Abstract
Existing reports concerning intervertebral disc disease (IVDD) have focused almost exclusively on dogs, although a small number of individual case reports of IVDD of cats has been published. The medical records of six cats with IVDD were reviewed. Radiographic studies confirmed narrowed intervertebral disc spaces, mineralised intervertebral discs, and one or more extradural compressive lesions of the spinal cord in each cat. All disc extrusions were located in the thoracolumbar region. Surgical decompression of the spinal cord was achieved in all cats by means of hemilaminectomy and removal of compressive extradural material confirmed to be degenerative disc material. Good to excellent neurological recovery was noted in five of the six cats included in this report. Based on this review, it appears that IVDD of cats has many similarities to IVDD of dogs, and that healthy cats with acute intervertebral disc extrusion(s) respond favourably to surgical decompression of the spinal cord.
Introduction
Intervertebral disc disease (IVDD) is a frequently recognised neurological problem of dogs, and numerous reports exist regarding aetiology and pathogenesis of IVDD in this species (Bray & Burbidge 1998a and 1998b). Acute intervertebral disc extrusion (Hansen's Type I) is seen most frequently in young to middle-aged chondrodystrophoid breeds of dog, secondary to chondroid metaplasia and mineralisation of the nucleus pulposus (Hansen 1952). Hansen's Type II disc disease is seen commonly in non-chondrodystrophoid breeds of dog, and is characterised by fibrous degeneration and protrusion of the anulus fibrosus (Hansen 1952). Several individual case reports of IVDD in cats (both Type I and II) resulting in clinical signs of a myelopathy have been published, however, diagnostic evaluation and approach to therapy have varied between reported cases, ranging from necropsy only, to diagnosis with myelography, decompressive surgery, and histopathologic evaluation (Heavner 1971, Seim & Nafe 1981, Gilmore 1983, Littlewood et al 1984, Sparkes & Skerry 1990, Bagley et al 1995, Kathmann et al 2000). Since IVDD should be considered a possible cause of spinal cord dysfunction in cats, further information regarding features of this disease in cats is necessary. The purpose of this report is to describe the history, neurological signs, myelographic findings, surgical therapy, pathological findings, and outcome of six cats with intervertebral disc extrusion (Hansen's Type I).
Materials and methods
Spontaneous disc extrusion that resulted in clinical signs of a myelopathy was diagnosed in six cats at the Veterinary Medical Teaching Hospital (VMTH) of the University of California, Davis, USA, between September 1995 and July 2000 (Table 1). Hospital records and radiographs were reviewed retrospectively. Each cat was evaluated by physical and neurological examinations. Complete blood count and serum chemistry panels were performed in four cats (Cats 2, 4, 5, and 6). Vertebral column radiographs, cerebrospinal fluid (CSF) analysis, and myelography via lumbar puncture, were completed under general anaesthesia in all cats. Surgical decompression by means of hemilaminectomy and removal of the compressive mass were done in all cats. Extradural material recovered at surgery was examined histopathologically All cats were re-examined at the VMTH by the authors from several weeks to years post-operatively (Table 1).
Table 1.
Summary of clinicopathologic features of six cats with intervertebral disc extrusion
| Cat# | Signalment | History | Neuroanatomical localisation | CSF analysis | Myelogram | Treatment | Pathology | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 9 yr FS DMH | Abnormal behaviour 2 days prior to being found acutely down Voluntary urination absent | T3-L3 myelopathy with possible additional L4-S1 myelopathy Non-ambulatory paraparetic, deep pain present | Lumbar: Protein=0.3 g/l RBC 0·014×109/l Nucleated cells 0.003 ×109/l Unremarkable CSF | Only 6 lumbar vertebrae; Extradural compressive lesions T13-L1, L4–5; calcification of T13-L1 disc | L4–5 left hemilaminectomy T13-L1 left hemilaminectomy | Mineralised fibrocartilage with granulomatous inflammation | 5 weeks post-op: very weakly ambulatory; owner expressing bladder 53 months post-op: mild monoparesis left pelvic limb; bladder expressed BID |
| 2 | 6 yr MC Persian | Acute back pain, pelvic limb paresis, lameness Voluntary urination present | T3-L3 myelopathy Ambulatory paraparetic | Lumbar: Protein=0.30 g/l RBC 0.5×109/l Nucleated cells 0.009 ×109/l Differential consistent with blood contamination | Extradural, ventral, and right-sided compression at T12–13 with chronic protrusions at C6–7, T2–3, T3–4, T4–5, T13-L1 | T12–13 right hemilaminectomy with durotomy | Fibrocartilage, degenerate, consistent with extruded disc material | 8 weeks post-op: ambulatory, pelvic limb ataxia 31 months post-op: euthanised because of large abdominal mass |
| 3 | 7 yr MC DSH | Found acutely paraplegic the day prior to presentation Voluntary urination absent | T3-L3 myelopathy Paraplegic, deep pain absent | Lumbar: Protein=0.85 g/l RBC 1.56×109/l Nucleated cells 0.252 ×109/l (92% neutrophils) Neutrophilic pleocytosis with mild haemorrhage | Extradural compression L2–3, with calcification evident | L2–3 left hemilaminectomy with durotomy | Extruded disc material, acute | 4 weeks post-op: weakly ambulatory 8 weeks post-op: mild paraparesis, deep pain absent 14 months post-op: mild paraparesis, deep pain present, superficial pain absent 35 months post-op: died while boarding |
| 4 | 3 yr FS DSH | 3 months progressive paraparesis Voluntary urination present | T3-L3 myelopathy Ambulatory paraparetic | Lumbar: Protein=0.39 g/l RBC 16.6×109/l Nucleated cells 0.020 ×109/l Blood contamination | Right sided extradural compressive lesion T13-L1 | T13-L1 right hemilaminectomy 2 days post-myelogram | Fibrocartilage consistent with extruded disc material | 12 months post-op: ambulatory 14 months post-op: acute onset paraparesis; L7-S3 myelopathy; multiple pulmonary vascular anomalies; marked improvement of neurological signs within 10 days 28 months post-op: lost to follow up, no recurrence of neurological deficits |
| 5 | 3 yr FS Himalayan | Abnormal pelvic limb gait, faecal incontinence; 1 month history paroxysmal episodes Voluntary urination present | T3-L3 myelopathy Ambulatory paraparetic | Lumbar: Protein=0.24 g/l RBC 0.303×109/l Nucleated cells 0.003 ×109/l Normal CSF | Ventral extradural compression T13-L1, L1–2, L4–5 | T13-L2 right hemilaminectomy L4–5 right hemilaminectomy 1 day post-myelogram | Degenerate intervertebral disc material with mineralisation | Phenobarbital 2.3 mg/kg PO BID started day 6 of hospitalisation 8 weeks post-op: weakly ambulatory, urinary incontinence Did not regain ability to ambulate without assistance, paroxysmal episodes increased 6 months post-op: died at home |
| 6 | 7 yr MC DMH | Found acutely paraplegic the night prior to presentation Voluntary urination absent | T3-L6 myelopathy Paraplegic, deep pain absent | Cisternal: Protein=0.30 g/l RBC 0.001 ×109/l Nucleated cells 0.001 ×109/l (64% neutrophils) Mild purulent inflammation | Extradural, right-sided compression L4–5; multiple mineralised intervertebral discs | L4-L6 right hemilaminectomy with durotomy; fenestration of L4–5 disc | Degenerate nucleus pulposus and acute haemorrhage | 3 weeks post-op: strongly ambulatory, pelvic limb ataxia, mild paresis, deep pain present |
MC: male, castrated; FS: female, spayed; DSH: domestic shorthair; DMH: domestic medium hair; PO: Per Os; BID: every 12 hours.
Results
Of the six cats included in this report, three were female. Breeds that were represented included domestic short hair (2), domestic medium hair (2), Persian (1), and Himalayan (1). A summary of presenting history neurological examination, and neuroanatomical localisation is provided in Table 1.
A complete blood count and serum chemistry panel completed preoperatively in four cats (Cats 2, 4, 5, and 6) were within normal limits, with the exception of a mild hypocalcaemia (2.2 mM/l; reference range 2.48–2.85 mM/l) in Cat 5. Results of lumbar CSF analysis were unremarkable in two cats (Cats 1 and 5). There was evidence of blood contamination in the CSF of two cats (0.5 ×109 RBC/l in Cat 2; 16.6×109 RBC/l in Cat 4; reference range 0 RBC/l). Analysis of CSF from Cat 3 revealed a CSF protein determination of 0.85 g/l (reference range [lumbar CSF] 0–0.3 g/l) and a neutrophilic pleocytosis (0.252 ×109 nucleated cells/l, 92% neutrophils; reference range 0–0.004 ×109 mononuclear cells/l) with mild haemorrhage (1.560 × 109 RBC/l) noted on cytology. Mild purulent inflammation (0.001 × 10 nucleated cell/l, 64% neutrophils) and a mildly elevated protein determination (0.3 g/l; reference range [cisternal CSF] 0–0.25 g/l) were noted in a cisternal CSF sample from Cat 6 (it was not possible to obtain lumbar CSF from this cat).
A single extradural compressive lesion of the spinal cord was identified on myelography of three cats (Cats 3, 4, and 6; Fig 1). Two extradural compressive lesions were identified in Cat 1 (Fig 2), and three extradural compressive lesions were seen in Cat 5 (Fig 3). A single severe extradural compressive lesion at T12–13 and multiple mild extradural compressive lesions were seen in Cat 2 (Fig 4). Radiographic evidence of mineralisation of intervertebral discs was identified in three cats (Cats 1, 3, and 6; Figs 2 and 5). Surgical decompression of the spinal cord by means of hemilaminectomy was done in all cats. A durotomy was done in Cats 3 and 6, because deep pain perception was (apparently) absent on neurological examination, and a durotomy was done in Cat 2 because marked discolouration of the spinal cord was noted at surgery.
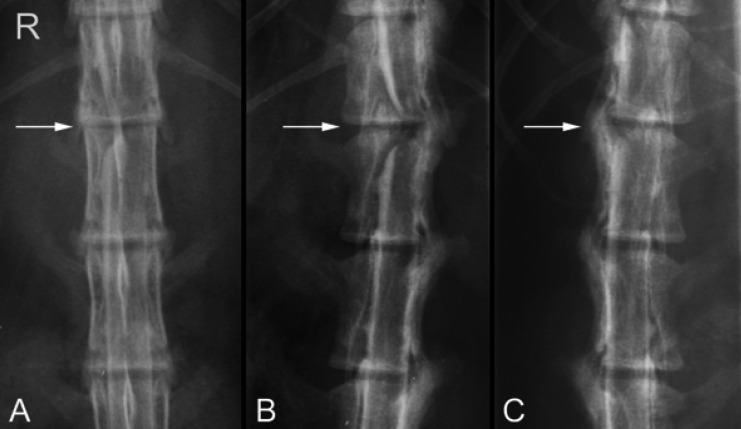
Fig 1.
Ventrodorsal (A), right oblique (B), and left oblique (C) views of the myelogram of Cat 4 demonstrating a right-sided intervertebral disc extrusion resulting in extradural spinal cord compression at T13-L1 (arrows). Deviation of the right contrast column and attenuation of the left contrast column are evident.
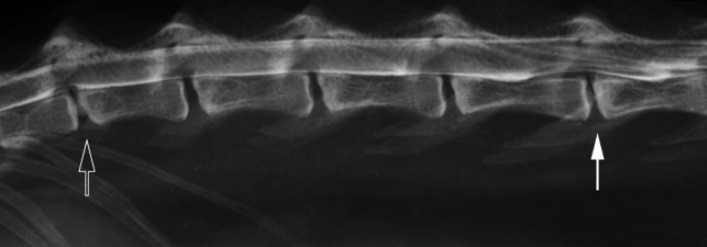
Fig 2.
Right lateral view of the myelogram of Cat 1. Mineralisation of the T13-L1 disc is apparent, combined with a ventral extradural spinal cord compression at that site (open arrow). A broad ventral extradural compression is seen at L4–5, with narrowing of that disc space and sclerosis of the vertebral endplates (closed arrow).
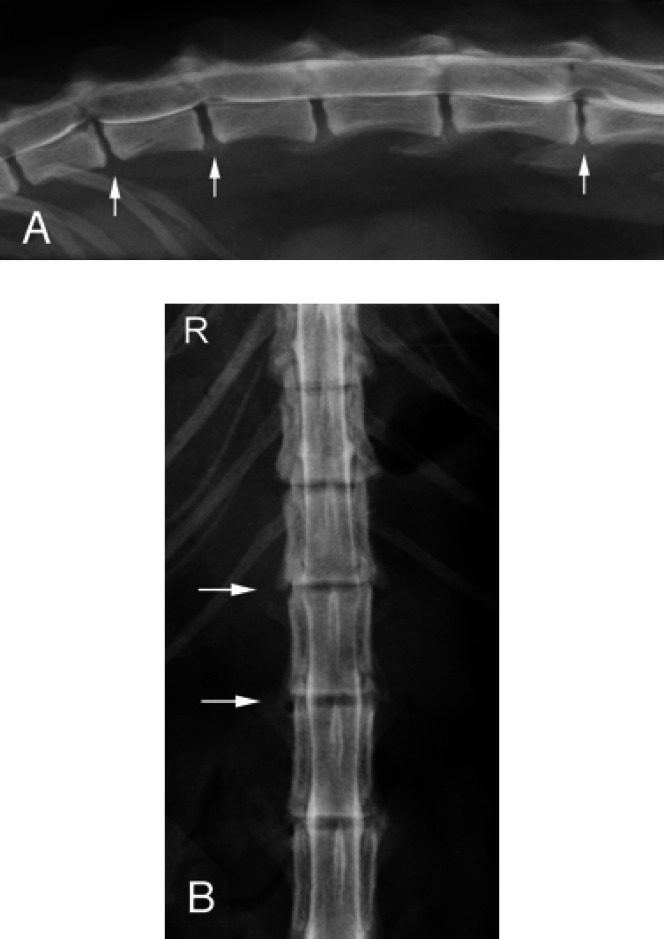
Fig 3.
(A) Right lateral view of the myelogram of Cat 5. Ventral extradural spinal cord compressions are seen at T13-L1, L1–2, and L4–5 (arrows). Narrowing of the T13-L1 and L4–5 disc spaces is noted. (B) Ventrodorsal view of the myelogram of Cat 5 demonstrating attenuation of the lateral contrast columns over the T13-L1 and L1–2 disc spaces.
Fig 4.
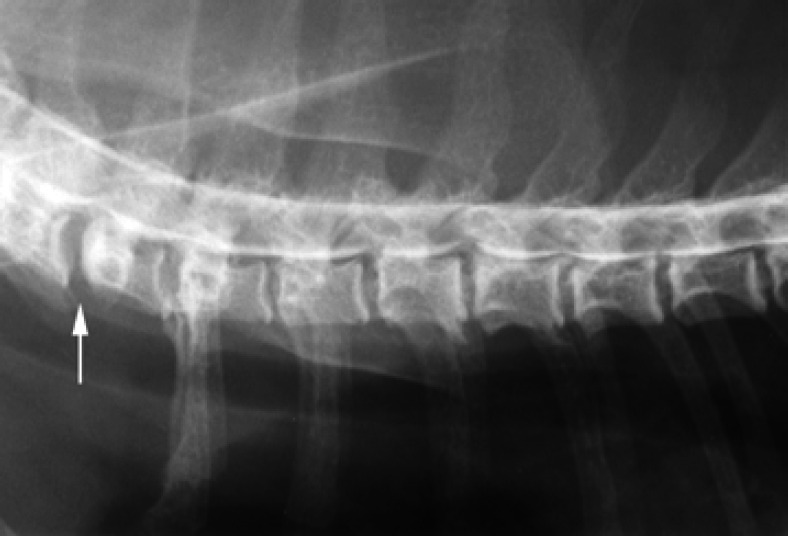
Right lateral view of the myelogram from Cat 2 showing multiple mild, ventral extradural spinal cord compressions, consistent with chronic disc protrusions at C6–7 (arrow), T2–3, T3–4, and T4–5.
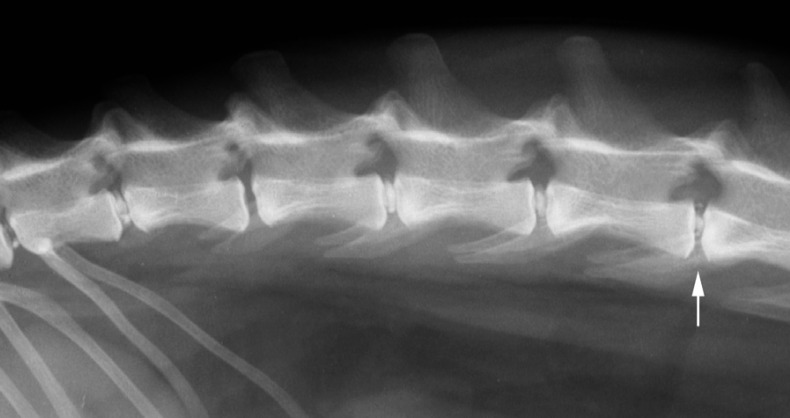
Fig 5.
Right lateral radiograph of the vertebral column of Cat 6. The intervertebral disc at L4–5 is mineralised and fragmented, with narrowing of the disc space (arrow). Mineralised material is noted in the region of the intervertebral foramen. Multiple mineralised discs in situ also are apparent.
Complete histopathological examination of extradural material removed at surgery was done in all cats. The presence of degenerating disc material was confirmed in all cases. Microscopic evidence of mineralisation was seen in the material removed from Cats 1 and 5.
Post-operative outcomes are summarised in Table 1. Five of six cats regained voluntary control of urination during the immediate postoperative hospitalisation period (mean 5 days; range 1–9 days). Cat 1 did not have voluntary control of urination at the time of discharge from hospital, and had not regained this function 5 weeks postoperatively, requiring manual bladder expression four times daily.
Cat 1 was weakly ambulatory 5 weeks post-operatively, with moderate to severe ataxia. At a recheck examination 53 months post-operatively, Cat 1 was strongly ambulatory with moderate generalised muscle atrophy and moderate monoparesis of the left pelvic limb. The owners were expressing the bladder twice daily. Cat 1 has experienced recurrent urinary tract infections (about every 1–2 months) that require intermittent antibiotic therapy. The owners state that Cat 1 is a functional pet and is not apparently in pain.
At a recheck examination 8 weeks post-operatively, Cat 2 had a mildly ataxic and paretic pelvic limb gait. At home, the cat was able to run and jump, with only a mild lameness in the left pelvic limb. Cat 2 was euthanised 31 months post-operatively because of clinical signs related to a large abdominal mass (cause undetermined) that was diagnosed by the referring veterinarian. A necropsy was not done.
At 8 weeks post-operatively, mild ambulatory paraparesis and ataxia were present in Cat 3 at a recheck examination. Superficial pain perception appeared to be normal in the tail, however deep pain perception was (apparently) absent in both pelvic limbs at that time. At examination 14 months post-operatively, mild pelvic limb ataxia was present, superficial pain perception was noted in the tail, and deep pain perception was present in the pelvic limbs, however, superficial pain in these limbs was apparently absent. The owner reported that the cat was ‘normal’, was urinary and faecally continent, and was able to go up and down stairs and jump reasonably well. Cat 3 died from unknown causes while at a boarding establishment, 35 months post-operatively. A necropsy was not done.
Cat 4 was presented to the VMTH 14 months post-operatively following an acute onset of paraparesis. Results of a neurological examination confirmed an L7-S3 myelopathy. A slight right-sided deviation of the contrast column at T13-L1 (site of previous surgery) was seen on a myelogram, however, compressive spinal cord lesions were not apparent. Results of a discogram and epidurogram at L7-S1 were within normal limits. A non-selective angiogram showed anomalies of pulmonary vasculature with absent arterial and venous supplies to the left lung and compensatory hypertrophy of the bronchoesophageal arterial supply. The clinical signs associated with the second episode improved without treatment, and neurological examination 10 days after the second episode revealed only decreased tail tone. The assumption was made that this incident was not related to disc disease. The owner reported that the cat was ambulating well at home, and that the cat was urinary and faecally continent. This cat was lost to follow-up 28 months post-operatively, at which time recurrence of clinical signs of spinal cord dysfunction had not been noted.
At the time of initial presentation, Cat 5 had a history of paroxysmal episodes of rolling, self-mutilation, and licking/biting nearby objects. These episodes were treated as complex partial seizures with 7.5 mg phenobarbital (2.3 mg/kg) orally, every 12 h. A good clinical response and decreased incidence of the episodes occurred initially. Cat 5 never regained good ambulatory function, however the paroxysmal episodes of cerebral dysfunction increased in frequency in spite of phenobarbital therapy. A primary brain disease (unrelated to the episode of IVDD) was suspected as a cause for the continuing clinical signs of cerebral dysfunction. Further investigation of a primary brain disease was not done. The cat died at home about 6 months post-operatively. A necropsy was not done.
Cat 6 was ambulatory with support at the time of discharge from the VMTH, and was ambulatory with mild ataxia at a recheck appointment 3 weeks post-operatively. The cat was able to jump, and the owners reported that the cat was strongly ambulatory and was not (apparently) in pain.
Discussion
Intervertebral disc disease in cats resulting in clinical signs of a myelopathy has been reported previously (Heavner 1971, Seim & Nafe 1981, Gilmore 1983, Littlewood et al 1984, Sparkes & Skerry 1990, Bagley et al 1995, Kathmann et al 2000), however the few reports of this disease in cats would suggest that the incidence of IVDD in cats is significantly lower than that in dogs. All cats in the present report were young to middle-aged (age range 3–9 years). A gender or breed predisposition was not apparent in this small group of cats. The six cats in this report were presented with a variety of clinical presentations, ranging from acute paraparesis/plegia to several months of pelvic limb ataxia or abnormal gait.
Results of CSF analysis were either normal or consistent with blood contamination in four cats (Cats 1, 2, 4, and 5). Cats 3 and 6 had evidence of neutrophilic inflammation on CSF cytology, but these cats also had (apparent) loss of deep pain perception on neurological examination, indicating severe spinal cord compromise. The neutrophilic component may have been consistent with spinal cord injury and inflammation. Further diagnostic evaluation for possible infectious aetiologies for the inflammation in the CSF was not done because of the rapid neurological improvement in each cat after surgical decompression. It was concluded that the disc extrusion was the primary problem, and the inflammation was secondary.
Myelography clearly delineated IVDD lesions in all cats. Most cats had a single compressive lesion, however Cats 1 and 5 had multiple levels of spinal cord compression that were confirmed by myelogram and at surgery (Figs 2 and 3). In addition to a single compressive lesion, Cat 2 had multiple mild extradural compressions of the spinal cord consistent with the appearance of chronic disc protrusions in dogs (Slocum et al 1998) (Fig 4). Protrusion of discs without associated clinical signs has been previously described in cats (King & Smith 1960a and 1960b). Although interpretation is limited by the small sample size, of the nine separate disc herniations identified in the present report, six (66%) were between T12 and L3. This is the region of the vertebral column of dogs where the majority of disc extrusions have been reported to occur (Hansen 1952).
In two cats (Cats 1 and 5), a hemilaminectomy was done at L4–5 to decompress the spinal cord, when in each cat the primary neuroanatomical localisation was to the T3-L3 spinal cord segments. On examination of Cat 1, the possibility of lower motor neuron involvement was not excluded, although the presenting signs were most consistent with a T3-L3 myelopathy. This cat had only six lumbar vertebrae, and it is possible that the spinal cord segments did not correspond to vertebral bodies as would be expected in a ‘normal cat’. Cat 5 had no overt lower motor neuron signs to the pelvic limbs, but in both cases, the myelographic lesion at L4–5 was considered significant, and was surgically decompressed.
In addition to hemilaminectomy for decompression of the spinal cord, a durotomy was done in three cats: Cat 2 because of discolouration of the spinal cord noted at surgery, and Cats 3 and 6 because deep pain perception was apparently absent on examination, indicating severe spinal cord compromise. The durotomy was done in these latter two cases in order to assess whether the spinal cord was anatomically intact, and to provide maximal decompression of the spinal cord.
Histopathological examination of the compressive material removed at surgery showed degeneration of the extruded nucleus pulposus and mineralisation in two cats. The finding of mineralised material within the compressive mass in two cats (Cats 1 and 5), and the observation of mineralisation of intervertebral discs on the radiographs of three cats (Cats 1, 3, and 6; Figs 2 and 5), parallel those of disc degeneration in chondrodystrophoid breeds of dog (Hansen 1952). Maturation and degeneration of the feline intervertebral disc has been described (King & Smith 1958 and 1964, Butler & Smith 1965 and 1967, Butler 1968), and chondrification and occasional apparent mineralisation has been noted, as well as microscopic evidence of ruptures through the anulus fibrosus. Mineralisation was also a feature of IVDD of cats in three previous reports (Seim & Nafe 1981, Gilmore 1983, Kathmann et al 2000).
All cats in this report were found to have residual neurological deficits when examined at various times post-operatively (Table 1). Following examination by the authors, four cats (Cats 2, 3, 4, and 6) were assessed to have made a good to excellent neurological recovery, and Cat 1 was assessed to have made a good recovery with the exception of requiring bladder expression. The owners of these cats were pleased with the outcome. Cat 5 was assessed to have made a poor recovery both by the authors and the owners, since this cat had never regained the ability to ambulate well without support. However, this cat, in contrast to the other cats, had a concurrent problem (paroxysmal episodes of rolling and biting) for which no further diagnostic evaluation was pursued. Progressive cerebral disease was suspected in this cat, and eventually the episodes of cerebral dysfunction became so frequent that the owners could not handle the cat without being bitten. This cat died at home, and a necropsy was not done.
The second episode of paraparesis in Cat 4, that was localised as a myelopathy between L7 and S3, was suspected to be secondary to a vascular event. This suspicion was based on the lack of significant abnormalities on myelogram and CSF analysis, the diagnosis of multiple unusual vascular anomalies in the thorax, and the rapid improvement in neurological status without specific treatment. Vascular causes of myelopathy (such as fibrocartilagenous embolic myelopathy) have been reported previously in cats (Zaki et al 1976, Turner et al 1995).
From the analysis of six cats with IVDD included in this report, it is concluded that while disc extrusion resulting in clinical signs of a myelopathy may be an infrequent problem of cats, it must be considered as a differential diagnosis in cats presenting with clinical signs of either an acute or chronic myelopathy. Surgical removal of extruded disc material in healthy cats appears to result in good neurological and functional recovery. Even in the two cats in this series in which deep pain perception was absent on initial examination (Cats 3 and 6), timely diagnosis and treatment resulted in a very good recovery of neurological function.
Acknowledgement
The authors would like to thank John Doval for his assistance in the preparation of the images.
References
- Bagley RS, Tucker RL, Moore MP, Harrington ML. (1995) Radiographic diagnosis: Intervertebral disk extrusion in a cat. Veterinary Radiology and Ultrasound 36, 380–382. [Google Scholar]
- Bray JP, Burbidge HM. (1998a) The canine intervertebral disk. Part One: Structure and function. Journal of the American Animal Hospital Association 34, 55–63. [DOI] [PubMed] [Google Scholar]
- Bray JP, Burbidge HM. (1998b) The canine intervertebral disk. Part Two: Degenerative changes—non-chondrodystrophoid versus chondrodystrophoid disks. Journal of the American Animal Hospital Association 34, 135–144. [DOI] [PubMed] [Google Scholar]
- Butler WF, Smith RN. (1965) Age changes in the annulus fibrosis of the non-ruptured intervertebral disc of the cat. Research in Veterinary Science 6, 280–289. [PubMed] [Google Scholar]
- Butler WF, Smith RN. (1967) Age changes in the nucleus pulposus of the non-ruptured intervertebral disc of the cat. Research in Veterinary Science 8, 151–156. [PubMed] [Google Scholar]
- Butler WF. (1968) Histological age changes in the ruptured intervertebral disc of the cat. Research in Veterinary Science 9, 130–135. [PubMed] [Google Scholar]
- Gilmore DR. (1983) Extrusion of a feline intervertebral disk. Veterinary Medicine 78, 207–209. [Google Scholar]
- Hansen H-J. (1952) A pathologic-anatomical study on disc degeneration in dog. Acta Orthopaedica Scandinavica, supplementum IX. [DOI] [PubMed] [Google Scholar]
- Heavner JE. (1971) Intervertebral disc syndrome in the cat. Journal of the American Veterinary Medical Association 159, 425–427. [PubMed] [Google Scholar]
- Kathmann I, Cizinauskas S, Rytz U, et al. (2000) Spontaneous lumbar intervertebral disc protrusion in cats: Literature review and case presentations. Journal of Feline Medicine and Surgery 2, 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AS, Smith RN. (1958) Protrusion of the intervertebral disc in the cat. Veterinary Record 70, 509–515. [Google Scholar]
- King AS, Smith RN. (1960a) Disc protrusion in the cat: Distribution of dorsal protrusions along the vertebral column. Veterinary Record 72, 335–337. [Google Scholar]
- King AS, Smith RN. (1960b) Disc protrusion in the cat: Age incidence of dorsal protrusions. Veterinary Record 72, 381–383. [Google Scholar]
- King AS, Smith RN. (1964) Degeneration of intervertebral disc in the cat. Acta Orthopaedica Scandinavica 34, 139–158. [DOI] [PubMed] [Google Scholar]
- Littlewood JD, Herrtage ME, Palmer AC. (1984) Intervertebral disc protrusion in a cat. Journal of Small Animal Practice 25, 119–127. [Google Scholar]
- Seim HB, Nafe LA. (1981) Spontaneous intervertebral disk extrusion with associated myelopathy in a cat. Journal of the American Animal Hospital Association 17, 201–204. [Google Scholar]
- Slocum B, Slocum TD, Biggart JF. (1998) Myelography of Disc Disease. In: Current Techniques in Small Animal Surgery, 4th ed. Bojrab MJ. (ed.). Philadelphia: Williams & Wilkins, pp. 803–808. [Google Scholar]
- Sparkes AH, Skerry TM. (1990) Successful management of a prolapsed intervertebral disc in a Siamese cat. Feline Practice 18, 7–9. [Google Scholar]
- Turner PV, Percy DH, Allyson K. (1995) Fibrocartilaginous embolic myelopathy in a cat. Canadian Veterinary Journal 36, 712–713. [PMC free article] [PubMed] [Google Scholar]
- Zaki FA, Prata RG, Werner LL. (1976) Necrotizing myelopathy in a cat. Journal of the American Veterinary Medical Association 169, 228–229. [PubMed] [Google Scholar]