Abstract
Practical relevance:
Fading kitten syndrome is a condition where one or more kittens of a litter are born apparently healthy but gradually become inactive, sick and die; typically faders are considered those that die during the first 2 weeks of life. Fading has many potential aetiologies, including a wide variety of infectious, toxic, traumatic, metabolic and genetic diseases. Regardless of the underlying cause, the approach to a sick neonate is similar, and initiating supportive care is the first priority, followed by a detailed physical examination. Where possible, the cause of disease should be determined, and this may inform adjustments to the treatment protocol.
Clinical challenges:
Most perinatal and neonatal diseases present similarly and a specific cause cannot usually be determined from clinical signs alone, which can make diagnosis challenging. When examining a kitten, it is important to remember that there are distinct physiological differences between adults and neonates. In addition, some procedures can be more difficult to perform than in adults, such as blood collection, and some diagnostic tests are harder to interpret, such as radiography. When treating kittens, differences compared with adults again need to be considered.
Aim:
The aim of this review is to provide guidance to veterinarians who are presented with a fading kitten. As well as reviewing the potential causes of fading kitten syndrome, the diagnostic approach and treatment options are discussed. Algorithms summarising possible pathways to neonatal mortality in kittens, and diagnostic and therapeutic options in fading kittens, are also provided.
Evidence base:
Information provided in this review is based on the published feline literature and papers discussing puppies and/or a range of species including cats, as well as the author’s own clinical experience.
Keywords: Faders, predisposing factors, pathways, hypoxaemia, immunodeficiency, environmental factors, infections, anomalies, diagnostic approach, physiological differences, treatment options
Why we say ‘syndrome’
Fading kitten syndrome is a condition where one or more kittens of a litter are born apparently healthy but gradually become inactive, sick and die. 1 Kittens that do not thrive and die between birth and weaning are frequently called ‘faders’. The highest mortality occurs within the first 2 weeks of life. 2 This is the time frame when breeders and veterinarians usually regard kitten mortality as ‘fading kitten syndrome’, and over half of all kitten deaths occur in this period.3,4 It is suspected that the number of cases is higher than the published percentages indicate, because many spontaneous kitten deaths go unreport-ed and post-mortem evaluation is rarely performed. As the term ‘syndrome’ implies, this is a clinical description rather than a diagnosis.
Causes of fading kitten syndrome
Fading has many aetiologies, including a wide variety of infectious, toxic, traumatic, metabolic and genetic diseases. 5 Neonatal kittens may die suddenly or ‘fade’ within a few days. Unfortunately, most perinatal and neonatal diseases present similarly and a specific cause cannot usually be determined from clinical signs alone.
In the peer-reviewed feline literature, the most common non-infectious cause of fading in kittens is neonatal isoerythrolysis (NI). The most frequently reported infectious cause is neonatal bacterial infection/sepsis, 5 with Escherichia coli, streptococci, Pasteurella species and staphylococci being especially involved.
Infections are the most common causes of fulminant disease. Factors that may predispose to infectious disease include hypothermia, hypoglycaemia, mismanagement, poor breeding strategies or immunological deficiencies. The last can be due to delayed colostrum intake, which is difficult for the breeder to determine, and is the reason why in some instances only one or two kittens of a litter are affected. All these conditions pla a part in ‘fading kitten syndrome’ (Figure 1).
Figure 1.
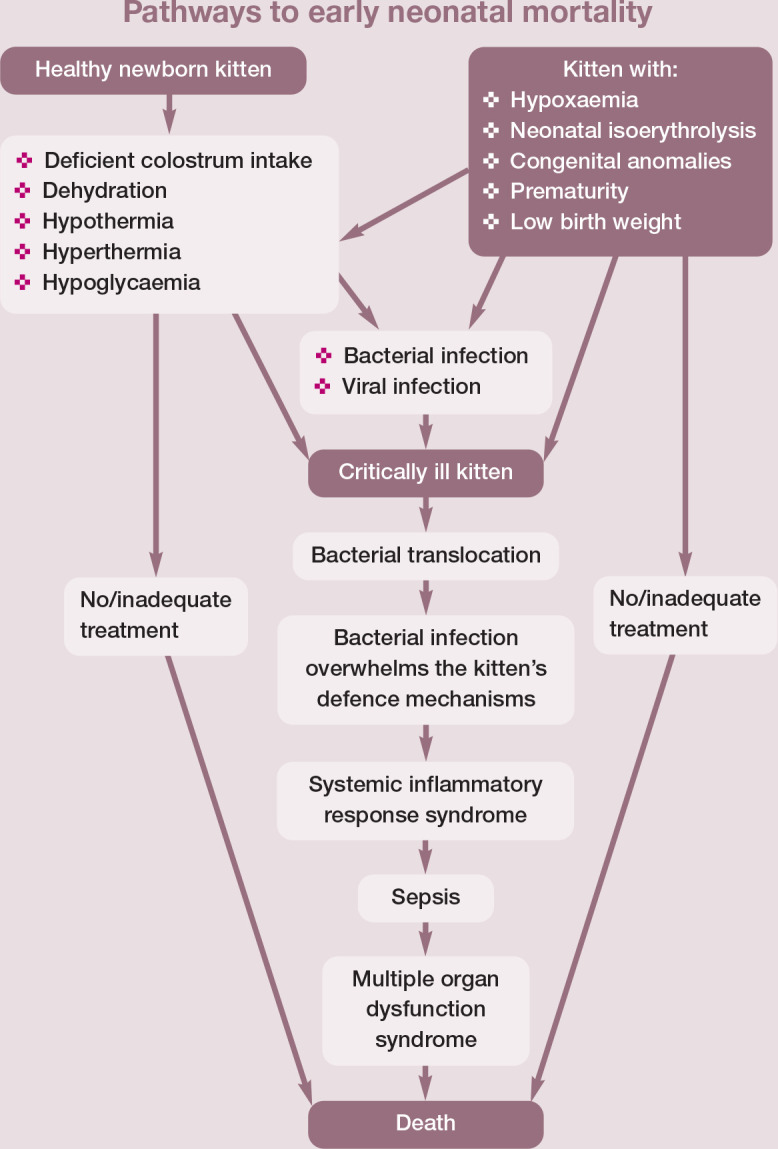
Possible pathways to neonatal mortality in kittens during the first week. Adapted from Münnich and Küchenmeister (2014) 6

Perinatal factors
Dystocia and fetal hypoxia
The birth process and maternal factors are important in the perinatal period (the first days of life). The birth process is a stressful event in itself. Marked hypoxia, due to dystocia, and hypothermia after birth are two additional physiological stresses; hypoxic conditions may also occur with bleeding due to birth
trauma. Hypoxic damage is found in organs that are most sensitive to oxygen deficit and can include varying degrees of small intestinal mucosal injury, degeneration of the heart, adrenal necrosis and hypoxic brain injury. 6
Although dystocia and prolonged labour in the queen are rarely observed compared with dogs, it has been shown that purebred queens, especially of breeds with abnormal conformation such as Persians and Siamese, experience much higher levels of dystocia (7-10%) than cats with normal conformation (<5%).7,8 Primiparous queens have higher kitten mortality than pluri-parous queens, likely related to poor mothering or inexperience. Older queens with more than five pregnancies have a higher rate of malformations and a higher incidence of faders and kitten losses. 8 The proportion of stillbirths and kitten mortality rates increase with litter size. 9
Kittens that survive severe hypoxia and resuscitation have a high risk of dying up to 48 h after birth. Such kittens are often unable to suckle, resulting in energy requirements not being met and the kitten missing out on colostrum (see box). It is crucial for the survival of these kittens that energy supplies and immunological support are provided. Administration of maternal serum is an alternative in immunocompromised kittens.12,13
Neonatal isoerythrolysis
NI is a rare but well-recognised condition most typically seen in purebred British Shorthair (60% type B blood phenotype) and Devon Rex (40% type B) kittens; it has also occasionally been seen in Persians, domestic shorthair cats, Somalis, Abyssinians, Birmans and Maine Coons. 14 NI occurs in blood type A or AB kittens born to type B queens. Type B cats develop a high level of naturally occurring alloantibodies against type A erythrocytes by the age of 3–4 months. When newborn type A or AB kittens suckle colostrum enriched with anti-A antibodies, these antibodies enter the kitten’s circulation and destroy the erythrocytes, resulting in anaemia, jaundice and haemoglobinuria (dark red-brown urine).2,8,15 The amount of antibody ingested influences the clinical course, which varies from fulminant to prolonged.2,8 It is with the latter chronic course, where there is a lower concentration of antibodies in the colostrum, that the typical faders will generally be observed. Signs begin a day or two after birth and include slow weight gain, failure to thrive (so-called ‘poor doers’), mild to severe anaemia and finally organ failure.1,15
Prevention of this condition involves avoiding mating a type B queen to a type A tom cat. Many breeding cats are now blood typed and breeders are well informed about the disease and its prevention. As well as certain breed predispositions, there is a geographical influence on the distribution of blood type.15,16 A study of non-pedigree cats from Italy 16 found that cats were 90% blood type A, 7% type B and 3% type AB.
Low birth weight and disturbed neonatal adaptation
Low birth weight is associated with premature birth and premature caesarean section, and is also seen in individual kittens at term (Figure 2). Birth weight should be considered low if it is >25% below the average for the breed. 17
Figure 2.
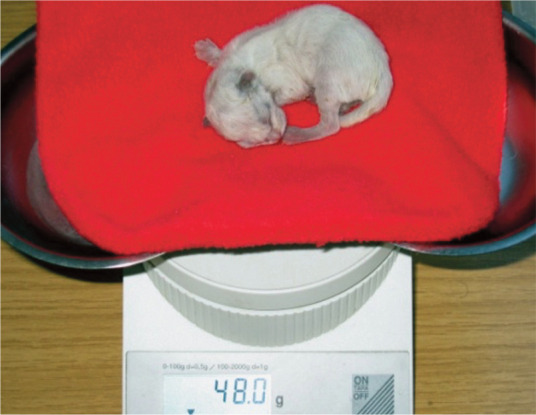
A kitten with low birth weight (48 g) after delivery at term. Support with additional feeding (gastric tube or bottle) and external warmth is essential to prevent hypothermia, hypoglycaemia and fading, and to increase chances of survival
Excessive weight loss in the first couple of days after birth in normally developed kittens has an effect similar to a low birth weight. Weight loss of >10% by day 2 requires supplemental feeding 18 and additional diagnostic procedures. In either scenario, with a low weight at day 2, kittens are likely to have a lack of sucking and/or swallowing reflex, and are at risk of developing hypoglycaemia. Small kittens may initially appear to be suckling all the time, changing from teat to teat, but may never gain weight. These kittens may be too weak to nurse adequately, despite appearing to be active. Small neonates often lack insulating fat and thermogenic brown fat, 8 and are particularly susceptible to hypothermia, dehydration, respiratory failure and sepsis. 17 If this vicious circle is not interrupted, the kittens fade.

Congenital anomalies
Some faders have congenital anomalies that may be visible or inapparent (see accompanying review on disorders of sexual development in this series). In general, most anomalies are unpredictable and unavoidable. The actual number of cases is likely higher than the proportions reported in the literature (where percentages typically range from 0.1 to 7 depending on the anomaly), especially in the light of internal defects. Anomalies can be genetic, caused by a terato-gen 5 or may represent a spontaneous mutation. If there is no genetic test available for the specific breed or disease, the cause remains unspecified. Anomalies leading to fading are often internal - a low-degree cleft palate, sternal defects, internal hydrocephalus, diaphragmatic hernia 2 or megaoesophagus.
✜ Microanatomic or biochemical anomalies usually go unreported and these cases are categorised as idiopathic stillbirths, faders or death from undetermined cause. Such anomalies cause death at birth or within the first days, weeks or months, with the time of death depending on the severity and organ system affected.
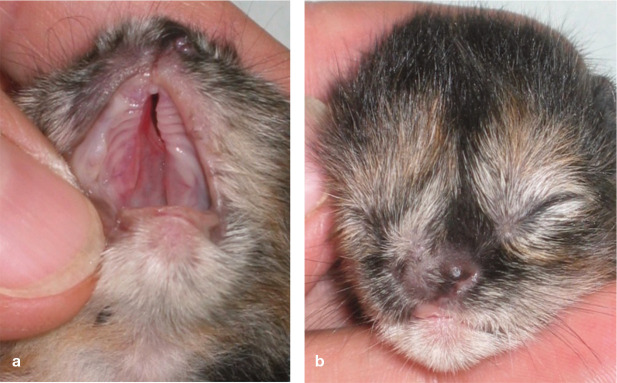
✜ Cleft palate is a common malformation (Figure 3), especially in Siamese cats. Incomplete closure of the palate may be inherited as a simple recessive or irregular dominant trait, or may be the result of teratogenesis; for example, due to hypervitaminosis A or the administration of corticosteroids, metronida-zole or griseofulvin during pregnancy.19,20 Even a small defect leads to insufficient weight gain and aspiration pneumonia, and is easily overlooked. Cleft palate should be ruled out by inspection of the oral cavity in all newborn kittens (the breeder should be advised to check in the home environment).
Figure 3.
(a,b) A newborn kitten with a cleft palate and microphthalmia of the right eye. The kitten was unable to suckle, and was euthanased because of the poor prognosis
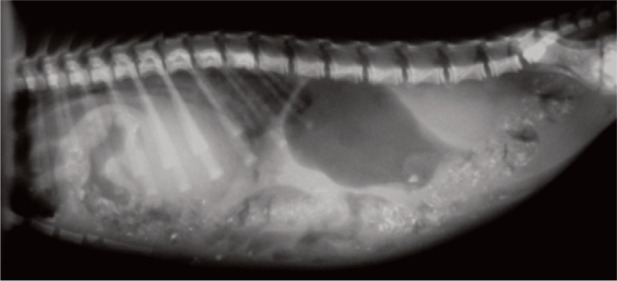
✜ Sternal malformations are well recognised in cats. In cases of pectus excavatum the sternum extends into the thoracic cavity and the ventral rib ends turn medially to meet the displaced sternum (Figure 4).20,21 The condition is seen in the Maine Coon, and sporadically in other breeds. Affected kittens, especially those with a flat chest, do not walk or breathe normally and mostly lie in ventral recumbency. The most important physiological effect of pectus excavatum is displacement of the heart and lungs, leading to pneumonia and difficulty breathing. Affected kittens often die after the appearance of the first clinical signs.
Figure 4.
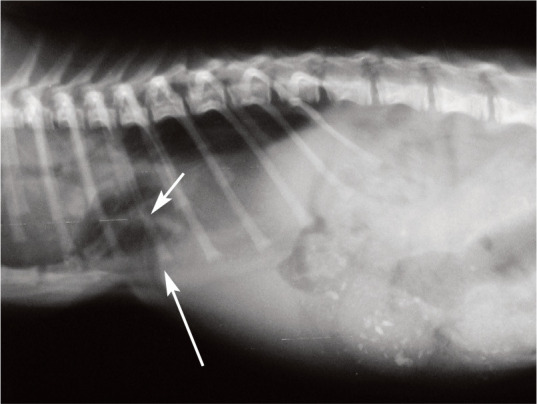
Radiographic examination of a 2-week-old kitten suffering from pectus excavatum, a sternal malformation in which the sternum encroaches into the thoracic cavity (short arrow). The ventral rib ends turn medially to meet the displaced sternum, resulting in a visible stricture (long arrow). In severe cases, such as this, with respiratory disease and pneumonia, surgical intervention is indicated
✜ Congenital hydrocephalus in kittens is of the internal type. 22 It results from structural defects that obstruct the outflow of cere-brospinal fluid (CSF). Some cases progress after birth by continued accumulation of CSF in the ventricular system. 23 Affected kittens have a dome-shaped calvarium and show developmental and growth retardation compared with littermates (Figure 5). Some have varying degrees of neurological dysfunction and, in severe cases, develop blindness, seizures and ventrolateral strabismus.
Figure 5.

Six-week-old kitten littermates. The kitten on the left has congenital hydrocephalus and is showing retarded development and growth - it is half the size and weight of its littermate. This kitten also had ventrolateral deviation of the eyes (so-called ‘setting sun’ phenomenon), a clinical abnormality encountered in neonates with increased intracranial pressure. Intracranial ultrasound evaluation at the point of the expected fontanel showed a lateral ventricular height of 6 mm (threshold value 3 mm). Such kittens often become faders and will be euthanased or die
Ventricular enlargement and internal hydrocephalus are encountered clinically with increasing frequency in modern Persian cats. A particular show line, the so-called ‘peke face’, which describes the triangular midface characterised by an extremely shortened nose that is aligned with the eyes, presents with a high incidence of internal hydrocephalus. 22
✜ Diaphragmatic hernia in the kitten presents as two types: peritoneopericardial and peritoneopleural. Both types can be traumatic in origin, but peritoneopericardial hernia is much more common in young cats, 21 suggesting it is also a developmental anomaly (Figure 6). A ventral diaphragmatic defect allows abdominal viscera to enter the pericardial sac. The defect does not appear to be inherited, 24 and indeed there is no known mode of inheritance for any breed, although some studies refer to a suspected genetic basis. The clinical signs depend on the protruded organ(s), but gastrointestinal or pulmonary signs are most common.
Figure 6.
Radiographic examination of a 3-week-old kitten that presented with retarded development, recurrent constipation and occasional dyspnoea. The kitten was treated with oral paraffin oil and underwent no further investigation. The intestinal loops in the thoracic cavity surrounding the heart and the increased cardiophrenic contact, with loss of distinction of the cardiac silhouette and diaphragm domes, are indicative of a congenital peritoneopericardial diaphragmatic hernia
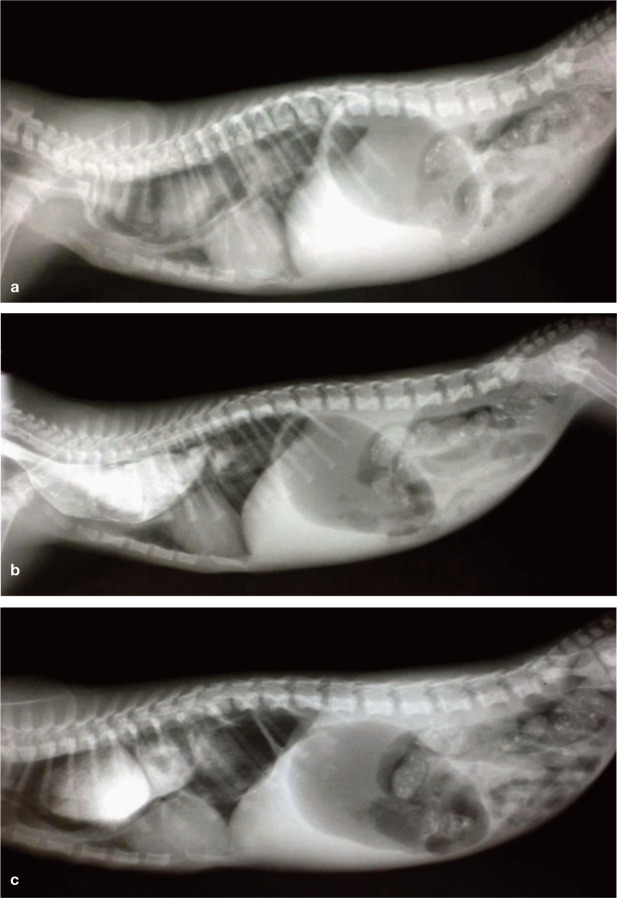
✜ Megaoesophagus can be congenital or acquired. It is caused by a dysfunction of the primary motor system of the oesophagus, 19 resulting in abnormal transportation of inges-ta between the pharynx and the stomach. Siamese and related breeds have the highest incidence of megaoesophagus. Infectious causes such as viral infections and toxoplasmosis contribute to the development of the condition. Some cases are seen in kittens (Figure 7). Clinical signs of regurgitation, halitosis and pneumonia occur, mostly after the third week of age and with changes in nutrition up to weaning. These signs of megaoesophagus are very similar to those exhibited by puppies of a comparable developmental stage. 25
Figure 7.
Series of radiographs showing idiopathic megaoesophagus in a 6-week-old Exotic Shorthair kitten; (a) prior to, (b) 5 mins after and (c) 10 mins after oral contrast medium administration. Regurgitation, growth retardation and coughing had been observed over the previous 10 days and since the introduction of a solid diet in addition to suckling. The kitten was treated with a liquid diet fed in a standing position, a vitamin B cocktail q8h and a week’s course of an antibiotic compound to prevent aspiration pneumonia. After 3 weeks the kitten had completely recovered. A specific cause was not found
Inadequate environment and nutrition
Hypothermia
In the author’s clinical experience, a healthy kitten has a temperature of 36–37°C around 24 h after birth. Pathological hypothermia occurs rapidly when the neonate remains wet in a cold environment or is separated from its mother.8,26 There is no hypothalamic control of body temperature in neonates. In addition, kittens lose heat easily because of the high ratio of body surface to body mass, minimal subcutaneous fat stores and an inability to shiver (until around day 6). In the first few days after birth, ‘non-shivering thermogenesis’ (transformation of chemical energy into thermic energy) is initiated by the release of catecholamines and metabolism of brown fat stores. This mechanism has high energy requirements, however, and a response to hypothermia can thus cause an ill neonate to decompensate.
Hypothermia is not necessarily lethal but it is harmful as it can trigger the onset of other problems. With a drop in temperature, the heart rate will fall from 200–260 beats per min at 36.0°C, to 40–50 beats per min at 21.1°C. This is a protective response, but, if sustained, the respiratory rate also decreases and may result in cardiopulmonary failure. 8 Abnormally low temperature in newborns (<35°C) can lead to a failure to suckle, dehydration, depressed gastrointestinal motility or intestinal paralysis/ileus, and increased susceptibility to bacterial infection. 6
Hypothermia develops because of mismoth-ering, cold environmental temperatures and underlying diseases. Untreated kittens often die from bacterial infections with E coli. If hypothermic kittens are forced to eat, regurgitation, aspiration pneumonia or ileus can occur. A decrease in bowel movements associated with hypothermia, lack of early colostrum ingestion and poor maternal care may lead to meconium retention (Figure 8). Hypothermia is believed to facilitate invasion of opportunistic pathogens through the intestine, resulting in endotoxaemia. In moderate hypothermia, neonates have a low heart rate and, despite attempts to suckle, milk will not be ingested. With severe hypothermia the kitten lies motionless in lateral recumbency and breathing slows to occasional gasps. 1
Figure 8.
Meconium (dark coloured) and milk faeces (yellowish) excreted by a hypothermic 2-day-old kitten following rewarming, rehydration and tube feeding
Hyperthermia
Hyperthermia from an overheated environment may predispose newborn kittens to respiratory failure. Exposure of neonatal kittens up to 48 h of age to high environmental temperatures reduces the ventilatory response to carbon dioxide; this is the opposite of what happens in adult cats. Abnormally high temperatures might arise, for example, during transportation of kittens by car in a small box with a very hot water bottle, or with use of an electric heating plate that covers the entire base of the kitten box. Exposure of newborns to prolonged high environmental temperatures leads to constipation due to dehydration. 8 Such kittens tend to cry continuously but stop when removed from the overheated environment.
Hypoglycaemia
Hypoglycaemia is a sequela of a negative energy balance, when the intake of glucose does not match the demand. The liver of the neonatal kitten is inefficient at metabolising glycogen and hepatic glycogen stores may be low.
A negative energy balance can result from, for example, inanition, a greater energy demand associated with cold temperatures, or concurrent disease or disorders. Sepsis, portosystemic shunts and glycogen storage abnormalities are all disorders that can cause a rapid decline in blood glucose saturation. Additionally, any kitten that is ill or stressed may develop hypo-glycaemia. Kittens with a low birth weight (see earlier) usually have a higher metabolism and thus higher energy requirements, and so too are at a greater risk of developing hypoglycaemia.
Clinical signs of hypoglycaemia include lethargy, failure to nurse, mental dullness, depression or stupor, seizures, tremor, vocalisation and irritability. 1
Dehydration
Dehydration and subsequent hypovolaemia can result from insufficient consumption of milk, or excessive fluid loss following overheating and diarrhoea. Neonates are predisposed to dehydration because, compared with adults, extracellular fluid volume is greater, the ratio of surface area to body weight is larger, renal capacity to conserve water is lower and fluid losses through immature skin are higher. Hypo-volaemic shock and subsequent hypotension result in organ failure due to tissue hypoxia.
Infectious disease
Infectious agents account for a large proportion of disease and kitten losses, with fading kitten syndrome often part of the clinical picture. Mortality is highest within the first few weeks after birth, 27 and a second peak is observed around weaning. Kittens may variously be infected during birth, immediately after parturition and during the first weeks of life, with sources of infection being the mother (milk [Figure 9], vaginal discharge, faeces or oral cavity), other cats in the cattery or the environment. 28 Many factors are involved and predispose kittens to infectious disease, including all of those discussed earlier (difficult birth, hypoxia, factors affecting passive immunity, hypothermia, low birth weight, premature birth, hypoglycaemia, dehydration and congenital anomalies, as well as environmental contamination 6 ). Bacteria, viruses and parasites enter via the oral cavity, and bacteria can also enter through the umbilicus. Clinical signs are usually non-specific, regardless of the cause, and include behavioural changes, dehydration, cold skin and/or altered skin colour, failure to gain weight and crying. Sudden death is possible.
Figure 9.
Sources of infectious disease for kittens include milk from the mother
Bacterial infections
Bacteria are among the most common infectious agents in the neonate prior to weaning. Kittens are colonised with bacteria during the first days of life. Under non-stressful conditions most bacteria are commensal, but they can sometimes induce mild, self-limiting disease. Moreover, some non-pathogenic and opportunistic organisms that lack virulence factors cause infections in newborns; these so-called ‘ubiquitous’ bacteria can sometimes induce severe disease. 28 Bacterial overgrowth, as well as hypoxia and impaired host immunity, also promotes bacterial translocation. 29 This is the process whereby viable resident bacteria and/or lipopolysaccharide (endotox-in) from the intestine cross the mucosal surface, which, in a healthy animal, is the major barrier preventing bacteria from gaining access to systemic organs and tissues. When a bacterial infection overwhelms defence mechanisms, neonatal sepsis develops. 27 Kittens in which there has been no passive transfer of immunoglobulins are at increased risk of sepsis (see ‘Colostrum and immunoglobulins’ box on page 244). 1
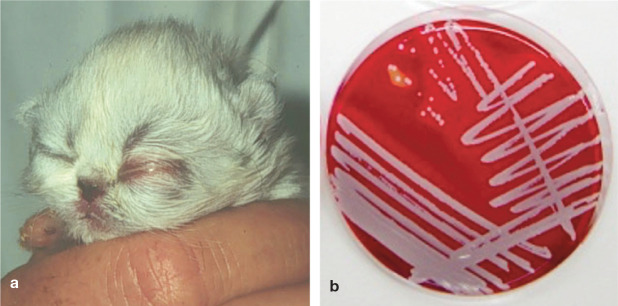
Sources of bacteraemia include the gastrointestinal tract, respiratory tract, urinary tract and skin wounds. E coli, streptococci (Streptococcus canis), staphylococci (Staphylococcus aureus, Staphylococcus pseudointermedius), Klebsiella species, Pseudomonas species,1,30 Campylobacter species, chlamydiae and anaerobic bacteria are regularly found in swabs (Figure 10) or during post-mortem examination.
Figure 10.
(a) Ophthalmia neonatorum in a 4-day-old kitten. One other littermate had the same appearance. Most often staphylococci (b) will be found in swabs taken from the secretion
Clinical signs depend on the site, nature and severity of infections, with most bacterial disease resulting in fading, sepsis and death.Poor sanitation often leads to neonatal dermatitis (crusting lesions), neonatal conjunctivitis with accumulation of purulent exudate behind the eyelids, umbilical infections or diarrhoea. 28
Prevention of bacterial infections in kittens should start by addressing predisposing factors; if infection does occur, supportive therapy should be provided. If there is recurrence of specific infections, detection of carrier animals within the cattery is necessary. Some specific infectious agents cross the species barrier and, as well as affecting cats, can affect dogs and also owners. 28
Viral infections
Viral infections usually occur after weaning because of the decreasing protection afforded by maternally derived antibodies at about that time. Post-weaning viral infections with feline calicivirus (FCV), feline herpesvirus-1 (FHV-1), feline panleukopenia virus (FPV) and feline coronavirus strains (feline infectious peritonitis virus; FIPV) represent the majority of cases. Kittens with a deficiency of colostrum intake and low passive immunity can develop viral infection much earlier in life, even during the first few weeks. Such neonates rarely survive. 8
The respiratory viruses FCV and FHV-1 are the most commonly seen viral infectious agents in kittens. 31 Infection may be mild in healthy kittens with sufficient passive immunity but, in weak animals, clinical signs can be severe. Secondary bacterial infection is common.
Parasitic infections
Infection with parasites can develop immediately after birth (eg, Toxocara species), and during the first few days or weeks of life (eg, Toxocara species, Giardia species, cryp-tosporidia, coccidia, Tritrichomonas foetus and ectoparasites). Infected kittens can become typical faders, developing diarrhoea, with poor body condition, soft or bloody faeces, lack of appetite, weight loss and/or a swollen abdomen up to death. A severe flea and hookworm infestation can result in significant anaemia. 32 The majority of breeders implement a regular programme of deworming after weaning (neonates), or at the earliest after 3 weeks of age (in contrast to puppies, there is no intrauterine transmission of ascarid larvae in kittens). Ectoparasites in young kittens are often a sign of poor management and hygiene. 32
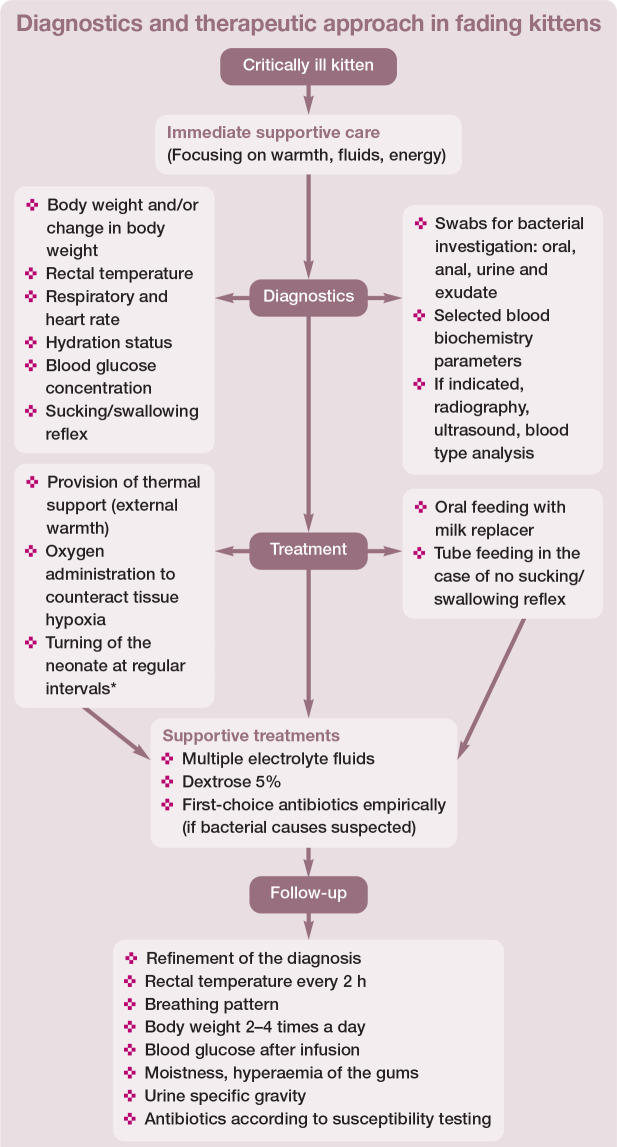
Diagnostic approach and treatment options
Diagnostic and therapeutic options in fading kittens are summarised in Figure 11 and discussed below. Regardless of the underlying cause, the approach to a sick neonate is simi-lar. 1 In terms of priorities, the first task is to initiate supportive care, and the second is to perform a detailed physical examination. The third priority is to try to find the cause of the disease, and to adjust the treatment if required, and finally the veterinarian needs to advise the breeder regarding ongoing individual care for the kitten or the whole litter.
Figure 11.
Summary of diagnostic and therapeutic options in fading kittens. *The author’s practical experience suggests that blood flow and wellbeing are supported by turning the newborn from side to side every few hours; for example, after feeding, administering medications or measuring rectal temperature. Adapted from Münnich and Küchenmeister (2014) 6
Diagnosis
Neonatal kittens tend to show limited clinical signs in disease (see box); their presenting problem or combination of signs is rarely indicative of a particular condition. Diagnosis of disease in neonatal kittens can therefore be challenging.

To correctly interpret diagnostic results it is important to know what is normal for neonates. There are distinct physiological differences between adults and neonates (see accompanying review on feline neonatology) and these variances make interpretation of clinical and laboratory findings problematic. 33 Additionally, performing diagnostic procedures such as blood collection in small kittens is difficult.
The signalment and history from the owner is often very helpful for the detection of possible causes, 2 and thus a detailed history should always be taken. 34
Examination of newborns should be performed on a heated table, looking for any trauma and visible congenital defects. The head and thorax should be of normal size and shape. As well as inspecting for a cleft palate, the colour and appearance of the oral mucous membranes should be assessed (Figure 12); the gums should be red during the first 7 days, and thereafter pink (sick kittens often have dry, pale grey or bluish [cyanotic] gums). The eyes and nose should be checked. Heart rate (200-260 beats per min) and respiratory rate (15-35 breaths per min) should be mea-sured. 34 Breathing should be regular and not laboured. There should be a sucking reflex. Muscle tone should be strong (a sick neonate is limp and relaxed). The umbilicus must not be red, moist or swollen, and there should be no umbilical discharge. The anus should be inspected for patency, swelling or redness (Figure 13). 8 For sick kittens younger than 3 weeks, the queen and (healthy) littermates act as controls and should also be examined. Further diagnostic tests are discussed below.
Figure 12.
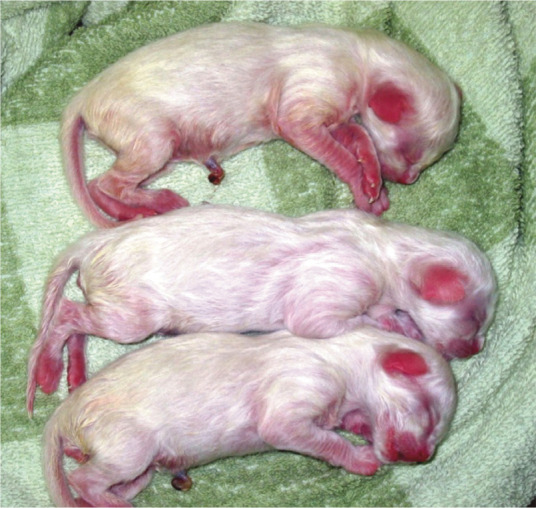
Hyperaemia of the oral mucosa is normal up to a week after birth. Thereafter, pink gums are observed. In white kittens especially, hairless areas of the skin (ears, around the nose or the tip of the paws) also have a hyperaemic appearance
Figure 13.
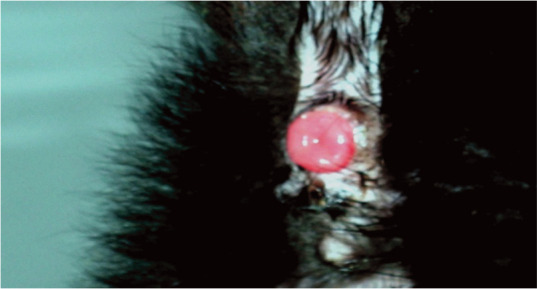
In heavily diarrhoeic kittens, the mucosa of the anus or caudal rectum can protrude and in some cases there may be complete rectal prolapse. Early treatment of diarrhoea is important to prevent such complications
✜ Rectal temperature Measurement of rectal temperature is one of the most important clinical examination procedures, and is easy to perform and non-invasive. A special low-reading digital thermometer with a flexible tip (designed for human neonates) is recommended. Most digital thermometers start at 32°C (some older mercury thermometers start at 30°C, but are not recommended for use).
✜ Blood sampling Haematological profile and serum biochemistry data in neonates differ from adult cats and need specific inter-pretation. 34 For blood sampling it is only possible to take about 0.7 ml (ie, 10% of total blood volume for a 100 g kitten). In the case of hypoglycaemia, which is very common regardless of the cause of illness, blood glucose measurement can be obtained easily with a dipstick or a blood glucometer using a droplet of blood taken from the ear or paw.
✜ Urinalysis It is easy to collect urine by stimulation of the perineal region. Urinalysis can give information on NI, urinary tract infection and hydration status.
✜ Body weight Birth weight is an important factor for survival and also reflects relative maturity. Daily weight measurement of the neonate is of critical importance for early recognition of disease and daily weight gain is an excellent indicator of general health. If the amount of milk being ingested is questionable, the neonate can be weighed before and after nursing. Crying occurs in response to failure to nurse (as well as pain and coldness). It is abnormal for a healthy neonate to cry for longer than 20 mins.
✜ Intestinal sounds Sick kittens lack normal bowel sounds on abdominal auscultation.
✜ Other tests Depending on the clinical course, further diagnostic procedures should be performed, such as radiography, ultra-sonography or bacterial culture (oral, faeces or urine swabs, or organs of deceased kittens during post-mortem examination). 1 Radiographic interpretation in neonatal patients poses a challenge as there is a loss of abdominal detail caused by lack of body fat; in addition, the heart appears relatively enlarged, the liver is located more caudally, and the lungs have a more opaque interstitium.35,36
Management of postnatal hypoxia
Hypoxic kittens born after dystocia are often weak, and slow or fail to suckle. Intervention is required, and is also indicated when a kitten is not breathing or the mother’s maternal instinct appears to be lacking. An approach to reduce birth-related neonatal mortality is Apgar scoring. A scheme that was developed for human neonates by Virginia Apgar37,38 was adapted for some animal species and will also work for kittens, as demonstrated in a very recent study. 39 Disturbances can be detected early, and treatment or intensive care can be initiated immediately. With the help of the Apgar score, effectiveness of resuscitation can also be monitored.
Resuscitation after a difficult birth, extraction (Figure 14) or caesarean section, or when a kitten does not start to breathe spontaneously, follows the A-B-C rules: Airway clearance, promotion of Breathing and activation of Circulation. 40
Figure 14.
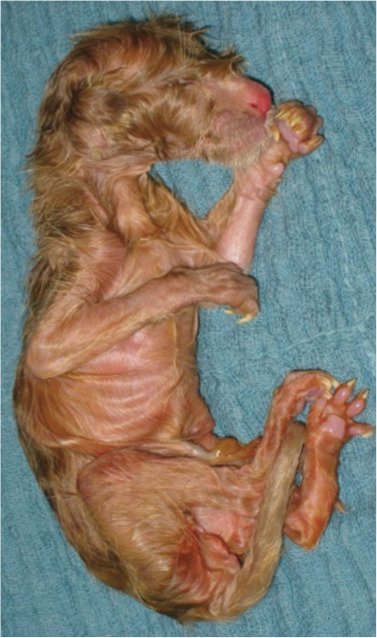
A singleton hypoxic Oriental Shorthair kitten. This neonate was born after a difficult extraction on day 72 of pregnancy, with parturition induced with aglepristone. Handling and management according to the A-B-C-rules is essential in cases such as this, as is provision of external warmth and surveillance of colostrum intake. Note the long hair and long claws, which are signs of prolonged pregnancy. A very small amount of fetal fluid was observed following rupture of the fetal membranes. The kitten survived and did well
Neonates experiencing hypoxic conditions (intrauterine or postnatally) have a comparatively long survival time. This is on account of an elective reduction in oxygen consumption and restrained function of chemoreceptors in states of mild hypoxia, with no effect on heart and respiratory rates. A kitten can be successfully resuscitated without subsequent problems. Cornerstones of successful resuscitation are described in the box.40,41
Management of neonatal isoerythrolysis
Queens of breeds predisposed to NI (eg, British Shorthair and Devon Rex) should be blood typed prior to breeding to prevent mating a type B queen with a type A tom cat. Both an in-house test card (Rapid Vet-H) and tests offered by commercial laboratories can help to identify blood types. 42 In newborn kittens, placental blood can be used to determine the blood type. If a kitten shows blood type A and the mother has type B, the kitten should be removed for the first 24 h to allow for closure of the intestinal mucosal barrier. 41 Such kittens are then either hand fed with milk replacer or fostered onto a lactating type A queen before being returned to their moth-er. 11 Although this will prevent NI, the lack of colostral antibodies will make the kitten susceptible to infectious disease. Milk from foster queens (also containing immunoglobulins) has been recommended as a replacement for colostrum in kittens younger than 24 h, 14 but a separate study found that colostrum-deprived neonatal kittens fostered onto queens in their milk phase of lactation failed to acquire maternal antibodies. 12 While the reason is not fully understood, colostral immunoglobulins resist destruction in the digestive tract, but non-colostral antibodies may not. 10 Antibody support can be provided via the administration of serum from a type A adult cat (intraosseous or subcutaneous route), albeit this produces a lower immuno-globulin concentration than ingestion of colostrum within the first fews hours of birth. The author has successfully performed subcutaneous administration of serum with no adverse effects.
Treatment (specific and supportive) of affected kittens depends on the severity of signs. If more severe clinical signs such as anaemia and jaundice occur, erythrocyte or blood transfusion will be required.15,41,42 Most kittens with NI die within the first week of life after removal from the dam and despite treatment.
Management of low birth weight with supplemental feeding
Kittens should receive supplemental feeding if they lose >10% of their birth weight within the first 24 h after birth, or if their birth weight is low (>25% below the average for the breed). The kitten’s stomach capacity is approximately 4–5 ml per 100 g.1,34 Overfeeding is the most common cause of non-infectious diarrhoea in orphaned kittens during the first 3 weeks after birth and must be avoided. The appearance of the faeces can be of diagnostic value: the colour changes from green (bile secretion) to white, suggesting a lack of digestive enzymes.
Figure 15.

These kittens have been rubbed dry and are being kept warm following delivery by caesarean section 30 mins earlier
As recommended in the accompanying review on feline neonatology, it is always better to feed small amounts but more frequently, never reaching maximum stomach capacity. The amount of milk replacer to be given in a 24 h period should be calculated as 20–30% of actual body weight and fed in 8–10 meals for the first 2 weeks after birth; 6 as a rule of thumb, 2–3 ml per feed for a 100 g kitten. Kittens that are still suckling to some extent should be weighed each day in order to determine how much milk has been consumed and the amount of milk replacer should be adjusted accordingly; for example, reducing the number of meals to five. In diarrhoeic kittens, the formula should be mixed at a 1:2 ratio with balanced electrolyte solution or isotonic sodium chloride.
Neonates can be fed with a bottle (if there is a sucking and swallowing reflex) or a feeding tube (if there is no swallowing reflex). Feeding (droplets of milk replacer) with a syringe should be avoided until around 8 days after birth as there is no gag reflex until this age. Stomach tubes must be soft and flexile, and generally not more than 2–3 mm diameter in size; a human neonatal nasogastric tube or soft rubber canine urethral catheter is suitable. The tube must be measured and marked to the correct length (nose to last rib). The catheter should be attached to the syringe and the air extracted from the tube so that no air enters the stomach. The tube passes down the oesophagus, and prewarmed milk replacer is delivered slowly into the stomach. 41 At the point of tube removal, pinching the tube closed with the fingers will avoid milk flowing into the pharynx and airways. When a sucking and swallowing reflex is established, bottle feeding in the case of orphan-reared kittens is possible.
It is best to feed a commercially available kitten formula and not a homemade formula, whether for supplemental or full replacement feeding. Most of the commercially available products are composed of cow’s milk that has been modified to the composition of queen’s milk, and they have a higher energy content and a lower proportion of lactose.
Congenital malformations
Clinically visible malformations are readily recognised. Radiography, ultrasonography, endoscopy or blood tests and finally postmortem evaluation can help to diagnose in-apparent defects. Live-born kittens with an untreatable condition should be euthanased.
In some faders that suffer from one of the following abnormalities, treatment could be attempted:
✜ A small cleft palate can be surgically repaired, but no earlier than at 8–10 weeks of age. Tube feeding is the only effective method of providing adequate nutrition until surgery. 20
✜ Surgical repair of pectus excavatum is indicated in severe cases; no specific treatment is required in mild cases. 20
✜ Ultrasonography or MRI are useful tools for diagnosis of congenital hydrocephalus. Treatment depends on the underlying cause.
✜ Diagnosis of diaphragmatic hernia can be made by radiography and ultrasonography. Treatment, if indicated, is usually surgical.20,24
✜ Kittens with idiopathic forms of mega -oesophagus have a 50% chance of recovery. Contrast radiography helps to confirm the diagnosis. 23 Treatment depends on the prognosis and usually consists of conservative therapy including feeding in a standing position and keeping the head up, vitamin B administration, and antibiotics in the case of aspiration/aspiration pneumonia.
Management of hypothermia
As mentioned earlier, a healthy kitten has a temperature of 36–37°C 24 h after birth and usually during the first week of life. 34 Kittens that are hypothermic should be rewarmed, but this rewarming process should be conducted very slowly because of the high energy demand it imposes. Warm electrolyte solution is given by the intraosseous, intravenous or subcutaneous route (depending on body weight) to rehydrate the kitten. Droplets of concentrated glucose solution (10–20%) placed on the oral mucosa help to ensure energy requirements are met. Given that bacterial infections regularly occur in hypothermic neonates, prophylactic antibiotics should be considered. The author’s first-choice antibiotic in these cases is injectable amoxicillin/clavulanic acid.
Oral feeding is only indicated when the body temperature has reached 36°C. 34 In the case of hand-rearing, and if kittens are separated from the queen, environmental temperatures must be maintained at specific levels to avoid hypothermia: 32°C for the first week of life and 26°C during the second week. The heat source can be a fleece-covered microwaveable heat pad (eg, SnuggleSafe), a hot water mattress or electric heating plate covered with towels. Kittens should always be able to select a temperature zone that is comfortable. An unheated area should be available in the kitten box, regardless of the method of providing heat. 6
Management of hyperthermia
Kittens that are hyperthermic should be kept in an area that is at room temperature for an hour. Other cornerstones of treatment include fluid and energy support. Recovery is gauged by the crying ceasing and the kitten becoming calmer and sleeping.
Management of hypoglycaemia
A blood glucose concentration <50 mg/dl in newborn kittens up to 2 weeks after birth is abnormal, especially when accompanied by clinical signs. In the literature, a range of 75–154 mg/dl on day 2 after birth is reported as normal. 12
The oral mucosa will absorb glucose readily, and a few droplets of 10–20% glucose solution applied to the gums of a hypoglycaemic kitten will increase the serum glucose concentration over the critical limit. Blood glucose concentration should be remeasured within an hour of bolus administration or at regular intervals during infusion over a long period, because of the immature regulatory and metabolic mechanisms in neonates. Hyperglycaemia will cause osmotic diuresis and electrolyte loss. The dosage of glucose for infusion is 0.5–1.0 g/kg diluted to a 5% to 10% solution. Glucose concentration should also be remeasured in cases that fail to respond to therapy. 2
Management of dehydration and hypovolaemia
Moistness of mucous membranes, packed cell volume, serum protein level and urine specific gravity and output are relatively sensitive methods of assessing hydration status in the neonate. A urine specific gravity of 1017–1020 can be used as a threshold value, with higher measurements, 43 and a concentrated dark yellow colour of the urine, indicating dehydration.
Fluid requirements are higher in neonates than in adults. In kittens during the first 2 weeks, maintenance rate is 120–180 ml/kg/24 h.1,17 The intravenous route is best to administer fluids, blood components (in NI) and medications. If an intravenous catheter cannot be placed, the medullary cavity of the large bones can be used for intraosseous infusion. 35 A small spinal needle or a butterfly needle needs to be placed aseptically into the medulla of the proximal femur or the humerus. Limiting factors are the size of medullary space in neonates and the technical experience required to perform catheter placement. Furthermore, there are risks associated with this procedure of damage to the sciatic nerve, bone or cartilage, extravasation of fluids around the puncture site, or the development of osteomyelitis. 37 An alternative is intra-peritoneal injection of fluid with an IV catheter placed through the midline (ventral abdomen) caudal to the umbilicus. The peritoneal surface reabsorbs fluids rapidly. 6 The catheter must be removed after infusion.
Neonates cannot accommodate volume overload as efficiently as older cats, and especial care should be taken because signs of vascular overload are difficult to detect at this young age. 35 Rapid volume replacement can lead to intracranial haemorrhage, as hyper-osmolarity increases the risk of myelinolysis from water loss in the brain.
Management of faders with infectious disease
Clinical signs observed in neonatal kittens with infectious disease are listed in the box; a number of these signs are also associated with other, non-infectious conditions (see box on page 250).1,6,8
As for other potential causes, a complete physical examination should always be performed, including assessment of weight and body condition. 2 Depending on the clinical picture, further investigations may include:
✜ Serology for viruses and swabs for bacterial culture
✜ Ultrasound examination
✜ Radiographic examination
✜ Faecal samples for detection of bacteria, protozoa or intestinal parasites
Swabs for bacterial culture should be taken from the oral cavity, faeces or affected areas in live kittens, and from the intestine, abdominal and/or thoracic organs and abdominal cavity in deceased kittens. 6
Regardless of the specific infection, supportive treatment is indicated and includes rewarming, and correction of dehydration and blood glucose concentration. If the neonate is hypothermic, oral feeding should be avoided until the body temperature reaches 36°C; parenteral support is required.
High hygiene standards should prevent most infections. Isolation of carriers may be required in some circumstances.

Bacterial infections
Ideally, antibiotics (Table 1) should be selected according to sensitivity tests, but in many situations antibiotics must be given empirically. Penicillins are generally not toxic and should be given parenterally. 40 Cephalosporins are another choice; they are well tolerated in kittens, and have a broad spectrum of antibacterial activity, especially ceftiofur (off-label use) and cephalexin (available for injection only in some countries). In exceptional cases mar-bofloxacin or enrofloxacin may be used. Aminoglycosides, tetracyclines and chloram-phenicol should be avoided because of the risk of severe adverse reactions. 35 Serum from adults, as a method of passive immunity, will support immune defence in some cases. Parenteral administration in neonates is preferred because of the variations in absorption and distribution of drugs administered orally. 35
Table 1.
Selected drug treatments for use in neonatal kittens
| Drug | Dosage |
|---|---|
| Ampicillin | 20 mg/kg q8h IM, SC |
| Amoxicillin/clavulanic acid | 20–30 (maximum 50) mg/kg q24h SC |
| Cephalexin | 20 mg/kg q24h SC |
| Ceftiofur | 2–4 mg/kg q12h SC |
| Marbofloxacin* | 2 mg/kg q24h SC, IV, IO |
| Enrofloxacin* | 5 mg/kg q12h SC, IM, IV, IO |
| Azithromycin | 5–10 mg/kg q24h 3–5 days PO |
| Inactivated Parapoxvirus ovis (Zylexis; Pfizer) | 2 consecutive days, thereafter on day 7 |
Should be used in exceptional cases only IM = intramuscular; SC = subcutaneous; IV = intravenous; IO = intraosseous
Viral infections
Kittens may be infected with FHV-1 and FCV from subclinically shedding adults/dams, leading to respiratory and ocular signs, and sometimes severe interstitial pneumonia. 31 Antiviral therapy, with aciclovir, ganciclovir or L-lysine, is an option for FHV-1 infection. Antiviral administration in infected queens may minimise shedding associated with the stress of parturition and lactation. 31
Antibiotics should be considered for secondary bacterial infection. 31 If available, commercial antibody preparations or serum with antibodies against specific viruses could be administered. Serum from vaccinated adult cats in the cattery can be given (intravenous or intraosseous administration) when passive transfer of immunoglobulins is known to have failed. Azithromycin (Zithromax; Pfizer; Table 1), licensed for human treatment, is effective against Chlamydia species. The immunomodulator Zylexis (Pfizer; Table 1) activates basic immunity and induces cytokine and interferon production.
Treatment of feline leukaemia virus-, FIPV- or FPV-infected neonates is often difficult and these kittens rarely survive. 8 The viruses will usually cause disease at weaning or later, but in occasional instances (in immunosuppressed kittens) also early in life. Vaccination programmes for the dam will be effective in preventing further occurrences (to be discussed in a later review in this series on reproductive management in catteries).
Parasites
The treatment of roundworms and hookworm consists of the application of pyrantel, fenben-dazole and febantel 32 (and selamectin or moxidectin later in life). The first treatment should be given after 3 weeks, as no transpla-cental transfer of larvae occurs. Giardia species can be treated with fenbendazole or febantel, 32 and T foetus with ronidazole 32 (30 mg/kg for a minimum of 10 days; off-label use).
For flea treatment, kittens from 2 weeks of age can be given fipronil (off label-use), but not ivermectin.
Key Points.
To prevent Nl, blood type B queens should not be mated with blood type A tom cats. Most kittens with NI die within the first week of life.
✜ Daily weight measurement of the neonate is of critical importance for early recognition of disease.
✜ Kittens should receive supplemental feeding if they lose >10% of their birth weight within the first 24 h after birth, or if their birth weight is low (>25% below the average for the breed).
✜ Overfeeding is the most common cause of non-infectious diarrhoea in orphaned kittens during the first 3 weeks after birth.
✜ Measurement of rectal temperature is one of the most important clinical examination procedures.
✜ If hypothermic kittens are forced to eat, regurgitation, aspiration penumonia or ileus can occur. Oral feeding is only indicated when the body temperature has reached 36°C.
✜ Kittens should always be able to select a temperature zone that is comfortable and an unheated area should be available in the kitten box. 1
✜ To correctly interpret diagnostic results it is important to know what is normal for neonates, as there are distinct physiological differences between neonates and adults.
Footnotes
The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author received no financial support for the research, authorship and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and therefore informed consent was not required. No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
- 1. Macintire DK. Pediatric intensive care. Vet Clin North Am Small Anim Pract 1999; 29: 971–985. [DOI] [PubMed] [Google Scholar]
- 2. Hoskins JD. Fading puppy and kitten syndrome. In: Kirk RW, Bonagura JD. (eds). Kirk’s current veterinary therapy Xii. Philadelphia: WB Saunders, 1995, pp 30–33. [Google Scholar]
- 3. Ström Holst B, Frössling J. The Swedish breeding cat: population description, infectious diseases and reproductive performance evaluated by a questionnaire. J Feline Med Surg 2009; 11: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fournier A, Masson M, Corbière F, et al. Epidemiological analysis of reproductive performances and kitten mortality rates in 5,303 purebred queens of 45 different breeds and 28,065 kittens in France. Reprod Domest Anim 2017; 52 Suppl 2: 153–157. [DOI] [PubMed] [Google Scholar]
- 5. Bucheler J. Fading kitten syndrome and neonatal isoerythro - lysis. Vet Clin North Am Small Anim Pract 1999; 29: 853–870. [PubMed] [Google Scholar]
- 6. Münnich A, Küchenmeister U. Causes, diagnosis and therapy of common diseases in neonatal puppies in the first days of life: cornerstones of practical approach. Reprod Domest Anim 2014; 49 Suppl 2: 64–74. [DOI] [PubMed] [Google Scholar]
- 7. FAB. Feline Advisory Bureau manual of cat breeding. Tisbury, FAB Publications; (cited by Gunn-Moore d, 20068). [Google Scholar]
- 8. Gunn-Moore D. Small animal neonatology: they look normal when they are born and then they die. Proceedings of the WSAVA/FECAVA World Congress 2006; Praque, pp 714–720. [Google Scholar]
- 9. Romagnoli S, Bensaia C, Ferré-dolcet L, et al. Fertility parameters and reproductive management of Norwegian Forest Cats, Maine Coon, Persian and Bengal cats raised in Italy: a questionnaire- based study. J Feline Med Surg 2019; 21: 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chastant-Maillard S, Aggouni C, Albaret A, et al. Canine and feline colostrums. Reprod Domest Anim 2017; 52 Suppl 2: 148–152. [DOI] [PubMed] [Google Scholar]
- 11. Casal ML, Jezyk PF, Giger U. Transfer of colostral antibodies from queens to their kittens. Am J Vet Res 1996; 57: 1653–1658. [PubMed] [Google Scholar]
- 12. Claus MA, Levy JK, Macdonald K, et al. Immunoglobulin concentrations in feline colostrum and milk, and the requirement of colostrum for passive transfer of immunity to neonatal kittens. J Feline Med Surg 2006; 8: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy JK, Crawford PC, Collante WR, et al. Use of adult cat serum to correct failure of passive transfer in kittens. J Am Vet Med Assoc 2001; 219: 1401–1405. [DOI] [PubMed] [Google Scholar]
- 14. Giger U, Casal ML. Feline colostrum – friend or foe: maternal antibodies in queens and kittens. J Reprod Fertil Suppl 1997; 51: 313–316. [PubMed] [Google Scholar]
- 15. Silvestre-Ferreira AC, Pastor J. Feline neonatal isoerythrolysis and the importance of feline blood types. Vet Med Int 2010; 2010. doi: 10.4061/2010/753726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tommaso MD, Miglio A, Crisi PE, et al. Frequency of blood types A, B and AB in a population of non-pedigree domestic cats from central Italy. Animals (Basel) 2020; 10. doi: 10.3390/ani10101937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mosier JE. The puppy from birth to 6 weeks. Vet Clin North Am Small Anim Pract 178; 8: 79–100. [DOI] [PubMed] [Google Scholar]
- 18. Mila H, Grellet A, Feugier A, et al. Differential impact of birth weight and early growth on neonatal mortality in puppies. J Anim Sci 2015; 93: 4436–4442. [DOI] [PubMed] [Google Scholar]
- 19. Hoskins JD. The digestive system. In: Hoskins JD. (ed). Veterinary pediatrics: dogs and cats from birth to six months. 3rd ed. Philadelphia: WB Saunders, 2001, pp 147–199. [Google Scholar]
- 20. Ruaux C. The respiratory system. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics. The first 12 months of life. St Louis, Mo: Saunders Elsevier, 2011, pp 328–334. [Google Scholar]
- 21. Hoskins JD. The respiratory system. In: Hoskins JD. (ed). Veterinary pediatrics: dogs and cats from birth to six months. 3rd ed. Philadelphia: WB Saunders, 2001, pp 97–98. [Google Scholar]
- 22. Schmidt M, ondreka N. Hydrocephalus in animals. Pediatric Hydrocephalus 2019: 53–95. doi 10.1007/978-3-319-27250-4_36. [DOI] [Google Scholar]
- 23. Hoskins JD, Shelton GD. The nevous and neuromuscular systems. In: Hoskins JD. (ed). Veterinary pediatrics: dogs and cats from birth to six months. 3rd ed. Philadelphia: WB Saunders, 2001, pp 425–462. [Google Scholar]
- 24. Johnson KA. Diaphragmatic, pericardial, and hiatal hernia. In: Slatter D. (ed). Textbook of small animal surgery. Vol 1, 2nd ed. Philadelphia: WB Saunders, 1993, pp 455–456. [Google Scholar]
- 25. Boudrieau RJ, Rogers WA. Megaesophagus in the dog: a review of 50 cases. J Am Anim Hosp Assoc 1985; 21: 33–40. [Google Scholar]
- 26. Poffenbarger EM, Chandler ML, Ralston SL, et al. Canine neonatology. Part 1: physiologic differences between puppies and adults. Comp Contin Educ Pract Vet 1990; 12: 1601–1609. [Google Scholar]
- 27. Hoskins JD. Puppy and kitten losses. In: Hoskins JD. (ed). Veterinary pediatrics: dogs and cats from birth to six months. 3rd ed. Philadephia: WB Saunders, 2001, 57–61. [Google Scholar]
- 28. Münnich A, Lübke-Becker A. Escherichia coli infections in newborn puppies – clinical and epidemiological investigations. Theriogenology 2004; 62: 562–575. [DOI] [PubMed] [Google Scholar]
- 29. Lelli JL, Jr, drongowski RA, Coran AG, et al. Hypoxia-induced bacterial translocation in the puppy. J Pediatr Surg 1992; 27: 974–981. [DOI] [PubMed] [Google Scholar]
- 30. daniels J, Spencer E. Bacterial infections. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics. The first 12 months of life. St Louis, Mo: Saunders Elsevier, 2011, pp 113–118. [Google Scholar]
- 31. Evermann JF, Kennedy MA. Viral infections. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics. The first 12 months of life. St Louis, Mo: Saunders Elsevier, 2011, pp 119–129. [Google Scholar]
- 32. datz C. Parasitic and protozoal diseases. In: Peterson ME, Kutzler MA. (eds). Small animal pediatrics. The first 12 months of life. St Louis, Mo: Saunders Elsevier, 2011, pp 154–160. [Google Scholar]
- 33. Münnich A. The pathological newborn in small animals: the neonate is not a small adult. Vet Res Commun 2008; 32: S81–S85. [DOI] [PubMed] [Google Scholar]
- 34. Little S. Feline pediatrics: how to treat the small and the sick. Comp Contin Educ Vet 2011; 33: E3. [PubMed] [Google Scholar]
- 35. McMichael M, dhupa N. Pediatric critical care medicine: physiologic considerations. Comp Contin Educ Pract Vet 2000; 22: 206–214. [Google Scholar]
- 36. Partington BP. Diagnostic imaging techniques. In: Hoskins JD. (ed). Veterinary pediatrics: dogs and cats from birth to six months. 3rd ed. Philadelphia: WB Saunders, 2001, pp 7–21. [Google Scholar]
- 37. Apgar A. A proposal for a new method of evaluation of the newborn infant. Curr Res Anaesth Analg 1953; 32: 261–267. [PubMed] [Google Scholar]
- 38. Apgar V, Holaday DA, James LS, et al. Evaluation of the new born infant; second report. J Am Med Assoc 1958; 168: 1985–1988. [DOI] [PubMed] [Google Scholar]
- 39. Hibaru VY, Pereira KHNP, Fuchs KdM, et al. Topics in the routine assessment of newborn kitten vitality: Apgar score, reflexes and complementary assessments. J Feline Med Surg. in Press 2022. doi is: 10.1177/1098612X221081404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. davidson AP. Neonatal resuscitation: improving the outcome. Vet Clin North Am Small Anim Pract 2014; 44: 191–204. [DOI] [PubMed] [Google Scholar]
- 41. Gunn-Moore D. Techniques for neonatal resuscitation and critical care. Proceedings of the WSAVA/FECAVA World Congress 2006; Prague, pp 707–713. [Google Scholar]
- 42. Taylor S, Spada E, Callan MB, et al. 2021 ISFM consensus guidelines on the collection and administration of blood and blood products in cats. J Feline Med Surg 2021; 23: 410–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Macintire DK. Pediatric fluid therapy. Vet Clin North Am Small Anim Pract 2008; 38: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]