Abstract
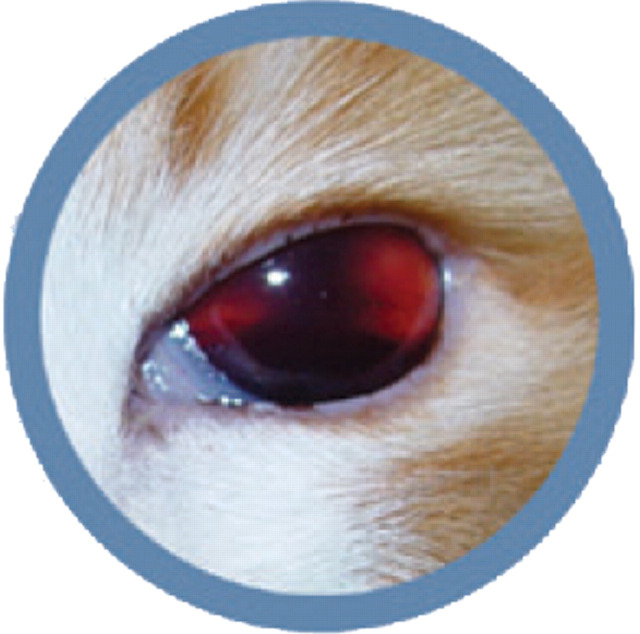
Practical relevance The increased availability of indirect blood pressure monitoring devices in clinical practice over the past decade has highlighted the significance of systemic hypertension in the feline population. Without routine monitoring and appropriate intervention, cats with undiagnosed systemic hypertension may first be presented with sudden-onset blindness as a consequence of either hyphaema or retinal detachment.
Clinical challenges The primary aim in the early diagnosis and treatment of systemic hypertension is prevention of hypertensive target organ damage (with respect to the eye, kidney, cardiovascular and central nervous systems, in particular). A prerequisite is a knowledge of the pathophysiological mechanisms and disease conditions that may contribute to the development of hypertension. This allows the clinician to determine those cases in which blood pressure assessment and longitudinal monitoring is essential and can assist in determining appropriate therapeutic strategies for control of blood pressure. Recent studies have also begun to explore the relationship that systemic hypertension may have with proteinuria and the progression of kidney disease.
Patient group The geriatric cat appears most susceptible to the development of systemic hypertension, and monitoring of systolic blood pressure is often advocated as part of a routine health screen in cats over 9–12 years old. Consideration must also be given to cats suspected of having an underlying disease such as chronic kidney disease or hyperthyroidism, or which are receiving therapeutic agents, irrespective of their age.
Evidence base Much of our understanding of the pathogenesis of feline hypertension is extrapolated from studies performed in experimental animal models or in human patients, and interspecies differences are often poorly understood.

Systemic hypertension can be defined as a persistent increase in systemic blood pressure. In human medicine, systemic hypertension can be subdivided into the presence of isolated or combined systolic and diastolic hypertension. However, in veterinary medicine, and particularly within a clinical setting, we are most often considering systolic hypertension. Integral to understanding why hypertension may develop in certain disease conditions is a knowledge of the regulatory mechanisms that, during health, maintain blood pressure within narrow limits.
Control of blood pressure
Blood pressure is determined by total peripheral resistance (TPR) and cardiac output, where cardiac output is the product of stroke volume and heart rate.
Blood pressure is under the control of the central nervous system which, in turn, receives feedback from sensory receptors located within the peripheral vasculature (eg, baroreceptors). The baroreceptors are located within the walls of the carotid sinus and aortic arch and respond to stretch within these vessels. Integration of baroreceptor discharge within the central nervous system facilitates short term blood pressure control, allowing for rapid adjustments in blood pressure to accommodate for variation in blood volume, cardiac output and TPR (see left).
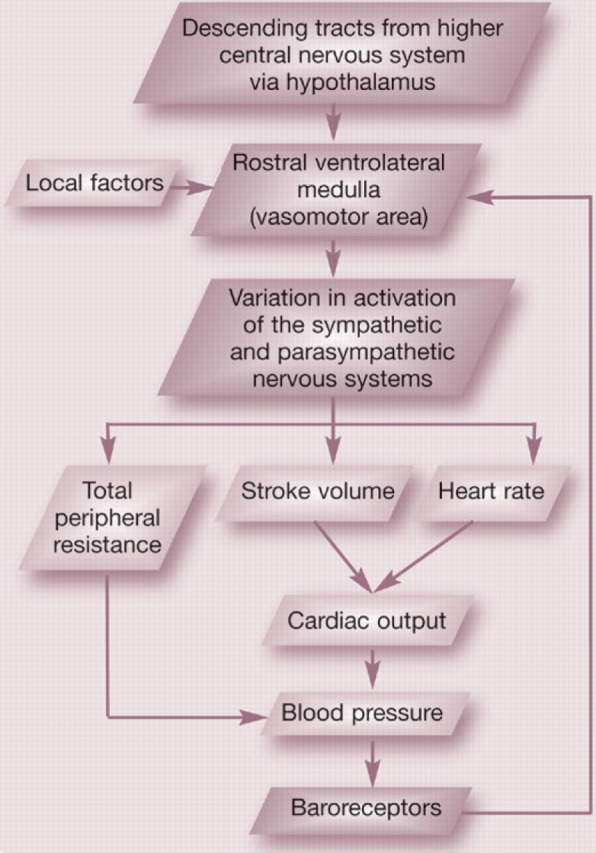
Baroreceptor feedback control of blood pressure
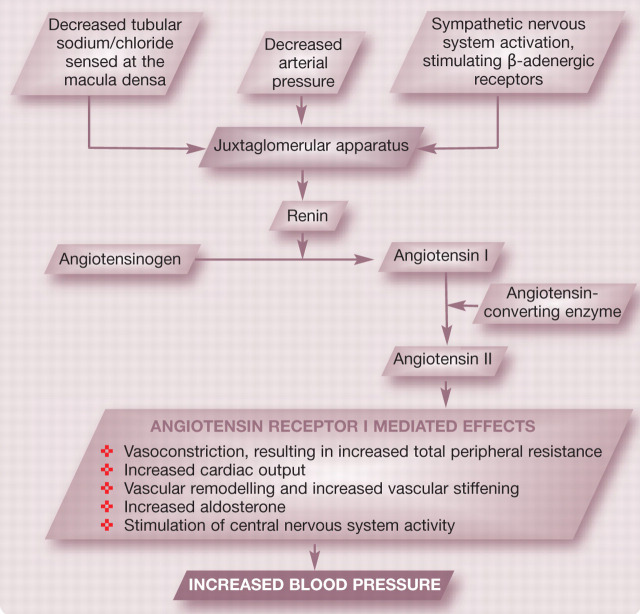
Blood pressure is also influenced by systemically circulating hormones (eg, renin—angiotensin—aldosterone system [RAAS], see right) and locally derived vasoconstrictor and vasodilatory metabolites. Arterioles are the major site of resistance to blood flow and are often termed the resistance vessels. Small changes in calibre, as a result of local, endothelial-derived, systemically circulating or neural factors, can cause large changes in TPR. Vasomodulatory factors that may influence TPR are summarised in Table 1.
TABLE 1.
Factors contributing to the modification of total peripheral resistance
| Origin | Vasodilation | Vasoconstriction |
|---|---|---|
| Circulating hormones |
|
|
| Local factors |
|
|
| Endothelialderived factors |
|
|
| Neurohormonal factors | Decreased activation of sympathetic nervous system | Increased activation of sympathetic nervous system |
DIAGNOSIS AND MANAGEMENT.
A sister article addressing the diagnosis and management of feline hypertension appears on pages 35–43 of this issue of J Feline Med Surg, and at: doi:10.1016/j.jfms.2010.11.008
In addition to neurohormonal modulation, some tissues and organs (eg, kidney) have a particularly well developed capacity to regulate their own blood pressure via a process referred to as autoregulation. Auto regulation results from a combination of the intrinsic myogenic and contractile response of smooth muscle within vessel walls to stretch (‘myogenic theory of auto regulation') and the concentration of local vaso dilator substances that accumulate in metabolically active areas (‘metabolic theory of autoregulation').
Within the kidney, autoregulation maintains renal blood flow and glomerular filtration rate (GFR) over a range of systemic pressures. However, hypertension may override this autoregulatory mechanism, leading to glomerular hypertension and the development of glomerulosclerosis. Glomerular hypertension can cause damage to the permselective nature of the glomerular filtration barrier and increased transfer of protein from the glomerular capillaries to the filtrate entering the proximal tubule. Evidence from experimental studies suggests that increased absorption of protein by the proximal tubular cells may stimulate the production of inflammatory mediators and cytokines, exacerbating interstitial nephritis and contributing to progressive kidney disease. The capacity of the kidney to autoregulate may also be adversely affected by the presence of pre-existing chronic kidney disease (CKD).
In health, these homeostatic mechanisms act in concert to maintain blood pressure within narrow limits (see right), maintaining adequate tissue perfusion and limiting potential target organ damage (TOD) from hypotension and hypoxia or hypertenson. In humans, an age-related increase in SBP is reported up to the ninth decade, while diastolic blood pressure (DBP) remains constant or declines after the fifth decade, resulting in a proportional increase in pulse pressure with age. 5 In cats, an association between age and SBP is controversial, with some studies suggesting an age-related increase may occur, and others suggesting a possible stepwise increase or no association at all. 4,6–7 Currently, no relationship has been established between gender, breed or body weight and blood pressure in cats.
The renin—angiotensin—aldosterone system and control of systemic blood pressure
Normal systolic pressure in cats.
In young healthy cats, studies using direct radiotelemetric blood pressure monitoring devices have reported normal systolic pressures of approximately 125 mmHg, although there can be considerable inter- and intra-individual variation. 1,2 Studies evaluating the healthy feline population within a clinical setting using indirect blood pressure monitoring devices report SBP ranging from 118 ± 11 mmHg (oscillometric) to 162 ± 19 mmHg (Doppler). 3,4
Currently, no relationship has been established between gender, breed or body weight and blood pressure in cats.
Defining systemic hypertension: what criteria should we use?
The definition of systolic hypertension has been the cause of some confusion within the veterinary literature. Early clinical studies used a wide range of cut-off values for the diagnosis of systolic hypertension (140–185 mmHg) and also used various techniques for the assessment of blood pressure. 3,4 These values were largely extrapolated from clinical experience and based on systolic pressures at which TOD, most commonly hypertensive retinopathy/choroidopathy, was appreciated.
In 2007, the American College of Veterinary Internal Medicine (ACVIM) consensus panel, composed of experts within the field of veterinary hypertension, produced a series of guidelines to aid in the definition, diagnosis and management of systemic hypertension in both dogs and cats. 8 Within this document a classification system for systemic hypertension was proposed based on the perceived relative risk of the development of TOD utilising both systolic and diastolic blood pressure (Table 2). This classification system is now widely accepted, although it should be appreciated that it remains to be clinically validated.
TABLE 2.
ACVIM consensus guidelines for classification of blood pressure ∗
| Risk category | Risk of TOD | SBP (mmHg) | DBP (mmHg) |
|---|---|---|---|
| I | Minimal | <150 | <95 |
| II | Mild | 150–159 | 95–99 |
| III | Moderate | 160–179 | 100–119 |
| IV | Severe | ≥180 | ≥120 |
∗Based on the risk of future target organ damage (TOD)
SBP = systolic blood pressure, DBP = diastolic blood pressure
Target organ damage
Eye
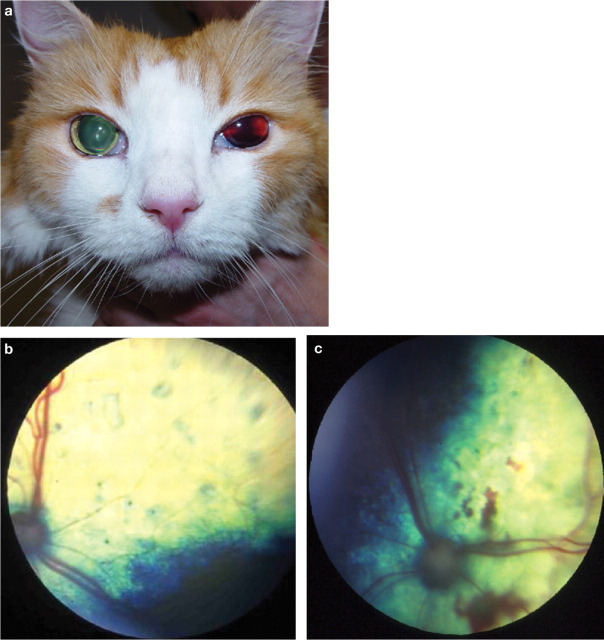
Clinical evidence suggests that hypertensive retinopathy/choroidopathy is a relatively common manifestation in cats with systemic hypertension, with studies suggesting a prevalence of 60–80% (Fig 1). 9–11 Often these ocular lesions are bilateral, although the severity may vary in each eye (Table 3). 12 Hyper tensive retinopathy/choroidopathy is most often reported with SBP measurements >170 mmHg. 9,13,14 In an experimental nephrectomy study hypertensive retinopathy/choroidopathy was reported in a cat with a direct arterial SBP of 168 mmHg. However, in such models the development of hypertension is acute and may not be directly comparable with the situation in naturally occurring disease. Recovery of vision after retinal detachment or substantial hyphaema, despite reattachment of the retina and resolution of haemorrhage with antihypertensive treatment, is rare. It may also be that fluctuations in systemic pressure, rather than absolute or incremental changes, are more damaging to the vessels of the eye, and that other factors such as age and concurrent disease may influence susceptibility to ocular TOD.
FIG 1.
(a) Unilateral hyphaema in a cat. (b) Fundic photograph showing multiple focal bullous retinal detachments in a cat. (c) Fundic photograph showing multiple areas of retinal haemorrhage in a cat. Courtesy of Dr D J Gould
TABLE 3.
Clinical manifestations of target organ damage
| Target organ | Clinical manifestations of damage |
|---|---|
| Eye | Hypertensive retinopathy/choroidopathy |
| Acute-onset blindness | |
| Focal bullous retinal detachment | |
| Retinal detachment | |
| Retinal haemorrhages | |
| Vitreal haemorrhage/hyphaema | |
| Retinal vessel tortuosity | |
| Papilloedema | |
| Secondary glaucoma | |
| Kidney | Development or progression of kidney disease |
| Development or progression of azotaemia | |
| Decline in glomerular filtration rate | |
| Microalbuminuria | |
| Proteinuria | |
| Central nervous system | Depressed/altered mentation |
| Seizures | |
| Vestibular signs/head tilt/nystagmus | |
| Lethargy | |
| Behavioural abnormalities | |
| Focal neurological deficits | |
| Cardiovascular system | Left ventricular hypertrophy |
| Gallop rhythm/arrhythmia | |
| Systolic murmur | |
| Epistaxis |
Kidney
In human medicine, an association between hypertension, proteinuria and progression of kidney disease has been established, although the role of hypertension as an initiating cause of kidney disease is less well defined. 15,16 Histopathological manifestations of renal TOD include glomerular hypertrophy, glomerulosclerosis, mesangial expansion and interstitial inflammation; clinically, in human medicine, TOD to the kidney may present as a serial increase in creatinine concentration, decline in GFR, development of microalbuminuria and progressive proteinuria.
Recovery of vision after retinal detachment or substantial hyphaema, despite reattachment of the retina and resolution of haemorrhage with antihypertensive treatment, is rare.
Clinical studies evaluating kidney tissue obtained post mortem from hypertensive cats (predominantly with concurrent renal disease) have revealed nephrosclerosis a medial thickening of renal arterioles. In experimental feline models of renal hyp tension, histopathology has reveal evidence of glomerulosclerosis, tubula atrophy and interstitial fibrosis. 18,19
An association between the magnitude of proteinuria and survival has been shown in cats both with CKD and systemic hypertension. 20,21 However, SBP may be a determinant of proteinuria and, therefore, proteinuria and SBP are unlikely to be independent contributors to the progression of kidney disease. Currently the relationship between systemic hypertension and progressive kidney disease, although suspected, remains unconfirmed cats.
Central nervous system
Hypertensive encephalopathy with hyperplastic arteriosclerosis of cerebral vessels, interstitial white matter oedema and parenchymal microhaemorrhages has been described in cats that have undergone partial nephrectomy or renal transplantation procedures. 22,23 In the clinic situation, hypertensive encephalopathy and associated neurological signs (Table 3) are most likely to occur with acute onset and/or severe hypertension. 12,17 Clients will often comment that their cat appears brighter or more inter active after starting antihypertensive medication. However, whether such subjective comments are evidence of subtle hypertensive encephalopathy is undetermined.
Cardiovascular system
In human patients a graded relationship exists between increasing blood pressure and cardiovascular morbidity and mortality. Cardiovascular manifestations are appreciated in cats with systemic hypertension (Table 3), with the prevalence of cardiovascular abnormalities reported to be significantly higher in cats with CKD and hypertension (65%) compared with normotensive cats with CKD (35%). 10 Echocardiographic studies have shown that 74–85% of hypertensive cats fulfil the criteria for left ventricular hypertrophy, with thickening of both the interventricular septum and the left ventricular free wall. 24,25 However, studies have not compared these findings with the normotensive geriatric feline population. A study by Elliott et al reported that up to 20% of cats with systemic hypertension may die from cardiovascular-related causes. 11 However, assigning cause of death is always problematic and no studies have rigorously evaluated cardiovascular morbidity or mortality longitudinally in cats with systemic hypertension.
Idiopathic hypertension
In human medicine, the terms essential and primary hypertension have been used interchangeably to refer to hypertension for which no underlying cause can be identified, accounting for between 95 and 99% of cases of systemic hypertension. Essential hypertension is considered a multifactorial problem occurring as a consequence of interaction between a large number of genetic and environmental factors. 31 The presence of persistently increased blood pressure implies that there is dysregulation of the neurohormonal and renal mechanisms that act to maintain blood pressure. 31 However, the identity of the genes that predispose to the development of essential hypertension, even in human medicine, remains largely undiscovered.
Idiopathic or secondary? Classification of systemic hypertension.
In veterinary patients, systemic hypertension is commonly classified as either idiopathic or secondary. Secondary hypertension may be attributable to an underlying disease process or associated with the administration of certain therapeutic agents (eg, erythropoietin, mineralocorticoids, glucocorticoids). Conditions that are implicated in the pathogenesis of secondary hypertension in cats include CKD, hyperthyroidism and, less commonly, primary hyperaldosteronism and, rarely, pheochromocytoma or chronic anaemia. 14,26,27 The phenomenon of white-coat hypertension is also recognised.
In human medicine there is a strong association between both obesity and diabetes mellitus and systemic hypertension, but to date these associations have not been documented in cats. 6,28–30
A diagnosis of primary or essential hypertension relies on thorough diagnostic screening to exclude all possible underlying disease conditions and to differentiate this condition from white-coat hypertension. In humans such investigations would usually include assessment or estimation of GFR. In veterinary patients such stringent criteria are rarely achieved and the term idiopathic hypertension is, therefore, preferred. 8
Idiopathic hypertension has been reported to occur in 13–20% of cats with systemic hyper-tension. 11,12,21 In one study, 20.3% (23/110) of cats diagnosed with systemic hypertension (SBP ≥175 mmHg, Doppler technique) were non-azota emic (plasma creatinine <177 μmol/l) and had no biochemical, clinical or historical findings consistent with hyperthyroidism. 32 Fifteen of these cats had aldosterone concentrations assessed in order to exclude hyperaldosteronism as an underlying aetiology, and aldosterone concentrations were considered normal in 14/15 cats. However, compared with a population of normotensive, healthy non-azotaemic geriatric cats, those with systemic hypertension had a significantly higher plasma creatinine concentration and lower urine specific gravity. 32 Suboptimal urine concentrating ability identified in this study may be the consequence of pressure diuresis secondary to hypertension; equally, without assessment of GFR and proteinuria, subclinical kidney disease cannot be excluded. Further study is warranted to determine whether such cases represent true essential hypertension or whether they are, in fact, cats with subclinical non-azotaemic kidney disease.
White-coat hypertension.
White-coat hypertension is the documentation of increased blood pressure as a consequence of stimulation of the autonomic and central nervous systems during situations of stress, anxiety or excitement. A study using radiotelemetric implant devices to monitor blood pressure in healthy cats during simulated clinic visits demonstrated that there was a significant increase in SBP during clinic visits (mean increase 17.6 mmHg ± 5.9 mmHg). The presence of tachycardia was not necessarily a reliable marker of the magnitude of the white-coat effect and there was great inter-individual variability in the increase in SBP. 2 The same study showed that cats subjected to repeat simulated visits to the veterinary clinic over a 6-week period did not show evidence of habituation, remaining susceptible to the white-coat effect on each revisit. This highlights the importance of acclimatisation, careful assessment of the cat's magnitude of stress and repeated measurements when interpreting blood pressure.
In human medicine use of 24 h ambulatory blood pressure monitoring enables representative assessment of blood pressure outside the clinic situation. 33 However, continuous blood pressure monitoring is rarely available outside of a research or critical care setting for our feline patients and great care must therefore be taken to ensure that anxiety-induced increases in blood pressure do not lead to an erroneous diagnosis of systemic hypertension. There is preliminary evidence that human patients with white-coat hypertension have more labile blood pressure and may be at increased risk of the development of hypertensive TOD. 34 Such an association has not yet been made in our veterinary patients and currently antihypertensive therapy is not recommended.
Great care must be be taken to ensure that anxiety-induced increases in blood pressure do not lead to an erroneous diagnosis of systemic hypertension.
Secondary hypertension
Chronic kidney disease
In feline patients, systemic hypertension is most commonly associated with CKD. Between 65% and 100% of cats that present with systemic hypertension and concurrent hypertensive retinopathy/choroidopathy have evidence of reduced renal function. 9,12,13,17 However, the relationship between systemic hypertension and renal function is complex, and there is little information currently available in cats to determine whether the presence of hypertension initiates renal damage or whether systemic hypertension develops as a consequence of reduced renal function. Although 20–65% of cats with CKD have evidence of systemic hypertension, many have normal blood pressure at diagnosis and never develop systemic hypertension. 3,10,13 Interestingly, in cats the severity of azotaemia does not appear to correlate with the presence of hypertension and, in most cases, azotaemia is only mild.
The pathogenesis of renal hypertension is complex and multifactorial but has traditionally been attributed to impaired excretion of sodium leading to excess intravascular volume, disruption of the pressure—natriuresis relationship and excessive activation of the RAAS. Other factors that have been associated in either experimental or human patient studies with the pathogenesis of renal hypertension include stimulation of the sympathetic nervous system, structural change to arteries, endothelial dysfunction, reduced bioavailability of the vasodilator nitric oxide, increased production of the vasoconstrictor endothelin, oxidative stress and an increase in reactive oxygen species. 35
Several studies have tried to evaluate the role of the RAAS in feline hypertensive CKD. Syme et al reported that plasma renin activity (PRA) was normal to low in hypertensive azotaemic cats. 36 However, in a study by Jensen et al no significant difference in PRA was found between hypertensive cats with CKD and a control group that consisted of non-azotaemic healthy young cats. 37
In human medicine, angiotensin-converting enzyme inhibitors (ACEi) are a first-line therapy in the management of systemic hypertension. Experimental studies in which the feline remnant kidney model has been used to induce mild renal hypertension have documented a reduction in blood pressure and improvement in glomerular hypertension with administration of ACEi. 38 However, such studies use direct arterial blood pressure monitoring, the reduction in blood pressure is small (<10 mmHg) and it is difficult to be certain that the results can be directly extrapolated to patients with naturally occurring disease. In the clinic situation, the use of ACEi as sole antihypertensive therapy rarely achieves adequate reduction in systemic blood pressure. This leads us to question whether stimulation of the RAAS is likely to be the primary determinant of the development of renal hypertension in cats. 17,37
Although 20–65% of cats with CKD have evidence of systemic hypertension, many have normal blood pressure at diagnosis and never develop systemic hypertension.
Recently there has been interest in the role of aldosterone in the pathogenesis of renal hypertension and the progression of renal disease. Experimental rat models have suggested that selective inhibition of aldosterone offers benefits beyond renin—angiotensin blockade. 39 A study by Syme et al reported an inverse association between systemic hypertension and plasma potassium concentration. 10 One potential explanation for this could be stimulation of the RAAS and increased aldosterone concentrations. Jensen et al reported a significant increase in plasma aldosterone concentrations in hypertensive azotaemic cats compared with normal healthy control cats. 37 However, other studies have failed to document a significant difference in plasma aldosterone concentration between normotensive and hypertensive cats with CKD. 40 Early work suggested that hypertensive cats may have micronodular hyperplasia of their adrenal glands, providing further evidence for a potential role of hyperaldosteronism in the generation of systemic hypertension. 41 However, more recent data evaluating adrenal glands from 67 cats did not reveal a significant difference in the degree of hyperplasia between those that were from normotensive (n = 30) cats versus those from hypertensive cats (n = 37). 42
To date, the studies performed in cats have focused on systemic activation of the RAAS and the role of local RAAS activation (eg, within the kidney) remains to be explored. The precise role of the RAAS in the pathogenesis of feline renal hypertension remains uncertain.
Hyperthyroidism
In humans hyperthyroidism is reported to result in a relatively small decrease in mean arterial blood pressure (MABP). This decrease is attributable to a decrease in DBP as a consequence of peripheral vasodilation and a 40–60% reduction in TPR. Vasodilation and a decrease in TPR in hyperthyroidism is the result of accumulation of local vasodilator metabolites and also a direct effect of triiodothyronine (T3) on vascular smooth muscle. 43,44 The decrease in TPR causes a reflex increase in heart rate, stroke volume and cardiac output. A decrease in TPR, reduced effective vascular filling and reduced renal perfusion pressure in turn stimulate the RAAS, augmenting sodium reabsorption and hence increasing plasma volume. 45 T3 may also stimulate erythropoietin and red blood cell production, leading to an increase in total blood volume. 46,47
An excess of circulating thyroid hormone causes positive inotropy and chronotropy. Despite the apparent hyperadrenergic state in hyperthyroidism, studies in human patients suggest that circulating catecholamine concentrations are either normal or decreased in hyperthyroidism. The relative increase in sympathetic tone is, therefore, likely to be the result of heightened sensitivity to catecholamines. 48 Systolic myocardial contractility, and thus cardiac output, can be influenced by both genomic and non-genomic cellular effects of T3 on cardiac myocytes. Once transported to the nucleus of cardiac myocytes, T3 causes either transcriptional activation or repression of specific target genes. These genes may code for structural (eg, α-myosin and β-myosin heavy chains of the cardiac myocytes) or functional cellular components (eg, calcium-activated ATP-ase or phospholamban), which when altered result in increased myocardial contractility. 48 Non-genomic effects of T3 include modulation of intracellular sodium, potassium and calcium concentrations, which may also alter myocardiac contractility. 47,48 However, these effects are likely to be minor compared with that of T3 on systemic vascular resistance.
Early feline studies suggested that systolic hypertension was a common finding in hyperthyroidism. 3,13 However, the case numbers in these studies were small and the criteria used for the diagnosis of hypertension, variable. Studies evaluating cats with evidence of hypertensive retinopathy/choroidopathy report relatively few cats with hyperthyroidism as an underlying aetiology; similarly, large retrospective studies of cats with hyperthyroidism have not reported a high prevalence of hypertensive retinopathy/choroidopathy. 12,17,21,49,50
The prevalence of systemic hypertension in newly diagnosed hyperthyroid cats has been reported to range from 9–23%, suggesting that systemic hypertension may be less prevalent in the hyperthyroid feline population than first anticipated.
Hypertension after treatment of hyperthyroidism does not always develop as a consequence of unmasking of underlying renal insufficiency, highlighting the importance of longitudinal assessment of blood pressure in cats treated for hyperthyroidism.
The prevalence of systemic hypertension in newly diagnosed hyperthyroid cats has been reported to range from 9–23%, suggesting that systemic hypertension may be less prevalent in the hyperthyroid feline population than first anticipated. 13,51,52 Interestingly, approximately 20% of cats may develop systemic hypertension after treatment. 51,52 The study by Morrow et al reported that the median time from initiating antithyroid medication to the subsequent development of hypertension was 5.3 months; and, of those cats that developed hypertension, only 35% were concurrently found to be azotaemic (plasma creatinine >177 μmol/l [>2mg/dl]). 52 This suggests that hypertension after treatment of hyperthyroidism does not always develop as a consequence of unmasking of underlying renal insufficiency and highlights the importance of longitudinal assessment of blood pressure in cats diagnosed with and treated for hyperthyroidism.
Primary hyperaldosteronism
Primary hyperaldosteronism, or Conn's syndrome, is defined as an excess of aldosterone independent of its chronic regulator, angiotensin II. 53 In human patients, primary hyperaldosteronism is most frequently associated with bilateral adrenal hyperplasia and aldosterone-producing adenomas, but has also been associated with unilateral adrenal hyperplasia, aldosterone-producing adrenocortical carcinomas and as a familial condition. 54
Aldosterone is a mineralocorticoid secreted by the zona glomerulosa of the adrenal cortex. Like many other steroid hormones, aldosterone binds to a cytoplasmic receptor and this receptor—hormone complex enters the nucleus of the cell where it alters gene transcription. 55 In the non-diseased state aldosterone secretion is under the control of the RAAS (see page 26) and a direct stimulatory effect of increasing potassium concentration. 53,55 Adrenocorticotrophic hormone (ACTH) may cause acute increases in aldosterone release, but this is a short-lived response. 55 Within the kidney, aldosterone acts on the principal cells of the collecting ducts, resulting in retention of sodium, potassium diuresis, fluid retention and an increase in extracellular volume. 55
Systemic hypertension in primary hyperaldosteronism may initially be the consequence of volume expansion leading to increased cardiac output. However, with sustained systemic hypertension, pressure diuresis develops leading to natriuresis. Ultimately, plasma volume returns to normal and, therefore, does not explain persistent hypertension in patients with primary aldosteronism. 53
Recently it has been recognised that aldosterone has important roles in modulating vascular tone and within the central nervous system. Mineralocorticoid receptors have been identified in non-epithelial tissues such as fibroblasts in the heart, endothelial cells, vascular smooth muscle cells and in the brain. 53,55 Experimental studies in rats indicate that increased aldosterone concentrations are associated with increased sympathoexcitatory responses and increased arterial pressures. 56 Circulating aldosterone can exert both genomic and non-genomic effects on different layers of blood vessel walls. Within the peripheral vasculature, aldosterone is considered to be pro-inflammatory, profibrotic and to play an integral role in vascular remodelling and vasoconstriction, ultimately resulting in endothelial dysfunction. Thus persistent arterial hypertension in primary hyperaldosteronism is considered to be the consequence of the synergism of renal sodium and fluid retention, increased sympathetic drive, endothelial dysfunction and increased TPR. 53,55
Primary hyperaldosteronism remains a relatively uncommon condition in feline medicine, although the prevalence of hypertension in cats with this condition is reported to be high. 57–64 A case series by Ash et al evaluated 13 cats with primary hyperaldosteronism. Twelve of the cats had assessment of SBP performed using the Doppler technique and 91.6% (11/12) were reported to have evidence of systemic hypertension (using the criterion SBP ≥170mmHg), with 38.5% (5/13) showing evidence of hypertensive retinopathy/choroidopathy. 58 Similarly, in a study by Javadi et al, 10/11 cats with primary hyperaldosteronism had assessment of SBP performed using the Doppler technique and, in all cases, SBP was >190 mmHg, with 58% (7/12) showing signs of hypertensive retinopathy/choroidopathy. 41
Histopathological examination of adrenal tissue from cases of feline primary hyperaldosteronism has revealed unilateral and bilateral adrenal adenomas, micronodular hyperplasia and unilateral adrenal carcinomas. 41,58,59,63
Pheochromocytoma
Pheochromocytoma is a neuroendocrine tumour originating from the chromaffin cells of the adrenal medulla. Secretion of excessive concentrations of catecholamines, usually epinephrine or norepinephrine, can result in sustained or paroxysmal hypertension. 27,65 Occasionally these tumours can arise from outside the adrenal gland, being referred to as extra-adrenal pheochromocytoma or paraganglioma. Pheochromocytomas are a rare cause of secondary hypertension in cats, with very few reports within the literature. 26,66–69
Case notes.
Nelson, a 10-year-old, male neutered domestic shorthair cat, presented with a history and clinical signs that were suspicious of hyperthyroidism. Initial diagnostics confirmed the diagnosis. After starting medical management with methimazole, Nelson's owners were keen to pursue radioactive iodine therapy as a long-term treatment strategy.
History Nelson was diagnosed with hyperthyroidism when his owners noticed that he was losing weight more rapidly than they expected after they started a weight reduction diet. It had also been noted that his appetite had increased in the past few weeks. His thirst and urination were considered normal but he had suffered from occasional bouts of vomiting over the same time period. His total thyroxine (tT4) concentration at the time of diagnosis of hyperthyroidism was 96 nmol/l (reference interval 19–65 nmol/l). Nelson had been started on methimazole (Felimazole; Dechra Veterinary Products, 2.5 mg q12h) and had responded well to this medication, with resolution of his ravenous appetite and appropriate weight gain. A repeat tT4 performed after 3 weeks showed that he had become euthyroid with treatment (tT4 = 32 nmol/l); a serum biochemical profile performed at the same time was unremarkable with no evidence of azotaemia. Nelson's methimazole therapy was stopped 14 days prior to referral for radioactive iodine therapy.
Physical examination and laboratory findings On presentation for radioactive iodine therapy, physical examination revealed that Nelson weighed 4.6 kg with a body condition score of 4/9. A unilateral left-sided goitre was palpable. On cardiac auscultation a grade I/VI parasternal systolic murmur was audible with a heart rate of 220 bpm. Lung auscultation, abdominal palpation and his remaining physical examination were unremarkable. Mean SBP assessed using the Doppler technique was 158 mmHg and an indirect fundic examination was unremarkable. A serum biochemical profile performed at that time revealed only a mild increase in alanine transferase activity (207 U/l; reference interval 25–130 U/l), and a tT4 of 85.1 nmol/l confirmed the presence of hyperthyroidism. Full urinalysis (semiquantitative dipstick analysis, sediment examination and urine specific gravity [USG]) performed on a cystocentesis sample revealed 1+ protein with a USG of 1.045, but was otherwise unremarkable.
Continued case management Nelson was acclimatised to the hospital environment and subsequently received 111 MBq of radioactive iodine (I131). He remained within the radioactive isolation unit for 2 weeks before discharge for continued isolation at home for a further fortnight. On the day of discharge his weight had increased by 200 g and a repeat tT4 was 10.2 nmol/l. A serum biochemical profile was unremarkable, his USG 1.035 and his urinalysis otherwise within normal limits. However, repeat assessment of his blood pressure revealed a mean SBP of 206 mmHg. Fundic examination at this point remained unremarkable.
Nelson was re-examined 2 weeks later. Clinically, he had continued to gain weight and his owners were very pleased with his progress. However, his mean SBP was again increased, at 196 mmHg. Fundic examination remained unremarkable.
WHAT IS YOUR ASSESSMENT?
-
1
According to the ACVIM hypertension guidelines, in which risk category would you place Nelson?
-
(a)
I — minimal
-
(b)
II — mild
-
(c)
III — moderate
-
(d)
IV — severe
-
(a)
-
2
How would you classify Nelson?
-
(a)
Essential hypertension, as there is no underlying cause.
-
(b)
Idiopathic hypertension, but it could be secondary to subclinical non-azotaemic kidney disease.
-
(c)
Secondary renal hypertension after unmasking reduced renal function with treatment of his hyperthyroidism and a reduction in GFR.
-
(d)
Hypertension occurring after treatment of hyperthyroidism, without evidence of reduced renal function.
-
(e)
White-coat hypertension.
-
(a)
KEY POINTS.
Systemic hypertension is a common problem in the geriatric feline population and is most often associated with impaired kidney function.
Systemic hypertension is less prevalent in the hyperthyroid feline population than once anticipated. Approximately 20% of cats treated for their hyperthyroidism may subsequently develop hypertension, but not all will have an associated azotaemia.
It remains undetermined whether idiopathic hypertension is a genuine phenomenon in cats or whether such cats have subclinical non-azotaemic kidney disease.
The ACVIM consensus guidelines have been widely adopted and classify systemic hypertension according to the relative risk of the future development or progression of target organ damage.
A better understanding of the pathogenesis of hypertension in cats may lead to more specific therapeutic interventions for the treatment of systemic hypertension and the prevention of target organ damage.
References
- 1. Brown SA, Langford K, Tarver S. Effects of certain vasoactive agents on the long-term pattern of blood pressure, heart rate, and motor activity in cats. Am J Vet Res 1997; 58: 647–52. [PubMed] [Google Scholar]
- 2. Belew AM, Barlett T, Brown SA. Evaluation of white-coat effects in cats. J Vet Intern Med 1999; 13: 134–42. [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi DL, Peterson ME, Graves TK, Lesser M, Nicols CE. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990; 4: 58–62. [DOI] [PubMed] [Google Scholar]
- 4. Sparkes AH, Caney SMA, King MCA, Gruffydd-Jones TJ. Inter- and intraindividual variation in doppler ultrasonic indirect blood pressure measurements in healthy cats. J Vet Intern Med 1999; 13: 314–18. [DOI] [PubMed] [Google Scholar]
- 5. Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. Circulation 1997; 96: 308–15. [DOI] [PubMed] [Google Scholar]
- 6. Bodey R, Sansom J. Epidemiological study of blood pressure in domestic cats. J Small Anim Pract 1998; 39: 567–73. [DOI] [PubMed] [Google Scholar]
- 7. Sansom J, Rogers KS, Wood JLN. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res 2004; 65: 245–52. [DOI] [PubMed] [Google Scholar]
- 8. Brown SA, Atkins CE, Bagley R, et al. Guidelines for the identification, evaluation and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–58. [DOI] [PubMed] [Google Scholar]
- 9. Sansom J, Barnett KC, Dunn KA, Smith KC, Dennis R. Ocular disease associated with hypertension in 16 cats. J Small Anim Pract 1994; 35: 604–11. [Google Scholar]
- 10. Syme HM, Barber PJ, Markwell PJ, Elliott J. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002; 220: 1799–804. [DOI] [PubMed] [Google Scholar]
- 11. Elliott J, Barber PJ, Syme HM, Rawlings JM, Markwell PJ. Feline hypertension: clinical findings and response to anti-hypertensive treatment in 30 cases. J Small Anim Pract 2001; 42: 122–29. [DOI] [PubMed] [Google Scholar]
- 12. Maggio F, Defrancesco TC, Atkins CE, Pizzirani S, Gilger BC, Davidson MG. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000; 217: 695–702. [DOI] [PubMed] [Google Scholar]
- 13. Stiles J, Polzin DJ, Bistner SI. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J Am Vet Med Assoc 1994; 30: 564–72. [Google Scholar]
- 14. Morgan RV. Systemic hypertension in four cats: ocular and medical findings. J Am Anim Hosp Assoc 1985; 22: 615–21. [Google Scholar]
- 15. Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end stage renal disease in men. New Engl J Med 1996; 334: 13–18. [DOI] [PubMed] [Google Scholar]
- 16. Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria and angiotensin-converting enzyme inhibition. Ann Intern Med 2003; 139: 244–52. [DOI] [PubMed] [Google Scholar]
- 17. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994; 8: 79–86. [DOI] [PubMed] [Google Scholar]
- 18. Mathur S, Brown CA, Dietrich UM, et al. Evaluation of a technique of inducing hypertensive renal insufficiency in cats. Am J Vet Res 2004; 65: 1006–13. [DOI] [PubMed] [Google Scholar]
- 19. Mathur S, Syme HM, Brown CA, et al. Effects of the calcium channel antagonist amlodipine in cats with surgically induced hypertensive renal insufficiency. Am J Vet Res 2002; 63: 833–39. [DOI] [PubMed] [Google Scholar]
- 20. Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–35. [DOI] [PubMed] [Google Scholar]
- 21. Jepson RE, Brodbelt D, Elliott J, Syme HM. Evaluation of the effects of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med 2007; 21: 402–9. [DOI] [PubMed] [Google Scholar]
- 22. Brown CA, Munday JS, Mathur S, Brown SA. Hypertensive encephalopathy in cats with reduced renal function. Vet Pathol 2005; 42: 642–49. [DOI] [PubMed] [Google Scholar]
- 23. Kyles AE, Gregory CR, Wooldridge JD, et al. Management of hypertension controls postoperative neurologic disorders after renal transplantation in cats. Vet Surg 1999; 28: 436–41. [DOI] [PubMed] [Google Scholar]
- 24. Snyder PS, Sadek D, Jones GL. Effect of amlodipine on echocardiographic variables in cats with systemic hypertension. J Vet Intern Med 2001; 15: 52–56. [DOI] [PubMed] [Google Scholar]
- 25. Chetboul V, Lefebvre HP, Blerc B, Boussouf M, Pouchelon J-L. Spontaneous feline hypertension: clinical and echocardio-graphic abnormalities and survival rate. J Vet Intern Med 2003; 17: 89–95. [DOI] [PubMed] [Google Scholar]
- 26. Henry CJ, Brewer WG, Montgomery RD. Adrenal pheochromocytoma in a cat. J Vet Intern Med 1993; 7: 199–201. [DOI] [PubMed] [Google Scholar]
- 27. Maher ERJ, McNiel EA. Pheochromocytoma in dogs and cats. Vet Clin North Am Small Anim Pract 1997; 27: 359–80. [DOI] [PubMed] [Google Scholar]
- 28. Moore WV, Fredrickson D, Brenner A, et al. Prevalence of hypertension in patients with type II diabetes in referral versus primary care clinics. J Diabetes Complications 1998; 12: 302–6. [DOI] [PubMed] [Google Scholar]
- 29. Aneja A, El-atat F, McFarlane SI, Sowers JR. Hypertension and obesity. Recent Prog Horm Res 2004; 59: 169–205. [DOI] [PubMed] [Google Scholar]
- 30. Sennello KA, Schulman RL, Prosek R, Siegel AM. Systolic blood pressure in cats with diabetes mellitus. J Am Vet Med Assoc 2003; 223: 198–201. [DOI] [PubMed] [Google Scholar]
- 31. Freel EM, Connell JMC. Mechanisms of hypertension: the expanding role of aldosterone. J Am Soc Nephrol 2004; 15: 1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elliott J, Fletcher M, Syme HM. Idiopathic feline hypertension: epidemiological study [abstract]. J Vet Intern Med 2003; 17: 754. [Google Scholar]
- 33. Imai Y. Prognostic significance of ambulatory blood pressure. Blood Press Monit 1999; 4: 249–56. [PubMed] [Google Scholar]
- 34. Kotsis V, Stabouli S, Tourmanidis S, et al. Target organ damage in ‘white coat hypertension’ and ‘masked hypertension’. Am J Hypertens 2008; 21: 393–99. [DOI] [PubMed] [Google Scholar]
- 35. Campese V, Mitra N, Sandee D. Hypertension in renal parenchymal disease: why is it so resistant to treatment? Kidney Int 2006; 69: 967–73. [DOI] [PubMed] [Google Scholar]
- 36. Syme HM, Markwell PJ, Elliott J. Aldosterone and plasma renin activity in cats with hypertension and/or chronic renal failure. J Vet Intern Med 2002; 16: 354. [Google Scholar]
- 37. Jensen J, Henik RA, Brownfield M, Armstrong J. Plasma renin activity and angiotensin I and aldosterone concentrations in cats with hypertension associated with chronic renal disease. Am J Vet Res 1997; 58: 535–40. [PubMed] [Google Scholar]
- 38. Brown SA, Brown CA, Jacobs G, Stiles J, Hendi RS, Wilson S. Effects of the angiotensin converting enzyme inhibitor benazepril in cats with induced renal insufficiency. Am J Vet Res 2001; 62: 375–83. [DOI] [PubMed] [Google Scholar]
- 39. Epstein M. Aldosterone as a mediator of progressive renal dysfunction: evolving perspectives. Intern Med 2001; 40: 573–83. [DOI] [PubMed] [Google Scholar]
- 40. Mishina M, Watanabe G, Fujii K, Maeda H, Wakao Y, Takahashi M. Non-invasive blood pressure measurements in cats: clinical significance of hypertension associated with chronic renal failure. J Vet Med Sci 1998; 60: 805–8. [DOI] [PubMed] [Google Scholar]
- 41. Javadi S, Djajadiningrat-Laanen SC, Kooistra HS, et al. Primary hyperaldosteronism, a mediator of progressive renal disease in cats. Domest Anim Endocrinol 2005; 28: 85–104. [DOI] [PubMed] [Google Scholar]
- 42. Keele SJ, Smith KC, Elliott J, Syme HM. Adrenocortical morphology in cats with chronic kidney disease (CKD) and systemic hypertension. J Vet Intern Med 2009; 23: 1328. [Google Scholar]
- 43. Park KW, Dai HB, Ojamaa K. The direct vasomotor effect of thyroid hormones on rat skeletal muscle resistance arteries. Anesth Analg 1997; 85: 734–38. [DOI] [PubMed] [Google Scholar]
- 44. Ojamaa K, Klempere JD, Klein I. Acute effects of thyroid hormone on vascular smooth muscle. Thyroid 1996; 6: 505–12. [DOI] [PubMed] [Google Scholar]
- 45. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001; 344: 501–9. [DOI] [PubMed] [Google Scholar]
- 46. Ma Y, Freitag P, Zhou J, Brune B, Frede S, Fandrey J. Thyroid hormone induces erythropoietin gene expression through augmented accumulation of hypoxia-inducible factor-1. Am J Physiol Regul Integr Comp Physiol 2004; 287: R600–R7. [DOI] [PubMed] [Google Scholar]
- 47. Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res 2004; 59: 31–50. [DOI] [PubMed] [Google Scholar]
- 48. Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation 1993; 87: 1435–41. [DOI] [PubMed] [Google Scholar]
- 49. van der Woerdt A, Peterson ME. Prevalence of ocular abnormalities in cats with hyperthyroidism. J Vet Intern Med 2000; 14: 202–3. [DOI] [PubMed] [Google Scholar]
- 50. Peterson ME, Kintzer PP, Hurvitz AI. Methimazole treatment of 262 cats with hyperthyroidism. J Vet Intern Med 1988; 2: 150–57. [DOI] [PubMed] [Google Scholar]
- 51. Syme HM, Elliott J. The prevalence of hypertension in hyper-thyroid cats at diagnosis and following treatment [abstract]. J Vet Intern Med 2003; 17: 754. [Google Scholar]
- 52. Morrow LD, Adams VJ, Elliott J, Syme HM. Hypertension in hyperthyroid cats: prevalence, incidence and predictors of its development [abstract]. J Vet Intern Med 2009; 23: 699. [Google Scholar]
- 53. Tomaschitz A, Pilz S, Ritz E, Obermayer-Pietsch B, Pieber TR. Aldosterone and arterial hypertension. Nature Rev Endocrinol 2010; 6: 83–93. [DOI] [PubMed] [Google Scholar]
- 54. Raynor B. Primary aldosteronism and aldosterone associated hypertension. J Clin Pathol 2008; 61: 825–31. [DOI] [PubMed] [Google Scholar]
- 55. Connell JMC, Davies E. The new biology of aldosterone. J Endocrinol 2005; 186: 1–20. [DOI] [PubMed] [Google Scholar]
- 56. Huang BS, Wang H, Leenen FH. Chronic central infusion of aldosterone leads to sympathetic hyperactivity and hypertension in Dahl S but not Dahl R rats. Am J Physiol Heart Circ Physiol 2005; 288: H517–H24. [DOI] [PubMed] [Google Scholar]
- 57. Schulman RL. Feline primary hyperaldosteronism. Vet Clin North Am Small Anim Pract 2010; 40: 353–59. [DOI] [PubMed] [Google Scholar]
- 58. Ash AR, Harvey AM, Tasker S. Primary hyperaldosteronism in the cat: a series of 13 cases. J Feline Med Surg 2005; 7: 173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flood SM, Randolph JF, Gelzer JF. Primary hyperaldosteronism in two cats. J Am Anim Hosp Assoc 1999; 35: 411–16. [DOI] [PubMed] [Google Scholar]
- 60. Eger CE, Robinson WF, Huxtable CRR. Primary aldosteronism (Conn's syndrome) in a cat; a case report and review of comparative aspects. J Small Anim Pract 1983; 24: 293–307. [Google Scholar]
- 61. Moore LE, Biller DS, Smith TA. Use of abdominal ultrasonography in the diagnosis of primary hyperaldosteronism in a cat. J Am Vet Med Assoc 2000; 217: 213–15. [DOI] [PubMed] [Google Scholar]
- 62. MacKay AD, Holt PE, Sparkes AH. Successful surgical treatment of a cat with primary aldosteronism. J Feline Med Surg 1999; 1: 117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rijnberk A, Voorhout G, Kooistra HS, et al. Hyperaldosteronism in a cat with metastasised adrenocortical tumour. Vet Q 2001; 23: 38–43. [DOI] [PubMed] [Google Scholar]
- 64. Briscoe K, Barrs VR, Foster DF, Beatty JA. Hyperaldosteronism and hyperprogesteronism in a cat. J Feline Med Surg 2009; 11: 758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Manger WM. An overview of pheochromocytoma: history, current concepts, vagaries and diagnostic challenges. Ann N Y Acad Sci 2006; 1073: 1–20. [DOI] [PubMed] [Google Scholar]
- 66. Wimpole JA, Adagra CFM, Billson MF, Pillai DN, Foster DF. Plasma free metanephrines in healthy cats, cats with non-adrenal disease and a cat with suspected phaeochromocytoma. J Feline Med Surg 2010; 12: 435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Calsyn JDR, Green RA, Davis GJ, Reilly CM. Adrenal pheochromocytoma with contralateral adrenocortical adenoma in a cat. J Am Anim Hosp Assoc 2010; 46: 36–42. [DOI] [PubMed] [Google Scholar]
- 68. Patnaik AK, Erlandson RA, Lieberman PH. Extra-adrenal pheochromocytoma (paraganglioma) in a cat. J Am Vet Med Assoc 1990; 197: 104–6. [PubMed] [Google Scholar]
- 69. Chun R, Jakovljevic S, Morrison WB. Apocrine gland adenocarcinoma and pheochromocytoma in a cat. J Am Anim Hosp Assoc 1997; 33: 33–36. [DOI] [PubMed] [Google Scholar]