Abstract
The aims were to assess dysautonomia in Alzheimer’s Disease (AD), clinically and electrophysiologically, using sympathetic skin response (SSR) test and R-R interval variation (RRIV) test and to analyze the relationship between symptoms of dysautonomia and SSR/RRIV results. A tota of 54 patients with AD and 37 controls were evaluated using Autonomic Symptoms Questionnaire and SSR/RRIV test. Clinical dysautonomia was observed in 66% of patients (eg, orthostatic hypotension in 34.5%, constipation in 17.2%, urinary incontinence in 13.8%). The SSR test was abnormal in 26%, but the RRIV test was abnormal in 97.7% of cases; there was significant difference in RRIV test results between AD and controls (R mean 8.05% and 14.6%, respectively). In AD, clinical dysautonomia occurs at a various degree, and the abnormal SSR and RRIV test results were not always related to the presence of clinical dysautonomia; this observation points that the tests could be used as a useful tool in the assessment of subclinical dysautonomia.
Keywords: Alzheimer’s disease, dysautonomia, sympathetic skin response, R-R interval variation, electroneurography, deep breathing R-R interval variation
Introduction
The autonomic nervous system (ANS) is extremely diffuse with its pathways permeating all organs and autonomic structures involved in the course of virtually all diseases. All pathophysiological processes involving the brain (both inherited and degenerative) can be the cause of autonomic syndromes. The ANS involvement in different degenerative diseases has been frequently discussed. It is well known that severe autonomic failure appears early in the course of multiple system atrophy (MSA), whereas clinical dysautonomia is moderate in Parkinson's disease (PD) and mild in motor neuron disease (MND). Clinically significant manifestations of dysautonomia are rarely seen in Alzheimer’s disease (AD).
Alzheimer’s disease is the most common cause of dementia in the elderly individuals. Alzheimer’s disease is a progressive neurodegenerative disease and its prevalence is about 1% of population at the age of 60 years and doubles every 5 years to reach 30% to 50% by the age of 85. The core symptom is slowly progressing impairment of memory and other cognitive functions. 1
The characteristic signs are also behavior and mood disturbances and psychotic symptoms such as hallucinations or delusions. Other abnormalities that typically occur are physical aggression, agitation, irritability, wandering, apathy, depression, and sleep disturbances. Except for the mental state, the neurological examination is usually normal, but extrapyramidal features like rigidity, bradykinesia, shuffling gait, and postural changes are relatively common in later stages of the diseases. Epileptic seizures occur in 10% of patients in the terminal stage of the disease. 2
Clinical and subclinical dysautonomia analyzed with different autonomic tests in patients with AD were in the focus of interest in many studies, but till now general or organ-specific involvement in AD is still poorly investigated. The findings are scarce and conflicting.
Clinical dysautonomia is not common in AD, although lack of control of the sphincters occurs in late and terminal phase of AD in nearly all cases. However, a few authors describe normal results of autonomic tests in AD, especially compared with patients having vascular dementia. 3 The results of recent studies indicated that the cholinergic deficit, crucial in AD for cognitive and memory impairment could also be reflected by ANS abnormalities for example pupil size changes and mobility. Patients with probable AD (n = 23) had abnormal pupillometric parameters. 4
Moreover, Toledo and Junqueira, investigated the relationship between ANS function and cognitive abilities in AD and concluded that the cognitive status and the cardiac sympathovagal modulation, estimated by heart interval variability in supine and standing positions (n = 22), appear to be correlated and hypothetically may influence one another in mild-to-severe AD. 5
Algotsson et al emphasize that tests for evaluation of subclinical dysautonomia reveal abnormalities in both sympathetic and parasympathetic system in most cases of AD. 6
Ferini-Strambi et al described in one-third of the patients with AD abnormal results of R-R interval variation test (RRIV) and spectral analysis during sleep and during spontaneous sleep movements. 7
Vitiello et al demonstrated abnormal reactions of sympathetic system for stress and erect position in patients with AD. These abnormalities were found in the early as well as in the late stage of the disease. 8
Wang et al found mild changes in the activity of the parasympathetic system, especially vagus nerve in patients with AD, while sympathetic functions remained normal. The sympathetic skin response (SSR) test results in AD were within normal range in this study, whereas the results of the RRIV test were abnormal. 9 However, Paradowski et al described abnormal results of the RRIV test as well as the SSR test in patients with AD. 10
Because the conclusions of current studies are still inconclusive, further studies are required. Moreover, in previous studies, the authors focused on autonomic test results but less on the analysis of relationship between clinical signs of dysautonomia in AD and assessment in autonomic, functional examinations as in our study.
The aims of our study were:
to assess the frequency of symptoms and intensity of dysautonomia in AD, by clinical assessment and autonomic functional tests to define the pattern of autonomic abnormalities found on SSR and RRIV and to analyze the usefulness of both tests in the evaluation of dysautonomia compared to clinical examination; and
to analyze the correlation between the results of both tests.
Materials/Patients
Clinical involvement of ANS, and autonomic, functional tests, SSR test, and RRIV test results were analyzed in 54 patients with probable diagnosis of AD (according to McKhann criteria), 1 the Caucasian white race, including 31 women (57.4%) and 23 men (42.6%) of age 58 to 86 years (mean age: 73.1 ± 6.3), with disease duration of 1 to 8 years (mean 3.6 ± 1.8). The degree of cognitive impairment was assessed using Mini-Mental State Examination (MMSE) scale according to Folstein et al, 11 with a mean MMSE score of 17.85 ± 5.49. The exclusion criteria from the study group under study were serious systemic diseases such as severe arterial hypertension (nontreated, or treated irregularly, with signs on the oculi fundus of at least second grade according to Keith Wagener scale), cardiac arrhythmia as atrial fibrillation or extra systole, renal or hepatic dysfunction, diabetes mellitus, marked trophic lesion of shins, and taking drugs influenced on autonomic functions markedly (high doses of beta-blockers, etc). Patients with symptoms of peripheral neuropathy were also excluded. The control group consisted of 37 healthy volunteers (21 women and 16 men) of age 25 to 91 years (mean age: 47.3 ± 16.4 years). None of the controls had clinical symptoms of cardiac failure, coronary disease, renal or hepatic dysfunction, diabetes mellitus, arterial hypertension, cardiac arrhythmia, and trophic skin lesions. None of them were taking medicines that could influence the autonomic system.
Methods
The clinical evaluation of dysautonomia was performed by 2 independent physicians the same day as RRIV and SSR tests were performed, after obtaining informed consent from the patient and caregiver. Medical history of patients with dementia (MMSE below 20 points) was taken from the patient’s caregiver or his or her relatives.
Dysautonomia was evaluated using modified Autonomic Symptoms Questionnaire proposed by Low. 12 For a semiquantitative evaluation of dysautonomia in AD, the authors modified the aforementioned questionnaire and quantitatively evaluated clinical dysautonomia intensity according to an arbitrally defined score system presented in Table 1.
Table 1.
Autonomic Symptoms Questionnaire for Semiquantitative Evaluation of Dysautonomia (in Scores)
| Clinical signs of dysautonomia | Intensity of changesa ( in points) |
|---|---|
| Orthostatic hypotension | (0-2) |
| - Mild | 1 |
| - Severe | 2 |
| Syncope | 0-1 |
| Anhidrosis | 0-1 |
| Hipohidrosis | 0-1 |
| Hiperhidrosis | 0-1 |
| Urinary incontinence | 0-1 |
| Urinary retention | 0-1 |
| Urinary urgency | 0-1 |
| Hypersalivation | 0-1 |
| Constipation | 0-1 |
| Diarrhea | 0-1 |
| Impotence (in men) | 0-1 |
a 0 = no symptoms; 1 = symptoms present; orthostatic hypotension: mild → present only in case of facilitating conditions; severe → present all the time.
Both RRIV and SSR tests were recorded in participants lying in a semi-darkened room, with temperature of 22°C to 26°C, after relaxing for several minutes. Tests were recorded at the same time of the day (between 10.00 and 13.00 hours) within a few hours after a light meal, using the Viking IV, Nicolet Biomedical Inc (Multi-Mode Program Plus, version 4.0). Both tests were performed according to a protocol recommended by International Federation of Clinical Neurophysiology (IFCN). 13
The SSR test was recorded with standard electromyogram (EMG) surface electrodes placed in the center of the palm and the sole, with the reference electrodes on the dorsal surfaces of the hand and the foot. Five consecutive electrical stimuli with 10 to 12 mA intensity and 0.2 ms duration were applied to the median nerve at the wrist. The stimuli were delivered at irregular intervals longer than 30 seconds to assure reproducibility. Recordings were made simultaneously from the upper and lower limbs with the Viking IV, Nicolet Biomedical Inc (Multi-Mode Program Plus, version 4,0) using a band-pass of 2 to 5000 Hz for upper and of 2 to 2000 Hz for lower limbs. The input sensitivity ranged from 50 to 500 mV per division depending on the amplitude of the response.
The latency and amplitude (peak to peak) of the highest response were measured (3 evoked responses were registered but only 1 was analyzed—that is of the shortest latency and the highest amplitude).
The SSR was considered abnormal if the latency deviation was more than 2 standard deviation (SD) compared to the control group or if the response was absent (not elicited by 3 consecutive stimulations). The degree of SSR abnormality was quantified using our laboratory scores, 11 defined as below:
0 = normal;
1 = increased latency in one limb;
2 = increased latency in the upper and lower limb or absence of the response from one limb;
3 = increased latency in one limb or absence of the response from the other limb; and
4 = absence of the response from the upper and lower limb.
The RRIV was recorded using 2 disc electrodes placed over the precordium (one in the apex area, the other in the second left intercostal space) and a ground electrode over the wrist. The electrocardiograph (ECG) tracing was obtained at rest and during deep breathing and the values of RRIV were calculated by the computer and expressed as a percentage (heart rate variation).
where HRV is the heart rate variability; HR max is the maximal heart rate; HR min is the minimal heart rate; and HR mean the mean heart rate.
The degree of RRIV abnormality was quantified using our laboratory scores defined as below:
0 = normal RRIV test at rest, during deep breathing, and an increase of the RRIV during deep breathing;
1 = abnormal (decreased) RRIV test at rest or during deep breathing, or no increase in the RRIV during deep breathing; and
2 = abnormal (decreased) RRIV test at rest and during deep breathing and no increase in the RRIV during deep breathing.
Statistical Analysis
Prior to analysis, the normality of distribution of the functional variables was tested by Shapiro-Wilk test. Considering that the variables showed nonnormal distribution, correlation analysis between different parameters were studied using Spearman correlation coefficient test. For group comparison, Wilcoxon rank sum, chi-square, and Fisher exact tests were used. Statistical significance was defined as P < .05. Values are presented as medians and ranges and also mean ± SD, to compare other studies results with normal distribution of variables. As an additional analysis, logistic regression was used to calculate the significance level, because of the presence of the confounding factor—age.
Results
Clinical symptoms of dysautonomia were found in 66% of our patients with AD but were relatively mild. The orthostatic hypotension, constipation, urinary incontinence, syncope, urinary urgency, and diarrhea were reported by this group of patients. Frequency of symptoms of dysautonomia in patients with AD is presented in Table 2.
Table 2.
Frequency of Symptoms of Dysautonomia in Patients With AD
| Signs of dysautonomia | Number of Patients With AD, n (%) |
|---|---|
| Orthostatic hypotension | 10 (34.5) |
| Constipation | 5 (17.2) |
| Urinary incontinence | 4 (13.8) |
| Syncope | 2 (6.9) |
| Hipohidrosis | 1 (3.5) |
| Urinary urgency | 1 (3.5) |
| Diarrhea | 1 (3.5) |
Abbreviation: AD, Alzheimer’s disease.
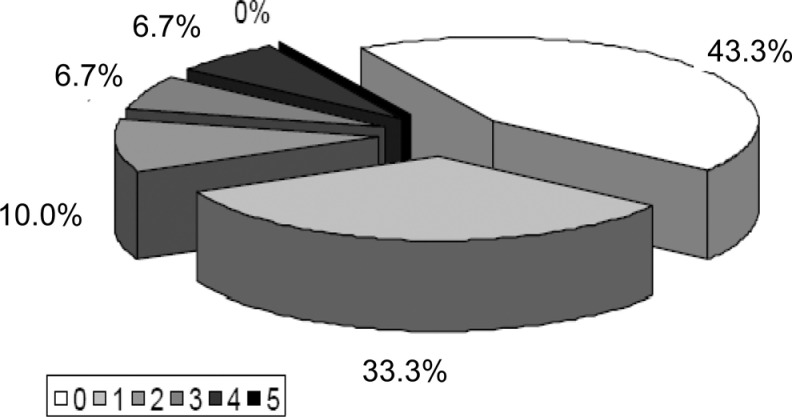
The orthostatic hypotension, constipation, and urinary incontinence were the most common signs of dysautonomia in patients with AD. The intensity of clinical dysautonomia was usually mild (Figure 1).
Figure 1.
Intensity of clinical dysautonomia in patients with AD. 0, 1, 2, 3, 4, and 5 indicate the sum of points of Autonomic Symptoms Questionnaire for semiquantitative evaluation of dysautonomia (Table 1). AD indicates Alzheimer’s disease.
The mean (and median) values of SSR latency from upper and lower limb in patients with AD and controls are presented in Table 3.
Table 3.
Latency of SSR in Patients With AD (n = 54) and Control Group (n = 37)
| Alzheimer’s disease | Control group | |||
|---|---|---|---|---|
| Parameter | Upper limb (seconds) | Lower limb (seconds) | Upper limb (seconds) | Lower limb (seconds) |
| Median (range) | 1.43 (1.12−1.89) | 1.97 (1.63−3.17) | 1.37 (1.12−1.67) | 1.83 (1.49−2.29) |
| Mean ± SD | 1.44 ± 0.17 | 2.03 ± 0.30 | 1.37 ± 0.12 | 1.87 ± 0.22 |
Abbreviations: SSR, sympathetic skin response; AD, Alzheimer’s disease; SD, standard deviation.
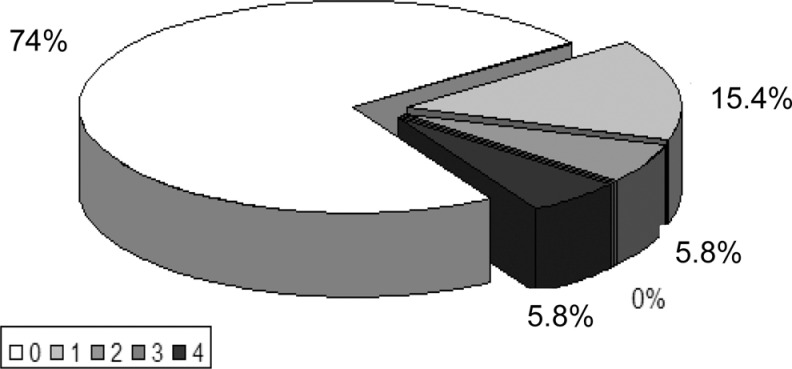
There were no differences between the mean values of SSR latency in patients with AD and controls. The distribution of the degree of SSR abnormalities (in scores) in patients with AD is shown on Figure 2.
Figure 2.
The intensity of SSR abnormalities in AD. 0 = normal; 1 = increased latency in one limb; 2 = increased latency in the upper and lower limb or absence of the response from one limb;3 = increased latency in one limb or absence of the response from the other limb; 4 = absence of the response from the upper and lower limb. AD indicates Alzheimer’s disease; SSR, sympathetic skin response.
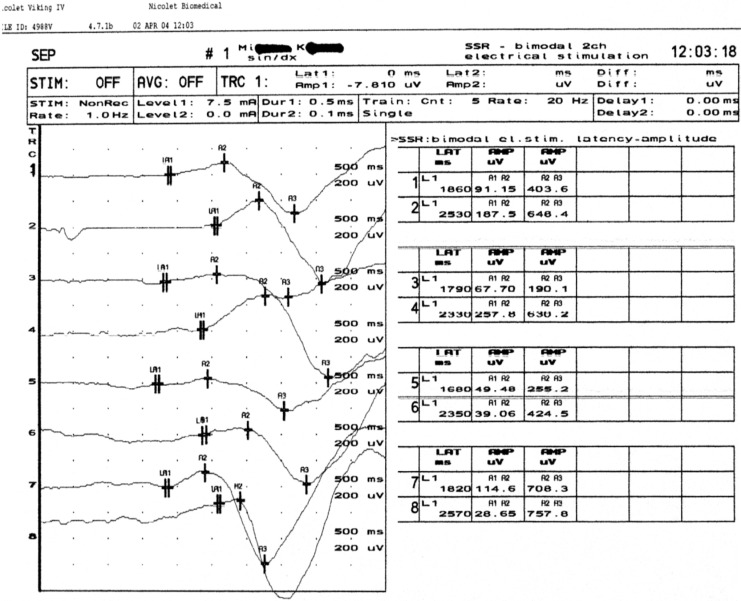
In about 25.9% (14 of 54) of the patients with AD, the SSR test results were abnormal but the intensity of those changes was relatively mild. In nearly 6% (3) of the patients with AD, there was no SSR response from the upper limbs and in another 6% from the lower limbs. The example of abnormal SSR test results in a patient with AD is presented in Figure 3.
Figure 3.
Prolonged latency of SSR from the upper and lower limbs in AD patient. AD indicates Alzheimer’s disease; SSR, sympathetic skin response.
The mean and median values of RRIV response at rest and during deep breathing in controls and patients with AD are presented in Table 4.
Table 4.
The RRIV Results in Patients With AD and Control Group
| RRIV results (%) | AD | Control group | |||
|---|---|---|---|---|---|
| Median (range) | Mean ± SD | Median (range) | Mean | P value | |
| R1 | 7.58 (3.40−18.40) | 7.78 ± 2.80 | 14.10 (4.83−31.0) | 15.15 ± 6.57 | .058 |
| R2 | 7.93 (2.97−14.90) | 7.81 ± 2.70a | 13.30 (4.13−26.0) | 14.07 ± 5.91 | .039 |
| R3 | 7.59 (2.85−16.20) | 7.60 ± 2.81a | 14.60 (4.32−25.80) | 13.97 ± 5.12 | .037 |
| Rmean | 8.05 (3.65−16.0) | 7.73 ± 2.35a | 14.60 (4,96−25.10) | 14.39 ± 5.45 | .03 |
| R-DB | 17.20 (3.62−32.70) | 17.87 ± 6.74a | 27.20 (8.40−61.40) | 31.57 ± 14.49 | .018 |
Abbreviations: RRIV, R-R interval variation; AD, Alzheimer’s disease.
a P < .05 (Wilcoxon test).
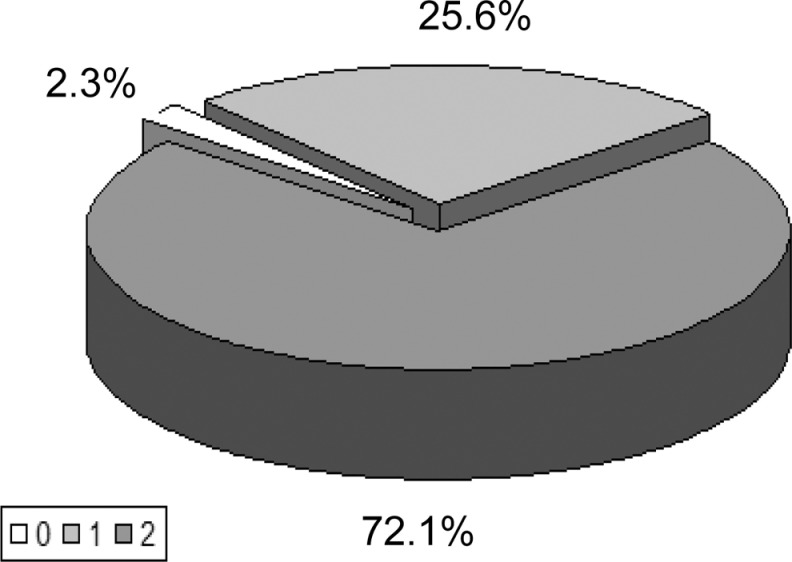
The differences between all mean values of RRIV parameters in patients with AD and controls were statistically significant (using Wilcoxon test). The distribution of the degree of RRIV abnormalities (in scores) in patients with AD is shown in Figure 4.
Figure 4.
The intensity of RRIV abnormalities in AD.
0 = normal RRIV test at rest, during deep breathing, and an increase of the RRIV during deep breathing; 1 = abnormal (decreased) RRIV test at rest or during deep breathing or no increase of the RRIV during deep breathing; 2 = abnormal (decreased) RRIV test at rest And during deep breathing And no increase of the RRIV during deep breathing. AD indicates Alzheimer’s disease; RRIV, R-R interval variation.
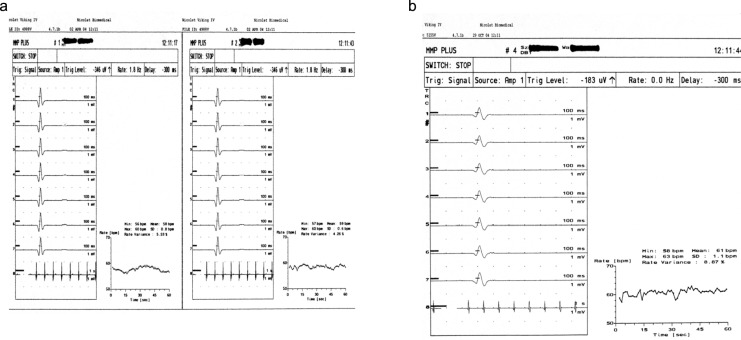
In 97.7% (53) of patients with AD, the RRIV test results were abnormal and in 72.1% (39) of patients the intensity of those changes was moderate (score of 2 points). Examples of abnormal RRIV test results in patients with AD are presented in Figure 5A and b. The relationship between the presence and degree of abnormalities (SSR and RRIV evaluated together and separately) and intensity of clinical symptoms in the group of patients with AD were analyzed. The significant correlation was not found (test chi-square; Fisher test). There was no correlation between the results of SSR and abnormalities in RRIV as well as between the intensity of dysautonomia evaluated by clinical examination (scale 0-5 points) and the results of the RRIV (0-2 points) and SSR (0-4 points) tests, analyzed separately and together (test chi-square, Fisher exact test).
Figure 5.
A, Abnormal RRIV at rest in patients with AD (two curves): decreased HRV (5.53% and 4.26%). B, Abnormal RRIV during deep breathing in patients with AD: decreased HRV (8.87%). AD indicates Alzheimer’s disease; RRIV, R-R interval variation; HRV, heart rate variability.
There was no correlation between the intensity of abnormalities found in patients with AD in the SSR test and the degree of changes in the RRIV test (the Fisher test). Relationships between age, duration of the disease, the degree of clinical and electrophysiological abnormalities and the values of the parameters analyzed in the SSR and RRIV were not revealed by Spearman’s correlation test.
Discussion
Dysautonomia is never a predominant clinical manifestation in AD. 3,6,14,15 In our study, 66% patients with AD presented clinical signs of dysautonomia. The most common were orthostatic disturbances and urinary impairment. The intensity of symptoms in most cases was mild or moderate.
The advantage of our study is numerous group of patients with AD under study. As was noticed previously, the selection of participants in this age range with no systemic, coexisting diseases is particularly difficult. Our AD group was significantly older than the controls, but the logistic model of analysis proved that the differences between RRIV results in AD and control group are real due to the effect of disease. 16
In literature, data on the prevalence and intensity of dysautonomia in AD are often controversial. Yamamoto et al emphasized the absence of signs of dysautonomia in AD. 3 Siennicki-Lantz et al. considered that orthostatic disturbances found in patients with AD could be partly related to advanced age of population, and they compared the results of AD with the group of older people without dementia. They found a certain percentage of orthostatic disturbances in both groups. 17 Jhee et al considered that orthostatic hypotension found in AD is directly related to the process of ageing. 18 Idiaquez et al described frequent hypotension after meals in patients with AD. 19 Many authors revealed abnormal results of cardiovascular tests in AD and obtained the objective confirmation of clinical dysautonomia by these methods. 8,20
Some authors consider that urinary incontinence, found also in our group with AD, should not be considered as a symptom of real dysautonomia. Sobów et al suggested that the so-called pseudoincontinence could be the effect of difficulties in finding the way to the toilet resulting from visual–spatial agnosia. 21 Del-Ser et al compared impairment of urinary bladder control between patients with AD and patients with Lewy body disease (LBD). In patients with AD, urinary incontinence occurred later in the course of the disease than in patients with LBD, and it was always accompanied by severe dementia; whereas in patients with LBD, incontinence was an early symptom of the disease and usually it was not associated with marked cognitive impairment. 14
Results of RRIV tests are often abnormal in AD. In our material, RRIV examination revealed abnormal results in the majority (98%) of patients with AD and the degree of changes was important. The significant difference between the median and the mean values of the RRIV parameters, that is the RRIV at rest and during deep breathing in AD and control group was found (P < .05 Wilcoxon test). Abnormal RRIV test results found in AD were also reported by other authors. 9,22 -24 Our results and data from literature confirm that dysautonomia associated with dysfunction of the cardiovascular system occur frequently in AD. The cardiovascular dysfunction could be a result of typical for AD neurodegeneration with overlapping degenerative changes associated with the process of ageing.
It is well known that AD is classically a neurodegenerative process with predominant involvement of the cholinergic system. The function of this system could be evaluated by SSRs, which was abnormal in 27% of patients with AD in our material. In our material, SSR abnormalities were mild in most cases. In literature similar results were obtained by Paradowski et al, while Wang et al identified normal SSR test results in AD. 9,24 In our study, the relationship between the degree of abnormalities in the electrophysiological tests and the clinical evaluation of the autonomic signs was not shown. Till now, there has been no similar comparison in literature.
In a study by Parvizi et al, the neuropathological confirmation of the abnormal results of the electrophysiological tests was provided. 25 They found beta-amyloid plaques and neurofibrillary tangles in periaqueductal gray matter. Parvizi et al supposed that the neuropathological background of dysautonomia in AD is neurodegeneration in the periaqueductal gray matter. Stimulation of the lateral columns in periaqueductal gray matter results in hypertension and tachycardia, while activation of ventral–lateral columns causes hypotension and bradycardia. 25
Kalman et al postulated that disturbances in cholinergic neurotransmission, specific for AD, are reflected in abnormal results of vasodilatation test, which depend on function of cholinergic fibers. 15
Auld et al demonstrated that beta-amyloid itself may impair cholinergic transmission independently of its neurotoxic properties. 26 The results of Bengoechea et al’s recent, experimental study about the effect of beta-amyloid on the peripheral nervous system in AD mice is very interesting. The authors provided evidence that neurotrophin receptor p75 is neuroprotective against beta-amyloid–induced neurotoxicity in the sympathetic nervous system. The sympathetic innervation defects in p75-deficient AD mice, which died before the age of death in controls, could be one of the factor responsible for observed lethality. 27
Conclusions
The autonomic electrophysiological tests may be abnormal in AD: the abnormal SSR was very rarely seen in AD, whereas the abnormal RRIV test was observed in majority of patients with AD.
The abnormal electrophysiological test results were frequently found in the absence of clinical dysautonomia, which support the hypothesis that these tests are useful in the assessment of subclinical dysautonomia.
If the autonomic electrophysiological tests were within normal limits, there was no clinical evidence of dysautonomia in the studied patient group. This fact is suggestive of significant sensibility of electrophysiological tests being used.
In AD population, the abnormal electrophysiological tests were not always related to the presence of clinical dysautonomia. This observation points out the complementary role of autonomic tests versus clinical evaluation.
In summary, sympathetic and parasympathetic involvement occurs in AD in a various degree and may be assessed by noninvasive electrophysiological tests.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease report of the NINCDS–ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 2. Kłoszewska I. Incidence and relationship between behavioural and psychological symptoms in Alzheimer’s disease. Int J Geriatr Psychiatry. 1998;13(11):785–792. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto T, Shimazu K, Tamura N, Watanabe S, Hamaguchi K. (1990) Autonomic nervous functions in Alzheimer type and multi–infarct dementia – a hemodynamic study. Rinsho Shinkeigaku. 1990;30(9):1020–1022. [PubMed] [Google Scholar]
- 4. Fotiou DF, Stergiou V, Tsiptsios D, Lithari C, Nakou M, Karlovasitou A. Cholinergic deficiency in Alzheimer’s disease and Parkinson’s disease: evaluation with pupillometry. Int J Psychophysiol. 2009;73(2):143–149. [DOI] [PubMed] [Google Scholar]
- 5. De Vilhena Toledo MA, Junqueira LF. Cardiac sympathovagal modulation evaluated by short-term heart interval variability is subtly impaired in Alzheimer's disease. Geriatr Gerontol Int. 2008;8(2): 109–118. [DOI] [PubMed] [Google Scholar]
- 6. Algotsson A, Viitanen M, Winblad B, Solders G. Autonomic dysfunction in Alzheimer’s disease. Acta Neurol Scand. 1995;91(1):14–18. [DOI] [PubMed] [Google Scholar]
- 7. Ferini–Strambi L, Smirne S. Cardiac autonomic function during sleep in several neuropsychiatric disorders. J Neurol. 1997;244 (suppl 1):29–36 [DOI] [PubMed] [Google Scholar]
- 8. Vitiello B, Veith RC, Molchan SE, et al. Autonomic dysfunction in patients with dementia of Alzheimer type. Biol Psychiatry. 1993;34(7):428–433. [DOI] [PubMed] [Google Scholar]
- 9. Wang SJ, Liao KK, Fuh JL. Cardiovascular autonomic functions in Alzheimer’s disease. Age Ageing. 1994;23(5):400–404. [DOI] [PubMed] [Google Scholar]
- 10. Paradowski B, Bilińska M, Koszewicz M, Pokryszko A. Evaluation of cardiovascular and sudomotor functions in Alzheimer’s disease. Pol Merkuriusz Lek. 1999;7(40):180–184. [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR. Mini Mental State. A practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 12. Low PA. Clinical Autonomic Disorders: Classification and Clinical Evaluation. In: Low PA, ed. Clinical Autonomic Disorders. Evaluation and Management. 2nd ed. New York, NY: Lippincott–Raven; 1997:3–13. [Google Scholar]
- 13. Claus D, Schondorf R. Sympathetic skin response. In: Deuschl G., Eisen A., eds. Recommendation for the Practice of Clinical Neurophysiology: Guidelines of the International Federation of Clinical Neurophysiology. 2nd ed. Elsevier; 1999:277–285. [PubMed] [Google Scholar]
- 14. Del-Ser T, Munoz DG, Hachinski V. Temporal pattern of cognitive decline and incontinence is different in Alzheimer’s disease and diffuse Levy body disease. Neurology. 1996;46(3):682–686. [DOI] [PubMed] [Google Scholar]
- 15. Kalman J, Szakacs R, Torok T, et al. Decreased cutaneous vasodilatation to isometric handgrip exercice in Alzheimer’s disease. Int J Geriatr Psychiatry. 2002;17(4):371–374. [DOI] [PubMed] [Google Scholar]
- 16. De Vilhena Toledo MA, Junquiera LF, Jr. Cardiac sympathovagal modulation evaluated by short-term heart interval variability in subtly impaired in Alzheimer’s disease. Geriatr Gerontol Int. 2008;8(2):109–118. [DOI] [PubMed] [Google Scholar]
- 17. Siennicki-Lantz A, Lilja B, Elmstahl S. Orthostatic hypotension in Alzheimer’s disease: result or cause of brain dysfunction? Aging. 1999;11(3):155–160. [PubMed] [Google Scholar]
- 18. Jhee SS, Sramek JJ, Wardle TS, Cutler NR. Orthostasis in Alzheimer disease: a retrospective analysis. Alzheimer Dis Assoc Disord. 1995;9(4):243–246. [PubMed] [Google Scholar]
- 19. Idiaquez J, Sandoval E, Seguel A. Association between neuropsychiatric and autonomic dysfunction in Alzheimer’s disease. Clin Auton Res. 2002;12(1):43–46. [DOI] [PubMed] [Google Scholar]
- 20. Szili-Török T, Kálmán J, Paprika D, Dibó G, Rózsa Z, Rudas L. Depressed baroreflex sensitivity in patients with Alzheimer’s and Parkinson’s disease. Neurobiol Aging. 2001;22(3):435–438. [DOI] [PubMed] [Google Scholar]
- 21. Sobów T, Barcikowska M, Mossakowski MM, Alzheimera Choroba. In: Neurodegeneracje. Liberski PP, Mossakowski MM. eds. Polish Academy of Sciences. Warsaw, Poland, 2003:101–116. [Google Scholar]
- 22. Giubilei F, Strano S, Imbimbo BP, et al. Cardiac autonomic dysfunction in patients with Alzheimer disease: possible pathogenetic mechanisms. Alzheimer Dis Assoc Disord. 1998;12(4):356–361. [DOI] [PubMed] [Google Scholar]
- 23. McLaren AT, Allen J, Murray A, Ballard CG, Kenny RA. Cardiovascular effects of donepezil in patients with dementia. Dement Geriatr Cogn Disord. 2003;15(4):183–188. [DOI] [PubMed] [Google Scholar]
- 24. Paradowski B, Bilińska M, Koszewicz M, Pokryszko A. Evaluation of cardiovascular and sudomotor functions in Alzheimer’s disease. Pol Merkuriusz Lek. 1999;7(40):180–184. [PubMed] [Google Scholar]
- 25. Parvizi J, Van Hoesen GW, Damasio A. Selective pathological changes of the periaqueductal gray matter in Alzheimer’s disease. Ann Neurol. 2000;48(3):344–353. [PubMed] [Google Scholar]
- 26. Auld DS, Kar S, Quirion R. Beta-amyloid peptides as direct cholinergic neuromodulators: a missing link? Trends Neurosci. 1998;21(1):43–49. [DOI] [PubMed] [Google Scholar]
- 27. Bengoechea TG, Chen Z, O'Leary DA, Masliah E, Lee KF. P75 reduces β-amyloid-induced sympathetic innervation deficits in an Alzheimer’s diseasemouse model. Proc Natl Acad Sci U S A. 2009;106(19):7870–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]