masson P, Nilsson-Ehle P. (1988) A simple chemical method for the quantification of the contrast agent iohexol, applicable to glomerular filtration rate measurements. Scandinavian Journal of Clinical Laboratory Investigation 48, 825–829. [DOI] [PubMed] [Google Scholar]
- Broshner-Mortensen J. (1972) A simple method for the determination of glomerular filtration rate. Scandinavian Journal of Clinical Laboratory Investigation 30, 271–274. [DOI] [PubMed] [Google Scholar]
- Brown SA, Finco DR, Boudinot FD, Wright J, Tarver SL, Cooper T. (1996) Evaluation of a single injection method, using iohexol, for estimating glomerular filtration rate in cats and dogs. American Journal of Veterinary Research 57, 105–110. [PubMed] [Google Scholar]
- Brown SA. (1999) Evaluation of chronic renal disease: A staged approach. Compendium on Continuing Education for the Practicing Veterinarian 21, 752–763. [Google Scholar]
- Dunn OJ. (1977) Describing a sample: Measures of location. In: Basic Statistics: A Primer for the Biochemical Sciences (2nd edn). New York, John Wiley & Sons, pp. 30–37. [Google Scholar]
- Elliott JE, Barber PJ. (1998) Feline chronic renal failure: Clinical findings in 80 cases diagnosed between 1992 and 1995. Journal of Small Animal Practice 39, 78–85. [DOI] [PubMed] [Google Scholar]
- Finco DR. (1995) Evaluation of renal functions. In: Canine and Feline Nephrology and Urology. Osborn CA, Finco DR. (eds). Baltimore, Williams & Wilkins, pp. 216–229. [Google Scholar]
- Goldston RT. (1995) Introduction and overview of geriatrics. In: Geriatrics & Gerontology of the Dogs and Cats. Goldston RT, Hoskins JD. (eds). Philadelphia, W. B. Saunders, pp. 1–8. [Google Scholar]
- Levey AS. (1990) Measurement of renal function in chronic renal disease. Kidney International 38, 167–184. [DOI] [PubMed] [Google Scholar]
- Levey AS, Madaio MP, Perrone RD. (1991) Laboratory assessment of renal disease: Clearance, urinalysis, and renal biopsy. In: Kidney (4th edn) Brenner BM, Rector FC. (eds). Philadelphia, W. B. Saunders, pp. 919–968. [Google Scholar]
- Lulich JP, Osborn CA, O'Brien TD, Pozin DJ. (1992) Feline renal failure: Question, answer, question. Compendium on Continuing Education for the Practicing Veterinarian 14, 127–152. [Google Scholar]
- Lund EM, Armstrong PJ, Kirk CA, Kolar LM, Klausner JS. (1999) Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. Journal of American Medical Association 214, 1336–1341. [PubMed] [Google Scholar]
- Miyamoto K. (2000) Use of plasma clearance of iohexol for estimating glomerular filtration rate in cats. American Journal of Veterinary Research 62, 572–575. [DOI] [PubMed] [Google Scholar]
- Ross LA, Finco DR. (1981) Relationship of elected clinical renal function tests to glomerular filtration rate and renal blood flow in cats. American Journal of Veterinary Research 42, 1704–1710. [PubMed] [Google Scholar]