Abstract
Practical relevance:
Despite considerable interest in the field of companion animal reproduction, feline neonatology remains largely unstudied. This contrasts with the need for a more professional veterinary approach to newborn kittens and feline husbandry, across the pet, breeding cattery and rescue shelter settings, to reduce kitten mortality.
Global importance:
Feline neonatology has relevance for veterinarians around the world as cats are continuing to become established as popular companion animals.
Clinical challenges:
Perinatal mortality in cats is remarkably high. Therefore, adequate neonatal evaluation and assistance at birth, careful monitoring of kittens in the vulnerable period until weaning begins, assessment of maternal factors and well-informed management of orphans are crucial in helping to reduce kitten losses.
Aim:
This review aims to deepen the basic knowledge of the veterinary clinical team regarding the characteristics of feline newborns under normal conditions at birth through to the commencement of weaning. Much of the information is also relevant to breeders and rescue/shelter caregivers.
Evidence base:
In compiling the present review, the authors have drawn on specific feline research data, where available, complemented by data extrapolated from scientific publications on newborn dogs, and also their own and their colleagues’ professional clinical experience.
Keywords: Neonatology, management, hand rearing, orphan

Introduction
As an altricial species, the offspring of the domestic cat (Fells catus) are completely dependent on the mother for multiple vital functions for the first 2 months of age (ie, up until the end of weaning). This makes kittens extremely vulnerable, especially at birth, but also subsequently, given that the maturation of most physiological processes occurs gradually. Therefore, although the neonatal period is dynamic, two main stages can be recognised: from the time of birth until 4 weeks of age (lactation period); and from 4 to about 8 weeks of age (weaning period).
Perinatal mortality
Rates and timing
Losses at birth (stillbirth), and between birth and weaning, together taken as perinatal mortality, are remarkably high in cats. Studies published between 2006 and 2019 have reported a stillbirth rate of between 5 and 12.5%.1-6 In a very recent study of 136 kittens born from 35 dys-tocic queens, only 66% survived, despite the provision of medical and/or surgical assistance. 7 Data on kitten mortality indicate that the majority of post-kittening deaths occur within the first week of age (this phenomenon affects 13% of litters), 4 with an average mortality rate of 27%. 8 The majority of losses (91%) occur during the first 3 days. 9 Some authors have reported that the mortality rate occurring between birth and weaning varies from approximately 8% to 14%.3,5
General causes
Although, in general, studies have not found an association between kitten mortality and the age of the queen, Ström Holst and Frössling reported that queens <1 year old had significantly higher kitten mortality rates, while the occurrence of stillbirths was found to be associated with the queen’s increasing age, especially when >5 years old. 4 Postpartum kitten mortality was also associated with litter size and caesarean section. 4 These authors reported that losses occurring within the first 12 weeks of age vary among breeds, 4 and Root Kustritz reported that Persian, Manx and Himalayan breeds are predisposed to kitten losses. 8
The aetiology of perinatal losses is often complex, remaining unknown in about a third of cases. 10 Potential causes include: intra-partum or postnatal trauma; infections; congenital defects; genetic diseases; low birth weight; maternal factors; and environmental factors.10-12 During the neonatal period, kittens can display sudden death, death within a short time of being born or the so-called ‘fading kitten syndrome’ (which is the focus of an accompanying review in this series), leading to morbidity and mortality. 11
Infections can be related to viral or bacterial agents; sometimes, parasitic diseases can also be observed. The impact of infectious diseases on neonatal kittens depends strongly on whether they are pets, belong to professional breeding facilities or are rescue shelter kittens. In pet and professional husbandry settings, queens are regularly vaccinated against most of the feline viruses, and regularly dewormed or treated for ectoparasites, thus limiting the impact of these agents on kittens. However, viral infections can still play a role in the aetiology of kitten mortality, with viral causes for stillbirth or neonatal death including feline herpesvirus-1 (FHV-1), feline calicivirus (FCV), feline coronavirus (FCoV), feline leukaemia virus (FeLV), feline immunodeficiency virus (FIV) and feline pan-leukopenia virus (FPV). 13 Viral infections predominantly affect kittens at weaning, but FHV-1 and FCV can also be responsible for neonatal infections. 10 In contrast, bacterial infections are mostly responsible for kitten mortality within the first week of life, mainly through the development of septicaemia, and can involve commonly isolated pathogens, such as Staphylococcus species, Streptococcus species, Escherichia coli, Campylobacter species and Salmonella species. 14 Toxoplasma gondii and Neospora can-inum are recognised as rare causes of neonatal mortality. In rescue shelter kittens, severe flea infestations and coccidiosis can also be responsible for severe anaemia and death. 14
Non-infectious causes of death include congenital defects, genetic diseases, low birth weight, maternal factors and neonatal isoery-throlysis. The breeding system and management can additionally have an impact on kitten losses. 10 Appropriate environmental conditions (temperature, humidity, ventilation and hygiene) are essential for kitten survival. Moreover, queens and their litters should be separated from other cats as this is important for limiting both infectious diseases and maternal behavioural problems.
Kitten death can also be the consequence of hypothermia, hypoglycaemia and dehydration. These conditions can, in turn, be sequelae of kitten illness, maternal neglect or simply prolonged fasting due to incorrect management of orphan kittens.
The kitten at birth
The principal vital physiological parameters of the kitten at birth are summarised in Table 1.
Table 1.
| Physiological parameters | Normal values (range) |
|---|---|
| Birth weight | 100 g (75-120) |
| Body temperature | 35.5°C (34.4-37.2) |
| Heart rate | 230 beats per minute (220-260) |
| Mucous membrane colour | Dark reddish pink |
| Respiratory rate | 15 breaths per minute (10-18) |
| Sleeping | Activated sleeping |
| Sucking reflex | Active |
| Swallowing reflex | Active |
| Righting reflex | Active, lifting the head within a few seconds |
| Rooting reflex | Active |
| Movement | Active |
| Urination and defecation | Involuntary |
| Daily urine output | 2.5 ml/100 g body weight |
Factors associated with kitten survival at birth
Gestation length
A normal gestation length is crucial to allow complete growth, development and maturation of fetuses before birth. Therefore, it is important to know the typical range and length of pregnancy in cats. The cat’s gestation is reported to last 52–76 days, 2 with an average of 65.3 days. 2 Breed influences are recognised, with Romagnoli et al 5 reporting a gestation length of 64.7 ± 2.4 days in purebred queens, and Socha et al 6 a mean duration of 65.5 ± 1.32 days in Maine Coon queens. Moreover, large litters are associated with shorter gestation lengths, 2 especially in Maine Coons 6 and Norwegian Forest Cats. 5
Parturition
Problems at parturition in the cat are considered rare, and most queens will kitten by spontaneous vaginal delivery, without the need for intervention. 18 The incidence of dystocia due to uterine inertia, inadequate size of the birth canal or maternal overweight is reported to range between 3% and 6%, with certain breeds showing a predisposition. 19 Notably, in a recent report of 35 queens presenting with dystocia, 69% of cases were of maternal origin, while the remaining 31% were attributed to the fetal component. 7 In general, only 3–7% of queens have been reported to require surgical intervention (caesarean section).4,5 In some breeds, such as Maine Coons and Persian cats, an increased risk for caesarean section is reported, as well as an association between caesarean section and the presence of one or more stillborn kittens. 5
Litter size
Litter size has been reported to range between one and nine kittens. 2 Of a total of 7075 litters, mean litter size was 4.0 ± 1.9 kittens. 3 Litter size was shown to decrease with increasing age of the queen (≥7 years old), 4 and has been reported to vary among breeds, with Persian/Exotic and Birman cats having smaller litters. 4 Romagnoli and colleagues, in a questionnaire study of purebred cats in Italy, found that increasing litter size was associated with both stillbirth and neonatal mortality. 5
Neonatal assessment and assistance at birth
Correct management of newborns and planning of assistance at (or after) birth depends on appropriate neonatal assessment. The following discussion aims to give practical pointers, but readers should note that some aspects relating to feline newborns are yet to be fully investigated.
Malformations and physical defects
All kittens must be checked as soon as possible after birth for malformations and physical defects. (See accompanying review on fading kitten syndrome in this series for a discussion of congenital anomalies.)
Maturity
Mature fetuses are able to cope with the dramatic changes occurring during the transition from fetal to neonatal life, known as ‘neonatal adaptation’. The concept of newborn maturity is still unclear in the dog and remains largely unknown in the cat. Prematurity in kittens can be suspected when the kitten’s coat is not developed to fully cover the tips of the ears, the tail and the paws, although breed-related differences in the characteristics of the coat must be considered. Based on what is known in the dog, 20 when birth occurs a few days before term, kittens can appear completely covered by the coat, but still be physiologically premature (especially organs such as lungs and kidneys), impacting their survival after birth. Thus, the clinical assessment of kitten maturity at birth remains a challenge.
Figure 1.
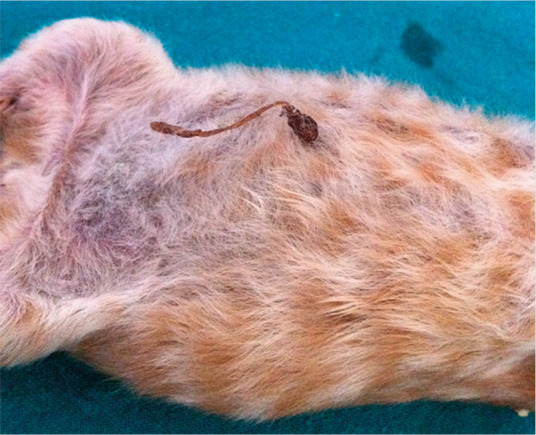
Dry umbilical stump in a 1-day-old Norwegian Forest Cat kitten
Viability
In newborn dogs, assessment of viability at birth, using the Apgar score, 24 has been demonstrated to be useful in improving neonatal outcome. A very recent study has proposed a modified form of Apgar scoring for the assessment of feline neonate vitality (see Addendum, page 241). By adapting some of the Apgar score parameters it is possible to check viability. Moreover, viability can be also assessed by the evaluation of reflex scores, as suggested by the same recent study in kittens. Indeed, in that study a positive correlation between Apgar score and reflex score was found. It is therefore advisable to assess the presence of vital reflexes such as the righting, rooting, sucking and swallowing reflexes, which enable the kitten to reach the nipple, attain the correct position for nursing within a few seconds and nurse effectively. Active movements and vocalisations are also indicators of newborn kitten viability. It is the authors’ opinion, however, that in comparison with puppies, newborn kitten reflexes and vocalisations are less robust; therefore, experience is needed to distinguish between normal viable and moderately less viable kittens.
Birth weight
Birth weight is reported to be associated with survival. 9 In a study by Mugnier et al, 25 birth weight less than the first defined quartile for the breed was associated with kitten mortality, not only in the first 2 days, but also thereafter at 2–42 days after birth. In a separate study, average birth weight in kittens was reported to be 104 g (range 65–165 g), and was found to increase with increasing weight and height (at the withers) of the queen. 26 Conversely, birth weight was reported to be inversely proportional to the number of pregnancies and litter size. 26 In a study by Musters et al, 2 mean birth weight was 98 g, with a range of 35–167 g; mean birth weight was higher in males than females. Birth weight less than 45 g was significantly associated with stillbirth. 2
The critical threshold for birth weight, which is useful in identifying kittens at risk of mortality, was defined in seven cat breeds by Mugnier et al, 25 and ranged from 77 g in Oriental breeds up to 120 g in the Maine Coon. It is interesting to note that, in Maine Coons, Socha et al 6 reported a mean weight of 119.6 ± 18.4 g in kittens born alive, with birth weight increasing in kittens born after prolonged ges-tational lengths (although within the reported ranges) and decreasing with larger litter sizes.
When a low birth weight kitten is presented, it should be strictly observed to make sure it will ingest an adequate amount of colostrum within the first hours of birth, 21 and a regular milk intake subsequently. Moreover, thermal and nutritional support must be provided, when necessary.
Kitten immunity
The cat’s colostral phase of lactation has been demonstrated by higher total concentrations of immunoglobulin (Ig) classes G and A in the mammary secretions collected on the day of parturition when compared with day 7 of lac-tation. 27 The major Ig class in both colostrum and milk is IgG. 27 Thus, the susceptibility of newborn kittens to many harmful conditions is counteracted by the acquisition of passive immunity from the mother. The endotheliocho-rial placenta of the queen largely prevents the transfer of antibodies from the maternal to the fetal compartment, 28 and thus passive transfer of immunity via the placenta is extremely limited. In a study by Crawford et al, all kittens were found to be agammaglobulinaemic at birth, 29 while Ig concentrations in queen’s colostrum were reported to be 40–50 g/l compared with <1 g/l in milk. 30 Therefore, the colostrum is the major source of passive immunity in kittens, providing immune protection throughout the neonatal period, with endogenous IgG production starting between 5 and 6 weeks of age. 28 Absorption of colostral immunoglobulins from the digestive tract and subsequent transfer to the bloodstream is recognised to be crucial for the survival of newborn kittens. 30 Enteric absorption of IgG peaks at birth and rapidly decreases in the following hours, ceasing at about 12–16 h after birth. 30
Although there is an old study showing that milk obtained any time during lactation from queens could be used as a colostrum replace-ment, 31 more recent studies have demonstrated the different concentrations of Ig between colostrum and milk. 27 Colostrum, produced during the first 2 days after parturition, represents the main source of immunoglobulins and nutrients for the newborn kitten, and also contributes to digestive tract maturation. 29
Kittens should nurse as soon as possible after birth, and promptly after resuscitation. This is particularly important for kittens born by caesarean section, as the mother cannot take care of the litter until completely recovered from anaesthesia. In the case of prolonged maternal recovery time, it advisable to place the kittens near to the nipples and stimulate the suckling of colostrum.
The only exception with respect to colostrum intake relates to the risk of neonatal isoerythrol-ysis, which is discussed in an accompanying review on fading kitten syndrome in this series.
Following colostrum intake, the acquisition of passive immunity by kittens can be assessed by measuring peak serum IgG concentrations (range 350–6000 mg/dl). A study by Claus and colleagues reported that this can be performed as early as day 1 after birth, although quantification is better at day 2. 27 These investigators collected 0.25 ml of blood from the jugular vein of the kittens; however, the present authors consider this procedure not to be routinely applicable, mainly due to the fragility of the veins in newborns.
Adequate passive immune transfer can also be indirectly assessed by measuring alkaline phosphatase activity in the blood, with a threshold of 1500 IU/ml at day 1. 29 The adequacy of passive immunity depends on several factors, such as individual maternal ability to produce colostrum containing different concentrations of specific immunoglobulins, and the varying efficiency of colostrum uptake within the litter. 28 To the authors’ knowledge, however, the kitten threshold for acquired protective passive immunity is currently not available. Nevertheless, in the study by Claus and colleagues, 27 serum IgG concentrations of <400 mg/dl were observed in colostrum-deprived kittens and, therefore, this was suggested as the threshold to detect failure of passive transfer of immunity, as also reported in large animal neonates. Furthermore, while the uptake of maternal passive immunity undoubtedly provides strong protection for newborns against possible life-threatening infections, it should also be considered a ‘double-edged sword’. The high concentrations of maternal antibodies, in fact, prevent the development of the neonatal endogenous immune response, with the maternal IgG half-life reported to be around 4.4 days in kittens. 31
Colostrum is crucial for neonatal adaptation, not only as a source of immunity, but for the maturation of several organs; it is also an important energy source. 31 The quantity of colostrum needed to cover energy requirements should be at least 16 ml for a 100 g body weight kitten/day, based on the reported requirement of 20–22 kcal/100 g/day. 32
From a clinical standpoint, maximising colostrum quality could represent a strategy to ensure the best outcome for newborn kittens. This can be achieved through careful attention to the management of breeding queens, including nutrition and hygiene. An increase in antibodies specifically directed against pathogens affecting newborns, such as FCV and FHV-1, 30 may be achieved through vaccination performed as close as possible to the beginning of heat in queens scheduled for mating. 30 When kittens do not ingest colostrum and passive immune transfer is known to have failed, it is possible to provide a defence through the administration of stored colostrum or serum obtained from an adult cat. Breeders can store colostrum obtained from queens by freezing it in small volume vials for a few months. However, harvested colostrum can be contaminated by bacteria harboured on the skin of the nipple, or, in some cases (ie, E coli contamination), the process of colostrum thawing can lead to bacterial membrane rupture and release of endotoxins. Therefore, colostrum must be collected asepti-cally and always coupled with bacterial culture to avoid possible harmful contamination.
Serum can be administered orally to kittens in the first 12–16 h 30 and up to 24 h of age, and subcutaneously or intraperitoneally in older kittens. Parenteral administration of 150 ml/kg adult cat serum subcutaneously or intraperitoneally to colostrum-deprived kittens has been reported to lead to serum IgG levels comparable with those of suckling kittens. 33 However, it should be underlined that, although subcutaneous administration is performed at different sites of inoculation, sometimes severe and potentially disastrous subcutaneous necrosis can occur, leading to kitten death or the need for euthanasia.
From birth to the commencement of weaning
Weaning in kittens usually begins around 4 weeks after birth, and as early as 3 weeks after birth in orphans. Monitoring from birth to the beginning of weaning implies regular observation of the queen, the whole litter and each kitten. Kitten monitoring is essential for the prompt recognition of any abnormality in weight gain, age-related development, behaviour and body temperature, and to promptly detect any sign of sickness.
Weight gain
The gold standard for kitten monitoring is regular weight measurement with an accurate 1 g scale and meticulous record-keeping. This, in turn, entails regular handling, allowing evaluation of behaviour, reactivity (reaction to external stimuli) and vigour, as well an approximation of body temperature. Weight gain is assured by regular and adequate milk uptake. Inadequate intake can be due to kitten weakness, illness, competition from siblings or various maternal-related factors (see below). Healthy kittens should be weighed daily until 2 weeks of age and then twice a week until fully weaned. Sick kittens will require more frequent weighing, depending on the severity of the illness.
A loss of <10% birth weight is not unusual in the first 24 h of life. However, in the authors’ experience, with healthy, viable kittens where queens exhibit normal prompt lactation and provide maternal care, this loss does not occur, and weight gain will have already started by day 1. Weight gain is an important and accurate indicator for assessing the newborns’ health. In kittens, a weight gain of 50–100 g per week is expected, 15 with differences mainly related to breed. Weight gain should be steady and progressive; any loss or stasis needs strict observation, and treatment as appropriate. When weight gain is lower than expected, supplemental feeding must be provided, and the kitten observed scrupulously so as to promptly detect any possible underlying disease. For orphan management, a high quality commercial milk replacer formula is the best option (see later). If the diet is not correctly balanced, multivitamin, energy or taurine supplementation may sometimes be needed.
Monitoring of development
The handling of kittens for daily weight measurement allows evaluation of flexor tone (Figure 2) initially, and then also extensor tone, both of which are good indicators of normal kitten development. Absence of flexor tone could indicate severe defects and often carries a poor prognosis. Multiple factors could be associated with delayed development of the kitten; among them are slow growth rate, inadequate colostrum intake and/or problems at birth (ie, hypoxia) or thereafter. The consequence is lack of preparation for weaning at 21–28 days; slow growth rate is also a risk factor for post-weaning wasting. 34
Figure 2.

Flexor tone in a 2-day-old Devon Rex kitten
Maternal role
Maternal dependence
Newborn kittens depend on the mother not only for nutrition, but also for the maintenance of correct body temperature, for grooming, protection and stimulation of urination/defecation. 37 Therefore, appropriate maternal behaviour and care at birth (and subsequently) is necessary for kitten survival. At birth, under normal conditions and with typical maternal behaviour, the queen is responsible for opening the fetal membranes, severing (biting) the umbilical cord and licking the newborns; the purpose of licking, aside from being important in stimulating respiration, is to remove fetal fluids and allow the kitten to dry. Licking the anal region and external genitalia is also crucial for the excretory functions of the kittens, as mentioned above.
Although these maternal actions are innate, some queens do not show a normal pattern of maternal behaviour and care for their newborns, especially young primiparous queens or those experiencing stress. Therefore, the authors would always advise discreet (no disturbance) surveillance to allow prompt intervention and assistance to the newborns, when necessary. Moreover, when a caesarean section is performed, maternal instincts may be disrupted in the first 24 h after surgery.
Maternal factors
It is important to note that besides genetic predisposition, age and parity of the queen, there are other maternal factors that can cause kitten loss, including nutritional or metabolic abnormalities such as diabetes mellitus, hypothyroidism, hypocalcaemia and pregnancy toxaemia. Kitten death at birth can also be related to poor maternal care and savaging behaviours.8,9 Kittens that have experienced intrapartum trauma usually show extensive regional haemorrhage and/or oedema; with inappropriate maternal care, amputation, skin lesions and/or skeletal fractures have also been observed (Figure 3). 14
Figure 3.
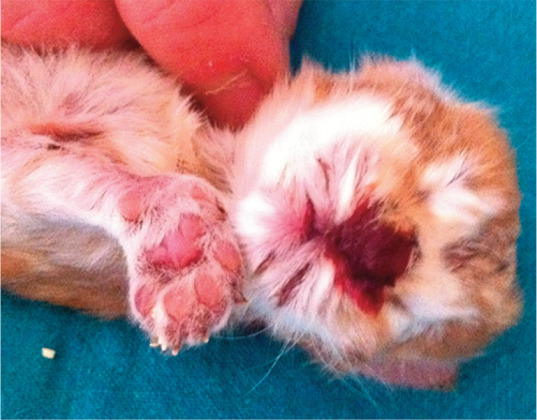
Trauma and haemorrhage due to infanticide in a newborn Norwegian Forest Cat kitten
A queen responsible for infanticide in a litter suggests an increased risk of infanticide in future litters. 14 Causes of infanticide are multiple and include genetic predisposition, extremes of maternal age, low viability of the newborn and environmental causes (in particular, interference from intra- or interspecific individuals). 38 Strict surveillance of maternal behaviour around parturition and during the first 48 h after birth is crucial, especially in primiparous queens, to promptly identify abnormalities and to save kittens. 21 The authors advise that each case of infanticide should be fully investigated to clarify, and where possible address, the underlying cause. If proved to be individually predisposed to infanticide, the queen should be removed from breeding. It has been reported anecdotally that using a different tom cat at the subsequent mating might help to reduce infanticide.
Maternal illness, trauma, inadequate nutrition, stress or toxin ingestion, and administration of certain drugs, can all cause stillbirth 14 or have consequences for the health and survival of the newborn kittens. Appropriate management of pregnant queens and focused clinical monitoring is important for the early detection of these conditions, reducing the impact both on the mother and the litter. There is a report in the literature describing the long haircoat of the queen as a possible life-threatening factor for neonates, leading to the recommendation of regular grooming of pregnant long-haired queens close to parturition. 18
Orphan management
Kittens may be orphaned or found abandoned, or may need to be hand-reared because of maternal illness, death, rejection or inadequate lactation. 36 As reported for dogs, inadequate or absent milk production can be due to multiple factors, such as the mother’s inexperience, nervousness, age, familial traits, systemic illness, dystocia, mastitis or malnourishment, or may result from an inadequate environment with intra- or interspecific disturbances. 39 Depend -ing on the underlying cause, the management of orphans may range from simple feeding to the substitution of full maternal care, including warming, feeding, protection, stimulation of excretion (urine and faeces), grooming and socialisation. 36 Finding a foster mother that accepts the orphan kittens is certainly the best option, but is rarely possible. It is reported that fostering of kittens from another dam is more likely to be successful if the queens involved are related and if this transition is carried out in the first week after parturition. 38 Otherwise it is advised that orphaned kittens are more likely to be correctly nourished if they are no more than 2 weeks younger than the ‘real’ litter, so as to avoid competition and an unfavourable size disadvantage. 36 In practical terms, most orphans must be hand-reared.
Hand-rearing of orphans is time-consuming, requiring the availability of simple instruments and an adequate environment, as well as an understanding of the main physiological features and milestones in kitten development; such knowledge is necessary not only to provide correct management, but also to allow prompt recognition of abnormalities and, in turn, timely intervention. In the authors’ experience, in the majority of cases, a fully dedicated and skilled person can be more influential in successfully raising orphan kittens than a fully equipped facility. Orphan management will be slightly different depending on whether the kitten is a singleton or if multiple kittens have to be reared. In addition, the specifics of orphan management strongly depend on the factors that led to the orphaning, as well as the health of the kittens. It is important to be aware that outcomes can be unpredictable, and sometimes frustrating, despite correct management.
Irrespective of whether orphans come from pet or breeding queens, or from a shelter, there are certain essential requirements (see box on page 239).
The age-based approach to management means that when the kitten’s age is unknown, it must be estimated by assessing body weight and age-dependent changes in physiology. The most important parameters, in addition to an average daily weight gain of 10 g (range 7–15 g), are summarised in Table 2.
Table 2.
| Parameters | Age (days) |
|---|---|
| Umbilical stump falls off | 3 |
| Eyelids opening | 8-10 |
| External ear canals opening | 6-14 |
| Crawling | 7-14 |
| Walking | 14-21 |
| Normal posture | 28 |
| Deciduous teeth eruption | 14-28 |
| Flexor tone | 0 to 3–4 |
| Extensor tone | 4 to 21–35 |
| Voluntary urination and defecation | 21-28 |
Environment
A quiet, clean, well ventilated room (ie, regular air changes, avoiding draughts at the same time as not having a completely closed room), with adequate humidity (55%) and a room temperature of 20–22°C is important for the management of orphan kittens.21,35 The litter must be maintained in a kitten box of a suitable size to keep the kittens nestled closely together (avoiding kitten spread), especially within the first week of age, and lined with soft, warm, clean, disposable bedding; ensuring the kittens do not become separated helps to avoid dropping of body temperature.
The temperature of the kitten box must be regulated appropriately according to the age of the kittens (32-34°C up to 1 week of age, gradually decreasing to 24–27°C by 3 weeks of age), 36 and must be kept constant as much as possible; sudden changes in temperature, as can happen, for example, when using a plastic bottle filled with warm water, must be avoided. Also, heating pads must be used cautiously, because temperature regulation is sometimes difficult with these devices, which risks causing damage to the kittens. 40 Independent of the chosen method of thermal support, a clean towel should always be used to cover the source of warmth so there is no direct contact with the kittens to avoid any risk of skin burns. An infrared heat lamp can also be used, provided it is positioned at a reasonable distance (at least 60 cm41) from the kittens, which must be closely monitored to avoid skin burns and dehydration.
Whichever method is used, the authors suggest restricting these sources of warmth to one side of the box only. Although considered neu-rologically immature in terms of their ability to move closer to or away from a heat source, 42 in a practical setting it is not uncommon to see litters migrating away from the heating device in the case of excessive warmth, and vice versa. Indeed, other authors have described healthy kittens as being able to escape from an excessive heat source, but this is something that must not be assumed, as it also relates to the viability of the newborns. 41 For all of these reasons, it is dangerous not to provide a means of ‘escaping’ excessive warmth. 36 Finally, where available, paediatric incubators can be used to create optimal conditions for the kittens. However, even these instruments require continual monitoring to verify the correct ambient conditions. 42
Feeding
Of all the issues surrounding hand-rearing of kittens, feeding is the foremost. In the authors’ experience, many kittens sadly die because of incorrect feeding by caregivers. Kittens must not be fed when their body temperature is below 35.5°C and, where warming of chilled kittens is necessary, this must be achieved gradually (the authors suggest no more than 1°C in 1 h).21,36,40,42,43 Often there is a mismatch between the amount and frequency of feeding and the physiological stage of newborn development.
Commercial milk replacers are largely better quality than homemade formulae (which should be limited to emergency situa-tions 36 ), ensuring that kittens’ specific nutritional requirements are met. Cow’s milk or milk from other animal species must not be fed. 41 The wrong choice of milk can lead to acute diarrhoea and regurgitation, gastrointestinal meteorism, abdominal colic and starvation and, in the long run, metabolic and/or developmental abnormalities (eg, secondary hyperparathyroidism). 44
The daily caloric requirement of a newborn kitten is 20–26 kcal/100 g body weight, and most commercial milk replacer formulae deliver about 1.0 kcal/ml. 43 Therefore, the daily requirement for commercial milk is about 20–26 ml/100 g body weight. 43 However, the frequency of feeding and the amount of milk per feed depends on the age of the kitten and on stomach capacity. 41 During the first 2 weeks, kittens must be fed every 2 h. Thereafter, the interval between feeds can be extended to 3–4 h. Although stomach capacity has been reported to be 4 ml/100 g body weight, 43 in the authors’ experience it is always better to feed smaller amounts (never reaching maximum stomach capacity), but more frequently (ie, 2 ml per feed a total of 12 times/day for a kitten of 100 g body weight). This is very important so as to avoid regurgitation, milk inhalation, nasal discharge, abdominal distension and diarrhoea. Orphans with an active sucking reflex can be bottle-fed. For sick orphans or kittens with a weak or absent sucking reflex, milk must be delivered via an orogastric tube.
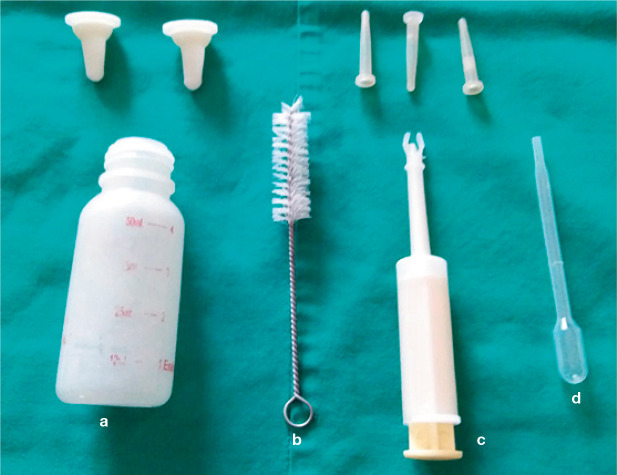
Milk replacers are available in liquid or powdered form, the latter needing correct dilution as detailed by the manufacturer. 35 Errors in dilution can lead to diarrhoea or constipation. Although many authors advise preparing and refrigerating enough milk for 24–48 h,15,32,36 the present authors recommend preparing only the amount needed for each feed to avoid contamination and possible fermentation. Before feeding, the milk must be warmed up (eg, using a water bath) to 35–38°C. All equipment (Figure 4) must be cleaned and sterilised at every feeding; 16 the present authors suggest the use of sterilising solutions for baby bottles for this purpose.
Figure 4.
Feeding instruments for kittens. (a) Bottle for feeding of newborn kittens; above it are two types of nipple, adequate for the size and vigour of newborns. (b) Brush for cleaning the feeding bottles. (c) Graduated syringe, ideal for feeding kittens in the first days. (d) Disposable Pasteur pipette, very useful for administering milk formula slowly, drop by drop
Orphans must be monitored for weight gain even more strictly than kittens being nursed by their mothers. Weight gain in orphaned kittens fed with commercial formula milk is slower than in kittens fed with queen’s milk. 16
Suckling
Suckling is important not only for feeding; the behaviour also satisfies an innate kitten desire. Early weaned and orphan kittens can develop cross-sucking behaviour. 45 This refers to non-nutritive sucking of the littermates (mostly external genitalia and umbilical stumps), a behaviour that can inflict severe injuries. Where this happens, kittens must be separated. If cross-sucking recurs when kittens are reintroduced, they must be separated again and kept alone. While there is much still to be learnt about this phenomenon, a recent study 45 documented an increased risk of cross-sucking development in orphan, bottle-fed and younger kittens, as well as in those precociously separated from the mother or belonging to all-male litters.
Excretion
Orphans that are less than 3–4 weeks of age should be stimulated to urinate and defecate by gently rubbing the anogenital area using soft, warm, white, dampened cotton cloths. Urine must be checked for normal appearance (almost transparent) and faeces for the normal characteristics (yellowish, soft). Excretion of urine should be stimulated at each feeding time, while faeces excretion might be slightly delayed. In kittens not defecating for 24 h, a small amount of paraffin oil (0.5 ml, irrespective of the weight of the kitten) can be added to the milk; if the condition is prolonged for more than 24 h, constipation must be counteracted by using a similar amount (0.5 ml) of newborn babies’ enema diluted (50:50) with warm water. Any alteration in urine or faecal colour and characteristics must be investigated.
Diarrhoea
Diarrhoea is a not uncommon condition in orphan kittens and consists of an increased frequency of evacuations and/or altered (diminished) consistency of the stool. Among the possible causes, feeding management mistakes, infectious agents and parasitic diseases are the most frequent. 42 Depending on the underlying cause, diarrhoea can be mild or severe, and sometimes fatal. It has been reported that about 15% of fostered kittens die before 8 weeks of age, mostly due to diarrhoea and with post-mortem findings of enteritis. 46
In the authors’ clinical experience, a sudden change in feeding is often the underlying cause; for example, when making the transition from maternal to artificial milk, or changing the milk replacer formula. Ingestion of milk of the incorrect temperature can also lead to vomiting and hypothermia, as well as issues with absorption, 40 in turn triggering colitis and diar-rhoea. 42 Environmental conditions can affect digestion, contributing to an altered faecal consistency. Moreover, ingestion of contaminated milk (eg, when not properly stored), and the presence of viruses and/or bacteria, could potentially act directly or in a synergistic way, leading to dysbiosis and diarrhoea. 12
In adults without any other clinical signs such as lethargy or vomiting, the presence of stools of altered consistency is something that can often be addressed less urgently. By contrast, the presence of diarrhoea in newborn kittens must be promptly addressed. Indeed, associated losses of substantial quantities of water, minerals and nutrients create the conditions for dehydration and hypoglycaemia. As the situation can worsen rapidly, in just a matter of hours, the authors recommend the provision of supportive therapy, which can include the administration of fluids, prebiotics, probiotics and sometimes adsorbents. 12 If the kitten’s appetite is maintained and the sucking reflex is present without vomiting, oral administration of milk and nutritional supplements might be enough to restore fluid losses. If there is loss of appetite, no sucking reflex and signs of dehydration are present, then the use of a feeding tube must be considered. While the real value of subcutaneous fluids in newborn kittens is still debated, intraosseous access should be used to provide parenteral fluids, 35 as it is preferable not to use newborn vessels given their small size and fragility. Where possible, however, the underlying cause of the diarrhoea must be identified with, for example, coprological tests, faecal smear and/or an accurate medical history to investigate possible nutritional errors.
As reported for adults, use of antibiotics for the medical treatment of diarrhoea is contro-versial.12,47,48 Specific antimicrobial therapy should be considered when there are signs of inflammation in the gastrointestinal tract (eg, numerous inflammatory cells in faecal smears), bloody faeces, fever, leukocytosis or when septicaemia is suspected. 47
Socialisation
It is reported that the sensitive period for socialisation towards humans occurs between 2 and 7 weeks of age, when multiple stimuli allow for the rapid growth of neural connections and, in turn, the development of social behaviour. 49 During this period, kittens should become accustomed to human contact. 50 For orphan kittens, an age-dependent progressive increase in handling is needed to allow for an appropriate degree of behavioural and social maturation.
Key Points.
✜ Kittens are highly vulnerable at birth and until weaning.
✜ An understanding of basic neonatal physiology is needed for the correct management of kittens at birth and up to the beginning of weaning.
✜ At birth, suitable maternal care and/or assistance improves neonatal outcome.
✜ Normal birth weight is a strong predictor of kitten survival.
✜ Colostrum intake, as soon as possible and no later than after 12 h of life, is needed for passive immune transfer.
✜ Steady and progressive weight gain is related to normal neonatal development.
✜ Maternal instinct and appropriate behaviour is critical for kitten survival and normal growth.
✜ The success of orphan management depends more on a skilled dedicated person than the availability of a fully equipped facility.
✜ Hand-rearing of orphans involves not only correct feeding, but also warming, protection, voiding, grooming and socialisation.
Addendum
At the time of going to press, a paper was accepted for publication in JFMS that discusses routine assessment of newborn kitten vitality. Readers are referred to Hibaru et al’s study, 51 which demonstrates significantly lower Apgar scores and reflex scores (ie, low vitality) in 13 neonates delivered by caesarean section compared with 19 neonates delivered by natural birth. The paper proposes the use of a modified feline Apgar score that allows the provision of immediate assistance at birth, increasing the chance of survival for these patients.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and therefore informed consent was not required. For any animals or people individually identifiable within this publication, informed consent (verbal or written) for their use in the publication was obtained from the people involved.
References
- 1. Sparkes AH, Rogers K, Henle WE, et al. A questionnaire- based study of gestation, parturition and neonatal mortality in pedigree breeding cats in the UK. J Feline Med Surg 2006; 8: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Musters J, de Gier J, Kooistra HS, et al. Questionnaire based survey of parturition in the queen. Theriogenology 2011; 75: 1596–1601. [DOI] [PubMed] [Google Scholar]
- 3. Fournier A, Masson M, Corbière F, et al. Epidemiological analysis of reproductive performances and kitten mortality rates in 5,303 purebred queens of 45 different breeds and 28,065 kittens in France. Reprod Domest Anim 2017; 52 Suppl 2: 153–157. [DOI] [PubMed] [Google Scholar]
- 4. Ström Holst B, Frössling J. The Swedish breeding cat: population description, infectious diseases and reproductive performance evaluated by a questionnaire. J Feline Med Surg 2009; 11: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romagnoli S, Bensaia C, Ferré-dolcet L, et al. Fertility parameters and reproductive management of Norwegian Forest Cats, Maine Coon, Persian and Bengal cats raised in Italy: a questionnaire- based study. J Feline Med Surg 2019; 21: 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Socha P, Lengling R, Bonecka J, et al. Obstetric and newborn parameters in the Maine Coon cats. Pol J Vet Sci 2019; 22: 439–443. [DOI] [PubMed] [Google Scholar]
- 7. Bailin HG, Thomas L, Levy NA. Retrospective evaluation of feline dystocia: clinicopathologic findings and neonatal outcomes in 35 cases (2009–2020). J Feline Med Surg. Epub ahead of print 14 June 2021. doi: 10.1177/1098612X211024154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Root Kustritz MV. Clinical canine and feline reproduction: evidence-based answers. Hobo ken, NJ: Wiley-Blackwell, 2010. [Google Scholar]
- 9. Lawler dF, Monti KL. Morbidity and mortality in neonatal kittens. Am J Vet Res 1984; 45: 1455–1459. [PubMed] [Google Scholar]
- 10. Cave TA, Thompson H, Reid SW, et al. Kitten mortality in the United Kingdom: a retrospective analysis of 274 histopathological examinations (1986 to 2000). Vet Rec 2002; 151: 497–501. [DOI] [PubMed] [Google Scholar]
- 11. Willoughby K. Paediatrics and inherited diseases. In: Chandler EA, Gaskell CJ, Gaskell RM. (eds). Feline medicine and therapeutics. 3rd ed. oxford: Blackwell Publishing, 2004, pp 355–377. [Google Scholar]
- 12. Veronesi MC. Patologie neonatali. In: Veronesi MC, Castagnetti C, Taverne MAM. (eds). Neonatologia veterinaria. Napoli, italy: EdiSES, 2013, pp 93–144. [Google Scholar]
- 13. Evermann JF, Kennedy MA. Viral infections. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics: the first 12 months of life. St Louis, Mo: Saunders, 2011, pp 119–129. [Google Scholar]
- 14. Lamm CG, Njaa BL. Clinical approach to abortion, stillbirth, and neonatal death in dogs and cats. Vet Clin North Am Small Anim Pract 2012; 42: 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Little S. Feline pediatrics: how to treat the small and the sick. Comp Contin Educ Pract 2011; 33: E3. [PubMed] [Google Scholar]
- 16. Zambelli d. Feline neonatal physiology, behavior, and socialization. In: Lopate C. (ed). Management of pregnant and neonatal dogs, cats, and exotic pets. New York: John Wiley & Sons, 2012, pp 145–158. [Google Scholar]
- 17. Veronesi MC. Esame clinico del neonato. In: Veronesi MC, Castagnetti C, Taverne MAM. (eds). Neonatologia veterinaria. Napoli, italy: EdiSES, 2013, pp 63–92. [Google Scholar]
- 18. Azari o, Akhtardanesh B. A clinical report of entangled neonates’ umbilical cord with queen’s fur in Persian cat. Asian Pac J Trop Biomed 2011; 1: 502–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. davidson A. Problems during and after parturition. In: England G, Heimendahl VA. (eds). Manual of small animal reproduction and neonatology. 2nd ed. Cheltenham: British Small Animal Veterinary Association, 2010, pp 127–142. [Google Scholar]
- 20. Bonte T, del Carro A, Paquette J, et al. Foetal pulmonary maturity in dogs: estimated from bubble tests in amniotic fluid obtained via amniocentesis. Reprod Domest Anim 2017; 52: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 21. Veronesi MC. Assistenza neonatale al parto. In: Veronesi MC, Castagnetti C, Taverne MAM. (eds). Neonatologia veterinaria. Napoli, italy: EdiSES, 2013, pp 31–48. [Google Scholar]
- 22. Traas AM. Resuscitation of canine and feline neonates. Theriogenology 2008; 70: 343–348. [DOI] [PubMed] [Google Scholar]
- 23. davidson AP. Approaches to reducing neonatal mortality in dogs. In: Concannon PW, England G, Verstegen J, et al. (eds). Recent advances in small animal reproduction. ithaca, NY: international Veterinary information Service, A; 1226.0303. [Google Scholar]
- 24. Veronesi MC. Assessment of canine neonatal viability – the Apgar score. Reprod Domest Anim 2016; 51 Suppl 1: 46–50. [DOI] [PubMed] [Google Scholar]
- 25. Mugnier A, Mila H, Guiraud F, et al. Birth weight as a risk factor for neonatal mortality: breed-specific approach to identify at-risk puppies. Prev Vet Med 2019; 171: 104746. doi: 10.1016/j.prevetmed.2019.104746 [DOI] [PubMed] [Google Scholar]
- 26. Gatel L, Rosset E, Chalvet-Monfray K, et al. Relationships between fetal biometry, maternal factors and birth weight of purebred domestic cat kittens. Theriogenology 2011; 76: 1716–1722. [DOI] [PubMed] [Google Scholar]
- 27. Claus MA, Levy JK, Macdonald K, et al. Immunoglobulin concentrations in feline colostrum and milk, and the requirement of colostrum for passive transfer of immunity to neonatal kittens. J Feline Med Surg 2006; 8: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. day MJ. Immune system development in the dog and cat. J Comp Pathol 2007; 137: S10–S15. [DOI] [PubMed] [Google Scholar]
- 29. Crawford PC, Levy JK, Werner LL. Evaluation of surrogate markers for passive transfer of immunity in kittens. J Am Vet Med Assoc 2006; 228: 1038–1041. [DOI] [PubMed] [Google Scholar]
- 30. Chastant-Maillard S, Guillemot C, Feugier A, et al. Reproductive performance and pre-weaning mortality: preliminary analysis of 27,221 purebred female dogs and 204,537 puppies in France. Reprod Domest Anim 2017; 52 Suppl 2: 158–162. [DOI] [PubMed] [Google Scholar]
- 31. Casal ML, Jezyk PF, Giger U. Transfer of colostral antibodies from queens to their kittens. Am J Vet Res 1996; 57: 1653–1658. [PubMed] [Google Scholar]
- 32. Prendergast H. Nutritional requirements and feeding of growing puppies and kittens. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics: the first 12 months of life. St Louis, Mo: Saunders, Elsevier, pp 58–66. [Google Scholar]
- 33. Levy JK, Crawford PC, Collante WR, et al. Use of adult cat serum to correct failure of passive transfer in kittens. J Am Vet Med Assoc 2001; 219: 1401–1405. [DOI] [PubMed] [Google Scholar]
- 34. Lawler dF. The role of perinatal care in development. Semin Vet Med Surg 1995; 10: 59–67. [PubMed] [Google Scholar]
- 35. Veronesi MC. Gestione del neonato patologico. In: Veronesi MC, Castagnetti C, Taverne MAM. (eds). Neonatologia veterinaria. Napoli, italy: EdiSES, 2013, pp 145–161. [Google Scholar]
- 36. Little S. Playing mum: successful management of orphaned kittens. J Feline Med Surg 2013; 15: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casal M. Management and critical care of the neonate. In: England G, Heimendahl VA. (eds). Manual of small animal reproduction and neonatology. 2nd ed. Cheltenham: British Small Animal Veterinary Association, 2010, pp 135–146. [Google Scholar]
- 38. Root Kustritz MV. Reproductive behavior of small animals. Theriogenology 2005; 64: 734–746. [DOI] [PubMed] [Google Scholar]
- 39. Marti JA, Fernandez S. Clinical approach to mammary gland disease. In: England G, Heimendahl VA. (eds). Manual of small animal reproduction and neonatology. 2nd ed. Cheltenham: British Small Animal Veterinary Association, 2010, pp 155–165. [Google Scholar]
- 40. Münnich A, Küchenmeister U. Causes, diagnosis and therapy of common diseases in neonatal puppies in the first days of life: cornerstones of practical approach. Reprod Domest Anim 2014; 49 Suppl 2: 64–74. [DOI] [PubMed] [Google Scholar]
- 41. Veronesi MC. Gestione dell’orfano. In: Veronesi MC, Castagnetti C, Taverne MAM. (eds). Neonatologia veterinaria. Napoli, italy: EdiSES, 2013, pp 49–61. [Google Scholar]
- 42. Fitzgerald KT, Newquist KL. Husbandry of the neonate. In: Peterson ME, Kutzler MA, (eds). Small animal pediatrics: the first 12 months of life. St Louis, Mo: Saunders, Elsevier, pp 44–52. [Google Scholar]
- 43. Lawler dF. Neonatal and pediatric care of the puppy and kitten. Theriogenology 2008; 70: 384–392. [DOI] [PubMed] [Google Scholar]
- 44. Prendergast H. Clinical approach to pediatric nutritional conditions. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics: the first 12 months of life. St Louis, Mo: Saunders, Elsevier, pp 483–491. [Google Scholar]
- 45. delgado MM, Walcher i, Buffington CAT. A survey-based assessment of risk factors for cross-sucking behaviors in neonatal kittens, Felis catus. Appl Anim Behav Sci 2020; 230. doi: 10.1016/j.applanim.2020.105069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghosh A, Borst L, Stauffer SH, et al. Mortality in kittens is associated with a shift in ileum mucosa-associated enterococci from Entero coccus hirae to biofilm-forming Entero coccus faecalis and adherent Escherichia coli. J Clin Microbiol 2013; 51: 3567–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peterson ME. The digestive system. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics: the first 12 months of life. St Louis, Mo: Saunders, Elsevier, pp 351–367. [Google Scholar]
- 48. Pignataro G, di Prinzio R, Crisi PE, et al. Comparison of the therapeutic effect of treatment with antibiotics or nutraceuticals on clinical activity and the fecal microbiome of dogs with acute diarrhea. Animals (Basel) 2021; 11: 1484. doi: 10.3390/ani11061484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karsh EB, Turner dC. The human–cat relationship. In: Turner dC, Bateson P. (eds). The domestic cat. The biology of its behavior. Cambridge: Cambridge University Press, 1988. [Google Scholar]
- 50. Radosta L. Feline behavioral development. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics: the first 12 months of life. St Louis, Mo: Saunders, Elsevier, pp 88–96. [Google Scholar]
- 51. Hibaru VY, Pereira KHNP, Fuchs KdM, et al. Topics in the routine assessment of newborn kitten vitality: Apgar score, reflexes and complementary assessments. J Feline Med Surg. in Press 2022. doi: 10.1177/1098612X221081404. [DOI] [PMC free article] [PubMed] [Google Scholar]