Abstract

Overview Feline ulcerative keratitis is a common presenting complaint and is frequently a sequela of feline herpesvirus 1 (FHV-1) infection; so much so, in fact, that it is fair to assume an FHV-1 aetiology until proven otherwise. Other potential causes of ulceration are trauma or underlying eyelid abnormalities (entropion, ectropion, agenesis, dermoids, neoplasia), lash abnormalities (ectopic cilia, trichiasis), tear film abnormalities or neurological deficiencies (trigeminal nerve paralysis, facial nerve paralysis).
Clinical challenges The management of corneal ulceration in cats is frequently challenging, and treatment needs to be tailored carefully to the individual cat, its temperament, and the disease process present.
Evidence base The scientific literature on feline ulcerative keratitis is extensive, particularly that related to FHV-1 infection. The aim of this article is to review the aetiology and diagnosis of corneal ulceration in cats with particular reference to the evidence base available.
Patient group All age groups and breeds can suffer with ulcerative keratitis. Breed predispositions are present for some forms of corneal ulceration, and these are discussed.
Corneal Anatomy and Physiology
The feline cornea is a transparent avascular window, 592 ± 80 μm (ie, approximately 0.5–0.7 mm) thick, forming the anterior-most barrier of the eye. It refracts and transmits light, and relies on limbal blood vessels, the preocular tear film and aqueous humour for nutrition.
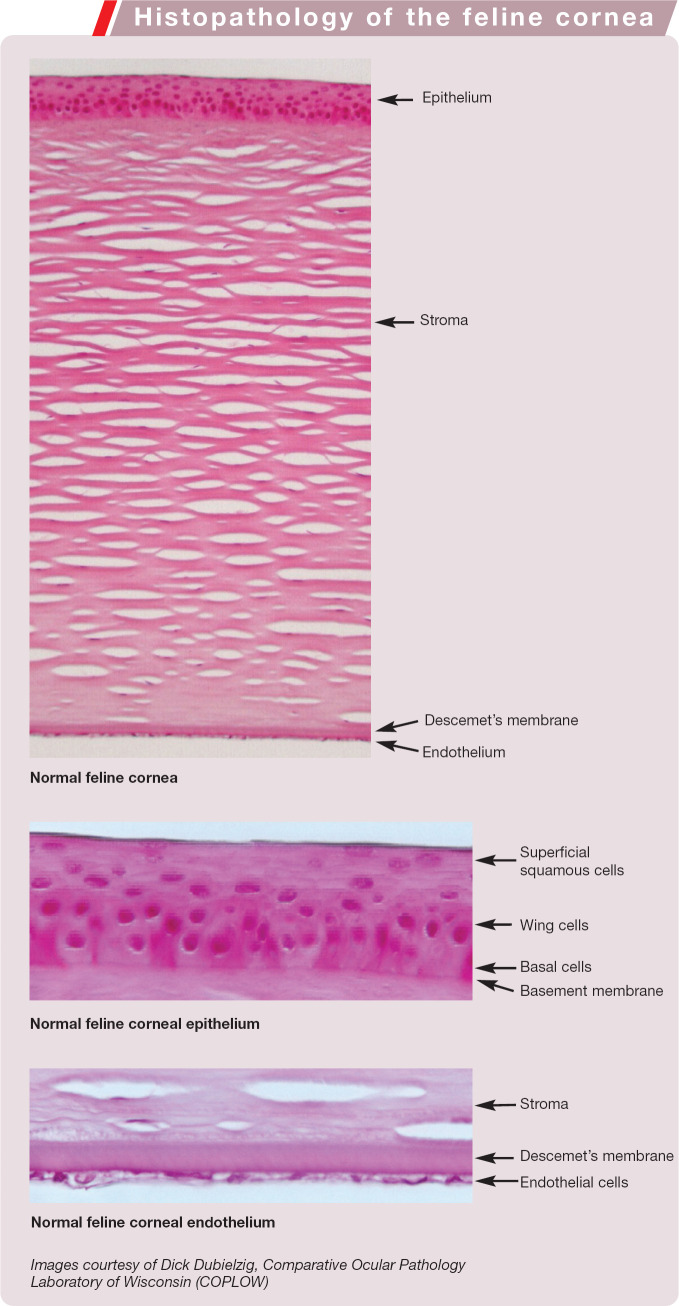
The corneal epithelium is six to 10 cell layers thick and is a non-keratinised stratified squamous epithelium (see box on page 25). The basal epithelial cells are firmly attached to a basement membrane and the underlying stroma by hemidesmosomes, anchoring fibrils and anchoring plaques. These attachment complexes bridge the basement membrane to the anterior stroma and are sufficiently strong that mechanical debridement usually results in basal epithelial cell rupture rather than separation from the basement membrane. Above this basal cell layer lie two to three layers of wing cells, above which are the superficial squamous layers.
When superficial differentiated cells are sloughed into the tear film they are replaced from the underlying proliferating basal cell layer. The basal cell layer is in turn replenished from limbal-based stem cells. These pluripotential cells occupy specialised areas known as crypts or palisades of Vogt. The entire corneal epithelium is replenished within an estimated 1–2 weeks.
The corneal stroma, which is responsible for 90% of corneal thickness, is composed of parallel bundles of collagen fibrils arranged into lamellae. This parallel arrangement of the lamellae, and uniform spacing of collagen fibrils within them, reduces light scatter and promotes corneal transparency. Keratocytes, proteoglycans and glycosaminoglycans intersperse these lamellae and, with the collagen fibrils, constitute 15–25% of the corneal stroma, the remainder being water. The corneal stroma is relatively dehydrated compared with other tissues. It is maintained in this state by the epithelial barriers of the anterior epithelium and posterior endothelium, as well as by the active transport of water from the cornea into the aqueous by the corneal endothelium. Entrance of water into the hydrophilic corneal stroma results in corneal oedema and loss of clarity.
The entire corneal epithelium is replenished within an estimated 1–2 weeks. By contrast the corneal endothelium has only very limited regenerative abilities.
Endothelial cells do not actively divide in the cat. Therefore, cell losses due to corneal perforation or surgical trauma are replaced by thinning and spreading of existing cells.
Histopathology of the feline cornea
Descemet's membrane forms the posterior boundary of the stroma and is an acellular exaggerated basement membrane of the corneal endothelium. The corneal endothelium lies posterior to Descemet's membrane (in direct contact with aqueous humour) in a single cell layer, and has only very limited regenerative abilities. Due to the active transport function of endothelial cells, they have a high metabolic requirement. The feline cornea has an endothelial cell density of 2846 ± 403 cells/mm2, as measured by in vivo confocal microscopy. 1 (In comparison, adult humans have 2720 ± 367 cells/mm2 and dogs have 3175 ± 776 cells/mm2.)1,2
Corneal Healing
Epithelial healing occurs in three phases: an initial lag phase is followed by a migratory phase and healing concludes with a proliferation phase. During the lag phase, cells neighbouring the wound alter their attachments to nearby cells and the underlying basement membrane. The epithelium bordering the defect becomes thin and an epithelial sheet begins to migrate towards the centre of the wound. Disassembly of attachment complexes, with formation of new temporary attachments, occurs with this migration. Once the epithelial sheet covers the denuded region the epithelial cells proliferate to re-establish the normal thickness and differen tiation of the anterior epithelium.
Stromal defects (Fig 1) stimulate keratocytes to undergo either apoptosis or transformation into repair phenotypes. Loss of the epithelial basement membrane is a critical factor in determining the fibrotic response of keratocytes and subsequent scarring.
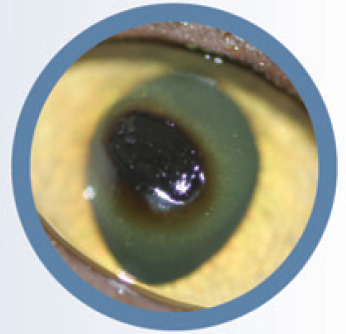
Fig 1.
Stromal ulcer
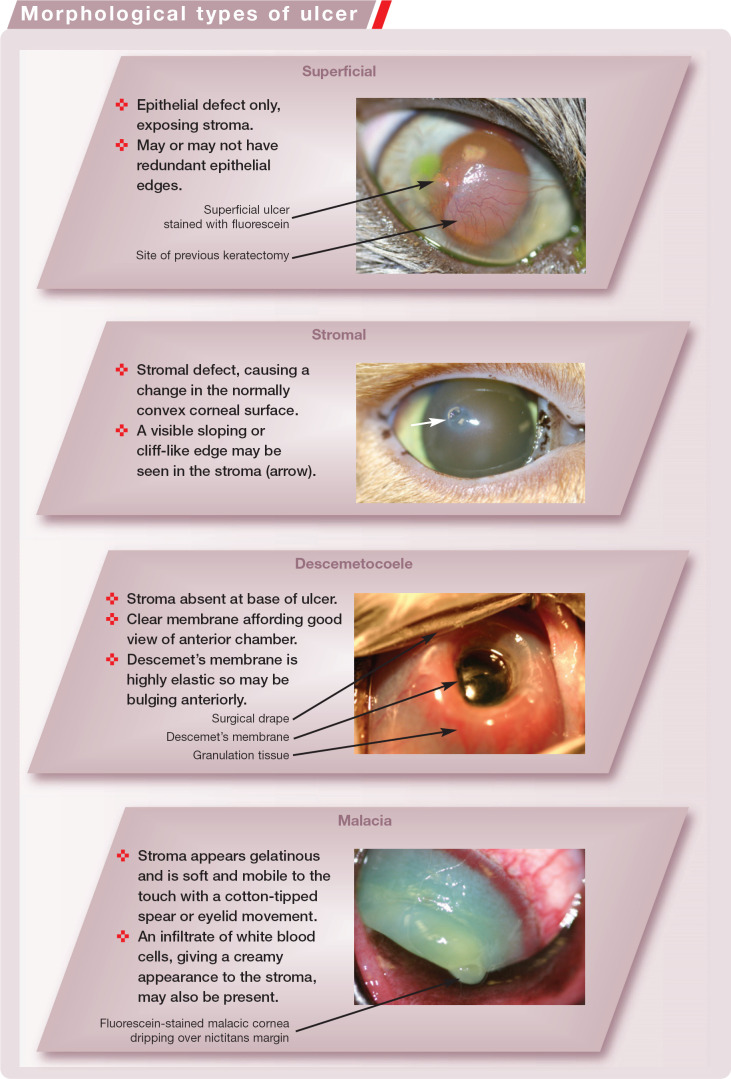
Morphological Types of Ulcer
Endothelial cells do not actively divide in the cat. Therefore, cell losses due to corneal perforation or surgical trauma are replaced by thinning and spreading of existing cells. If cell loss is such that the remaining cells cannot maintain a functional monolayer, corneal decompensation occurs producing diffuse corneal oedema (Fig 2).
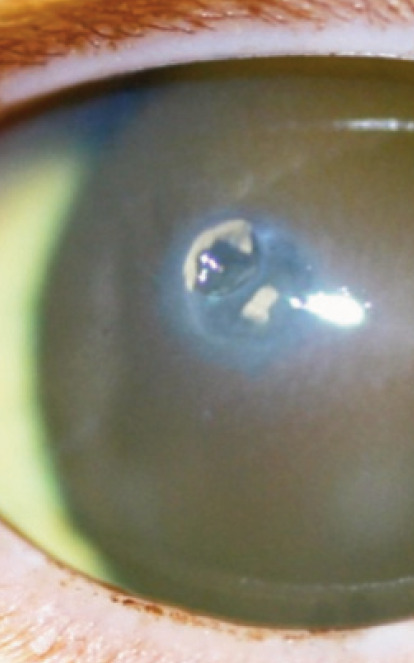
Fig 2.
Diffuse oedema of the ventral cornea associated with keratic precipitate deposition, leading to endothelial dysfunction
What is the Cause of the Ulceration -Reduced Protection or Direct Tissue Loss?
Broadly, corneal ulceration in any animal can result from reduced corneal protection or direct epithelial/stromal loss. Reduced corneal protection may be a consequence of inadequate tear production, composition, retention or distribution, as well as eyelid factors such as inadequate blinking (eg, lagophthalmos, facial nerve paralysis). Epithelial or stromal loss may occur as a result of abrasion from entropion, lash abnormalities (eg, ectopic cilia, trichiasis) or eyelid masses. Exogenous insults such as foreign bodies and trauma (mechanical and chemical) can also lead to corneal ulceration. Arguably the most important cause of corneal ulceration in cats is FHV-1 infection.
Diagnostic Approach
Faced with a cat with ocular discomfort, a logical and thorough approach to investigation will greatly facilitate appropriate treatment and resolution of the condition.
Visual inspection Thorough examination of the eyelids, conjunctiva (including the posterior surface of the third eyelid) and cornea is required to exclude lash abnormalities, eyelid masses or foreign bodies.
Assessment of tear production and composition This is frequently overlooked in cats. The tear film is composed of three intermingled layers: the inner mucin layer, which aids adhesion of the tear film to the hydrophobic corneal epithelium; the middle aqueous layer, which makes up the bulk of the tear film; and the thin outer lipid layer, which retards tear evaporation and creates a smooth optical surface.
Schirmer tear test This test assesses both basal and reflex (via corneal and/or conjunctival sensory nerve stimulation) tear production. Normal results in cats are reported as being 14.3 ± 4.7 mm/min. 3 Keratoconjunctivitis sicca is diagnosed when results below 10 mm/min are recorded in conjunction with appropriate clinical signs (eg, tacky mucoid discharge, conjunctival hyperaemia and thickening).
Tear film break-up time This test is an objective means of assessing tear film quality; it measures tear film stability and indirectly evaluates the mucin and/or lipid component of the preocular tear film. The tear film is stained with fluorescein and the time taken for this to evaporate from the corneal surface is recorded. After fluorescein instillation a forced blink is made; the eyelids are then held open and the interval until the first dark unstained area is observed is timed. The break-up time is reported as being 16.7 ± 4.5 s in normal cats. 3
Rose bengal staining This will identify areas of the corneal surface not covered by the preocular tear film. Rose bengal is an irritant dye, however, with epitheliotoxic effects. For this reason, it is best reserved for clinical cases where tear film deficiencies cannot be identified by other means.
Blink reflexes Appraisal of the palpebral blink reflex, and corneal blink reflex if appropriate, is important in order to appreciate any conformational lagophthalmos (incomplete eyelid closure) or eyelid function abnormalities. Such functional abnormalities may be secondary to a lack of sensory drive to blink due to trigeminal nerve paralysis, or lack of motor function to achieve orbicularis oculi contraction and effect eyelid closure. Additionally cicatricial ectropion may prevent normal eyelid closure; and ulcerative blepharitis following severe bacterial infection or trauma (in particular, chemical or thermal) may cause sufficient scarring to distort the eyelids or prevent normal function.
Cytology and bacterial culture Corneal cytology and bacterial culture and sensitivity testing are worthwhile additional diagnostics. Cytological preparations are easy to make using a Kimura spatula under topical local anaesthesia. The blunt handle end of a scalpel blade can be used (with care) where a Kimura spatula is not available. Valuable information that can be gathered from examination of such smears includes the presence or absence of bacteria, the type of bacteria involved (eg, Gram-positive or negative, rods or cocci), and the presence of intracellular inclusions (eg, chlamydophilal or viral) and inflammatory cell infiltrate (eg, neutrophilic, eosinophilic, monocytic or mixed).
Fluorescein staining Use of the vital dye fluorescein to highlight corneal defects will ensure that subtle lesions are not missed. Dendritic ulcers are virtually impossible to identify without the use of fluorescein and cobalt light illumination. Fluorescein will not stain Descemet's membrane; thus the absence of stain uptake in an ulcer bed, in the presence of stained margins, is pathognomonic for a descemetocoele.
Fluorescein will not stain Descemet's membrane; thus the absence of stain uptake in an ulcer bed, in the presence of stained margins, is pathognomonic for a descemetocoele.
FHV-1 replication within epithelial cells has a cytopathic effect, resulting in epithelial erosion and inflammation.
FHV-1 Infection and its Ocular Consequences
FHV-1 infection is widespread in domestic cat populations throughout most of the world, and is believed to be the most common cause of feline ocular disease.4,4 In fact, it has been suggested that it is fair to assume that any corneal ulceration in a cat is secondary to FHV-1 infection until proven otherwise. 6
The virus is a double-stranded DNA alpha-herpesvirus with similarities to canine herpesvirus and phocine (seal) herpesvirus, as well as to herpes simplex virus in humans (HSV-1). 7 Neuronal latency is characteristic of alphaherpesviruses and has been shown in FHV-1 infections.8,9 The virus has been demonstrated to establish latency in the trigeminal ganglia, from where recrudescence via anterograde axonal transport is postulated.8–11 Corneal latency has been demonstrated in rabbits, mice and humans, but has not been proven in cats.11,11
Primary infection typically occurs in kittens, with an estimated 80% progressing to latent infection, and 45% of those experiencing spontaneous reactivation of the virus later in life. 13 Predicting which cats within a population will suffer recrudescent disease is not currently possible, similar to the situation in humans with HSV-1. 6 FHV-1 has a tropism for the conjunctival, nasal and pharyngeal epithelium. 8 Transmission between cats is by close contact and via bodily fluids, particularly respiratory secretions. 14 Acutely infected cats shed the largest numbers of viral particles; however, latently infected cats may also shed virus and infect susceptible cats. 15 Overcrowded conditions and close housing greatly increase the likelihood of viral transmission as FHV-1 is short-lived in the environment. 16 Fomite transmission is possible; however, the virus is destroyed by most disinfectants, so strict attention to hygiene should be sufficient to prevent this route of infection.15,15 Reactivation of FHV-1 can follow corticosteroid treatment, pregnancy, lactation and other stressors, and can be delayed in onset for up to 10 weeks following a stressful event.13,13 Given that parturition has been shown to precipitate viral shedding in 40% of queens, neonatal infection is highly probable. 13 FHV-1 is responsible for ophthalmia neonatorum and secondary bacterial invasion makes early opening of eyelid margins essential to avoid corneal perforation. 18
Symblepharon
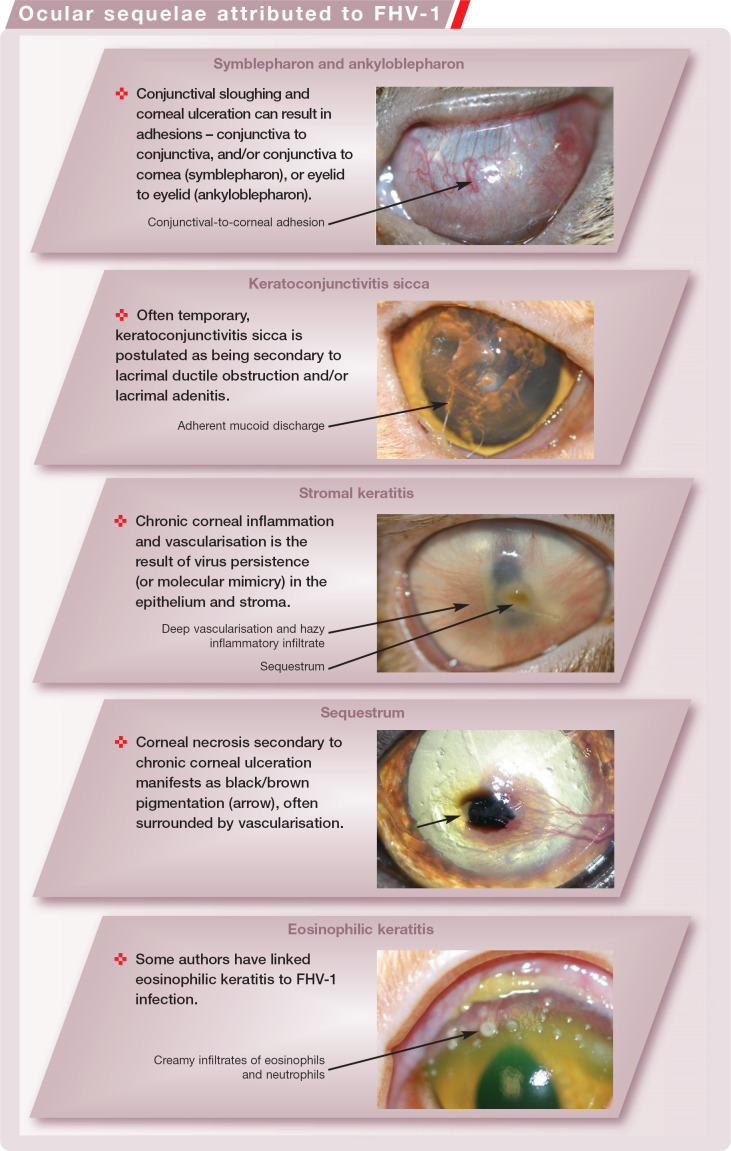
FHV-1 replication within epithelial cells has a cytopathic effect, resulting in epithelial erosion and inflammation.8,8 Epithelial sloughing and necrosis can lead to symble-pharon formation in acute infections (Fig 3). This may reduce the palpebral fissure and conjunctival fornices, cause visual difficulties, and occlude the lacrimal ductules and nasolacrimal punctae. 20
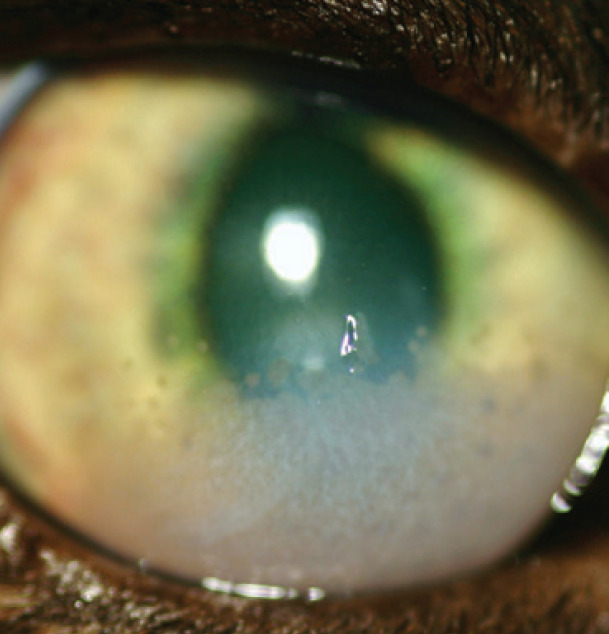
Fig 3.
Symblepharon formation; in this case, there is an adhesion from the dorsal conjunctiva and third eyelid to the cornea
Ulceration
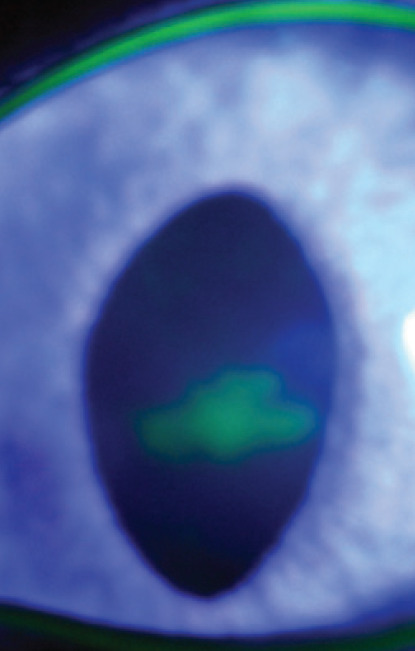
Corneal epithelium invasion by FHV-1 is associated with epithelial ulceration, initially of a pathognomonic dendritic form (Fig 4), but progressing rapidly to a larger irregular ‘geographical’ form (Fig 5 and Fig 6). 19 Occasionally these ulcers may progress to involve the stroma, or lead to descemetocoele formation or corneal perforation. 20
Fig 4.
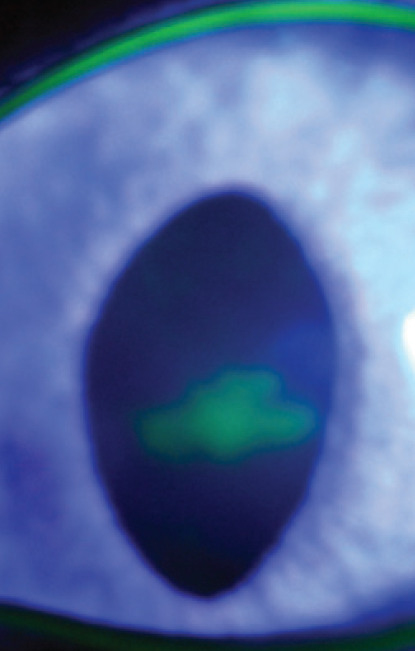
Dendritic corneal ulcer — a pathognomonic feature of FHV-1 infection
Fig 5.
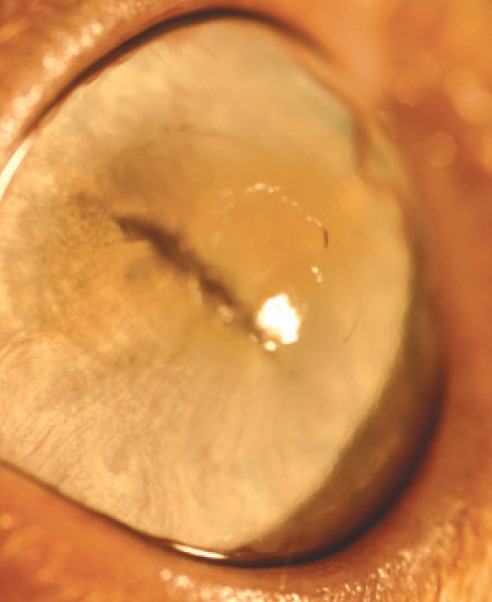
FHV-1 geographical ulcer. Note the faint corneal pigmentation, which represents early sequestrum formation
Fig 6.
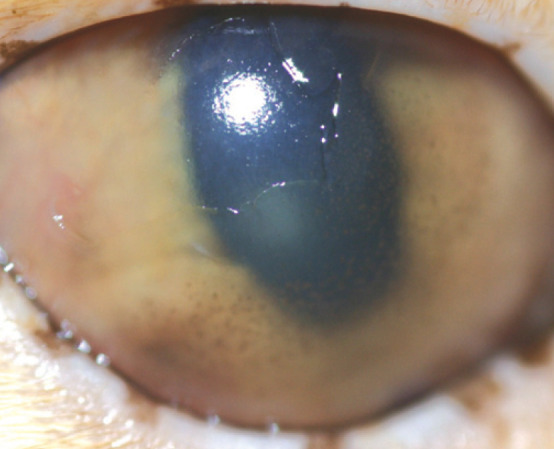
Geographical superficial corneal ulcer with anterior uveitis
Reactivation of FHV-1 latency is usually associated with conjunctivitis and may be accompanied by superficial corneal ulceration. 20 It can be unilateral or bilateral, is not usually associated with general malaise, and is generally milder than primary infections. 6 Again, pathognomonic dendritic ulcers are a feature early in recrudescence, but many progress so quickly to geographical ulcers that the presence of dendritic ulcers is never witnessed. Vital stains such as rose bengal and fluorescein are required to diagnose dendritic ulceration, often in combination with magnified ophthalmic examination (eg, slit lamp biomicroscopy). 19 Many of these ulcers are slow to heal, leading to a more chronic evolution than seen in primary disease. 6 Angiogenesis due to chronic ulceration results in corneal vascularisation. Inflammatory cell infiltrates may also accompany chronic disease. 19
Keratoconjunctivitis Sicca
Keratoconjunctivitis sicca is occasionally seen secondarily to either acute FHV-1 infection or recurrent FHV-1 conjunctivitis, and is believed to result from lacrimal ductule occlusion and/or lacrimal adenitis. 21
Anterior Uveitis
Recently a causative link between FHV-1 infection and anterior uveitis has been demonstrated via identification of local FHV-1 antibody production within the eye (Goldmann Witmer coefficient). 23 For some years, HSV-1 has been known to cause anterior uveitis and endothelialitis in humans. 24
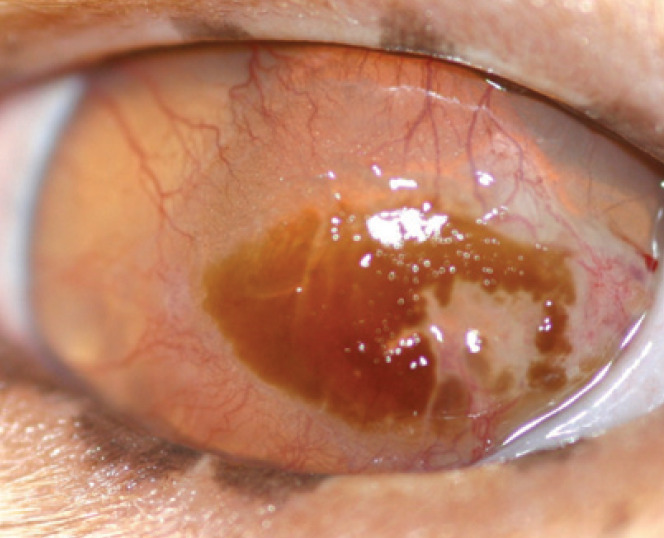
Stromal Keratitis
Stromal keratitis is a secondary immunemediated condition postulated to be the result of virus antigen persistence within the stroma, initiating and perpetuating corneal inflammation.18,18 Deep corneal vascularisation with an inflammatory cell infiltrate and corneal fibrosis is seen (Fig 7), and is frustrating to treat. The cornea may or may not be ulcerated (and retain fluorescein stain) depending on whether active epithelial disease is present.18,18 Recent evidence suggests a similar mechanism may be involved in feline chronic rhinosinusitis. 25
Fig 7.
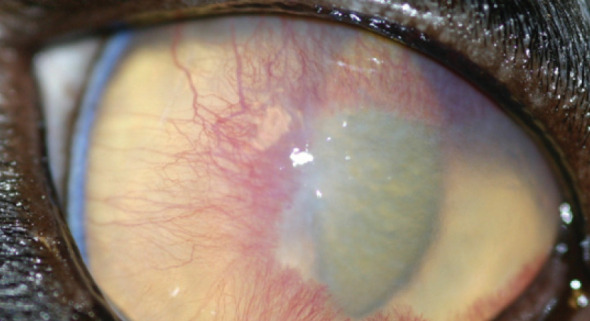
Stromal keratitis. Note the vascularisation and hazy infiltrate at the leading edge of the vascularisation
Sequestra
The formation of corneal sequestra can occur secondarily to any chronic ulcerative keratitis, including FHV-1 ulceration. 26 A breed predisposition for sequestrum formation has been reported in Persians, Himalayans and Burmese cats. FHV-1 infected cats receiving topical or subconjunctival corticosteroids are more likely to develop stromal keratitis and sequestra. 19
A sequestrum is a plaque of corneal necrosis (Fig 8). 27 Laboratory analysis of sequestra using ultraviolet-visible light absorbance spectroscopy and optical microscopy indicates that the pigmentation is likely to be melanin. 28
Fig 8.
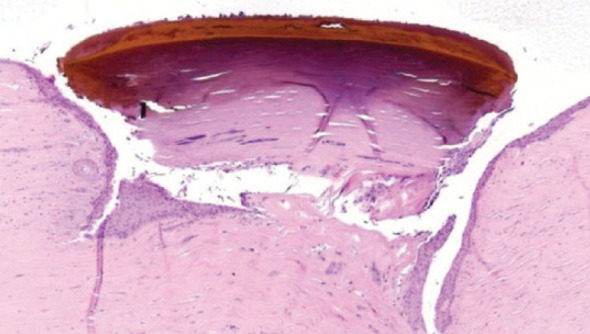
Histopathology of corneal sequestrum. Courtesy of Dick Dubielzig, COPLOW
Analysis of excised sequestra revealed 18% to be positive for FHV-1 DNA in one study using nested PCR, 26 and 55.1% to be positive in another study using single-round PCR. 29 A third study demonstrated the presence of FHV-1 DNA by PCR in 44% (4/9) of keratectomy samples from sequestrum cases; more peculiarly, 44% of samples were also positive for Toxoplasma gondii DNA. 27 It was speculated that T gondii reached the cornea haematogenously in all but one case. Neither of these infectious agents were demonstrated on ultrastructural examination in this study. 27 These results sparked the theory that FHV-1 infection was causally associated with sequestrum formation.
However, two further studies found that the distribution of FHV-1 PCR positive cases was not statistically significant between cats with and without sequestra.26,26 Also, within the subset of brachycephalic breeds, the percentage of FHV-1 PCR positive sequestra was lower. 29 Despite this, brachycephalic breeds (eg, Persians, Colourpoints, Himalayans and Burmese) are overrepresented for sequestra (Fig 9 and Fig 10).31–34 This appears to contradict the hypothesis that FHV-1 infection causes sequestrum formation. It seems more probable that chronic irritation and ulceration is responsible for sequestra, at least in brachycephalic breeds (Fig 10).
Fig 9.
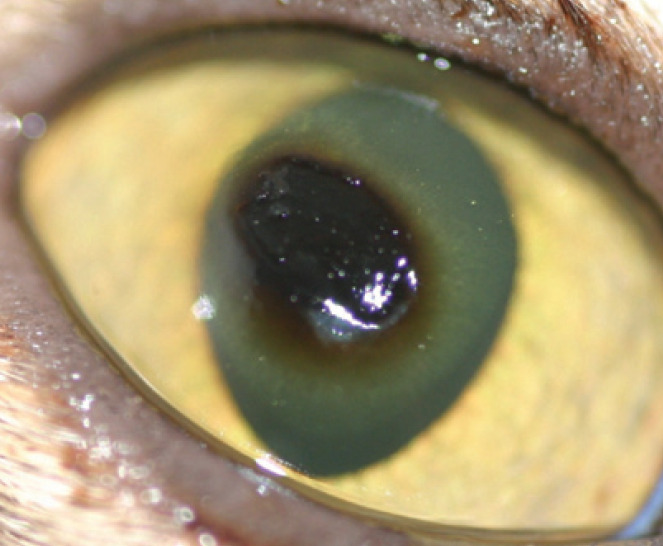
Central corneal sequestrum (plaque of corneal necrosis) in a Burmese cat. Note also the darkly pigmented dried discharge on the eyelids. The pigmentation is likely to be melanin 28
Fig 10.
Stromal keratitis and sequestrum formation in a Persian. This cat had been treated with a grid keratotomy; the grid lines are visible within the sequestrum
Differentials for FHV-1 Conjunctivitis
Differentials for feline conjunctivitis include Chlamydophila felis and feline calicivirus infection, but involvement of the cornea has not been described in these infections. 22
Ocular Sequelae Attributed to FHV-1
Eosinophilic Keratitis
Eosinophilic keratitis (or keratoconjunctivitis) has also been linked to FHV-1 infection, and may be associated with corneal ulceration as eosinophil granules contain many cytotoxic chemicals. 35 Studies are conflicting; some have demonstrated a significant association between the presence of FHV-1 DNA and eosinophilic keratitis (76.3% of cases of eosinophilic keratitis were positive for FHV-1 DNA in one study, 29 and 85.7% in another 30 ), while others did not.26,26 An important contradiction of this hypothesis is the fact that the mainstay of treatment for eosinophilic keratitis is corticosteroids (topical or systemic), which would be likely to promote viral replication; but rather than exacerbating the condition, this treatment is very successful at resolving it.37,37 A novel chlamydia (Neochlamydia hartmannellae) has been described associated with eosinophilic keratitis, but no direct causation has to date been demonstrated. 39
Eosinophilic keratitis can have a variable presentation with perilimbal vascularisation and proud accretions of inflammatory cells, often at the leading edge of the vascularisation, with or without involvement of the conjunctiva (Fig 11). The lesion can also vary in its location on the cornea, and may progress to involve the entire cornea.29,29 Cytological evaluation of corneal scrapes is instrumental to diagnosis. Eosinophils with mast cells, plasma cells, lymphocytes and neutrophils are seen, with neutrophils and eosinophils being the most conspicuous. 35
Fig 11.
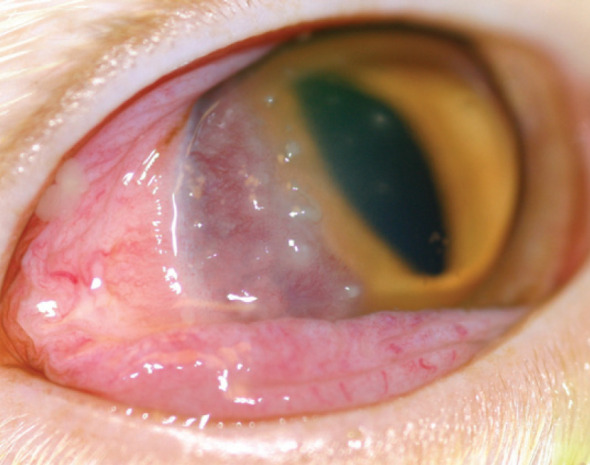
Eosinophilic keratoconjunctivitis in a Maine Coon
Keratomalacia
Rarely, FHV-1 ulceration may progress to stromal involvement, and even to corneal liquefaction (ie, keratomalacia or corneal ‘melting’). The cytopathic effect of viral replication induces inflammation primarily of a neutrophilic nature. 18 Endogenous proteases (released from neutrophils and wounded corneal epithelial cells) are a more significant source of collagenases in keratomalacia than bacterial-derived proteases. 40
Feline Herpetic Dermatitis
Just as HSV-1 has been associated with dermatitis in humans,6,41,42 feline herpetic dermatitis has been described. The condition in humans has been associated with immunosuppression or compromise, and there is speculation that a similar pathophysiology exists in cats, although support for this hypothesis is lacking.43–45 FHV-1 dermatitis is characterised by a facial and nasal dermatitis with vesicles, ulcers and crusting; stomatitis may or may not be present. 46
Diagnosis of FHV-1
Prior to the development of PCR diagnostics, virus isolation was considered the gold standard for diagnosis of FHV-1. 47 As virus culture and isolation relies on the presence of viable viral particles, careful collection and handling of samples is required to avoid false negative results. Immunofluorescent antibody testing (IFAT) of conjunctival or corneal scrapings requires less stringent sample handling. However, the use of vital stains, particularly fluorescein, may result in false positive results.
Serology, virus isolation and IFAT are of limited value diagnostically in ocular FHV-1. Serology is complicated by vaccine virus and positive titres are independent of clinical ocular signs. 48 FHV-1 can be detected by virus isolation and IFAT in clinically normal cats (presumably due to intermittent subclinical viral shedding) and therefore neither test appears to aid in the clinical diagnosis of FHV-1 infection. 48
PCR (single or nested) is now the mainstay technique for diagnosis of feline herpetic disease.49,49 PCR identifies the presence of FHV-1 DNA and therefore does not require viable virus for a positive result. 51 Given this, distinguishing between FHV-associated disease and healthy FHV carriers is problematic. 50 Nested PCR is 4.8 times more sensitive than single PCR. 52 Sample handling and postage requirements do not need to be rigorous for PCR submissions compared with those for viral culture (see table). 51
Importantly, as viral reactivation from the trigeminal ganglion can be triggered by trigeminal nerve stimulation, positive results need to be interpreted in the knowledge that viral presence may be a result of corneal pathology and not the cause of that pathology. Detection of virus may indicate either coincidental reactivation of latent infection, a consequential reactivation due to another disease process or the cause of the ocular disease.17,53,54
Given the complexity of interpretation of laboratory findings, many ophthalmologists rely on consistent clinical signs with a history of respiratory signs to make a diagnosis of FHV-1 associated ocular disease.
Comparison of laboratory diagnostic tests
| Serology | Virus isolation | IFAT | PCR | |
|---|---|---|---|---|
| Detects virus or host response? | Host | Virus | Virus | Virus |
| Viable virus required? | No | Yes | No | No |
| Test affected by vital stains? | No | Yes | Yes | Possible |
| Collection and transport | Simple | Complicated Simple | Simple |
Courtesy of D Maggs, University of California-Davis
Other Causes of Corneal Ulceration
Corneal Trauma
Cats appear to be a great deal more adept at avoiding corneal trauma than their canine counterparts. According to some authors, cats possess similar corneal sensitivity to dogs,55,55 while others report lower sensitivity in dogs.57,57 Brachycephalic cats have reduced corneal sensitivity compared with domestic shorthair cats; likewise reduced corneal sensation has been reported in brachycephalic dogs compared with their mesocephalic and dolichocephalic cousins. 58 Cats also have some active control over third eyelid movement, unlike dogs. In most mammals, third eyelid protrusion is effected passively by retraction of the globe, displacing orbital fat. 59 Strands of smooth muscle have been identified extending into the third eyelid in the cat, and are believed to be responsible for active protrusion and retraction. 60 Both increased corneal sensitivity and active third eyelid protrusion could afford the feline cornea better protection. Dogs do, however, blink more frequently than cats. 61
Nonetheless, corneal trauma does occur in the cat, often perpetrated by another cat (Fig 12Fig 14). With an injury sustained in a cat fight there is a significant infection risk. If presentation for veterinary attention has been delayed, an inflammatory cell infiltrate is to be expected; the cell infiltrate is derived from both limbal blood vessels and, more significantly, the tear film.
Fig 12.
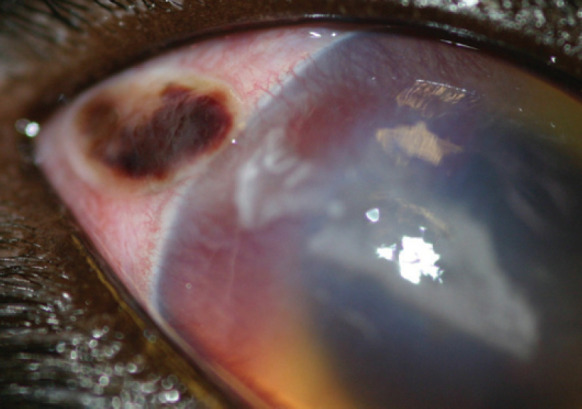
Scleral and corneal lacerations in a domestic shorthair cat
Fig 13.
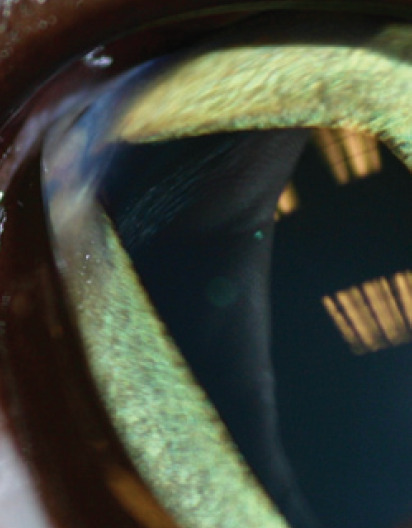
Full thickness corneal perforation with prolapsed iris. Note the dyscoria due to iris entrapment in the corneal wound
Fig 14.

Corneal foreign body. Fluorescein has been applied to assess for aqueous leakage (Seidel's test)
Chemical Trauma
Keratomalacia is a common sequela where stromal inflammatory cell infiltrate is conspicuous. Chemical trauma of the cornea may also result in keratomalacia through direct action of the chemical irritant as well as the inflammation it provokes. In general, alkaline injury of the cornea is more serious than acidic injury, as acids tend to denature corneal protein on contact, which impedes deeper penetration. 62
Alkaline agents can penetrate deeply, including into the anterior chamber, and may result in full thickness corneal perforation by corneal liquefaction. Intraocular inflammation is more intense with alkaline injury due to the potential for anterior chamber penetration. 62 Corneal stem cell injury is common with chemical trauma, and, where this is extensive, conjunctivalisation of the corneal surface ensues. Cicatricial abnormalities are also commonly seen secondarily to conjunctival burns, and may result in trichiasis and/or reduced conjunctival fornices, necessitating correction. 62
Entropion
Chronic trauma as a result of entropion is less frequent in cats than dogs, but is encountered, particularly in brachycephalic breeds.65,65 Spastic entropion resulting from chronic intense blepharospasm can initially be resolved if the primary cause is identified and removed. However, given time, it is postulated that fibrous changes occur within the eyelid such that the entropion becomes non-reducible permanently without surgical intervention (Fig 15). 22
Fig 15.
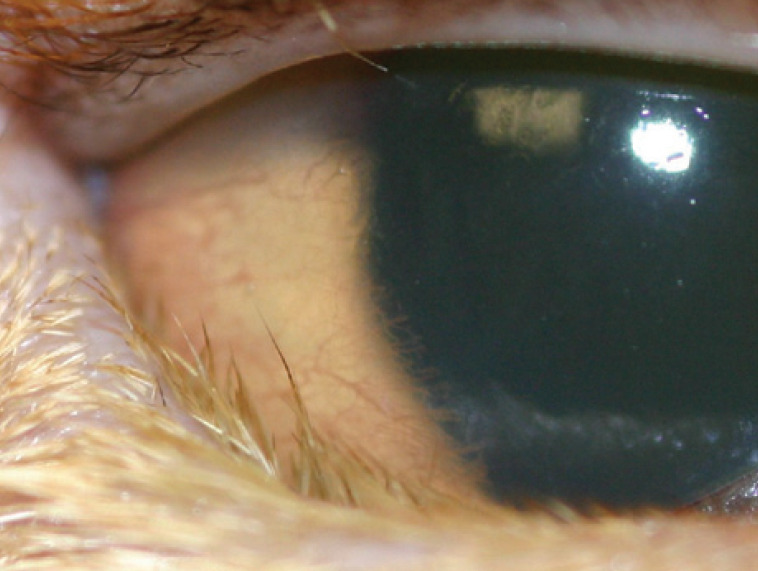
Lower lid entropion in an Abyssinian cat secondary to chronic ulcerative keratitis
Eyelid Agenesis
Eyelid agenesis (or coloboma) is an uncommon congenital defect in which the dorsolateral (or rarely medial can-thal) portion of the eyelid is absent to some degree (Fig 16).65,65 As well as occurring in domestic cats, particularly in Birmans and Burmese, this abnormality has also been seen in Snow Leopards 68 and a Texas cougar. 69 It does not always occur in isolation and other ocular defects such as persistent pupillary membranes, cataracts and other colobomatous defects may be evident.66–68,70 Trichiasis from adjacent haired skin, in conjunction with altered tear film dynamics, often results in chronic exposure and irritation of the cornea closest to the defect. 22 Keratitis, with or without ulceration, usually occurs and sequestrum formation may also be seen.
Fig 16.
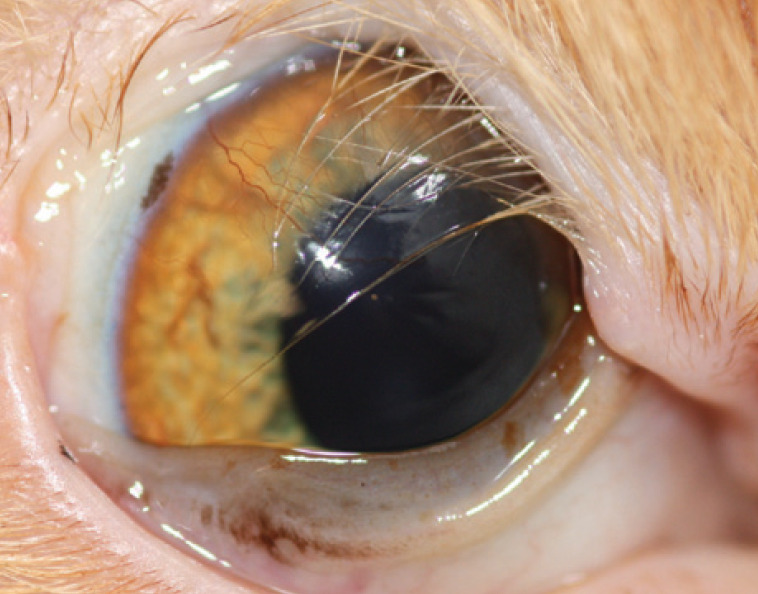
One-year-old domestic shorthair cat with unilateral eyelid agenesis. The left eye was microphthalmic with a micropalpebral fissure
Stromal Abscess -An Occasional Sequela of Ulceration
Stromal abscessation can occur following stromal ulceration, where overlying epithelial healing sequesters bacterial or fungal infection within the stroma. 63 Fortunately, these abscesses are not common in cats as treatment of the microorganism is hindered by the epithelial barrier to drug penetration. 64
Dermoids
Dermoids, which are foci of epidermal and dermal tissue in an abnormal location (ie, choristoma), occur infrequently and are a rare cause of ulceration. They tend to be restricted to the Burmese and Birman breeds but reports in domestic shorthair cats do exist (Fig 17).65,71,72 The hairs on dermoids are typically long, and those that contact the cornea tend to float on the tear film rather than abrade the epithelium (Fig 17).
Fig 17.
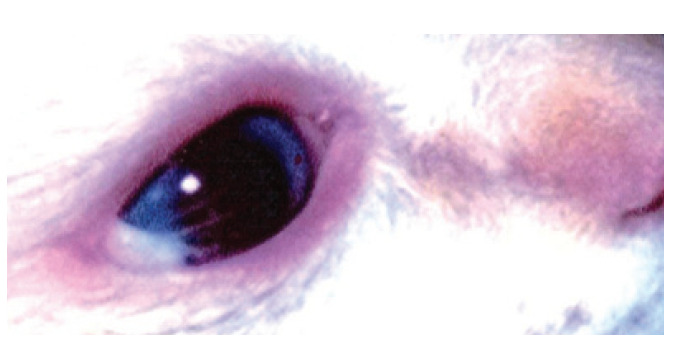
Three-month-old Birman kitten with epibulbar dermoid. Note the long hairs across the cornea
Distichiasis and Ectopic Cilia
Distichiasis is very rare in cats,73,73 and a single case report of an ectopic cilia in a Siamese cat exists. 75 An ectopic cilium is more likely to cause ulceration as the hair is directed through the conjunctiva, and perpendicular to the corneal surface, whereas distichia arise from meibomian gland openings along the eyelid margin.
Eyelid Neoplasms
Eyelid neoplasms, in particular erosive lesions of squamous cell carcinoma, may in rare cases alter tear film dynamics sufficiently to result in exposure and ulcerative keratitis.
Cranial Neuropathy
It is important that cranial neuropathies are not overlooked as a cause of corneal ulceration. Although less common in cats than in dogs, failure to identify a neurological cause can mean increased recovery time and a poorer prognosis.
Facial Nerve Paralysis
Facial nerve paralysis (cranial nerve VII) results in an efferent deficiency of the palpebral blink reflex. The parasympathetic fibres of the lacrimal gland may also be damaged in cats with facial nerve pathology and, therefore, Schirmer tear test measurements are indicated in all cases. As lacrimal innervations distally join the trigeminal nerve (cranial nerve V), a Schirmer tear test may reveal normal tear production if the facial nerve lesion is distal to the pterygopalatine ganglion. In addition, third eyelid (nictitans membrane) movement is primarily under indirect abducens nerve (cranial nerve VI) control via globe retraction and passive protrusion of the third eyelid. Hence, tear production and distribution may be relatively unaffected (Table 1).
Table 1.
Comparison of cranial neuropathies
| Innervation of cranial nerve | Corneal ulceration | Tear production | |
|---|---|---|---|
| Trigeminal nerve paralysis | Afferent to eye and adnexae | Common | Often affected |
| Facial nerve paralysis | Efferent to eyelids | Uncommon | May be affected |
It could be argued that animals with facial paralysis are at increased risk of corneal trauma as one facet of corneal protection is deficient. Where keratitis develops, it is referred to as neuroparalytic keratitis.
Causes of facial paralysis in cats include lesions of the middle ear or petrous temporal bone (otitis media, surgically induced such as tympanic bulla osteotomy, trauma) and facial trauma due, for example, to a road traffic accident (RTA).
Trigeminal Nerve Paralysis
Trigeminal nerve paralysis (cranial nerve V) is associated with severe corneal ulceration in nearly all cases, even when aggressive tear replacement is instituted (Fig 18). The trigeminal nerve is responsible for sensory innervation of the cornea and adnexae, the cornea being more densely innervated with sensory nerve endings than any other tissue in the body. 76
Fig 18.
(a) Domestic shorthair cat with left-sided trigeminal nerve paralysis secondary to an RTA. Note the severe central corneal exposure with stromal ulceration (tropicamide [Mydriacyl; Alcon Laboratories] had been applied to both eyes for retinal examination). (b) Close-up image of the left eye demonstrating severe stromal ulceration, deep corneal vascularisation, sequestrum formation and inflammatory cell infiltrate
Corneal innervation is essential for homeostasis as well as healing of the cornea. Stimulation of sensory nerve endings initiates release of epitheliotrophic factors, in particular substance P, 77 which has been shown to be pivotal in epithelial healing; in a mouse model, denervation of the cornea decreased the concentration of substance P by 40%. 78 Absence of trigeminal innervation is responsible for neutrophic keratitis, which tends to be much more severe than neuroparalytic keratitis (Table 1).
The trigeminal nerve is also responsible for sensory stimulation of the lacrimal gland, and therefore paralysis is commonly associated with keratoconjunctivitis sicca (Table 1). 78 False tear preparations, even when applied hourly, may not be sufficient to prevent ulceration; and, once ulcerated, the cornea is unlikely to heal without surgical intervention.
Key Points
Feline ulcerative keratitis should be assumed to be caused by FHV-1 until proven otherwise.
Essentially, all cats will have been exposed to FHV-1 as kittens; 45% will become latently infected and half of these will suffer recrudescent disease.
Ocular sequelae to FHV-1 include symblepharon, keratoconjunctivitis sicca, stromal keratitis, sequestrum and eosinophilic keratitis.
PCR is now the mainstay diagnostic technique for FHV-1.
When assessing an ulcer, identify if it is superficial, stromal, deep stromal or a descemetocoele, as this will influence the approach to treatment.
Monitor an ulcer for signs of progression or keratomalacia as these may suggest a change of treatment or surgery is required.
Causes of trigeminal paralysis include trauma (eg, RTA), central nervous system neo-plastic, infectious or inflammatory lesions (usually associated with other signs of central disease such as decreased mentation and depression), and orbital neoplasia, infections or inflammation.
Treatment of Corneal Ulcers
An article to be published in a future ‘clinical practice’ issue of J Feline Med Surg will review medical and surgical treatment of corneal ulcers.
References
- 1.Kafarnik C, Fritsche J, Reese R. In vivo confocal microscopy in the normal corneas of cats, dogs and birds. Vet Ophthalmol 2007; 10: 222–30. [DOI] [PubMed] [Google Scholar]
- 2.Niederer RL, Perumal D, Sherwin T, McGhee CNJ. Age-related differences in normal human cornea: A laser scanning in vivo confocal microscopy study. Br J Ophthalmol 2009; 91: 1165–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cullen CL, Lim C, Sykes J. Tear film breakup times in young healthy cats before and after anaesthesia. Vet Ophthalmol 2005; 8: 159–65. [DOI] [PubMed] [Google Scholar]
- 4.Low HC, Powell CC, Veir JK, Hawley JR, Lappin MR. Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res 2007; 68: 643–48. [DOI] [PubMed] [Google Scholar]
- 5.Di Martino B, Di Francesco CE, Meridiani I, Marsilio F. Aetiological investigation of multiple respiratory infections in cats. New Microbiol 2007; 30: 455–61. [PubMed] [Google Scholar]
- 6.Maggs DJ. Update on pathogenesis, diagnosis and treatment of feline herpesvirus type 1. Clin Tech Small Anim Pract 2005; 20: 94–101. [DOI] [PubMed] [Google Scholar]
- 7.Rota PA, Maes RK, Ruyechan WT. Physical characterisation of the genome of feline herpesvirus 1. Virology 1986; 154: 168–79. [DOI] [PubMed] [Google Scholar]
- 8.Gaskell RM, Povey RC. Feline viral rhinotracheitis: Sites of virus replication and persistence in acutely and persistently infected cats. Res Vet Sci 1979; 27: 167–74. [PubMed] [Google Scholar]
- 9.Gaskell RM, Dennis PE, Goddard LE, Cocker FM, Wills JM. Isolation of felid herpesvirus 1 from the trigeminal ganglia of latently infected cells. J Gen Virol 1985; 66: 391–94. [DOI] [PubMed] [Google Scholar]
- 10.Nasisse MP, Davis BJ, Guy JS. Isolation of feline herpesvirus-1 from the trigeminal ganglia of acutely and chronically infected cats. J Vet Intern Med 1992; 6: 102–3. [DOI] [PubMed] [Google Scholar]
- 11.Townsend WM, Stiles J, Guptill-Yoran L, Krohne SG. Development of a reverse transcriptase-polymerase chain reaction assay to detect feline herpesvirus-1 latency-associated transcripts in the trigeminal ganglia and corneas of cats that did not have clinical signs of ocular disease. Am J Vet Res 2004; 65: 314–19. [DOI] [PubMed] [Google Scholar]
- 12.Stiles J, Pogranichniy R. Detection of virulent feline herpesvirus-1 in the corneas of clinically normal cats. J Feline Med Surg 2008; 10: 154–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaskell RM, Povey RC. Experimental induction of feline rhinotracheitis virus re-excretion in FVR-recovered cats. Vet Rec 1977; 100: 128–33. [DOI] [PubMed] [Google Scholar]
- 14.Stiles J. Feline herpesvirus. Vet Clin North Am Small Anim Pract 2000; 30: 1001–14. [DOI] [PubMed] [Google Scholar]
- 15.Gaskell RM, Povey RC. Transmission of feline viral rhinotracheitis. Vet Rec 1982; 111: 359–62. [DOI] [PubMed] [Google Scholar]
- 16.Povey RC, Johnson RH. Observations on the epidemiology and control of viral respiratory disease in cats. J Small Anim Pract 1970; 11: 485–94. [DOI] [PubMed] [Google Scholar]
- 17.Gaskell RM, Povey RC. Re-excretion of feline viral rhinotracheitis virus following corticosteroid treatment. Vet Rec 1973; 93: 204–5. [DOI] [PubMed] [Google Scholar]
- 18.Bistner SI, Shivley JN, Scott FW. Ocular manifestations of feline herpesvirus infection. J Am Vet Med Assoc 1971; 159: 1223–37. [PubMed] [Google Scholar]
- 19.Nasisse MP, Guy JS, Davidson MG, Sussman WA, Fairley NM. Experimental ocular herpesvirus infection in the cat. Sites of replication, clinical features and effects of corticosteroid administration. Invest Ophthalmol Vis Sci 1989; 30: 1758–68. [PubMed] [Google Scholar]
- 20.Nasisse MP. Manifestations, diagnosis, and treatment of ocular herpesvirus infection in the cat. Compend Cont Educ Pract Vet 1982; 4: 962–70. [Google Scholar]
- 21.Stiles J. Treatment of cats with ocular disease attributable to herpesvirus infection: 17 cases (1983–1993). J Am Vet Med Assoc 1995; 5: 599–603. [PubMed] [Google Scholar]
- 22.Stiles J, Townsend WM. Feline ophthalmology. In: Veterinary ophthalmology. 4th edn. II. Iowa: Blackwell Publishing, 2007: 1095–114. [Google Scholar]
- 23.Maggs DJ, Lappin MR, Nasisse MP. Detection of feline herpesvirus-specific antibodies and DNA in aqueous humour from cats with and without uveitis. Am J Vet Res 1999; 60: 932–36. [PubMed] [Google Scholar]
- 24.Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea 1999; 18: 127–43. [DOI] [PubMed] [Google Scholar]
- 25.Johnson LR, Foley JE, De Cock HE, Clarke HE, Maggs DJ. Assessment of infectious organisms associated with chronic rhinosinusitis in cats. J Am Vet Med Assoc 2005; 227: 579–85. [DOI] [PubMed] [Google Scholar]
- 26.Stiles J, McDermott M, Bigsby D, et al. Use of nested polymerase chain reaction to identify feline herpesvirus in ocular tissue from clinically normal cats and cats with corneal sequestra or conjunctivitis. Am J Vet Res 1997; 58: 338–42. [PubMed] [Google Scholar]
- 27.Cullen CL, Wadowska DW, Singh A, Melekhovets Y. Ultrastructural findings in feline corneal sequestra. Vet Ophthalmol 2005; 8: 295–303. [DOI] [PubMed] [Google Scholar]
- 28.Featherstone HJ, Franklin VJ, Sansom J. Feline corneal sequestrum: Laboratory analysis of ocular samples from 12 cats. Vet Ophthalmol 2004; 7: 229–38. [DOI] [PubMed] [Google Scholar]
- 29.Nasisse MP, Glover TL, Moore CP. Detection of feline herpesvirus 1 DNA in corneas of cats with eosinophilic keratitis or corneal sequestration. Am J Vet Res 1998; 59: 856–58. [PubMed] [Google Scholar]
- 30.Volopich S, Benetka V, Schwendenwein I, Möstl K, Sommerfeld-Stur I, Nell B. Cytologic findings, and feline herpesvirus DNA and Chlamydophila felis antigen detection rates in normal cats and cats with conjunctival and corneal lesions. Vet Ophthalmol 2005; 8: 25–32. [DOI] [PubMed] [Google Scholar]
- 31.Vawer GD. Corneal mummification in colourpoint cats. Vet Rec 1981; 109: 413. [DOI] [PubMed] [Google Scholar]
- 32.Morgan RV. Feline corneal sequestration. A retrospective study of 42 cases. J Am Anim Hosp Assoc 1994; 30: 24–28. [Google Scholar]
- 33.Startup FG. Corneal necrosis and sequestration in the cat: A review and record of 100 cases. J Small Anim Pract 1988; 29: 476–86. [Google Scholar]
- 34.Featherstone HJ, Sansom J. Feline corneal sequestra: A review of 64 cases (80 eyes) from 1993 to 2000. Vet Ophthalmol 2004; 7: 213–27. [DOI] [PubMed] [Google Scholar]
- 35.Prasse KW, Winston SM. Cytology and histopathology of feline eosinophilic keratitis. Vet Comp Ophthalmol 1996; 6: 74–81. [Google Scholar]
- 36.Allgoewer I, Schaffer EH, Stockhaus C, Vogtlin A. Feline eosinophilic conjunctivitis. Vet Ophthalmol 2001; 4: 69–74. [DOI] [PubMed] [Google Scholar]
- 37.Paulsen ME, Lavach JD, Severin GA. Feline eosinophilic keratitis: A review of 15 cases. J Am Anim Hosp Assoc 1987; 23: 63–69. [Google Scholar]
- 38.Morgan RV, Abrams KA, Kern TJ. Feline eosinophilic keratitis: A retrospective study of 54 cases: (1989–1994). Vet Comp Ophthalmol 1996; 6: 131–34. [Google Scholar]
- 39.Von Bomhard W, Polkinghorne A, Huat Z, et al. Detection of novel chlamydiae in cats with ocular disease. Am J Vet Res 2003; 64: 1421–28. [DOI] [PubMed] [Google Scholar]
- 40.Olliver FJ, Gilger BC, Barrie KP, et al. Proteinases of the cornea and preocular tear film. Vet Ophthalmol 2007; 10: 199–206. [DOI] [PubMed] [Google Scholar]
- 41.Holland JL, Outerbridge CA, Affolter VK, Maggs D. Detection of herpesvirus 1 DNA in skin biopsy specimens from cats with or without dermatitis. J Am Vet Med Assoc 2006; 229: 1442–46. [DOI] [PubMed] [Google Scholar]
- 42.Fivenson DP, Breneman DL, Wander AH. Kaposi's varicelliform eruption. Absence of ocular involvement. Arch Dermatol 1990; 126: 1037–39. [PubMed] [Google Scholar]
- 43.Gross TL, Ihrke PJ, Walder EJ, Afflolter VK. Ulcerative and crusting diseases of the epidermis. In: Skin diseases of the dog and cat: clinical and histopathologic diagnosis. Oxford: Blackwell Science, 2005: 124–26. [Google Scholar]
- 44.Vestey JP, Howey SE, Norval M, Maingay JP, Neill WA. Severe eczema herpeticum is associated with prolonged depression of cell-mediated immunity to herpes simplex virus. Current Probl Dermatol 1989; 18: 158–61. [DOI] [PubMed] [Google Scholar]
- 45.Scott D, Miller WH, Griffin CE. Viral, rickettsial, and protozoal skin diseases. In: Small animal dermatology. Philadephia: Saunders, 2001: 524–25. [Google Scholar]
- 46.Guaguere E, Declercq J. Viral dermatoses. In: A practical guide to feline dermatology. Lyon: Merial, 1999: 7.10. [Google Scholar]
- 47.Lutz H, Leutenegger C, Hofmann-Lehmann R. The role of poly-merase chain reaction and its newer developments in feline medicine. J Feline Med Surg 1999; 1: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maggs DJ, Lappin MR, Reif JS, et al. Evaluation of serologic and viral detection methods for diagnosing feline herpesvirus-1 infection in cats with acute respiratory tract or chronic ocular disease. J Am Vet Med Assoc 1999; 214: 502–7. [PubMed] [Google Scholar]
- 49.Stiles J, McDermott M, Willis M, Roberts W, Greene C. Comparison of nested polymerase chain reaction, virus isolation, and fluorescent antibody testing for identifying feline herpesvirus in cats with conjunctivitis. Am J Vet Res 1997; 58: 804–7. [PubMed] [Google Scholar]
- 50.Burgesser KM, Hotaling S, Schiebel A, Ashbaugh SE, Roberts SM, Collins JK. Comparison of PCR, virus isolation and indirect fluorescent antibody staining in the detection of naturally occurring feline herpesvirus infections. J Vet Diagn Invest 1999; 11: 122–26. [DOI] [PubMed] [Google Scholar]
- 51.Clarke HE, Kado-Fong H, Maggs DJ. Effects of temperature and time in transit on polymerase chain reaction detection of feline herpesvirus DNA. J Vet Diagn Invest 2006; 18: 388–91. [DOI] [PubMed] [Google Scholar]
- 52.Hara M, Fukuyama M, Suzuki Y, et al. Detection of feline herpesvirus 1 DNA by the nested polymerase chain reaction. Vet Microbiol 1996; 48: 345–52. [DOI] [PubMed] [Google Scholar]
- 53.Maggs DJ. Update on pathogenesis, diagnosis and treatment of feline herpesvirus type 1. Clin Tech Small Anim Pract 2004; 20: 94–101. [DOI] [PubMed] [Google Scholar]
- 54.Maggs DJ, Clarke HE. Relative sensitivity of polymerase chain reaction assays used for detection of feline herpesvirus type 1 DNA in clinical samples and commercial vaccines. Am J Vet Res 2005; 66: 1550–55. [DOI] [PubMed] [Google Scholar]
- 55.Good KL, Maggs DJ, Hollingsworth SR, Randall H, Scagliotti RH, Nelson RW. Corneal sensitivity in dogs with diabetes mellitus. Am J Vet Res 2003; 64: 7–11. [DOI] [PubMed] [Google Scholar]
- 56.Blocker T, Van de Woerdt A. A comparison of corneal sensitivity between brachycephalic and domestic shorthaired cats. Vet Ophthalmol 2001; 4: 127–30. [DOI] [PubMed] [Google Scholar]
- 57.Chan-Ling T. Sensitivity and neural organization of the cat cornea. Invest Ophthalmol Vis Sci 1989; 30: 1075–82. [PubMed] [Google Scholar]
- 58.Barrett PM, Scagliotti RH, Meredith RE, Jackson PA, Alarcon FL. Absolute corneal sensitivity and corneal trigeminal nerve anatomy in normal dogs. Prog Vet Comp Ophthalmol 1991; 1: 245–54. [Google Scholar]
- 59.Berthier NE. The role of the extraocular muscles in the rabbit nictitating membrane response: A re-examination. Behav Brain Res 1984; 14: 81–4. [DOI] [PubMed] [Google Scholar]
- 60.Nuyttens JJ, Simoens PJ. Morphologic study of the musculature of the third eyelid in the cat (Felix catus). Lab Anim Sci 1995; 45: 561–63. [PubMed] [Google Scholar]
- 61.Gum GG, Gelatt KN, Esson DW. Physiology of the eye. In: Gelatt KN, ed. Veterinary ophthalmology. 4th edn. Oxford, Blackwell Publishing, 2007: 149–82. [Google Scholar]
- 62.Wagoner MD. Chemical injuries of the eye: Current concepts in pathophysiology and therapy. Surv Ophthalmol 1997; 41: 275–313. [DOI] [PubMed] [Google Scholar]
- 63.Hendrix DV, Brooks DE, Smith PJ, et al. Corneal stromal abscesses in the horse: A review of 24 cases. Equine Vet J 1995; 27: 440–47. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki H, Yamamura K, Mukai T, et al. Enhancement of ocular drug penetration. Crit Rev Ther Drug Carrier Syst 1999; 16: 85–146. [PubMed] [Google Scholar]
- 65.Koch SA. Congenital ophthalmic abnormalities in the Burmese cat. J Am Vet Med Assoc 1979; 174: 90–1. [PubMed] [Google Scholar]
- 66.Narfstrom K. Hereditary and congenital ocular disease in the cat. J Feline Med Surg 1999; 1: 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belhorn RW, Barnett KC, Henkind P. Ocular colobomas in domestic cats. J Am Vet Med Assoc 1971; 159: 1015–21. [PubMed] [Google Scholar]
- 68.Barnett KC, Lewis JC. Multiple ocular colobomas in the snow leopard (Uncia uncia). Vet Ophthalmol 2002; 5: 197–99. [DOI] [PubMed] [Google Scholar]
- 69.Cutler TJ. Bilateral eyelid agenesis repair in a captive Texas cougar. Vet Ophthalmol 2002; 5: 143–48. [DOI] [PubMed] [Google Scholar]
- 70.Martin CL, Stiles J, Willis M. Feline colobomatous syndrome. Vet Comp Ophthalmol 1997; 7: 39–43. [Google Scholar]
- 71.Hendy-Ibbs P.M. Familial feline epibulbar dermoids. Vet Record 1985; 116: 13–14. [DOI] [PubMed] [Google Scholar]
- 72.Ketring KL, Glaze MB. Dermoid. In: Atlas of feline ophthalmology. Trenton: Veterinary Learning Systems, 1994: 41. [Google Scholar]
- 73.Ketring KL, Glaze MB. Distichiasis. In: Atlas of feline ophthalmology. Trenton: Veterinary Learning Systems, 1994; 27. [Google Scholar]
- 74.Barnett KC, Crispin SM. Upper and lower eyelids. In: Feline ophthalmology: an atlas & text. London: WB Saunders, 1998: 44–54. [Google Scholar]
- 75.Hacker DV. Ectopic cilia in a Siamese cat. Comp Anim Pract 1989; 19: 29–31. [Google Scholar]
- 76.Rozsa AJ, Beuerman RW. Density and organisation of free nerve endings in the corneal epithelium of the rabbit. Pain 1982; 14: 105–20. [DOI] [PubMed] [Google Scholar]
- 77.Lu L, Reinach PS, Kao WW-Y. Corneal epithelial wound healing. Exp Biol Med 2001; 226: 653–64. [DOI] [PubMed] [Google Scholar]
- 78.Gilbard JP, Rossi SR. Tear film and ocular surface changes in a rabbit model of neurotrophic keratitis. Ophthalmology 1990; 97: 308–12. [DOI] [PubMed] [Google Scholar]