Abstract

Practical relevance Clinicians who deal with diabetic cats can have mixed experiences. Some patients are ‘textbook cases’, responding very well to insulin administration; others prove to be more challenging. Recent studies have shown a significant proportion of problem diabetic cats to have underlying acromegaly (hypersomatotropism). Recognising this syndrome in these cats will be key to successfully managing the concurrent diabetes.

Patient group Just like the ‘normal’ (non-acromegalic) diabetic cat, the acromegalic diabetic cat tends to be a middle-aged to older male neutered domestic short hair. However, with increasing case experience, this signalment may change. Most patients are insulin resistant, although this may not be the initial presenting sign. No breed predispositions have been recognised to date.
Clinical challenges There is no single diagnostic test for feline acromegaly — a confident diagnosis relies on a combination of clinical signs, feline growth hormone and insulin-like growth factor 1 levels, and intracranial imaging. Additionally, the ideal treatment protocol has yet to be established. Currently, radiotherapy is considered by many to be the best treatment; however, costs, the need for multiple anaesthetics, and the often delayed and unpredictable treatment response represent serious limitations of this modality. Previously, medical treatment has proven unsuccessful. Recent studies provide some evidence in favour of, and some against, the use of newer long-acting somatostatin analogue preparations in a proportion of acromegalic cats.
Evidence base Two recent studies have revealed a relatively high prevalence of acromegaly among diabetic cats. One also specifically assessed the value of hormonal tests, computed tomography and magnetic resonance imaging during the diagnostic process.
Aetiology of Acromegaly
The predominant cause of feline acromegaly is a functional somatotropic adenoma in the pars distalis of the anterior pituitary gland resulting in excessive growth hormone (GH) secretion.1–3 Research into the underlying mechanism of this adenomatous change has revealed overexpression of the cell cycle gene cyclin B2 (ccnb2) in human pituitary adenomas. 4 The exact initiating factor for this overexpression remains to be elucidated. In mouse models it has been shown that direct binding of so-called high mobility group A (HMGA) proteins to the promoter of the ccnb2 gene occurs during pituitary tumour formation. Additionally HMGA proteins were shown to be able to up-regulate ccnb2 promoter activity. Whether these human and mouse model findings correlate with the situation in the cat is unclear.
While adenoma formation seems to be the predominant cause of acromegaly in cats, the possibility of occasional acromegalic cats with somatotrophic hyperplasia has recently been suggested.1,5 One could therefore speculate that this represents either a separate disease process or perhaps a pre-adenomatous change or state.
Tip of the Iceberg?
In the UK, a large screening study among diabetic cats with variable glycaemic control revealed that 59 out of 184 (ie, 1 in 3) individuals had insulin-like growth factor 1 (IGF-1) values strongly suggestive of acromegaly (>1000 ng/ml). 1 A subpopulation of these 59 cats was submitted for further tests in order to conclusively establish a diagnosis. Intracranial contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) helped to establish a definitive diagnosis of acromegaly in 94% of these more carefully assessed cases. This proved two things: first, IGF-1 appears to be a useful screening tool; secondly, the original estimation of the prevalence of acromegaly among the larger diabetic cat population seems accurate and therefore likely to be close to the true prevalence in the diabetic cat population in the UK, especially as samples were recruited from primary (non-referral) practices and glycaemic control ranged widely from reasonable to inadequate. Further support for this detected high prevalence was delivered by a retrospective North American study indicating that 26% of diabetic cats met clinical criteria for the diagnosis of acromegaly. 3 The two studies therefore suggest that acromegaly is less rare than previously thought. There is a genuine possibility that this syndrome is currently underdiagnosed — or even, as has been suggested elsewhere, that we are only recognising ‘the tip of the iceberg’. 12
Pituitary adenomas secreting multiple hormones are recognised in human endocrinology. Most frequently, they produce GH, prolactin, thyroid stimulating hormone and/or the alpha subunit of the glycoprotein hormones. Other uncommon combinations may also be apparent. 6 The potential for plurihormonality in at least some feline pituitary adenomas is suggested by a case report describing a cat with a double pituitary adenoma causing both hyperadrenocorticism and acromegaly. 7 Feline cases of progestin-induced GH secretion in the mammary gland causing clinical acromegaly, as seen in the dog, have yet to be described; however, expression of GH mRNA in feline mammary gland tissue has been demonstrated, which indicates that mammary GH expression could also occur in the cat. The mammary gene was found to be identical to the pituitary-expressed gene, as well as being driven by the same promoter.8,9
Pathophysiology — and Its Manifestations
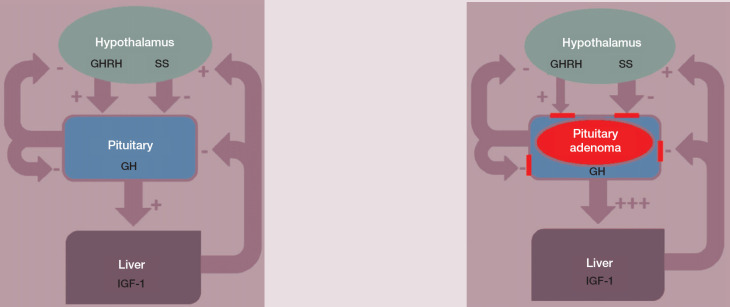
Normally, GH is secreted in a pulsatile fashion by the pars distalis of the pituitary gland and this is subject to negative feedback control mechanisms (Fig 1). In the case of hypersomatotropism due to a pituitary adenoma normal feedback mechanisms are disturbed, and this pulsatile release of feline GH appears not only increased in amplitude but also in frequency and duration. 10
Fig 1.
Production of GH and IGF-1 is normally finely regulated thanks to feedback mechanisms (left). In the case of a GH-producing pituitary adenoma (right), feedback mechanisms tend to fail to control GH and, in turn, IGF-1 production. GHRH = growth hormone releasing hormone, SS = somatostatin, GH = growth hormone, IGF-1 = insulin-like growth factor 1; + = stimulates, — = inhibits
The syndrome of acromegaly results from a generalised overexposure of tissues to not only GH, but also IGF-1, the latter because of a significant elevation in GH-induced production, predominantly by the liver (Fig 1). The classical clinical signs of acromegaly (see later) are the result of the anabolic and catabolic effects of GH, the anabolic effects of IGF-1 and, in some cases, the additional effect of a gradually expanding pituitary tumour.
Extensive research has shown that GH is an important modulator of insulin sensitivity. A multitude of mechanisms seem to be involved in this process, including hyperinsulinaemia-induced reduction of insulin receptor levels and impairment of insulin's kinase activity. 11 Growth hormone and insulin have many post-receptor events in common. Both the liver and striated muscle are thought to be important sites for GH-induced insulin resistance. In striated muscle GH-induced insulin resistance has been suggested to involve an increase in the p85 subunit of phosphatidylinositol 3-kinase, resulting in reduced insulin signalling. Growth hormone also induces suppressors of cytokine signalling and reduces insulin sensitivity by enhancing stimulation of serine phosphorylation of insulin receptor substrate 1, decreasing its affinity for the insulin receptor. Finally, GH has been shown to decrease the expression of the insulin-sensitising adipocytokines adiponectin and visfatin. 11
In the light of the above, it seems logical that, thus far, almost all reported cats with acromegaly have had concurrent insulin-resistant diabetes mellitus.
Adenoma formation seems to be the predominant cause of acromegaly in cats.
Extensive research has shown that GH is an important modulator of insulin sensitivity … In the light of this, it seems logical that, thus far, almost all reported cats with acromegaly have had concurrent insulin-resistant diabetes mellitus.
Insulin-like growth factor 1 has extensive anabolic potential and, when abnormally elevated for extended periods of time, can lead to excessive tissue growth and deformations. This might display itself as renal or myocardial changes (resulting in nephropathy or cardiomyopathy), thyroid enlargement, adrenomegaly, hepatomegaly, thickening of oropharyngeal tissues (Fig 2 and Fig 3), and bone and cartilage remodelling and thickening (resulting in arthropathy, broad facial features and enlarged, or so-called ‘clubbed’, paws).1,2,12–16
Fig 2.
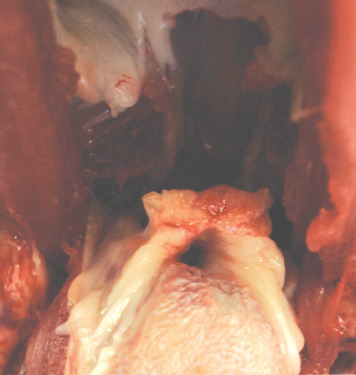
Intraoral view of a cat with confirmed acromegaly showing thickening of oropharyngeal tissues, in particular the soft palate. This is thought to underlie the inspiratory stridor that is encountered in these cats. Bar = 1 cm. Courtesy of Dr Julia Beatty
Fig 3.
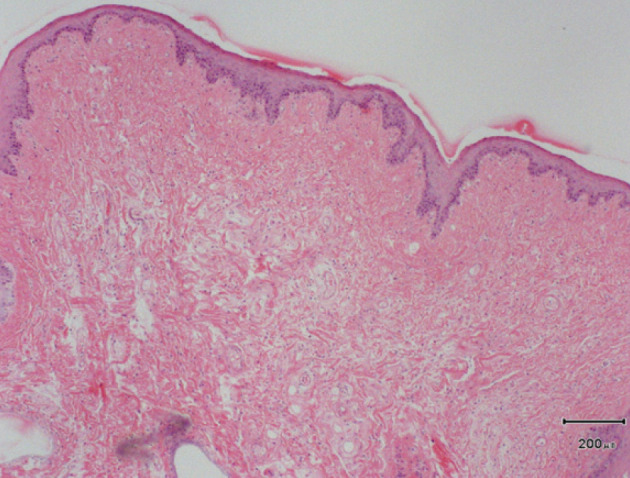
Histopathology of the soft palate of the cat shown in Fig 2 reveals severe thickening, mostly due to hyperplasia of mucous (salivary) glands with irregular connective tissue between some acini and a wide zone of loose connective tissue beneath the oral mucosa. Courtesy of Dr Julia Beatty
Renal histopathology in acromegalic cats has shown diffuse thickening of the glomerular basement membrane, thickening of Bowman's capsule, periglomerular fibrosis, adipose and hydropic change with epithelial degeneration and regeneration of tubules. 1
Echocardiography in confirmed acromegalic cats reveals a spectrum of changes, ranging from no structural abnormalities, to atrial enlargement, generalised or focal interventricular septal thickening, and/or left ventricular free wall hypertrophy with thinning towards the apex. Diastolic dysfunction, systolic anterior motion of the mitral valve, mitral valve insufficiency and increased left ventricular outflow velocity, poor left atrial wall motion, spontaneous echo contrast, and a restrictive pattern of pulmonary venous flow have all been reported. 1 Overall, myocardial changes seem common in acromegaly and can lead to congestive heart failure as a sequela.
Hypertension has been reported, 2 yet the largest study to date did not detect an increased prevalence of hypertension in acromegalic cats on the basis of Doppler systolic blood pressure measurement and fundus examination. 1 Should it occur, ocular haemorrhages, sudden blindness or neurological abnormalities might be expected as presenting signs.
Pancreatic abnormalities were previously documented as incidental findings in acromegalic cats, and were also evident in two cats that underwent postmortem examination in the most recent British study on this disease.1,13 However, since pancreatic pathology has been reported to be potentially common among non-acromegalic diabetic cats, further studies are needed to confirm a specific predisposition in acromegalic diabetic cats. 17
Signalment
The predominant signalment of an acromegalic cat is that of a male neutered middle-aged to older domestic short hair with insulin-resistant diabetes mellitus.1,2,13–16 However, since relatively few cases have been described in the veterinary literature, clinicians need to remain open-minded. Although in the Royal Veterinary College's (RVC's) Acromegalic Cat Clinic insulin resistance is frequently encountered in acromegalic cats, it is not consistently present, and especially not at the time of initial diagnosis. The insulin dose of 59 acromegalic cats seen by the author ranged from 1–35 iu bid (median 7 iu bid). 1 Among these 59 cats there were 47 neutered males, six neutered females, five intact males and one of unknown gender; 52 were domestic short hair cats, three were domestic long hair, and four were of unknown breed. The median age was 11 years (range 6–17 years) and median body weight was 5.8 kg (range 3.5–9.2 kg).
The classical clinical signs of acromegaly are the result of the anabolic and catabolic effects of GH, the anabolic effects of IGF-1 and, in some cases, the additional effect of a gradually expanding pituitary tumour.
The absence of a typical acromegalic appearance should not decrease the clinician's index of suspicion for the condition in an insulin-resistant cat.
When is a High Index of Suspicion Warranted?
Feline acromegaly has (like its human counterpart) a slow and gradual onset, often resulting in delayed recognition by owners, or possibly a failure to recognise the syndrome altogether. The onset of clinical signs in the acromegalic cats seen by the author ranged broadly from 2–42 months (mean±SD, 11.2±11.4). 1
Table 1 lists the historical signs and clinical examination findings of 17 confirmed acromegalic cats, as reported by owners and veterinarians, respectively. The clinical signs seen, and hence the index of suspicion, will be partly dependent on the timing of diagnosis. If diagnosed early, acromegalic cats might well look like any other diabetic cat, whereas a more ‘classic’ acromegalic picture will be seen as the syndrome progresses (Fig 4 and Fig 5).
Table 1.
Historical signs and clinical examination findings in 17 confirmed acromegalic cats
| Historical signs (owner reported) | Clinical examination findings (attending clinician reported) |
|---|---|
| Polyuria (n=17) | Abdominal organomegaly (liver and kidneys) (n=15) |
| Polydipsia (n=17) | |
| Polyphagia* (n=16) | Broad facial features (n=14) |
| Weight gain (n=10) | Respiratory stridor (n=9) |
| Lameness (n=5) | Prognathia inferior with increased distance between upper and lower canine teeth (n=8) |
| Central nervous system signs (n=2) | |
| Increase in paw size (n=2) | Multiple limb lameness (n=5) |
| Broad facial features (n=1) | Systolic cardiac murmur (n=4) |
| Abdominal enlargement (n=1) | Clubbed (enlarged) paws (n=3) |
| Plantigrade stance — hindlimbs (n=1) (diabetic neuropathy) | Central nervous system signs (n=2) |
| Immature bilateral cataracts (n=2) | |
| Gallop rhythm (n=1) | |
| Plantigrade stance — hindlimbs (n=1) | |
| Periods of open-mouth breathing and tachypnoea when stressed (due to congestive heart failure) (n=1) |
Frequently described as ‘extreme’ From Niessen et al (2007) 1
Fig 4.
Two confirmed acromegalic cats with very different phenotypical appearances. (a) This cat looks like any other domestic short hair, whereas the cat pictured in (b) displays clear morphological facial changes induced by overexposure to GH and IGF-1
Fig 5.
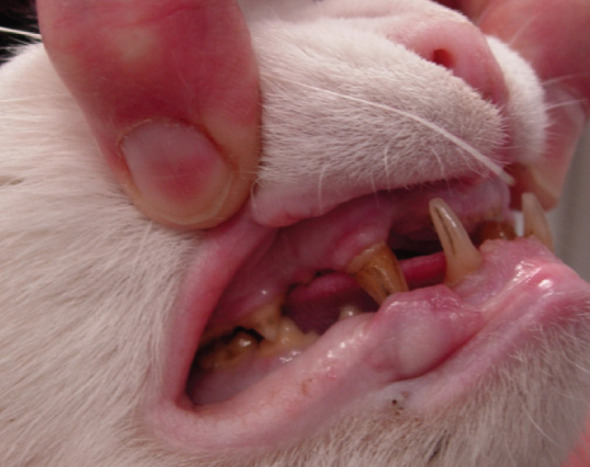
Prognathia inferior (protrusion of the mandible) in an acromegalic cat. Note the increased distance between the upper and lower canine teeth
As discussed earlier, uncontrolled GH secretion can result in poorly controlled diabetes mellitus in affected cats. However, very rare non-diabetic cats have been described. 2 The anabolic effects of IGF-1 may result in marked changes in the phenotypical appearance of an acromegalic cat. However, a recent study suggests that this typical acromegalic phenotype is not consistently present (Fig 4). 1 Certainly, the absence of a typical acromegalic appearance should not decrease the clinician's index of suspicion for the condition in an insulin-resistant cat.
Interestingly, a commonly encountered symptom in the cats seen at the RVC's Acromegalic Cat Clinic (53%) has been respiratory stridor, which is assumed to occur due to IGF-1 induced generalised thickening of oropharyngeal tissues (Fig 2 and Fig 3).
Once the presence of true insulin resistance is established in a cat (see box on page 19), acromegaly must be considered. Any weight gain in the light of poor diabetic control should further increase the suspicion of the presence of acromegaly or perhaps hyperadrenocorticism, although the latter is reported to be more often associated with weight loss.
How Best Should a Suspected Case Be Confirmed?
Routine Clinical Pathology
A comparison of routine clinical pathology parameters (haematology, biochemistry, urinalysis) between acromegalic diabetic and non-acromegalic diabetic cats demonstrated that only total protein levels were significantly elevated in the acromegalic cats. 18 Although haematocrit, glucose and fructosamine showed a trend towards being more elevated in the acromegalic cats, statistical significance was not attained for these parameters and there was considerable overlap with the values recorded in non-acromegalic diabetic cats. 18 Anecdotally, hyperphosphataemia has also been seen in acromegalic cats due to increased renal reabsorption, 19 although it was not documented in recent studies.1,18
True or Apparent Insulin-Resistance?
It is important to keep in mind that apparent insulin resistance can arise as a result of a number of management and disease factors, examples of which are given below. Undoubtedly, the most common cause of problems with diabetic control is a management issue, usually owner-related. Clinicians should, therefore, try their utmost best to exclude management issues prior to undertaking more elaborate and expensive specialist investigations. Owners should be encouraged to demonstrate an adequate insulin injection technique, and insulin handling and storage habits should be carefully revisited.
Before arriving at a diagnosis of true insulin resistance, the clinician is strongly advised to carefully interpret a combination of clinical (ie, persistent diabetes-related) signs and a range of diagnostic data (eg, fructosamine, multiple at-home collected urine glucose samples, multiple hospital and/or home blood glucose curves). A single blood glucose curve indicating consistently high blood glucose levels does not prove the presence of insulin resistance!
Management Associated
Incorrect insulin administration
Incorrect insulin storage
Inactive insulin preparation
Underdosing
Overdosing and subsequent hyperglycaemia from Somogyi phenomena
Short duration of insulin action
Stress hyperglycaemia
Endocrinopathy/Iatrogenic Hormone Administration
Hyperthyroidism
Hyperadrenocorticism
Hypersomatotropism (acromegaly)
Corticosteroid/megestrol acetate administration
Infectious Disease
Urinary tract infection
Dental disease
Inflammatory Disease
Pancreatitis
Inflammatory bowel disease
Gingivostomatitis
Other Disease
Obesity
Neoplasia
Nephropathy
Cardiovascular disease
In view of the lack of screening potential of routine clinical pathology parameters, more specific laboratory tests are necessary to diagnose acromegaly, as discussed below.
IGF-1
Measurement of IGF-1 provides a practical diagnostic aid, being both readily available and reasonably reliable. However, false positive and false negative results have been reported.1,16 Documenting an elevated IGF-1 concentration therefore does not permit a definitive diagnosis, but indicates when further testing is warranted. Indeed elevated IGF-1 levels have been reported in apparently non-acromegalic diabetic cats.20,21
GH
Feline GH estimation has proved an unpopular diagnostic aid in the past in view of limited assay availability, and assumed impractical sample handling requirements and lack of specificity and sensitivity. In human endocrinology, a single GH determination has proven unreliable; however, the likelihood of acromegaly is great if a GH concentration >10 ng/ml is recorded, which does illustrate the value of the assay.22,23
A recently validated feline GH radioimmunoassay proved useful in distinguishing normal cats from acromegalic cats, with no overlap occurring between the two groups studied. 5 Furthermore, stability studies showed that feline GH concentrations remained stable in circumstances similar to overnight transport at room temperature. 5 In a separate study that used 10 ng/ml as a cut-off, two out of 34 non-acromegalic diabetic cats were wrongly classified as being acromegalic. 1 Nevertheless, the specificity and sensitivity of this assay at that particular cut-off level proved a reasonable 95% and 84%, respectively.
Which Combination of Tests?
Unfortunately, no single diagnostic test with sufficiently high specificity and sensitivity exists for acromegaly, and so a combination of tests must be relied on to diagnose cases with a reasonable level of confidence. In the practical setting, pituitary-dependent hyperadrenocorticism is an important differential diagnosis, and differentiation from acromegaly can prove difficult (both can cause insulin-resistant diabetes mellitus, are associated with a pituitary abnormality and can cause adrenomegaly). Ultimately, however, the combination of clinical signs, feline GH and/or IGF-1 determination, and intracranial imaging will provide a confident diagnosis in most cases.
Intracranial Imaging
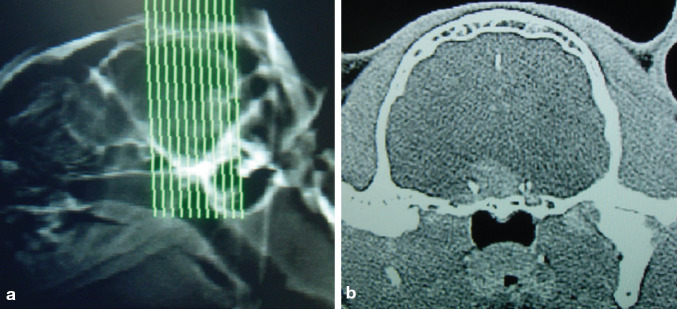
Once IGF-1 or feline GH determination (or preferably a combination of the two — see page 19) suggest acromegaly, intracranial imaging is indicated (Fig 6). Contrast-enhanced CT and MRI have proven useful in the past and in recent reports, although false negative results have been documented and differentiation of pituitary abnormalities is not possible on the basis of such imaging alone.1,15
Fig 6.
Computed tomography should be aimed at visualising the pituitary fossa before and after contrast administration. (b) Transverse section of the brain of an acromegalic cat, showing a mass at the level of the pituitary fossa
Post-Mortem Examination
Ultimately, definitive diagnosis relies on postmortem examination by a neuroendocrine-oriented experienced pathologist, evaluating the pituitary gland using specific staining protocols.
Treatment Approaches
Radiotherapy
Recently attention has been focused on the use of radiotherapy to treat pituitary tumours.24–27 Resolution or improvement of diabetes mellitus and neurological signs has been seen in cats undergoing a variety of radiotherapy protocols, suggesting that radiotherapy is probably the best treatment option that clinicians can currently offer owners of acromegalic cats. However, not all patients will respond to radiotherapy, with some showing improvement of diabetes mellitus but persistence of other acromegalic signs; 27 the author has documented a decrease in feline GH in one cat subsequent to radiotherapy, while IGF-1 remained elevated. 5 Given also the need for multiple anaesthetics, the unpredictable response, minor side-effects such as depigmentation (Fig 7) and the costs of such therapy, it is clear that efforts need to be directed at developing superior alternative treatment methods.
Fig 7.

Depigmentation in an acromegalic cat as a result of radiotherapy treatment
Targeting the pituitary radiotherapy more carefully (using stereotactical software) might, however, prove useful.
Hypophysectomy
Hypophysectomy, the gold standard treatment for many human acromegaly patients, would appear to be a logical approach that could result in fast and complete normalisation of feline GH levels. Transsphenoidal hypophysectomy has been employed as a treatment method for pituitary tumours in cats at the University of Utrecht. 28 However, it is a highly specialised procedure, which is not likely to be available to many clinicians in the near future. It is also not without risk, with two out of seven cats with a pituitary tumour dying within 4 weeks of surgery, albeit of apparently unrelated disease. 28
Cryohypophysectomy has been described in two cases to date, but needs further evaluation and longer-term follow-up before any general recommendations can be made.29,30
Medical Therapy
Medical therapy has thus far proven disappointing, with dopamine agonists and somatostatins not producing detectable clinical improvement.2,31 Long-acting formulations of somatostatin analogues, requiring only once-monthly injection, have greatly improved the convenience, efficacy and popularity of this class of drugs in human medicine. Growth hormone/ IGF-1 hypersecretion is controlled by these drugs in about 50% of patients, with the remaining patients requiring additional treatment. 32 Pegvisomant, a GH-receptor antagonist, is effective in most of these latter patients, but has not yet been assessed in acromegalic cats.
Is Conservative (Insulin) Treatment a Valid Approach?
Conservative treatment represents a genuine alternative option, especially in cases where definitive treatment is simply not possible (eg, too costly). In the author's experience it has proved possible to obtain a reasonable quality of life employing high doses of insulin (dose determined by gradual increases, each time guided by blood glucose curves and clinical image). However, some authors suggest limiting insulin doses to a maximum of 15 iu per injection. Although this might prevent hypoglycaemia in some cats, the clinical picture of excessive polyuria, polydipsia and polyphagia might be unacceptable to cat and owner without resorting to higher insulin doses.
When using unconventionally high insulin doses, home urine and/or blood glucose monitoring, and good communication between attending clinician and owner will be essential in the prevention and early detection of hypoglycaemia, especially in the post-radiotherapy period. Since GH secretion remains pulsatile in the acromegalic state, some cats can indeed suddenly suffer from life-threatening hypoglycaemia. If acceptable to the owner, specifically measuring blood glucose concentration at home before each dose of insulin can be considered in order to reduce the risk of clinical hypoglycaemia. Monitoring for negative glycosuria might prove a more acceptable alternative for some owners.
A pilot trial with a long-acting somatostatin analogue, which is currently ongoing at the RVC's Acromegalic Cat Clinic, has proved disappointing. However, a recent report documented suppression of GH levels in acromegalic cats following intravenous octreotide administration. 33 Adminis -tration of octreotide and measurement of pituitary hormones, specifically GH, was suggested as a test to aid selection of cats that would benefit from somatostatin treatment.
Concurrent Morbidities
Prompt attention to acromegaly-associated concurrent morbidities is essential and will make all the difference in establishing a good quality of life. Arthropathies require analgesia and congestive heart failure, nephropathy and hypertension require specific therapy.
Case Notes
Boris, a 9-year-old male neutered domestic short hair cat, presented with a 5-month history of diabetes mellitus that was proving difficult to control. Increasing insulin doses had been ineffective in controlling the diabetic signs.

History Boris was diagnosed with diabetes mellitus after he developed typical clinical signs, including polyuria, polydipsia and severe polyphagia, as well as weight gain. His owners had not noticed any change in his physical appearance. At the time of examination, he was being treated with 5 iu of PZI (protamine zinc insulin) twice daily, but this was having no significant impact on the clinical and laboratory control of the diabetes mellitus. The owner's insulin injection technique and insulin storage habits were reviewed and found to be adequate. Replacing the old insulin vial with a new vial did not result in improved control.
Physical examination and laboratory findings Physical examination proved fairly unremarkable, with no obvious features suggestive of acromegaly. Boris's weight was 5.6 kg. Cardiovascular assessment revealed no abnormalities, as did fundus examination. Interestingly, respiratory stridor was noted during the cat's hospitalisation. Haematology was unremarkable, whereas biochemistry revealed mild hypercholesterolaemia. Urinalysis showed glucosuria; urine culture did not yield any growth. Fructosamine assessment confirmed the lack of control (598 μmol/l, reference range 205–322 μmol/l). Systolic blood pressure measurement (Doppler) was normal.
-
WHAT IS YOUR ASSESSMENT?
-
How does Boris's presentation differ from that of a ‘normal’ diabetic cat?
He is not showing any response to the administered insulin
He is a male neutered middle-aged cat
He is gaining weight while his diabetes remains uncontrolled
A ‘normal’ diabetic cat would always show ‘hypoglycaemia’ on 5 iu of PZI bid
-
Respiratory stridor is not commonly associated with uncomplicated diabetes mellitus
Further investigations Abdominal ultrasound was performed to exclude further causes of insulin-resistant diabetes mellitus. The liver was diffusely hyperechoic, possibly compatible with the diabetic state. Adrenal morphology was unremarkable, as were the urinary tract, abdominal lymph nodes and the remainder of the abdomen. Total thyroxine level assessment suggested sick euthyroid syndrome (7.2 nmol/l; reference range 19–65 nmol/l). A sample for IGF-1 concentration was submitted for analysis and returned significantly elevated (1170 ng/ml; acromegaly suspected when >1000 ng/ml).
On the basis of the presence of insulin-resistant diabetes mellitus, the absence of any common reasons to explain problematic diabetic control (eg, owner-related management errors, urinary tract infection) and an elevated IGF-1, intracranial imaging was performed. Computed tomography proved unremarkable, but the presence of a pituitary abnormality was demonstrated using MRI (see below). Hyperadrenocorticism was excluded as a differential (in view of the cat's normal skin and fur, no bruising tendency, and unremarkable adrenal morphology), and acromegaly was deemed most likely.
Radiotherapy was initiated, but proved unsuccessful, and the cat is currently part of a novel long-acting somatostatin analogue trial in an attempt to control his disease.
-
Which one or more endocrinopathies are most useful to consider when dealing with a cat with diabetes mellitus that proves difficult to control?
Hyperthyroidism
Hyperadrenocorticism
Acromegaly
Hyperparathyroidism
Phaeochromocytoma
-
Answers 1 (a), (c), (e); 2 (a), (b), (c)
Key Points
Feline acromegaly is more common thanpreviously thought and represents an essential differential diagnosis for the insulin-resistant diabetic cat.
The phenotypical appearance of acromegalic cats can range from classical to unremarkable.
Further cases need to be described in order to provide a more comprehensive description of disease presentation and progression.
A confident diagnosis requires a combination of clinical examination findings, hormonal tests (particularly IGF-1 and feline GH) and intracranial imaging of the pituitary fossa.
Treatment options consist of conservative (treating the diabetes mellitus) or definitive modalities (radiotherapy, hypophysectomy or medical treatment).
Radiotherapy seems currently to the best and mostaccessible option, but is expensive and results are not consistently successful.
Medical treatment was previously thought to be ineffective; however, newer longer-acting somatostatin analogues will hopefully prove useful in at least a subset of acromegalic cats.
Prognosis — Experience to Date
The prognosis is difficult to establish in view of the gradual onset of the disease and lack of objective information on its duration. If untreated with a definitive method, most cats eventually succumbto, or are euthanased as a resultof, acromegaly or associated disease or treatment morbidities (congestive heart failure, renal failure, hypoglycaemia, respiratory distress, pituitary mass-induced neurological signs). Some cats survive for only a few weeksfollowing diagnosis, whereas others live for many years before dying from causes that may be unrelated to their endocrinopathy.
Gaps to Be Filled
Further researchis essential and will hopefully yield a more thorough understanding ofthis endocrinopathy, especially in view of its recently recognised prevalence. Placebo-controlled randomised clinical trials comparing definitively treated (medical, surgical, radiotherapy) with conservatively treated (insulin-only) patients are needed to pass a balanced judgement on the benefits of these different treatment modalities. Such research would hopefully result in a better quality of life foracromegalic cats, as well as for their owners.
Acknowledgements
The author would like to thank all involved in the RVC's AcromegalicCat Clinic, in particularthe owners and their cats, and also the nurses at the Clinical Investigations Centre ofthe RVC, David Church, Mohammed Khalid, Jane Eastwood, Lucy Goodwin and Yaiza Forcada, as well as PetPlan for its support in validating the feline GH assay. Dr Julia Beatty of the Valentine CharltonCat Centre, The Universityof Sydney, Australia, is thanked for providing the oropharyngeal tissue pictures.
References
- 1.Niessen SJ, Petrie G, Gaudiano F, et al. Feline acromegaly: An underdiagnosed endocrinopathy? J Vet Intern Med 2007; 21: 899–905. [DOI] [PubMed] [Google Scholar]
- 2.Feldman EC, Nelson RW. Disorders of growth hormone. In Feldman EC, Nelson RW. (eds). Canine and feline endocrinology and reproduction. 3rd edn. St Louis, Missouri: Saunders, 2004: p 69. [Google Scholar]
- 3.Berg RI, Nelson RW, Feldman EC, et al. Serum insulin-like growth factor-I concentration in cats with diabetes mellitus and acromegaly. J Vet Intern Med 2007; 21: 892–98. [DOI] [PubMed] [Google Scholar]
- 4.De Martino I, Visone R, Wierinckx A, et al. Fusco A proteins up-regulate CCNB2 gene in mouse and human pituitary adenomas. Cancer Res 2009; 69: 1844–50. [DOI] [PubMed] [Google Scholar]
- 5.Niessen SJ, Khalid M, Petrie G, Church DB. Validation and application of an ovine radio-immunoassay for the diagnosis of feline acromegaly. Vet Rec 2007. 160: 902–7. [DOI] [PubMed] [Google Scholar]
- 6.Salehi F, Cohen S, Syro LV, et al. Plurihormonality in pituitary adenomas associated with acromegaly. Endocr Pathol 2006; 17: 291–96. [DOI] [PubMed] [Google Scholar]
- 7.Meij BP, Van der Vlugt-Meijer RH, van den Ingh TS, Rijnberk A. Somatotroph and corticotroph pituitary adenoma (double adenoma) in a cat with diabetes mellitus and hyperadrenocorticism. J Comp Pathol 2004; 130: 209–15. [DOI] [PubMed] [Google Scholar]
- 8.Mol JA, van Garderen E, Selman PJ, Wolfswinkel J, Rijnberk A, Rutteman GR. Growth hormone mRNA in mammary gland tumors of dogs and cats. J Clin Invest 1995; 95: 2028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mol JA, van Leeuwen Lanting I, van Garderen E, Rijnberk A. Progestin-induced mammary growth hormone (GH) production. Adv Exp Med Biol 2000; 480: 71–76. [DOI] [PubMed] [Google Scholar]
- 10.Barkan AL, Stred SE, Reno K, et al. Increased growth hormone pulse frequency in acromegaly. J Clin Endocrinol Metab 1989; 69: 1225–33. [DOI] [PubMed] [Google Scholar]
- 11.Dominici FP, Argentino DP, Muñoz MC, Miquet JG, Sotelo AI, Turyn D. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res 2005; 15: 324–36. [DOI] [PubMed] [Google Scholar]
- 12.Peterson ME. Acromegaly in cats: Are we only diagnosing the tip of the iceberg? J Vet Intern Med 2007; 21: 889–91. [PubMed] [Google Scholar]
- 13.Gunn-Moore D. Feline endocrinopathies. Vet Clin North Am Small Anim Pract 2005; 35:171–210, vii. [DOI] [PubMed] [Google Scholar]
- 14.Hurty CA, Flatland B. Feline acromegaly: A review of the syndrome. J Am Anim Hosp Assoc 2005; 41: 292–97. [DOI] [PubMed] [Google Scholar]
- 15.Peterson ME, Taylor RS, Greco DS, et al. Acromegaly in 14 cats. J Vet Intern Med 1990; 4: 192–201. [DOI] [PubMed] [Google Scholar]
- 16.Norman EJ, Mooney CT. Diagnosis and management of diabetes mellitus in five cats with somatotrophic abnormalities. J Feline Med Surg 2000; 2: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forcada Y, German AJ, Steiner JM, et al. Determination of fPLI concentrations in cats with diabetes mellitus. J Feline Med Surg 2008; 10: 480–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niessen SJ, Petrie G, Gaudiano F, et al. Routine clinical pathology findings in feline acromegaly. Proceedings of the British Small Animal Veterinary Association (BSAVA) congress 2007, Birmingham, UK, 2007.
- 19.Casanueva FF. Physiology of growth hormone secretion and action. Endocrinol Metab Clin North Am 1992; 21: 483–517. [PubMed] [Google Scholar]
- 20.Lewitt MS, Hazel SJ, Church DB, Watson ADJ, Powell SE, Tan K. Regulation of insulin-like growth factor-binding protein-3 ternary complex in feline diabetes mellitus. J Endocrinol 2000; 166: 21–27. [DOI] [PubMed] [Google Scholar]
- 21.Starkey SR, Tan K, Church DB. Investigation of serum IGF-I levels amongst diabetic and non-diabetic cats. J Feline Med Surg 2004; 6: 149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler J. Biochemical tests of growth hormone status in short children. Ann Clin Biochem 2001; 38: 1–2. [DOI] [PubMed] [Google Scholar]
- 23.Chang-DeMoranville BM, Jackson IMD. Diagnosis and endocrine testing in acromegaly. Endocrinol Metab Clin North Am 1992; 21: 649–68. [PubMed] [Google Scholar]
- 24.Brearley MJ, Polton GA, Littler RM, Niessen SJM. Coarse fractionated radiation therapy for pituitary tumours in cats: A retrospective study of 12 cases. Vet Comp Oncol 2006; 4: 209–17. [DOI] [PubMed] [Google Scholar]
- 25.Mayer MN, Greco DS, LaRue SM. Outcomes of pituitary tumor irradiation in cats. J Vet Intern Med 2006; 20: 1151–54. [DOI] [PubMed] [Google Scholar]
- 26.Kaser-Hotz B, Rohrer CR, Stankeova S, Wergin M, Fidel J, Reusch C. Radiotherapy of pituitary tumours in five cats. J Small Anim Pract 2002; 43: 303–7. [DOI] [PubMed] [Google Scholar]
- 27.Littler RM, Polton GA, Brearley MJ. Resolution of diabetes mellitus but not acromegaly in a cat with a pituitary macroadenoma treated with hypofractionated radiation. J Small Anim Pract 2006; 47: 392–95. [DOI] [PubMed] [Google Scholar]
- 28.Meij BP, Voorhout G, Van Den Ingh TS, Rijnberk A. Transsphenoidal hypophysectomy for treatment of pituitary-dependent hyperadrenocorticism in 7 cats. Vet Surg 2001; 30: 72–86. [DOI] [PubMed] [Google Scholar]
- 29.Abrams-Ogg ACG, Holmberg DL, Stewart WA, Claffey FP. Acromegaly in a cat: Diagnosis by magnetic resonance imaging and treatment by cryohypophysectomy. Can Vet J 1993; 34: 682–85. [PMC free article] [PubMed] [Google Scholar]
- 30.Blois SL, Holmberg DL. Cryohypophysectomy used in the treatment of a case of feline acromegaly. J Small Anim Pract 2008; 49: 596–600. [DOI] [PubMed] [Google Scholar]
- 31.Abraham LA, Helmond SE, Mitten RW, Charles JA, Holloway SA. Treatment of an acromegalic cat with the dopamine agonist L-deprenyl. Aust Vet J 2002; 80: 479–483. [DOI] [PubMed] [Google Scholar]
- 32.Chanson P. Emerging drugs for acromegaly. Expert Opin Emerg Drugs 2008; 13: 273–93. [DOI] [PubMed] [Google Scholar]
- 33.Slingerland LI, Voorhout G, Rijnberk A, Kooistra HS. Growth hormone excess and the effect of octreotide in cats with diabetes mellitus. Domest Anim Endocrinol 2008; 35: 352–61. [DOI] [PubMed] [Google Scholar]