Abstract
Background/Aims:
Spatial memory can be impaired in amnestic mild cognitive impairment (aMCI). The present study investigates categorical spatial memory deficits using a virtual navigation-based reorientation task.
Methods:
Twenty-eight amnestic single domain and 23 amnestic multiple domain patients were compared with 53 healthy elderly controls on the performance of the virtual reorientation test (VReoT).
Results:
The reorientation performance of participants in both aMCI groups was significantly worse than that of controls suggesting that VReoT detects spatial memory deficits. No significant difference emerged between the 2 groups of patients. A subsequent receiver–operating characteristic analysis showed that a score of 8 had a sensitivity of 80.4% and a specificity of 94.3% (area under the curve = 0.90).
Conclusion:
The VReoT seemed to be accurate in differentiating patients with aMCI from controls and may represent an evaluation supplement for spatial memory deficits in prodromal stages of Alzheimer’s dementia.
Keywords: mild cognitive impairment, spatial memory, categorical spatial task, reorientation paradigm, virtual reality, topographical disorientation, navigation
Introduction
Mild cognitive impairment (MCI) is known to be a transitional stage of cognitive impairment between normal aging and early dementia. The MCI cases range from 2% to 30% in normal population and from 6% to 85% (with an average value of 40%) in clinical setting. 1 Following Petersen’s MCI classification, it is possible to distinguish 4 subtypes, amnestic MCI single domain ([aMCIsd]; memory impaired only), amnestic MCI multiple domain ([aMCImd] memory impaired plus 1 or more other cognitive domains), nonamnestic MCI single domain ([naMCIsd]; impairment in 1 nonmemory domain), and nonamnestic MCI multiple domain ([naMCImd]; impairment in 2 or more nonmemory domains). 2,3 These subtypes show differences in clinical outcomes. Both aMCIsd and aMCImdconditions are more likely to convert to Alzheimer’s disease (AD), with remarkable differences in progression rates among them. Non-aMCI conditions are instead more likely to convert in other types of dementia, such as vascular dementia, frontotemporal dementia, or dementia with Lewy body. 1
In individuals having MCI, the conversion to dementia is often predicted by different cognitive impairments such as mnemonic and executive dysfunction. 4 –6 Persons with MCI, specifically aMCI, often show difficulties in spatial orientation and in way-finding, such as learning unfamiliar routes and remembering familiar ones, selecting and remembering landmarks, inferring distances, and directions among locations (topographical disorientation). 7,8
The interest on an accurate evaluation in spatial skills is increasing also with the need to undertake interventions aimed to support patients’ independence in orientation 9 –14 and improve the layout of dementia care units. 15,16 Recent studies have investigated spatial orientation and navigation deficits in MCI, some of them adopting virtual reality as a profitable methodology in the assessment process. This methodology has a major advantage and offers practical and economic opportunities to evaluate the spatial memory and cognition in clinical samples. 7,17 –20 Hort and colleagues 21 studied egocentric (ie, adopting a self-based frame of reference 22 ) and allocentric (ie, adopting an environment-based frame of reference 23 ) navigation in MCI subtypes: they used a virtual version of the hidden goal task, a human analogue of the Morris water maze task which required to process mainly coordinate (ie, defining the euclidean properties of object position) than categorical spatial relations (ie, capturing basic information such as above/below, left/right). 24 They found that, in allocentric navigation subtest, the performance of both aMCI groups was significantly worse compared to that of the control group. Laczó and colleagues 25 found largely similar results adopting the same task in a real environment, with greater impairments of aMCI groups in 3 subtests compared with the control group. Consistently with previous findings, Irish and colleagues 6 found that their everyday spatial tasks requiring navigation (both mental and physical) dissociated MCI participants and controls. Pengas and colleagues 26 assessed topographical memory in MCI, AD, semantic dementia, and control participants, using a Virtual Route Learning Test. Patients with AD and MCI were significantly impaired in route learning performance, while patients with semantic dementia and control participants performed accurately. Similarly, Bird and colleagues, 27 adopting computer-generated landscapes, found an impaired performance in topographical short-term memory and nonspatial perception in aMCI and AD groups and an impaired nonspatial short-term memory only in AD group.
It must be noted here that all the tasks described so far, and specifically those based on Morris Water Maze paradigm, are searching tasks of a target in a continuous space. Nonetheless, there are also spatial memory tasks with a finite number of possible positions for the target to assume, and they can be valuably captured by simple spatial dichotomies such as “left/right” and “near to/far from.” In the latter condition, the encoding of spatial information requires less effort than the former one in which the target can assume, in principle, an infinite number of positions. 28 –30 In addition, coordinate spatial relations rely more heavily than categorical ones on attention and executive processes. 31
These issues have been previously addressed in a study by Kessels and colleagues. 28 They showed that the learning curves of patients with MCI were similar (although overall less efficient) than those of control participants on a spatial memory task in which participants had to study and remember 10 common objects within 5 × 5 matrices (ie, with a finite number of possible positions). By contrast, the learning curves of AD participants were flat when compared with those of both MCI and controls. This allowed to conclude that remembering objects and their positions in space within a grid (thus within a limited number of locations) is still plausible for people with MCI but no longer for AD. To the best of our knowledge, there are no studies that have specifically addressed the issue of memory for objects within an empty square (ie, without grid or marked positions) in people with MCI. In addition, there are no navigational studies with people showing cognitive decline, employing environments where searching tasks are strictly categorical. The present study aims to fill this vacuum adopting the reorientation paradigm, 32 –38 which might constitute an evaluation supplement in the assessment of spatial memory deficits in normal and pathological aging.
The Reorientation Paradigm
People reorient themselves in an environment following 2 sources of information: (a) geometric information consisting of basic metric data (ie, the difference in length between the walls of a room) and direction sense (the ability to distinguish the left to the right with respect to the observer and among objects) and (b) landmark information, which refers to distinctive features of an environment (visual, auditory, haptic, and olfactory cues). 33 Healthy individuals can rely flexibly on both geometric and landmark information during the reorientation process, in order to find a hidden target previously seen during a learning phase. Several characteristics of the environment affect reorientation strategies, such as shape (ie, rectangular, square, and rhombic), size (small/large indoors, outdoor landscapes), visual characteristics of the landmark (shape, size, color, and stability), and more importantly for the present study, the relative position of the landmark with respect to the target, that is, directly associated with the target (namely, positional landmark) or nondirectly associated with the target (namely, directional target). The latter type of landmark–target relationship can be clearly more demanding than the former, also for young people controls. 32 A common feature of reorientation tasks is the discrete number of positions in which the target can be located. The target is usually placed in the corners of the environment or in other noteworthy positions (eg, adjacent to a landmark, on the center of a wall), which are usually marked by a patch on the floor. Reorientation tasks, especially in 3-demensional (3D) virtual version, have already been demonstrated effective and consistent in discriminating men and women and also in healthy young and old people. 35,37,38 Knowledge about virtual reorientation tasks has recently been further improved by Sutton et al, 39 which provided evidence for differences in neural processing depending on whether a landmark was present or not.
The first aim of the present study was to describe and evaluate the virtual reorientation task ([VReoT] ie, a categorical spatial task based on a cued recall procedure) in differentiating elderly people with or without memory deficits. As described above, VReoT was developed to test the role of single (ie, landmark only and layout only) and conjoined spatial cues. Moreover, it allows to evaluate the role of landmark and target relationship. We expected that, at least, when landmark and target are directly associated with each other, the recovery of target position might be plausible and eventually spared in MCI patients. A second aim was to find out which arrangement of the tasks (or which shortest combination of them) could best serve as a supplement of evaluation in differentiating between healthy controls and MCI participants as well as between subtypes of amnestic deficits in the prodrome of AD, recurring to receiver–operating characteristic (ROC) analysis. Indeed, the unique functional magnetic resonance imaging study recording neural network activation during VReoT 39 demonstrated that several areas within the medial temporal lobe showed increased activation when a landmark was present. In contrast, reliance on layout (ie, the shape of the environment) significantly activated areas not related to medial temporal lobe, such as prefrontal cortex and inferior temporal gyrus. Following these suggestions, our expectation was that layout cue might be a source of differentiation between aMCIsd and aMCImd cases.
Methods
Participants
MCI participants recruitment. The MCI sample (N = 51) of the present study was established by including outpatients demanding for memory assessment (directly or referred from other clinics, neurologists, and general practitioners) at the Memory Clinic of the Department of Neuroscience and Sense Organs of the University of Bari, Italy, a tertiary care referral center. In the final sample, 13 patients and 8 other people were excluded from further analysis following the consensus conference criteria (see at the end of Diagnosis paragraph).
Healthy controls recruitment. In order to include cognitively unimpaired controls, relatives or acquaintances of patients, as well as volunteers from other sources, were invited to participate in the study. The final sample size of healthy controls was 53.
Exclusion criteria
Preliminarily to the recruitment, candidates were evaluated for the following exclusion criteria: evidence of other neurological, psychiatric, systemic conditions or potentially reversible causes of cognitive impairment that could cause cognitive and functional impairments (eg, stroke, alcoholism, medication, major depression, heart failure, renal or hepatic dysfunction, and pulmonary disease), and vision loss.
Data were collected between February 2010 and June 2011. All participants signed their informed consent prior to enrolment in the present study. The review board of the institution approved the study protocol, and the whole study was performed in accordance with the Helsinki Declaration.
Clinical and Neuropsychological Assessment
Participants received a clinical evaluation and a standardized neuropsychological battery. In particular, aMCI participants were examined by neurologists, neuropsychologists, and clinicians specialized in aging cognitive disorders. Global cognitive function was evaluated by the Mini-Mental State Examination. 40 Severity of dementia was assessed with the Clinical Dementia Rating (CDR) 41 and the 30-item Geriatric Depression Scale 42 was administered to rule out depressive symptomatology.
Verbal episodic memory was evaluated by the Short Story Immediate and Delayed Recall Test, 43 and by the Rey Auditory Verbal Learning Test. 44,45 Nonverbal episodic memory was evaluated by the delayed recall of the Rey-Osterrieth Complex Figure Test. 46 Short-term verbal and visuospatial working memory were assessed by the Digit Span Forward, and by the Corsi Block Tapping Test, respectively. 47 Visuospatial function was checked by the copy of the Rey-Osterrieth Complex Figure Test. 46 Attention and psychomotor speed were investigated by the Attentive Matrices, 46 and by the Trail Making Test A, 48,49 respectively. Language function and comprehension were assessed by Boston Naming Test 50 and by Token Test, respectively. 46 Executive function was evaluated by the Semantic Word Fluency (animal naming), 46 the Phonological Word Fluency, 51 the Trail Making Test B, 48,49 the Frontal assessment Battery, 52 and the Clock Drawing Test. 46 Evidence of functional decline was based on the scores of the activities of daily living (ADL) 53 and instrumental ADL, 54 as reported by a close relative or caregiver, and on the patient’s self-report.
Diagnosis
Consensus diagnosis was reached at expert multidisciplinary conference, attended by the study physician, neuropsychologist, neurologist, and study coordinator. Diagnosis of MCI was made according to Petersen’s 3 criteria: (1) subjective cognitive complaint, preferably confirmed by an informant, (2) objective impairments in the performance on the cognitive tests of the assessment battery, at least greater than 1.5 standard deviation below the scores of age- and education-matched normal aged individuals, but not severe enough to reach dementia diagnosis, (3) preserved global cognitive function, (4) intact or minimal impairments in ADL, and (5) not demented.
According to the pattern of impairment on neuropsychological evaluation, all patients with aMCI had memory deficits in at least 2 episodic memory tests. In addition, participants classified as aMCImd experienced deficits in at least 2 nonmemory tests in one or more of the following cognitive domains: semantic, language, visuospatial, and attention and executive functions.
A small number of naMCI participants (n = 7) met criteria for executive function deficit as single domain (n = 3) or together with deficits in other domains (n = 4) excluding memory. Another small group of participants (n = 6) met criteria for probable dementia. Eight other people showed borderline scores at or below the critical threshold in only 1 neuropsychological test. Data of these people were excluded by further analysis since the sample size of their clinical groups was too small compared with that of the other groups or they were unable to be considered as MCI participants. The final arrangement of the study sample was as follows: 28 aMCIsd, 23 aMCImd, and 53 healthy controls.
Apparatus and Materials
An extended description of the apparatus used in this study was included elsewhere. 32,36 Freeware software, the C-G Arena was used. 55 A computer monitor (19 in wide) displayed an environment in a first-person perspective view. The environment had an internal structure composed by a circular, invisible arena, in which the participants could move and explore freely controlling their movements with a joystick. Five kinds of environments were created, one for each subtest described below. The VReoT is an instrument designed to evaluate reorientation performance in people with cognitive impairment, following a procedure similar to that employed in comparative studies with rats, 56 with children, 57,58 and with older adults. 37,38 The VReoT is composed of 5 subtests, each carrying a different combination of spatial information: (1) layout only (a rectangular room with all white walls), (2) layout and positional landmark (a rectangular room with 3 white walls and 1 blue wall, landmark directly associated with the target), (3) layout and directional landmark (a rectangular room with 3 white walls and 1 blue wall, landmark not directly associated with the target), (4) positional landmark only (a square room with 3 white walls and 1 blue wall, landmark directly associated with the target), and (5) directional landmark only (a square room with 3 white walls and 1 blue wall, landmark not directly associated with the target).
Procedure
Each participant entered the laboratory and sat on a chair in front of a computer screen and a joystick. Then, participants received standardized verbal instructions about how to move within the space in each task (for a description of instructions, see Bosco et al 32 ). A suitable training phase was designed. Prior to the start of the experiment, all participants were requested to gain experience with the desktop virtual environment in order to reach an adequate level of confidence with the apparatus. When participants felt comfortable with controlling their movements through the environment, they were allowed to start to the experimental session. A learning and a testing phase were separated by a 2-second black screen interval. Participants entered the learning environment, which contained a yellow sphere placed in 1 corner and 4 black response patches located on the floor. They faced randomly one of the 4 walls. In this phase, participants were explicitly requested to look for the yellow sphere and to remember the corner in which the sphere was placed. When they felt comfortable with the task, they gave a signal to the research assistant, who promptly pressed the space bar and the software virtually brought the participant into the testing environment. The participants’ facial position was randomly changed in order to interfere with their egocentric frame of reference. Entering the testing room, the participants could reorient and find the hidden sphere adopting a cue-based strategy. The testing environment had the same characteristics of the learning environment with the following exceptions: the sphere was replaced by a blue box and 3 identical boxes were located in other corners. Participants were informed that the yellow sphere was hidden but not moved from the original location and they were requested to discover the box housing the sphere by reaching the response patch corresponding to that box. If the participant chose the correct corner, a subroutine of the software brought him in a new testing environment, identical to the previous except for the participant’s facial orientation, again randomly changed by the software. The participant was then again asked to find the target. If the participant chose the wrong corner, a subroutine brought him in the learning room, and she or he was asked to look again for the sphere and to learn its position, remembering the corner in which the sphere was placed. Each subtest ended when the participant chose the correct corner for 3 times consecutively. Once this criterion was reached, there was a 2-minute break and then another subtest was presented. A new subtest could begin if the participants had reached criterion on the previous one or they had already spent all their 12 learning opportunities available for it. The dependent variable was the number of repetitions of the learning phase needed by the participant to reach the criterion.
Statistical Analysis
The data were analyzed using SPSS 16.0 statistical software. We obtained demographic data (gender, age, and years of education) and scores on screening instruments and VReoT. A one-tailed value of P < .05 was determined to be statistically significant. Pearson’s chi-square analysis was performed to assess for differences in the distribution of gender and CDR scores among different diagnostic groups. A series of univariate analysis of variance (ANOVA) were carried out to compare means from 3 diagnostic groups for demographic data, scores on cognitive and neuropsychological tests used to establish a diagnosis, and scores on VReoT. The confounding effects of potentially disturbing variables, such as depression, were controlled for by analysis of covariance (ANCOVA). Pairwise comparisons were carried out with Scheffè post hoc tests. Effect size estimates were reported by calculating partial eta-squared for each significant effect. The diagnostic accuracy and the optimal cutoff scores of the VReoT were assessed by calculating the area under the ROC curve (AUC). Positive likelihood ratios were calculated from the sensitivity and specificity values.
Results
Table 1 illustrates demographic characteristics, mean age/education adjusted scores on the neuropsychological screening tests, and statistical significance for comparisons between diagnostic groups. There were no significant differences of gender, age, and years of education among the 3 groups. As expected, the frequency of 0 and 0.5 in CDR scores are differently distributed across the 3 groups (P < .001). The comparisons between the 2 MCI and control groups on Geriatric Depression Scale were also significant. Further analysis was required in order to control for this variable, potentially associated with poorer performance. The average scores of neuropsychological measures for each group were compatible with Italian normative data, 43, 45 –49,51,52 even though they were rather low; this result was probably associated with the low level of education of the study sample.
Table 1.
Sociodemographic Characteristics of Participants, Mean Scores on the Neuropsychological Screening Tests and, According to Diagnostic Groups, Statistical Tests for Their Differences.
| aMCIsd (N = 28) | aMCImd (N = 23) | Healthy Control (N=53) | aMCI vs HC | aMCImd vs HC | aMCI vs aMCImd | |
|---|---|---|---|---|---|---|
| Age, years | 69.89 ± 5.17 | 73.91 ± 4.72 | 68.06 ± 5.96 | NS | NS | NS |
| Gender (F/M) | 17/11 | 15/8 | 29/24 | |||
| Education, years | 9.46 ± 5.21 | 5.74 ± 3 | 9.7 ± 4.31 | NS | NS | NS |
| CDR (0/0.5) | 6/22 | 3/20 | 53/0 | |||
| MMSE | 26.15 ± 2.03 | 25.35 ± 3.23 | 28.19 ± 1.88 | <.001 | <.001 | NS |
| ADL | 5.82 ± 0.48 | 5.87 ± 0.34 | 5.98 ± 0.14 | NS | NS | NS |
| IADL | 7.77 ± 0.59 | 7.74 ± 0.69 | 7.94 ± 0.23 | NS | NS | NS |
| Geriatric Depression Scale | 9.07 ± 6 | 10.65 ± 4.56 | 4.36 ± 3.37 | <.001 | <.001 | NS |
| Short Story Immediate Recall | 5.96 ± 3.46 | 4.7 ± 2.01 | 12.55 ± 5.65 | <.001 | <.001 | NS |
| Short Story Delayed Recall | 8.57 ± 4.07 | 7 ± 3.98 | 14.91 ± 4.17 | <.001 | <.001 | NS |
| Rey’s 15 Words Immediate Recall | 31.21 ± 7.5 | 32.3 ± 6.79 | 39.91 ± 6.27 | <.001 | <.001 | NS |
| Rey’s 15 Words Delayed Recall | 4.46 ± 1.1 | 3.91 ± 1.31 | 8.3 ± 2.57 | <.001 | <.001 | NS |
| Rey’s Complex Figure Delayed Recall | 10.25 ± 3.11 | 9.13 ± 4.44 | 17.02 ± 4.91 | <.001 | <.001 | NS |
| Digit Span | 4.89 ± 0.83 | 4.65 ± 0.78 | 5.42 ± 1.01 | NS | <.001 | NS |
| Corsi Block | 4.54 ± 0.88 | 5.04 ± 0.82 | 5.79 ± 5.26 | NS | NS | NS |
| Rey’s Complex Figure Copy | 29.75 ± 2.98 | 26.39 ± 8.41 | 29.36 ± 5.65 | NS | NS | NS |
| Visual Search (Attentive Matrices) | 42.86 ± 7.26 | 41.52 ± 6.16 | 47.11 ± 12.46 | NS | NS | NS |
| Trail Making Test A | 63.11 ± 24.03 | 119.57 ± 63.02 | 55.04 ± 22.43 | NS | <.001 | <.001 |
| Boston Naming Test | 115.75 ± 3.35 | 116.13 ± 3.45 | 115.62 ± 14.19 | NS | NS | NS |
| Token Test | 30.48 ± 1.24 | 29.35 ± 1.56 | 30.8 ± 2.29 | NS | <.05 | NS |
| Frontal Assessment Battery | 16.11 ± 1.69 | 13.7 ± 1.33 | 16.13 ± 1.13 | NS | <.001 | <.001 |
| Phonological Word Fluency | 28 ± 8.66 | 29.17 ± 8.23 | 30.98 ± 15.64 | NS | NS | NS |
| Semantic Word Fluency | 19.14 ± 7.12 | 16.39 ± 6.11 | 20.19 ± 7.21 | NS | NS | NS |
| Trail Making Test B | 171.13 ± 59.52 | 242.26 ± 70.28 | 148.37 ± 50.17 | NS | <.05 | <.05 |
| Clock Drawing Test | 8.77 ± 1.28 | 6.26 ± 2.85 | 9.11 ± 1.7 | NS | <.001 | <.001 |
Abbreviations: MCI, mild cognitive impairment; aMCIsd, amnestic MCI single domain; aMCImd, amnestic MCI multiple domain; HC, healthy controls; CDR, Clinical Dementia Rating; MMSE, Mini-Mental State Examination; ADL, activities of daily living; IADL, instrumental ADL; NS, not significant.
Two omnibus ANOVAs and 2 corresponding ANCOVAs (controlling for scores on Geriatric Depression Scale) were conducted on number of learning trials needed to correctly reach the criterion of 3 consecutive correct responses (Table 2). According to the aims of the present study, only the main and interaction effects related to the variable groups were reported. Analysis 1 was performed to assess the difference in performance among the 3 groups of participants in the rectangular environment with all white walls (layout only condition). This environment has a chance level of 0.5 for each trial (and a binomial probability to reach the criterion of three consecutive correct responses of 0.125); consequently, it has to be analyzed independently from the other environments showing a different pattern of chance level probabilities. Analysis 2 was performed on the other 4 environments. Independent variables were groups as between-participants variable and shape (rectangular and square) and landmark–target relationship (positional and directional) as within-participants variables. All these 4 environments have a chance level of 0.25 for each trial (and a binomial probability to reach by chance the criterion of 0.015).
Table 2.
Means and Standard Deviations of Number of Trials Needed to Reach the Criterion for Each Environment and for Each Group of Participants.
| aMCIsd | aMCImd | Healthy Control | Total | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Layout only | 2.61 ± 2.15 | 2.78 ± 2.39 | 1.30 ± 1.76 | 1.98 ± 1.76 |
| Layout + positional landmark | 1.36 ± 0.68 | 2.00 ± 1.00 | 1.02 ± 0.14 | 1.33 ± 0.70 |
| Layout + directional landmark | 2.54 ± 1.43 | 3.13 ± 2.63 | 1.19 ± 0.48 | 1.98 ± 1.68 |
| Positional landmark only | 1.68 ± 0.82 | 2.04 ± 1.43 | 1.08 ± 0.33 | 1.45 ± 0.91 |
| Directional landmark only | 2.82 ± 1.93 | 3.70 ± 2.93 | 1.15 ± 0.36 | 2.16 ± 2.01 |
Abbreviations: aMCIsd, amnestic MCI single domain; aMCImd, amnestic MCI multiple domain; SD, standard deviation.
Analysis 1
The main effect of groups was significant (F 2,101 = 9.47, P < .001; ηP 2 = 0.16). Scores of Geriatric Depression Scale showed to be different among the 3 groups. After controlling for level of depression, the main effect of groups was still significant (F 2,100 = 3.84, P < .05; ηP 2 = 0.07). The post hoc (Scheffé method) showed that both groups of aMCI participants performed worse (both P < .01) than healthy control (1.3 ± 1.76). Nonetheless, difference between them was not significant (aMCIsd 2.61 ± 2.15, aMCImd 2.78 ± 2.39).
Analysis 2
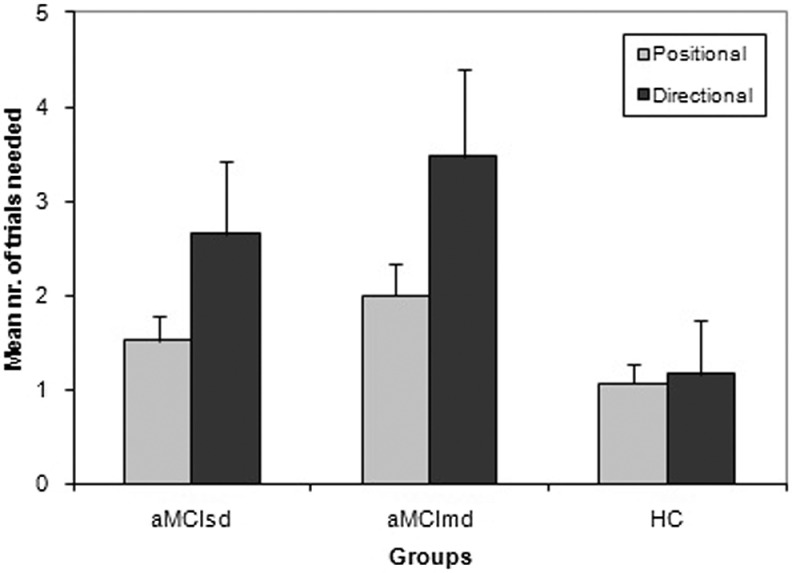
The main effect of groups was significant (F 2,101 = 33.74, P < .01; ηp 2 = 0.40). The interaction between groups and landmark–target relationship was also significant (F 2,101 = 8.67, P < .001; ηp 2 = 0.15). Again, an ANCOVA was performed to control for the scores on the Geriatric Depression Scale. The main effect of groups (F 2,100 = 20.66, P < .001; ηp 2 = 0.29) and the interaction between groups and landmark/target relationship (F 2,100 = 3.61, P < .05; ηp 2 = −0.07) were still significant. Post hoc analysis of the main effect (Scheffé method) showed that aMCIsd (2.1 ± 0.85) and aMCImd (2.72 ± 0.81) differed (both P < .01) by healthy controls (1.11 ± 0.80). However, they did not differ between them. Finally, as showed in Figure 1, healthy control learned in a comparable way both positional and directional relationships. On the contrary, the aMCI groups appeared much impaired when the relationship between landmark and target was directional rather than positional.
Figure 1.
Mean number of trials needed to reach the criterion (standard deviations in bars) in positional and directional VReoT subtests, for aMCIsd, aMCImd, and HC. VReoT indicates virtual reorientation test; MCI, mild cognitive impairment; aMCIsd, amnestic MCI single domain; aMCImd, amnestic MCI multiple domain; HC, healthy controls.
The ROC Curves
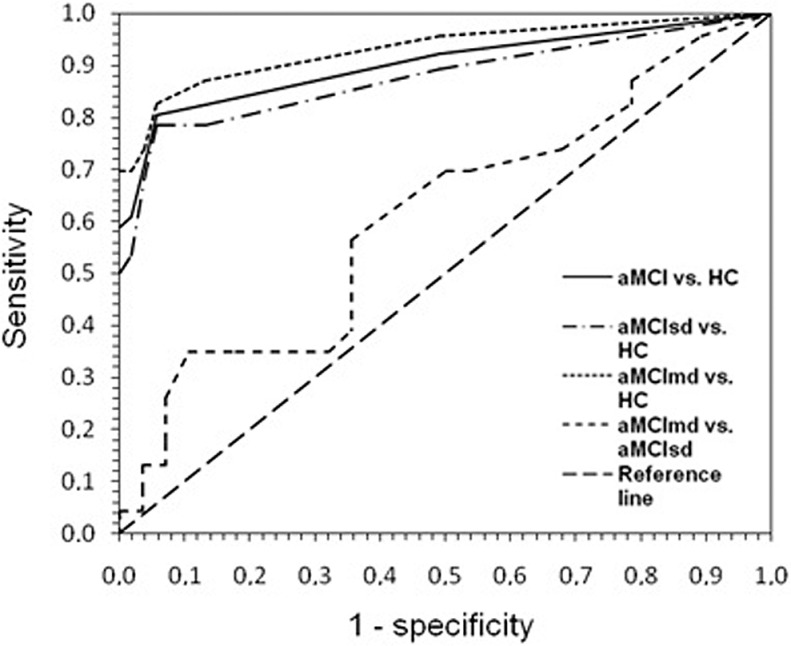
As described briefly in the closing part of the introductory paragraph, one of the aims of the present study was to carry out an initial evaluation of the diagnostic potential of VReoT both in differentiating patients from healthy controls, as well as the 2 groups of MCI participants. A related aim was to determine the best short version of VReoT. Table 3 shows AUC values for VReoT total score and for the shortest best subtest arrangements, between each diagnostic group versus controls and between the 2 aMCI groups. A cutoff greater than 7 on the total score proved to be the best threshold to discriminate both aMCI patients and the 2 subgroups of aMCIsd and aMCImd from healthy controls, with sensitivity ranging from 78.6% to 82.6% and specificity equal to 94.3%. The AUC values for VReoT total score between each diagnostic group versus controls varied from 0.877 to 0.931 (P < .001). The AUC value for the comparison between aMCIsd and aMCImd was 0.609 (P > .1). Likelihood ratio values varied from 13.9 to 14.6. The ROC curves are shown in Figure 2.
Table 3.
Cutoff, Sensitivity, Specificity, Likelihood Ratio, Area Under Curve (AUC), Standard Error and Associated Probability for Both VReoT Scores (ie, Sum of Scores of All Subtests), and the Shortest Best Subtest Arrangements (ie, knew that A = Layout Only, B = Layout and Positional Landmark, C = Layout and Directional Landmark, D = Positional Landmark Only, E = Directional Landmark Only), for Each Group Comparison. Z Scores and Associated Probabilities Were Calculated to Compare the AUCs for Each VReoT Score and the Shortest Best Subtest Arrangements, Following Hanley and McNeil's formula. 59 .
| Cutoff | Sensibility, % | Specificity, % | Likelihood ratio | AUC | SE | P | z | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| aMCI vs HC | VReoT score | >7 | 80.4 | 94.3 | 14.2 | 0.902 | 0.032 | <.001 | 0.81 | NS |
| Sum of D and E | >2 | 84.3 | 81.1 | 4.5 | 0.883 | 0.035 | <.001 | |||
| aMCIsd vs HC | VReoT score | >7 | 78.6 | 94.3 | 13.9 | 0.877 | 0.048 | <.001 | 0.22 | NS |
| Sum of D and E | >2 | 82.1 | 81.1 | 4.4 | 0.870 | 0.048 | <.001 | |||
| aMCImd vs HC | VReoT score | >7 | 82.6 | 94.3 | 14.6 | 0.931 | 0.038 | <.001 | 1.10 | NS |
| Sum of A, C and E | >5 | 78.3 | 86.8 | 5.9 | 0.907 | 0.041 | <.001 | |||
| aMCImd vs aMCIsd | VReoT score | >7 | 82.6 | 21.4 | 1.0 | 0.609 | 0.081 | NS | −1.12 | NS |
| Sum of B and D | >3 | 78.3 | 42.9 | 1.4 | 0.700 | 0.075 | <.05 |
Abbreviations: aMCI, amnestic mild cognitive impairment; aMCIsd, amnestic MCI single domain; aMCImd, amnestic MCI multiple domain; SE, standard error; AUC, area under the curve; HC, healthy control; VReoT, virtual reorientation test.
Figure 2.
The ROC analysis for VReoT: comparisons between the diagnostic groups and the healthy controls group and between the 2 diagnostic groups. ROC indicates receiver-operator characteristics; VReoT, virtual reorientation test.
Discussion
Two groups with aMCI and single or multiple domain impairment and a group of healthy controls were compared in a virtual reorientation task. Following Kessels et al, 28 our results extended to a 3D navigation task the notion that a categorical 2-dimensional spatial task is suitable for patients with aMCI. They generally reached the learning criteria largely within the limit of 12 repetitions. Nonetheless, they were prevented to use basic spatial cues with the same efficacy as healthy controls. Our findings are consistent with those documenting that spatial memory deficits in orientation/navigation task occur not only in AD26,60 but also in aMCI, 25,61,62 and also with the suggestion that the assessment of orientation deficits might be useful in monitoring the progression of the disease to AD. 21,63,64 Our experimental procedure also evaluated the function of a landmark in both positional (directly associated with the target) and directional (indirectly associated with the target) condition. The interaction effect between the 2 types of landmark and the groups showed that healthy controls can use both types effectively. On the contrary, patients with aMCI failed significantly more in the subtest characterized by directional landmark. This result can be explained by theoretical models which point out that the salience of a spatial cue might be proportional to its associative strength: the more the landmark is contingent to the target, the more it will be crucial for the retrieval of the target itself. 65 The low associative strength of a landmark far from the target seemed to be critical for aMCI participants, who could be probably affected by an initial degenerative damage in the medial temporal lobe structures. 39
As shown by the ROC analysis, VReoT had a relevant diagnostic function (in terms of sensitivity and specificity) for differentiating between all aMCI, regardless of its subtypes, and healthy controls. Unfortunately, the utility of VReoT was limited in differentiating aMCIsd from aMCImd. In particular, no evidences emerged on the possible role of layout/geometry information in such kind of distinction. Likelihood ratio values showed that VReoT might significantly improve aMCI diagnostic process. Indeed, for patients who have a positive result, positive Likelihood Ratio (LR+s) greater than 10 significantly increase the probability of disease. 66 In other terms, a score of 8 or higher is about 14 times more likely to come from a patient with aMCI than from a control participant. In order to evaluate the usefulness of abbreviated forms of VReoT, operative characteristics were calculated also on shortest best subtest arrangements. Although AUCs for the abbreviated forms did not differ significantly from AUCs for the full forms for each group comparison, there was a considerable loss in specificity, and consequently, a significant lowering of likelihood ratios. These considerations led to conclude that administration of the full form of VReoT is always preferable to any other of the shortest forms. Such forms failed to provide appropriate diagnostic outcomes, although more economical in terms of fatigue for the patient and time commitment for the clinician.
This study has some critical points. First, the recruitment of MCI participants took place in a tertiary care referral center. These patients are typically more symptomatic and impaired than those recruited from the community, and the distribution of cognitive impairment in clinical population may differ from that in the community. Moreover, our sample of patients showed a level of depression higher than that showed by our healthy controls. This difference was statistically controlled by the ANCOVA that has attenuated the effects of group membership without eliminating them. This result is compatible with the data reported by Doniger et al, 67 which showed that a computerized battery for the detection of MCI and mild dementia were marginally affected by higher levels of depression in patients than in controls. Second, there were small numbers of cases in the 2 aMCI subgroups, and this may have led to some inaccuracies (under- or overestimation) in the calculations of operating characteristics of the test.
Further studies are required in order to extend the investigation to a community-based sample 68 and to calculate positive and negative predictive values, using the prevalence of MCI from the general population. 69 Moreover, adding a group of naMCI and a group of AD would lead to a better understanding of spatial orientation deficits, also in those patients showing impairment in cognitive domains other than memory and in patients with an advanced stage of cognitive decline, respectively. Finally, it would be interesting to introduce a 10 minutes delayed task to compare performance in the retrieving and in the maintenance of spatial memory cues.
In conclusion, the VReoT presented here showed to be an easy-to-use tool recurring to very basic spatial information; it evaluated spatial orientation and navigation abilities emphasizing the use of categorical spatial relations (ie, right/left to, near to/far from) and minimizing the contribution of coordinate spatial relations (ie, euclidean distance from a target), as mainly assessed by previous works. 21,61,70 The failure of patients with aMCI in retaining spatial information, in a very simple reorientation task, may suggest that episodic categorical (nonverbal) spatial memory evaluated by VReoT could be eligible as an additional measure characterizing aMCI decline. It could be used to supplement current standard clinical protocols 17 usually characterized by extensive evaluation of verbal memory and by tasks strongly mediated by language.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119(4):252–265. [DOI] [PubMed] [Google Scholar]
- 2. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. [DOI] [PubMed] [Google Scholar]
- 3. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 4. Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58(9):853–858. [DOI] [PubMed] [Google Scholar]
- 5. Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63(8):916–924. [DOI] [PubMed] [Google Scholar]
- 6. Irish M, Lawlor BA, Coen RF, O'Mara SM. Everyday episodic memory in amnestic mild cognitive impairment: a preliminary investigation. BMC Neurosci. 2011;4;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iachini T, Iavarone A, Senese VP, Ruotolo F, Ruggiero G. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci. 2009;2(1):43–59. [DOI] [PubMed] [Google Scholar]
- 8. Pai MC, Lee CC, Yang YC, et al. Development of a questionnaire on everyday navigational ability to assess topographical disorientation in Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2012;27(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGilton KS, Rivera TM, Dawson P. Can we help persons with dementia find their way in a new environment? Aging Ment Health. 2003;7(5):363–371. [DOI] [PubMed] [Google Scholar]
- 10. Provencher V, Bier N, Audet T, Gagnon L. Errorless-based techniques can improve route finding in early Alzheimer's disease: a case study. Am J Alzheimers Dis Other Demen. 2008;23(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lancioni GE, Perilli V, Singh NN, et al. Persons with mild or moderate Alzheimer's disease use a basic orientation technology to travel to different rooms within a day center. Res Dev Disabil. 2011;32(5):1895–1901. [DOI] [PubMed] [Google Scholar]
- 12. Maci T, Pira FL, Quattrocchi G, Nuovo SD, Perciavalle V, Zappia M. Physical and cognitive stimulation in Alzheimer disease. The GAIA project: a pilot study. Am J Alzheimers Dis Other Demen. 2012;27(2):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moro V, Condoleo MT, Sala F, Pernigo S, Moretto G, Gambina G. Cognitive stimulation in a-MCI: an experimental study. Am J Alzheimers Dis Other Demen. 2012;27(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Provencher V, Bier N, Audet T, Gagnon L. Errorless-based techniques can improve route finding in early Alzheimer's disease: a case study. Am J Alzheimers Dis Other Demen. 2008;23(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marquardt G, Johnston D, Black BS, et al. Association of the spatial layout of the home and ADL abilities among older adults with dementia. Am J Alzheimers Dis Other Demen. 2011;26(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibson MC, MacLean J, Borrie M, Geiger J. Orientation behaviors in residents relocated to a redesigned dementia care unit. Am J Alzheimers Dis Other Demen. 2004;19(1):45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maki Y, Yoshida H, Yamaguchi H. Computerized visuo-spatial memory test as a supplementary screening test for dementia. Psychogeriatrics. 2010;10(2):77–82. [DOI] [PubMed] [Google Scholar]
- 18. Tippett WJ, Lee JH, Zakzanis KK, Black SE, Mraz R, Graham SJ. Visually navigating a virtual world with real-world impairments: a study of visually and spatially guided performance in individuals with mild cognitive impairments. J Clin Exp Neuropsychol. 2009;31(4):447–454. [DOI] [PubMed] [Google Scholar]
- 19. Weniger G, Siemerkus J, Schmidt-Samoa C, et al. The human parahippocampal cortex subserves egocentric spatial learning during navigation in a virtual maze. Neurobiol Learn Mem. 2010;93(1):46–55. [DOI] [PubMed] [Google Scholar]
- 20. Weniger G, Ruhleder M, Lange C, Wolf S, Irle E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia. 2011;49(3):518–527. [DOI] [PubMed] [Google Scholar]
- 21. Hort J, Laczó J, Vyhnálek M, Bojar M, Bureš J, Vlček K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci U S A. 2007;104(10):4042–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97(8):4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. [DOI] [PubMed] [Google Scholar]
- 24. D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60–90. [DOI] [PubMed] [Google Scholar]
- 25. Laczó J, Vlček K, Vyhnálek M, et al. Spatial navigation testing discriminates two types of amnestic mild cognitive impairment. Behav Brain Res. 2009;202(2):252–259. [DOI] [PubMed] [Google Scholar]
- 26. Pengas G, Patterson K, Arnold RJ, Bird CM, Burgess N, Nestor PJ. Lost and found: bespoke memory testing for Alzheimer's disease and semantic dementia. J Alzheimer's Dis. 2010;21(4):1347–1365. [DOI] [PubMed] [Google Scholar]
- 27. Bird CM, Chan D, Hartley T, Pijnenburg YA, Rossor MN, Burgess N. Topographical short-term memory differentiates Alzheimer's disease from frontotemporal lobar degeneration. Hippocampus. 2010;20(10):1154–1169. [DOI] [PubMed] [Google Scholar]
- 28. Kessels RP, Rijken S, Joosten-Weyn Banningh LW, Van Schuylenborgh VAN Es N, Olde Rikkert MG. Categorical spatial memory in patients with mild cognitive impairment and Alzheimer dementia: positional versus object-location recall. J Int Neuropsychol Soc. 2010;16(1):200–204. [DOI] [PubMed] [Google Scholar]
- 29. Amorapanth PX, Widick P, Chatterjee A. The neural basis for spatial relations. J Cogn Neurosci. 2010;22(8):1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Postma A, Kessels RP, van Asselen M. How the brain remembers and forgets where things are: the neurocognition of object-location memory. Neurosci Biobehav Rev. 2008;32(8):1339–1345. [DOI] [PubMed] [Google Scholar]
- 31. Martin R, Houssemand C, Schiltz C, Burnod Y, Alexandre F. Is there continuity between categorical and coordinate spatial relations coding? Evidence from a grid/no-grid working memory paradigm. Neuropsychologia. 2008;46(2):576–594. [DOI] [PubMed] [Google Scholar]
- 32. Bosco A, Picucci L, Caffò AO, Lancioni GE, Gyselinck V. Assessing human reorientation ability inside virtual reality environments: the effects of retention interval and landmark characteristics. Cogn Process. 2008;9(4):299–309. [DOI] [PubMed] [Google Scholar]
- 33. Cheng K, Newcombe NS. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon Bull Rev. 2005;12(1):1–23. [DOI] [PubMed] [Google Scholar]
- 34. Cheng K. Whither geometry? Troubles of the geometric module. Trends Cogn Sci. 2008;12(9):355–361. [DOI] [PubMed] [Google Scholar]
- 35. Picucci L, Caffò AO, Bosco A. Besides navigation accuracy: gender differences in strategy selection and level of spatial confidence. J Environ Psychol. 2011;31(4):430–438. [Google Scholar]
- 36. Caffò AO, Picucci L, Di Masi MN, Bosco A. Working memory components and virtual reorientation: a dual-task study. In: Levin ES, ed. Working Memory: Capacity, Developments and Improvement Techniques. New York, NY: Nova Science Publishers; 2011. [Google Scholar]
- 37. Picucci L, Caffò AO, Bosco A. Age and sex differences in a virtual version of the reorientation task. Cogn Process. 2009;10(suppl 2):S272–S275. [DOI] [PubMed] [Google Scholar]
- 38. Picucci L, Bosco A, Caffò AO, D’Angelo G, Di Masi MN, Lancioni GE. A new methodology to assess individual differences in spatial memory: The computer generated version of the Reorientation Paradigm. In: Salvati G, Rabuano V, eds. Perspectives on Cognitive Psychology. New York, NY: Nova Science Publishers; 2011. [Google Scholar]
- 39. Sutton JE, Joanisse MF, Newcombe NS. Spinning in the scanner: neural correlates of virtual reorientation. J Exp Psychol Learn Mem Cogn. 2010;36(5):1097–1107. [DOI] [PubMed] [Google Scholar]
- 40. Magni E, Binetti G, Bianchetti A, Rozzini R, Trabucchi M. Mini-Mental State Examination: a normative study in italian elderly population. Eur J Neurol. 1996;3(3):198–202. [DOI] [PubMed] [Google Scholar]
- 41. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140(6):566–572. [DOI] [PubMed] [Google Scholar]
- 42. Brink TL, Yesavage JA, Lum O, Heersema PH, Adey M, Rose TL. Screening tests for geriatric depression. Clin Gerontol. 1982;1(1):37–43. [DOI] [PubMed] [Google Scholar]
- 43. Carlesimo GA, Buccione I, Fadda L, et al. Standardizzazione di due test di memoria per uso clinico: Breve Racconto e Figura di Rey. Nuova Rivista di Neurologia. 2002;12:1–13. [Google Scholar]
- 44. Rey A. Mémorisation d'une serie de 15 mots en 5 répétitions. In: Rey A, ed. L'examen Clinique in Psychologie. Paris, France: Presses Universitaries de France; 1958. [Google Scholar]
- 45. Carlesimo GA, Caltagirone C, Gainotti G. and The Group for the Standardization of the Mental Deterioration Battery. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol. 1996;36(6):378–384. [DOI] [PubMed] [Google Scholar]
- 46. Spinnler H, Tognoni G, et al. Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci. 1987;6(suppl 8):47–50 [PubMed] [Google Scholar]
- 47. Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G. Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital J Neurol Sci. 1987;8(6):539–548. [DOI] [PubMed] [Google Scholar]
- 48. Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17(4):305–309. [DOI] [PubMed] [Google Scholar]
- 49. Mondini S, Mapelli D, Vestri A, Bisiacchi PS. Esame neuropsicologico Breve. Milano, Italy: Raffaello cortina Editore; 2003. [Google Scholar]
- 50. Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Experimental Edition. Boston, MA: Aphasia Research Center, Boston University; 1976. [Google Scholar]
- 51. Avanzi S, Postrearo L, Gugliotta M, Lombardi F, Cavatorta S, Mazzucchi A. Aspetti quantitativi e qualitativi della fluenza verbale: I. Nei soggetti normali. In: Archivio di Psicologia Neurologia e Psichiatria. 1997;58:85–108. [Google Scholar]
- 52. Appollonio I, Leone M, Isella V, et al. The frontal Assesment Battery (FAB): normative values in an Italian population sample. Neurol Sci. 2005;26(2):108–116. [DOI] [PubMed] [Google Scholar]
- 53. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. [DOI] [PubMed] [Google Scholar]
- 54. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 55. Jacobs WJ, Laurance HE, Thomas KGF. Place learning in virtual space I: acquisition, overshadowing, and transfer. Learn Motiv. 1997;28(4):521–541. [Google Scholar]
- 56. Cheng K. A purely geometric module in the rat's spatial representation. Cognition. 1986;23(2):149–178. [DOI] [PubMed] [Google Scholar]
- 57. Hermer L, Spelke E. Modularity and development: the case of spatial reorientation. Cognition. 1996;61(3):195–232. [DOI] [PubMed] [Google Scholar]
- 58. Hermer L, Spelke ES. A geometric process for spatial reorientation in young children. Nature. 1994;370(6484):57–59. [DOI] [PubMed] [Google Scholar]
- 59. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. [DOI] [PubMed] [Google Scholar]
- 60. Monacelli AM, Cushman LA, Kavcic V, Duffy CJ. Spatial disorientation in Alzheimer's disease: the remembrance of things passed. Neurology. 2003;61(11):1491–1497. [DOI] [PubMed] [Google Scholar]
- 61. Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: getting lost between aging and AD. Neurology. 2003;60(5):802–808. [DOI] [PubMed] [Google Scholar]
- 62. Laczó J, Andel R, Vyhnalek M, et al. Human analogue of the morris water maze for testing subjects at risk of Alzheimer's disease. Neurodegener Dis. 2010;7(1-3):148–152. [DOI] [PubMed] [Google Scholar]
- 63. Lim TS, Iaria G, Moon SY. Topographical disorientation in mild cognitive impairment: a voxel-based morphometry study. J Clin Neurol. 2010;6(4):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alescio-Lautier B, Michel BF, Herrera C, et al. Visual and visuospatial short-term memory in mild cognitive impairment and Alzheimer disease: role of attention. Neuropsychologia. 2007;45(8):1948–1960. [DOI] [PubMed] [Google Scholar]
- 65. Ratliff KR, Newcombe NS. Reorienting when cues conflict: evidence for an adaptive-combination view. Psychol Sci. 2008;19(12):1301–1307. [DOI] [PubMed] [Google Scholar]
- 66. Deeks JJ, Altman DG. Statistics notes-diagnostic tests 4: likelihood ratios. BMJ. 2004;329(7458):168–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Doniger GM, Dwolatzky T, Zucker DM, et al. Computerized cognitive testing battery identifies mild cognitive impairment and mild dementia even in the presence of depressive symptoms. Am J Alzheimers Dis Other Demen. 2006;21(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Inoue M, Jinbo D, Nakamura Y, Taniguchi M, Urakami K. Development and evaluation of a computerized test battery for Alzheimer's disease screening in community-based settings. Am J Alzheimers Dis Other Demen. 2009;24(2):129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ravaglia G, Forti P, Montesi F, et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc. 2008;56(1):51–58. [DOI] [PubMed] [Google Scholar]
- 70. Baumann O, Chan E, Mattingley JB. Distinct neural networks underlie encoding of categorical versus coordinate spatial relations during active navigation. Neuroimage. 2012;60(3):1630–7. [DOI] [PubMed] [Google Scholar]