Abstract
Streptococcal pyrogenic exotoxin B (SPE B) is a cysteine protease produced by Streptococcus pyogenes. In this study, the differences in virulence between protease-positive clinical isolates and their protease-negative mutants were examined in a mouse model. Isogenic protease-negative mutants were constructed by homologous recombination, using integrational plasmids to disrupt the speB gene. These mutants caused less mortality and tissue damage than protease-positive strains when inoculated into BALB/c mice via air pouch, suggesting that SPE B cysteine protease plays an important role in the pathogenesis of S. pyogenes infection. Reconstitution of SPE B in the air pouches increased the mortality of mice receiving the speB mutant strain. Infiltrated cell numbers in the exudates from the air pouches of mice infected with SPE B-producing S. pyogenes were higher than those from mice infected with protease-negative mutants at 12 h. However, despite pretreatment with vinblastine to deplete neutrophils, injection of protease-positive bacteria still resulted in severe tissue injury, indicating that neutrophil infiltration may not be the major factor involved in SPE B-enhanced tissue damage. The role of SPE B was further confirmed by demonstrating that SPE B immunization of mice conferred protection from challenge with a lethal dose of protease-positive bacteria.
The group A streptococcus Streptococcus pyogenes causes serious diseases in humans, including streptococcal toxic shock syndrome and necrotizing fasciitis (17, 22, 24). Streptococcal pyrogenic exotoxin A (SPE A), SPE B, and SPE C, as well as the M-protein types, have been studied for their association with the severity of S. pyogenes infection. Among them, SPE A, which belongs to the bacterial superantigen family, has been implicated as an important factor contributing to the virulence of S. pyogenes. However, many isolates from patients with invasive disease lack the speA gene, indicating that other factors may also be involved in severe invasive streptococcal infections (4, 8, 24, 25, 28).
The chromosomally encoded speB gene is carried by every strain of S. pyogenes. SPE B, which functions as a cysteine protease, is expressed as a 40-kDa precursor and is subsequently cleaved to a 28-kDa molecule (3, 6, 18). Several lines of evidence suggest that this protease may be a critical virulence factor in streptococcal infections (7, 14, 29). Patients with low levels of antibody against SPE B are more likely to succumb to invasive group A streptococcal infections than individuals with high antibody titers (29). SPE B was shown in vitro to cleave interleukin-1β (IL-1β) precursor to produce biologically active IL-1β, a major mediator of inflammation (12). Additionally, SPE B cleaves human fibronectin and degrades vitronectin, which may help bacterial dissemination, colonization, and invasion and inhibit wound healing (13). These observations led to the interest in investigating the ability of SPE B to facilitate tissue injury in vivo.
Animal models have been used to study various aspects of infection by S. pyogenes (1, 9, 19, 20). Different routes of bacterial infection, including cutaneous, intranasal, intratracheal, intraperitoneal (i.p.), and skin air sac, have been adopted for the experiments. A recent study using i.p. inoculation showed that speB mutants lost or had a decrease in their ability to cause death in mice (14). In this study, we used an air pouch model in BALB/c mice for group A streptococcal infection. The isogenic protease mutants were generated by speB disruption (27). We compared the virulence of protease-negative mutants to that of protease-producing S. pyogenes strains. Mortality, tissue damage, and local cell infiltration were evaluated when protease-positive clinical isolates and their speB mutants were injected into the air pouches of mice. The efficacies of protection conferred by different types of SPE B immunization were also assessed.
MATERIALS AND METHODS
Mice.
BALB/cByJ mice were purchased from The Jackson Laboratory, Bar Harbor, Maine, and maintained on standard laboratory food and water ad libitum in our animal center. Their progeny, ranging from 6 to 8 weeks of age, were used for experiments.
Bacteria.
S. pyogenes A-20 (type M1, T1; opacity factor negative) was isolated from a culture of blood from a patient with necrotizing fasciitis in National Cheng Kung University Hospital. S. pyogenes NZ131 (type M49, T14) was a gift from D. R. Martin, New Zealand Communicable Disease Center, Porirua. A fresh colony was inoculated into tryptic soy broth containing 0.5% yeast extract (TSBY) (Difco Laboratories, Detroit, Mich.) and cultured for 8 h at 35°C. The bacteria were harvested by centrifugation and resuspended in sterile saline, and bacterial density was determined by measuring the absorbance at 600 nm (A600). The bacterial suspension was then diluted to 2 × 1010 CFU/ml with saline, using a standard growth curve to relate measured A600 to the bacterial concentration. For quantification of bacteria, samples were plated on blood agar or TSBY plates and incubated for 24 h at 35°C.
Purification of SPE B.
SPE B was purified from S. pyogenes A-20 by purification procedures described previously (27). A-20, a protease-producing clinical isolate, was grown overnight at 35°C in TSBY medium. A 10-ml bacterial culture was added to 500 ml of TSBY medium and then incubated at 35°C. After 24 h, the bacteria were removed by centrifugation and the supernatant was filtered through a 0.45-μm-pore-size membrane filter (Sartorius GmbH, Goettingen, Germany). The filtrate was diluted with 4 volumes of cold distilled water, the pH was adjusted to 8.0, and then 1/20 volume of DEAE-Sephadex equilibrated with 20 mM Tris-HCl (pH 8.0) was added. After 30 min of incubation with occasional mixing, the unbound material was collected by filtration. The filtrate was concentrated to 100 ml by a 3-kDa-cutoff ultrafiltration cartridge (Amicon Division, W. R. Grace & Co., Beverly, Mass.). Buffer exchange by ultrafiltration was conducted at 4°C with 1 liter of 20% ethanol–20 mM Tris-HCl, pH 7.0 (buffer A). The ultrafiltered solution was passed through a matrix gel Red A column (1.5 by 15 cm) (Amicon Division, W. R. Grace & Co., Danvers, Mass.) equilibrated with buffer A. The column was washed with buffer A until the absorbance (280 nm) returned to baseline, and the protein was eluted with buffer A containing 2 M NaCl. The eluted material was collected and concentrated by ultrafiltration. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining showed a single band with an apparent molecular mass of 28 kDa (27). The N-terminal sequence of this 28-kDa protein was confirmed as SPE B by an Applied Biosystems 477A autosequencer. The protein was run on a sodium dodecyl sulfate–12.5% polyacrylamide gel and transferred to polyvinylidene difluoride membrane (Millipore) for sequence analysis.
Assay for protease activity.
The assay procedure described previously was followed with modifications (18). The reaction was initiated by adding 100 μl of S. pyogenes culture supernatant or purified SPE B to 400 μl of a reaction mixture containing 2.7 mg of azocasein (Sigma Chemical Co., St. Louis, Mo.)/ml in 50 mM Tris-HCl (pH 8.0). After 20 min of incubation at 37°C, the reaction was stopped by adding 100 μl of 15% ice-cold trichloroacetic acid. After 15 min on ice, the mixture was centrifuged, and an equal volume of 0.5 M NaOH was added to the supernatant. The absorbance of the sample at 450 nm was measured with a V-max microplate reader (Molecular Devices Corporation, Menlo Park, Calif.). The specificity of protease activity was confirmed by using cysteine protease inhibitor E64.
speB mutation.
Disruption of the speB gene was described by Tsai et al. (27). Briefly, the insertion vectors pMW152 and pMW153 were constructed from the pSF151 and pDL286 vectors (provided by L. Tao, University of Missouri, Kansas City [26]). The insertion vectors containing 0.4- and 0.7-kb fragments of the speB gene from nucleotides 578 to 1005 and 347 to 1005, respectively, were ligated with the pSF151 and pDL286 shuttle vectors after KpnI and PstI or HindIII and PstI digestion. pMW152 and pMW153 were introduced into S. pyogenes A-20 and NZ131, respectively, by electroporation, and the successful inserts were selected on Luria-Bertani plates (Difco Laboratories) containing 50 μg of kanamycin and 100 μg of spectinomycin/ml. Insertions of pMW152 and pMW153 into the speB gene were confirmed by Southern blotting with the 0.4- and 0.7-kb speB gene fragments as the probes. Plasmid pMW152 integrated into A-20 to obtain an isogenic protease mutant was designated SW507, and plasmid pMW153 integrated into NZ131 was designated SW510. The absence of proteolytic activity of these inserts was determined by both casein plate assay, as described by Hynes and Tagg (10), and azocasein assay as described earlier. The culture supernatants were collected, and the absence of SPE B protein production by SW507 and SW510 was confirmed by Western blot analysis with rabbit anti-SPE B antibody as described in reference 27.
Air pouch model of infection.
Mice were anesthesized by ether inhalation and then injected subcutaneously with 1 ml of air to form an air pouch. Bacterial suspension (0.1 ml) was inoculated into the air pouch. The mortality rates were monitored every day during the experimental periods. Tissues around the air pouch were excised 48 h after bacterial inoculation, fixed in 10% formaldehyde, and embedded in paraffin. The fixed tissues were sliced 5 μm thick and stained with hematoxylin and eosin. In some experiments, the mice were treated intravenously with 20 μg (in 100 μl) of vinblastine for four consecutive days to deplete neutrophils before bacterial inoculation. The infiltrated cells in the air pouch were collected by injecting 1 ml of phosphate-buffered saline into the air pouch and aspirating the exudate by syringe with an 18-gauge needle. Cell numbers were determined with a hemocytometer.
Active immunization with SPE B.
Mice were immunized i.p. with 50 μg of purified SPE B mixed with complete Freund’s adjuvant, followed by three further immunizations with 25 μg of SPE B mixed with incomplete Freund’s adjuvant over a 9-week period. Mice that showed titers of anti-SPE B 100-fold or more higher than those from the nonimmunized group were selected for experiments. These mice were inoculated in the air pouches with 109 CFU of S. pyogenes A-20, and mortality was monitored over 20 days.
Preparation of anti-SPE B IgG.
Rabbits were injected intramuscularly with 50 μg of purified SPE B mixed with complete Freund’s adjuvant. Four subsequent immunizations with 25 μg of SPE B mixed with incomplete Freund’s adjuvant were given at 2-week intervals. The serum anti-SPE B titers were measured by enzyme-linked immunosorbent assay 4 days after the final boost. Anti-SPE B immunoglobulin G (IgG) was purified by passing it through a protein A column (Zymed Laboratories Inc., South San Francisco, Calif.). The neutralizing activity of anti-SPE B IgG was determined by azocasein assay as described earlier.
Passive immunization with rabbit anti-SPE B IgG.
Three groups of mice each received 109 CFU of S. pyogenes A-20 via air pouch inoculation. In one group, 180 μg of rabbit anti-SPE B IgG in 0.2 ml of sterile saline was injected into the air pouch immediately after bacterial inoculation. The other two groups, which received purified IgG from preimmune serum or sterile saline, served as the controls. Mortality was monitored over 14 days.
RESULTS
Virulence of S. pyogenes A-20 and NZ131 and their speB mutants in mice.
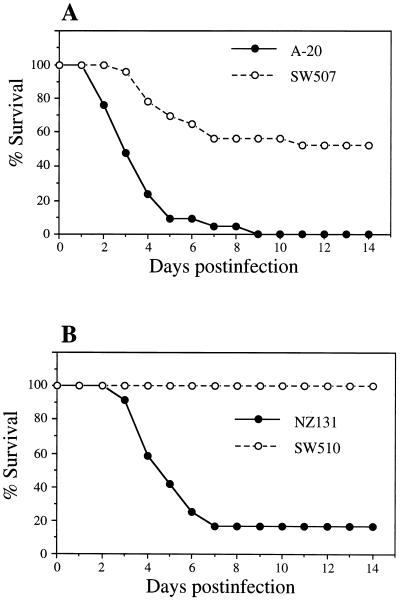
The protease activities of S. pyogenes A-20 and NZ131 and their speB mutants were determined by casein plate and azocasein assays, and the production of protease was confirmed by Western blot analysis with rabbit anti-SPE B antiserum (27). Studies revealed that the lethal doses of strains A-20 and NZ131, which exhibited protease activity, were in the range of 109 CFU via air pouch inoculation. At 12 h after injection with 4 × 109 CFU of protease-producing strains via the air pouch, mice became lethargic and developed ruffled fur. After 48 h, visible hair loss and bleeding on the skin around the air pouch were observed. No changes or only slight changes were seen in mice injected with the same number of CFU of speB mutants and monitored over a 14-day period. Figure 1 shows the percent survival of mice after injection with S. pyogenes A-20 and NZ131 and their speB mutants. The A-20 wild-type strain caused about 90% mortality on day 5 and 100% mortality on day 9, whereas its speB mutant, SW507, caused approximately 50% mortality over 14 days. Mice infected with NZ131 had only a 17% survival rate, whereas those injected with SW510 showed 100% survival.
FIG. 1.
Survival of S. pyogenes-infected mice after inoculation in air pouches with 4 × 109 CFU of wild-type A-20 (n = 21) or its speB mutant, SW 507 (n = 23) (A), or wild-type NZ131 (n = 12) or its speB mutant, SW510 (n = 15) (B).
The effect of SPE B on mouse mortality was tested directly by speB mutant strain inoculation with or without further addition of purified SPE B. When mice were injected with 4 × 108 CFU of SW507, the survival rate was 100%. Reconstitution of SPE B in the air pouch increased the lethality to 78% (14 of 18) and 100% (10 of 10) by day 6 (Table 1, experiments 1 and 2). However, treatment with SPE B alone did not cause a lethal effect (data not shown). These results revealed that SPE B caused an enhancing effect on mouse mortality. When SPE B was heat inactivated, the increased mortality rate was no longer observed (Table 1, experiment 2).
TABLE 1.
Mortality in SW507-infected mice with or without SPE B in the air pouch
| Expt no. | Inoculation | Mortality (no. dead/total)
|
||
|---|---|---|---|---|
| Day 2 | Day 4 | Day 6 | ||
| 1a | SW507 + saline | 0/12 | 0/12 | 0/12 |
| SW507 + SPE B | 7/18 | 14/18 | 14/18 | |
| 2b | SW507 + saline | 0/6 | 0/6 | 0/6 |
| SW507 + SPE B | 4/10 | 9/10 | 10/10 | |
| SW507 + SPE B (heat inactivated) | 0/8 | 0/8 | 0/8 | |
BALB/c mice were inoculated in the air pouches with 4 × 108 CFU of A-20 speB mutant SW507, and 150 μl of purified SPE B (1 mg/ml) or an equivalent volume of saline was also inoculated into the air pouches, at both 0 and 3 h.
In one group, SPE B was heat inactivated at 60°C for 20 to 30 min before inoculation into the air pouches.
Histologic studies.
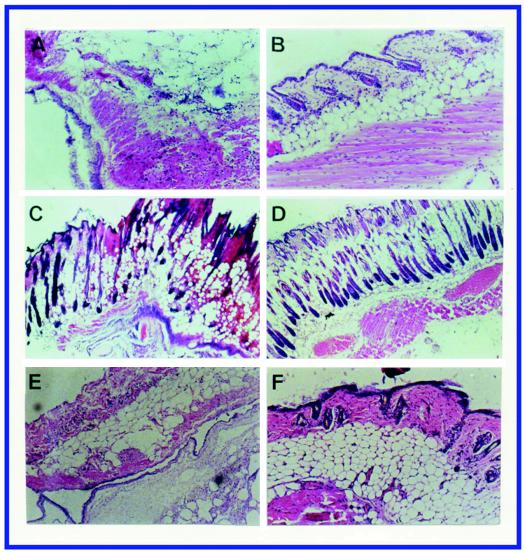
Forty-eight hours after infection with S. pyogenes A-20, skin tissue sections showed necrosis of epidermis, dermis, and subcutaneous fat (Fig. 2A). Similar findings were observed in NZ131-infected mice (Fig. 2C). This kind of tissue damage was not found at 48 h in mice infected with SW507 (Fig. 2B) or SW510 (Fig. 2D).
FIG. 2.
Skin tissue sections from mice 48 h after inoculation in air pouches with 109 CFU of S. pyogenes A-20 (A), SW507 (B), NZ131 (C), SW510 (D), A-20 in mice pretreated with vinblastine (E), and SW507 in mice pretreated with vinblastine (F). Magnification, ×24 (A, B, E, and F) and ×60 (C and D). The epidermis, subcutaneous fat, and muscle fibers were severely damaged or destroyed in skin tissues infected by wild-type strains with or without vinblastine pretreatment but not in those infected by the speB mutants.
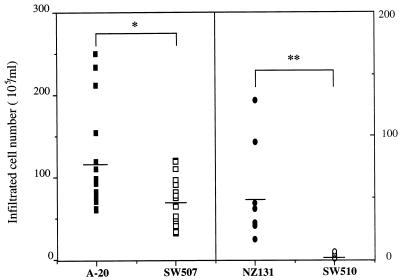
At 12 h a mass of infiltrated cells was seen in the exudates from mouse air pouches infected with A-20 and NZ131. The majority (>90%) of infiltrated cells had the morphology of neutrophils. A smaller number of infiltrated cells was observed in mice infected with speB mutants (Fig. 3). The difference in infiltrated cell numbers was particularly prominent in mice infected with NZ131 and its mutant, SW510. Even with treatment with vinblastine to deplete neutrophils before injection, A-20 still caused severe tissue injury (compare Fig. 2E and A). The depletion of neutrophils was confirmed by histological studies (Fig. 2E and F) and by both the peripheral blood cell count and the exudates of the air pouch. The A-20 speB mutant, SW507, did not cause tissue injury with or without vinblastine treatment (compare Fig. 2F and B).
FIG. 3.
Numbers of cells collected from the exudates of the air pouches of mice injected with 109 CFU of A-20 (n = 18), NZ131 (n = 7), and their speB mutants, SW507 (n = 18) and SW510 (n = 7), 12 h postinjection. ∗, P < 0.05; ∗∗, P < 0.01 (by Student’s t test).
Protective effects after active and passive SPE B immunization.
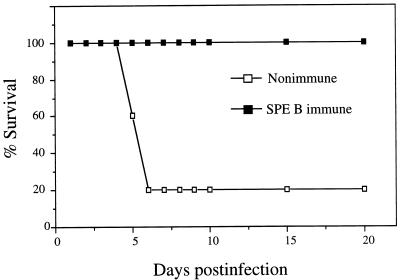
The protective efficacy of SPE B was tested by active vaccination. Mice were immunized with purified SPE B, and those that showed a serum anti-SPE B titer 100-fold or more higher than that of the nonimmunized group were selected for experiments. In the SPE B-immunized group 100% survival was recorded after A-20 challenge, while only 20% survival was observed in the nonimmunized group (Fig. 4). The severity of skin lesions in the vaccinated group was less than that in the nonimmunized control group, and the appearance with tissue injury was delayed (not shown). Passive immunization with anti-SPE B IgG conferred partial protection, with delayed death and about 50% survival 14 days after A-20 infection compared to 28% survival in the control group immunized with preimmune purified IgG and 22% survival in the control group immunized with saline. These results suggested the role of SPE B as an enhancing factor for the disease. The observation that administering purified SPE B alone did not result in tissue damage and lethality confirms this notion (data not shown).
FIG. 4.
Protection against skin infection by protease-positive S. pyogenes by immunization with SPE B. Mice were immunized with purified SPE B, with nonimmunized mice as controls, before A-20 challenge. Only those that showed titers of anti-SPE B 100-fold or more higher than those of the nonimmunized group were used for experiments. n = 5 mice/group.
DISCUSSION
Using an air pouch model, we showed in this study that rates of mortality and severe skin injury were lower in mice infected with speB mutants than in those infected with protease-positive S. pyogenes strains. This is consistent with a recent report demonstrating that streptococcal cysteine protease mutants had decreased lethality in mice infected by i.p. injection (14). Results with the insertion mutants thus provide direct evidence that SPE B may serve as an important virulence factor in group A streptococcal infection. We also showed that reconstitution of SPE B in the speB mutant inoculum caused an increase in the mortality rate (Table 1) and tissue damage (not shown).
Previous studies showed that both passive immunization with rabbit antibody directed against cysteine protease and active vaccination with cysteine protease protected mice against i.p. challenge with heterologous S. pyogenes (11). In the present study, protective effects were also observed for both active and passive immunization against protease-positive S. pyogenes challenge in the air pouch. SPE B appears to play a role in the pathogenesis of S. pyogenes infection, and immunity directed to this toxin could confer protection. However, SPE B alone did not have an effect. SPE B thus acts as an enhancing factor for the disease. In this study, active immunization conferred substantial protective effect whereas passive immunization provided only partial protection. Explanations for this include the possibility that the dose and timing of administration of the anti-SPE B antibodies were not optimal and also that, in addition to antibodies, other factors stimulated by SPE B immunization, such as T cells, may contribute to the protective effect. In contrast to SPE B, passive immunization against SPE A did not provide protection, and active vaccination with SPE A actually enhanced mortality in a mouse model (23).
Neutrophil influx was observed in rat lung injury induced by products of group A streptococci, including SPE B and streptococcal cell wall antigen (21). Our results also showed a higher level of neutrophil infiltration after inoculation with protease-positive S. pyogenes strains than after inoculation with the speB mutants. Figure 3 shows that the infiltrated cell numbers were far lower in mice infected with NZ131 speB mutant SW510 than in those infected with the wild-type strain. However, the difference between A-20 and SW507 was less prominent. In fact, the infiltrated cell numbers in the SW507-treated group showed a level similar to that of the NZ131 wild type-treated group. Genotyping of A-20 revealed the presence of speA and speC, while NZ131 lacks these two genes. In addition to SPE B, other molecules, such as SPE A, SPE C, and M protein types, may contribute to the infiltration of neutrophils. Further, despite treatment with vinblastine to deplete neutrophils before injection, protease-positive bacteria still caused tissue injury (Fig. 2), with an earlier onset than in the group without vinblastine treatment (not shown). These results indicated that neutrophil infiltration may not be the major factor involved in tissue damage after S. pyogenes infection. SPE B was shown to degrade the extracellular matrix proteins fibronectin and vitronectin (13). A recent report indicated that SPE B activated a 66-kDa matrix metalloprotease that is a type IV collagenase produced by endothelial cells (2). These functions of SPE B may contribute to endothelial cell damage, tissue destruction, and bacterial invasion and dissemination.
Studies by Lukomski et al. (15) showed that for 4 h following i.p. injection there were approximately equivalent amounts of neutrophil influx in the animals receiving the wild-type and speB mutant strains. However, by 22 h animals receiving the speB mutant actually had higher peritoneal neutrophil counts than those injected with the wild-type strain. They suggested that neutrophils effectively cleared the speB mutant from the peritoneum whereas the wild-type strain had the ability to kill phagocytes and continuously disseminated, resulting in host death. In this study, neutrophil infiltration in the air pouch was measured 12 h after inoculation with bacteria. Our preliminary experiment showed a higher level of infiltrated cell death in the group treated with the wild-type strain; whether this would result in a lower neutrophil count at a later time deserves further investigation.
The use of a rat lung injury model demonstrated that SPE B may act synergistically with other S. pyogenes products, such as streptococcal cell wall antigen and streptolysin O, to increase tissue injury. The mechanism of tissue injury at least in part involved increased tumor necrosis factor alpha (TNF-α) production, which was evident in bronchoalveolar lavage fluid (21). Matrix metalloproteases have been shown to cause the release of TNF-α (5, 16). Whether the activation of metalloprotease(s) by SPE B results in an increase in TNF-α production remains unknown. SPE B was shown to be capable of cleaving pre-IL-1β to generate the active form of IL-1β (12). The involvement of IL-1β in group A streptococcal disease should also be taken into consideration. To further delineate the pathological role of SPE B, the presence of various cytokines in the serum and local tissues following infection by protease-positive S. pyogenes strains and their speB mutants needs to be assessed. The use of NZ131 and its mutant should serve as an excellent model, ruling out the involvement of SPE A and SPE C.
The air pouch model described in this report showed that the cysteine protease, SPE B, appears to act as an important virulence factor in local tissue damage. Our preliminary observation showed the production of SPE B in the air pouch after A-20 inoculation. The presence of SPE B may cause degradation of host proteins for metabolism, have an effect on phagocytosis, and subsequently facilitate bacterial invasion. If the levels of anti-protease antibodies were increased by active or passive immunization, SPE B activity would be inhibited in local environments where bacteria enter. Our results did show that these animals could resist lethal doses of S. pyogenes and experienced less local tissue injury mediated by protease-positive strains of bacteria. The previous observation that an inverse relationship exists between anti-SPE B antibody titers in patient sera and disease severity also supports this notion (29).
ACKNOWLEDGMENTS
This work was supported by grants NSC85-2331-B006-020 and NSC86-2314-B006-056 from the National Science Council, Republic of China.
We thank Woei-Jer Chuang and Shu-Hui Chen for assistance in SPE B protein N-terminal sequencing.
REFERENCES
- 1.Bunce C, Wheeler L, Reed G, Musser J, Barg N. Murine model of cutaneous infection with gram-positive cocci. Infect Immun. 1992;60:2636–2640. doi: 10.1128/iai.60.7.2636-2640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns E H, Jr, Marciel A M, Musser J M. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaussee M S, Gerlach D, Yu C E, Ferretti J J. Inactivation of the streptococcal erythrogenic toxin B gene (speB) in Streptococcus pyogenes. Infect Immun. 1993;61:3719–3723. doi: 10.1128/iai.61.9.3719-3723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaussee M S, Liu J, Stevens D L, Ferretti J J. Genetic and phenotypic diversity among isolates of Streptococcus pyogenes from invasive infections. J Infect Dis. 1996;173:901–908. doi: 10.1093/infdis/173.4.901. [DOI] [PubMed] [Google Scholar]
- 5.Gearing A J H, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson A H, Drummond A H, Galloway W A, Gilbert R, Gordon J L, Leber T M, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood L M, Woolley K. Processing of tumour-necrosis factor-α precursor by metalloproteinases. Nature (London) 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 6.Hauser A R, Schlievert P M. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol. 1990;172:4536–4542. doi: 10.1128/jb.172.8.4536-4542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holm S E, Norrby A, Bergholm A M, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988–1989. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh P R, Wu J J, Tsai P J, Liu J W, Chuang Y C, Luh K T. Invasive group A streptococcal disease in Taiwan is not associated with the presence of streptococcal pyrogenic exotoxin genes. Clin Infect Dis. 1998;26:584–589. doi: 10.1086/514567. [DOI] [PubMed] [Google Scholar]
- 9.Husmann L K, Dillehay D L, Jennings V M, Scott J R. Streptococcus pyogenes infection in mice. Microb Pathog. 1996;20:213–224. doi: 10.1006/mpat.1996.0020. [DOI] [PubMed] [Google Scholar]
- 10.Hynes W L, Tagg J R. A simple plate assay for detection of group A streptococcus proteinase. J Microbiol Methods. 1985;4:25–31. [Google Scholar]
- 11.Kapur V, Maffei J T, Greer R S, Li L L, Adams G J, Musser J M. Vaccination with streptococcal extracellular cysteine protease (interleukin-1β convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 12.Kapur V, Majesky M W, Li L L, Black R A, Musser J M. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapur V, Topouzis S, Majesky M W, Li L L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 14.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Invest. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukomski S, Burns E H, Jr, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeehan G M, Becherer J D, Bast R C, Jr, Boyer C M, Champion B, Connolly K M, Conway J G, Furdon P, Karp S, Kidao S, McElroy A B, Nichols J, Pryzwansky K M, Schoenen F, Sekut L, Truesdale A, Verghese M, Warner J, Ways J P. Regulation of tumour-necrosis factor-α processing by a metalloproteinase inhibitor. Nature (London) 1994;370:558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- 17.Murray D L, Ohlendorf D H, Schlievert P M. Staphylococcal and streptococcal superantigens: their role in human diseases. ASM News. 1995;61:229–235. [Google Scholar]
- 18.Ohara-Nemoto Y, Sasaki M, Kaneko M, Nemoto T, Ota M. Cysteine protease activity of streptococcal pyrogenic exotoxin B. Can J Microbiol. 1994;40:930–936. doi: 10.1139/m94-149. [DOI] [PubMed] [Google Scholar]
- 19.Piepmeier E, Jr, Hammett-Stabler C, Price M, Peters J, Kemper G, Davis M., Jr Myositis and fasciitis associated with group A beta hemolytic streptococcal infections: development of a rabbit model. J Lab Clin Med. 1995;126:137–143. [PubMed] [Google Scholar]
- 20.Raeder R, Boyle M D P. Association between expression of immunoglobulin G-binding proteins by group A streptococci and virulence in a mouse skin infection model. Infect Immun. 1993;61:1378–1384. doi: 10.1128/iai.61.4.1378-1384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanley T P, Schrier D, Kapur V, Kehoe M, Musser J M, Ward P A. Streptococcal cysteine protease augments lung injury induced by products of group A streptococci. Infect Immun. 1996;64:870–877. doi: 10.1128/iai.64.3.870-877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer, R. C. 1995. Invasive streptococci. Eur. J. Clin. Microbiol. Infect. Dis. 14(Suppl.):S26–S32. [PubMed]
- 23.Sriskandan S, Moyes D, Buttery L K, Krausz T, Evans T J, Polak J, Cohen J. Streptococcal pyrogenic exotoxin A release, distribution, and role in a murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes. J Infect Dis. 1996;173:1399–1407. doi: 10.1093/infdis/173.6.1399. [DOI] [PubMed] [Google Scholar]
- 24.Stevens D L. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 25.Talkington D F, Schwartz B, Black C M, Todd J K, Elliott J, Breiman R F, Facklam R R. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect Immun. 1993;61:3369–3374. doi: 10.1128/iai.61.8.3369-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao L, LeBlanc D J, Ferretti J J. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene. 1992;120:105–110. doi: 10.1016/0378-1119(92)90016-i. [DOI] [PubMed] [Google Scholar]
- 27.Tsai P-J, Kuo C-F, Lin K-Y, Lin Y-S, Lei H-Y, Chen F-F, Wang J-R, Wu J-J. Effect of group A streptococcal cysteine protease on invasion of epithelial cells. Infect Immun. 1998;66:1460–1466. doi: 10.1128/iai.66.4.1460-1466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyler S D, Johnson W M, Huang J C, Ashton F E, Wang G, Low D E, Rozee K R. Streptococcal erythrogenic toxin genes: detection by polymerase chain reaction and association with disease in strains isolated in Canada from 1940 to 1991. J Clin Microbiol. 1992;30:3127–3131. doi: 10.1128/jcm.30.12.3127-3131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler M C, Roe M H, Kaplan E L, Schlievert P M, Todd J K. Outbreak of group A streptococcus septicemia in children: clinical, epidemiologic, and microbiological correlates. JAMA. 1991;266:533–537. [PubMed] [Google Scholar]