During the past 12 months chloroquine and hydroxychloroquine have been touted as miracle cures for COVID‐19 and introduced into COVID‐19 treatment protocols in Asia, Africa, and North and South America (see Figure 1). This has led to massive increases in demand such that patients with rheumatoid arthritis and lupus have been deprived of effective treatments.
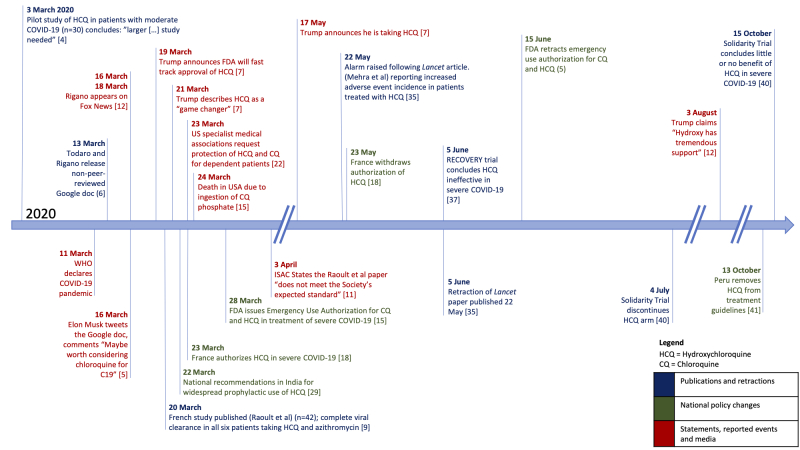
Timeline of key events in the story of chloroquine/hydroxychloroquine and COVID‐19
A Cochrane Review by Bhagteshwar Singh and colleagues definitively concludes that hydroxychloroquine has no clinical benefit in treating COVID‐19 in hospitalized patients. The dissemination of information on these drugs in the scientific press and other media has been rapid and tumultuous with strong and polarized opinions among scientists, politicians, and the general public, building a climate of mistrust. Potential resulting harms included wasted resources (including research capacity) and drug shortages for evidence‐based indications. The false hope instilled may have also led to unsupervised use of potentially harmful medications. While most national and health system‐level guidance is evidence based, how did we get into such a chaotic and confusing situation with the assessment of chloroquine and hydroxychloroquine efficacy?
Chloroquine was proposed as a potential treatment for severe acute respiratory syndrome (SARS) in 2003, but transmission of SARS was stopped before trials were started. As cases of COVID‐19 exploded in China in early 2020 some small studies of treatment with hydroxychloroquine reported benefit. Speculation regarding these two drugs began in January 2020, spreading globally from China (including statements issued by Chinese state media) and South Korea via Facebook and Instagram, reaching, for example, Nigeria, Vietnam, and France by February.
On 13 March 2020 a Google document on hydroxychloroquine was released by two cryptocurrency investors (Greg Rigano and James Todaro), and Elon Musk tweeted a link to the Google document to more than 40 million followers. Within days, President Trump made his first public comments in support of hydroxychloroquine. US‐based searches for online sale of chloroquine or hydroxychloroquine surged after these endorsements from Musk and Trump.
On 20 March 2020 a small study from France was published, reporting 100% cure of COVID‐19 in people treated with hydroxychloroquine and azithromycin. The primary outcome reported was time to virological clearance, assessed by daily PCR of nasopharyngeal swabs; all six patients receiving the two drugs had a negative PCR by day 6 of treatment. The paper was heavily criticized (but not corrected or retracted), with one review describing it as “fully irresponsible”. On 3 April, the journal's society owners stated that “the article does not meet the Society's expected standards”.
Fox News Channel, a US‐based news broadcaster, interviewed Rigano (on 16 and 18 March), and hosts and guests made frequent reference to chloroquine and hydroxychloroquine between 23 March and 6 April: 275 of these references were positive and 29 were negative or doubtful. Dr Oz, a television personality and cardiothoracic surgeon, appeared on the network supporting the 100% cure claim multiple times over the same period, and on 23 March he echoed Trump's description of hydroxychloroquine as a “game changer”.
The US Food and Drug Administration (FDA) was criticized by former leadership for issuing an Emergency Use Authorization for chloroquine and hydroxychloroquine on 28 March 2020 in response to Trump's endorsement of hydroxychloroquine. In France, a former Minister for Health and cardiologist led those demanding wider access to hydroxychloroquine. France authorized the use of hydroxychloroquine in hospitalized patients on 26 March. President Macron formally visited the institute of Dr Raoult, who led the French study referred to above, on 9 April. By 6 April, 98% of the French population had heard about chloroquine‐based treatment regimens for patients infected with SARS‐CoV‐2, and 59% of the French population believed that chloroquine‐based regimens were effective against this virus.
A significant increase in prescriptions of hydroxychloroquine or chloroquine above expected levels was seen between February and May 2020 in France. This peaked between 23 and 29 March with the volume of prescriptions for hydroxychloroquine or chloroquine alone and hydroxychloroquine in combination with azithromycin respectively 145% and 7000% higher than predicted. On 23 March, a joint statement from US medical specialist associations, including rheumatologists and dermatologists, requested protected supplies for those reliant on hydroxychloroquine treatment. These patients had been impacted by major shortages as demand for hydroxychloroquine also rose in the United States, with associated reports of prescription fraud. In the Dominican Republic, reduced availability drove such patients to solicit the drug via social media from those who had stockpiled it for COVID‐19. New manufacturers and suppliers quickly emerged, leading to concerns regarding quality control.
Countries around the world introduced chloroquine or hydroxychloroquine into guidelines. For example, India quickly directed all frontline healthcare workers and household contacts to take prophylactic hydroxychloroquine. A few days after Trump's initial hydroxychloroquine promotion, President Bolsonaro ordered an increase in production of chloroquine in Brazil.
Those expressing caution regarding the early use of hydroxychloroquine outside of clinical trials included Stephen Hahn, US FDA Commissioner, and Anthony S Fauci, director of the US National Institute of Allergy and Infectious Diseases, who described the evidence as “anecdotal” (20 March 2020). Concern arose about already known potential side effects of chloroquine and hydroxychloroquine, including cardiac arrythmias.
More than 100,000 COVID‐19 related papers and preprints were published by December 2020. A preprint released on 11 May 2020 suggested that hydroxychloroquine and azithromycin might reduce hospital morbidity in COVID‐19 patients. It was withdrawn 10 days later but had already been referenced by Fox News. A paper in The Lancet describing a high incidence of adverse events in hospitalized patients treated with hydroxychloroquine provoked alarm before retraction following controversy about the reliability of the data source. In an attempt to expose poor practice by a suspected predatory journal, a ‘Trojan horse’ article was submitted, published and only retracted once the nature of it was identified by readers.
Criticisms and retractions of widely disseminated publications engendered confusion and distrust. More robust data were released from large, randomized control trials in June and October 2020.
By July 2020 one in six COVID‐19 treatment trials included chloroquine or hydroxychloroquine, potentially at the expense of other drug candidates. Meanwhile, following authorizations by national agencies such as the US FDA, an unknown number of people received hydroxychloroquine outside of trials, with potentially useful data going unrecorded. Difficulties recruiting to studies during the initial enthusiasm for hydroxychloroquine were reported. Many patients in France reportedly declined randomization to any other treatment arm when approached for recruitment to the large DISCOVERY trial. After the tide of opinion turned, concern regarding adverse effects or perceived inefficacy restricted recruitment to trials addressing outstanding questions regarding pre‐ or post‐exposure prophylaxis.
The intense global discussion of the role of chloroquine‐based treatments in the management of COVID‐19 has highlighted the risks and repercussions of misunderstanding and politicization of the uncertainties which can arise in clinical medicine and public health. Uncertainties arise particularly when the need for effective treatments is urgent, information and evidence is scant and rapidly evolving, and the scenario highly complex. Challenges for the scientific community included the efficient prioritization of potential novel or repurposed treatments, the execution and publication of valid and generalizable research studies in short periods of time, and the effective communication of study findings and any attendant uncertainties with a diverse and global audience.
Scientists achieving control of the narrative, rather than politicians, seems improbable, but could provide clearer distinction between facts and subjective interpretation. Efforts should be made to identify substandard studies prior to dissemination, and consideration given to whether a change in approach to preprints is necessary, to incentivize researchers to avoid premature publication. High‐quality peer review using such principles as those underpinning Cochrane Reviews is vital. However, although Cochrane has done much to expedite this process, sifting through the data to produce a careful systematic review, even when rapid, nonetheless requires time.
With the urgent need for solutions to a global catastrophe, researchers responded with immense efforts, producing and sharing new ideas and information at an unprecedented rate. Scientific research was under a spotlight and politicians, and traditional and social media showed their power to expedite the spread of both information and disinformation. However, there was never a greater need for critical appraisal, peer review, clear communication of facts and any uncertainties in the data and evidence, and a distinction between evidence and its interpretation.
Scientists, politician and media organizations share a responsibility to combat ‘fake news' and to promote rational discourse, access to reliable information, and discussion of uncertainties. This will help to ensure the credibility of the scientific community and, most importantly, benefit individual and population health.
Acknowledgements
Susan Gould is supported by the National Institute for Health Research (NIHR) Health Protection Research Unit in Emerging and Zoonotic Infections, a partnership between Public Health England, The University of Liverpool, The University of Oxford and The Liverpool School of Tropical Medicine. The views expressed are those of the authors and not necessarily those of the NIHR, Public Health England or the Department of Health and Social Care. Susan Gould is supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies.
Feedback on this editorial and proposals for future editorials are welcome.
End Notes
Declarations of interest
SG carried out clinical work as an infectious diseases and medical microbiology specialist trainee at Liverpool University Hospitals Foundation Trust, which included care of patients with COVID‐19.
This publication is associated with the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies.
Provenance and peer review
This editorial was commissioned based on a proposal by the authors and was externally peer reviewed.
References
- Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID‐19. Cochrane Database of Systematic Reviews 2021;(2):CD013587. https://doi.org/10.1002/14651858.CD013587.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology Journal 2005;2:1–10. https://doi.org/10.1186/1743-422X-2-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. BioScience Trends 2020;14(1):1–2. https://doi.org/10.5582/bst.2020.01047 [DOI] [PubMed] [Google Scholar]
- Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID‐19. Journal of Zhejiang University (Medical Sciences) 2020;49(2):215–9. http://doi.org/10.3785/j.issn.1008-9292.2020.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels E, Kelly M. How false hope spread about hydroxychloroquine to treat covid‐19 — and the consequences that followed. The Washington Post, 13 April 2020. www.washingtonpost.com/politics/2020/04/13/how-false-hope-spread-about-hydroxychloroquine-its-consequences [Google Scholar]
- Robins‐Early N. The strange origins of Trump's hydroxychloroquine obsession. HuffPost, 13 May 2020. www.huffingtonpost.co.uk/entry/trump-hydroxychloroquine-coronavirus-fox-news_n_5ebaffdbc5b65b5fd63dac80 [Google Scholar]
- Solender A. All the times Trump has promoted hydroxychloroquine. Forbes, 22 May 2020. www.forbes.com/sites/andrewsolender/2020/05/22/all-the-times-trump-promoted-hydroxychloroquine [Google Scholar]
- Liu M, Caputi TL, Drezde M, Kesselheim AS, Ayers JW. Internet searches for unproven COVID‐19 therapies in the United States. JAMA Internal Medicine 2020;180(8):1116–8. http://doi.org/10.1001/jamainternmed.2020.1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents 2020;56(1):105949. https://doi.org/10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rosendaal FR. Review of: “Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial Gautret et al 2010 DOI: 10.1016/j.ijantimicag.2020.105949. International Journal of Antimicrobial Agents 2020;56(1):106063. https://doi.org/10.1016/j.ijantimicag.2020.106063 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Voss A. Official Statement from International Society of Antimicrobial Chemotherapy (ISAC): Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial (Gautret P et al. PMID 32205204). 3 April 2020. www.isac.world/news-and-publications/official-isac-statement [DOI] [PMC free article] [PubMed] [Retracted]
- Bump P. The rise and fall of Trump's obsession with hydroxychloroquine Forty days of promotion, hype – and eventual retreat. The Washington Post, 24 April 2020. www.washingtonpost.com/politics/2020/04/24/rise-fall-trumps-obsession-with-hydroxychloroquine [Google Scholar]
- Power L, Savillo R. Fox News has promoted hydroxychloroquine nearly 300 times in a two‐week period. Media Matters for America, 4 July 2020. www.mediamatters.org/fox-news/fox-news-has-promoted-hydroxychloroquine-nearly-300-times-two-week-period [Google Scholar]
- Korownyk C, Kolber MR, McCormack J, Lam V, Overbo K, Cotton C, et al. Televised medical talk shows—what they recommend and the evidence to support their recommendations: a prospective observational study. BMJ 2014;349:g7346.https://doi.org/10.1136/bmj.g7346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin J. Why does the President keep pushing a malaria drug? What is actually known about hydroxychloroquine, the medication that Trump is fixated on recommending for COVID‐19. The Atlantic, 6 April 2020. www.theatlantic.com/health/archive/2020/04/hydroxychloroquine-trump/609547 [Google Scholar]
- Piller C. Former FDA leaders decry emergency authorisation of malaria drugs for coronavirus. Science, 7 April 2020. https://doi.org/10.1126/science.abc1337 [Google Scholar]
- Sciama Y. Is France's president fueling the hype over an unproven coronavirus treatment?’ Science, 9 April 2020. https://doi.org/10.1126/science.abc1786 [Google Scholar]
- Le Haut Conseil à la Santé Publique. Communiqué de presse – hydroxychloroquine. 27 May 2020. solidarites-sante.gouv.fr/actualites/presse/communiques-de-presse/article/communique-de-presse-hydroxychloroquine-27-mai-2020
- Ledsom A. Hydroxychloroquine: Europe turns away from doctor who championed drug with ‘irresponsible’ study. Forbes, 19 July 2020. www.forbes.com/sites/alexledsom/2020/07/19/hydroxychloroquine-europe-turns-away-from-doctor-who-championed-drug-with-irresponsible-study [Google Scholar]
- IFOP. Chloroquine: miracle ou mirage? 6 April 2020. www.ifop.com/publication/chloroquine-miracle-ou-mirage
- Weill A, Drouin J, Desplas, Cuenot F, Dray‐Spira R, Zureik M. Usage des médicaments de ville en France durant l’épidémie de la Covid‐19 – point de situation après les 8 semaines de confinement et une semaine de post‐confinement (jusqu'au 17 mai 2020): Étude pharmaco‐épidémiologique à partir des données de remboursement du SNDS. Rapport 3 EPI‐PHARE Groupement d’ intérêt scientifique (GIS) ANSM‐CNAM. 9 June 2020. Available from www.ansm.sante.fr/uploads/2020/10/13/20201013-epi-phare-rapport-covid-3-1usage-medic.pdf
- Gibson SW, Gravallese E, Hruza GJ, Palmer AM. Joint statement urging White House Coronavirus Task Force, the leadership of the U.S. Congress and Nation's Governors to ensure hydroxychloroquine access during COVID‐19 Crisis. March 2020. www.lupus.org/news/joint-statement-urging-white-house-coronavirus-task-force-and-nation-s-governors-to-ensure
- Tuccori M, Convertino I, Ferraro S, Cappello E, Valdiserra G, Focosi D, et al. The impact of the COVID‐19 “Infodemic” on drug‐utilization behaviors: implications for pharmacovigilance. Drug Safety 2020;43(8):699–709. https://doi.org/10.1007/s40264-020-00965-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia L. COVID‐19 and fake news in the Dominican Republic. American Journal of Tropical Medicine and Hygiene 2020;102(6):1172–4. http://doi.org/10.4269/ajtmh.20-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D. Malaria drug promoted by Trump did not prevent Covid infections, study finds. The New York Times, 3 June 2020. www.nytimes.com/2020/06/03/health/hydroxychloroquine-coronavirus-trump.html [Google Scholar]
- Danish Medicines Agency. COVID‐19: facts about chloroquine and hydroxychloroquine. 7 April 2020. laegemiddelstyrelsen.dk/en/news/2020/covid-19-facts-about-chloroquine-and-hydroxychloroquine
- Belayneh A. Off‐label use of chloroquine and hydroxychloroquine for COVID‐19 treatment in Africa against WHO recommendation. Research and Reports in Tropical Medicine 2020;11:61–72. http://doi.org/10.2147/RRTM.S269936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YK, Yang J, He Y. Caution and clarity required in the use of chloroquine for COVID‐19. Lancet Rheumatology 2020;2(5):e255. http://doi.org/10.1016/S2665-9913(20)30093-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Sinha B, Ghosal S. Compliance of the Indian National Task Force guidelines for COVID‐19 recommendation by Indian doctors – a survey. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020;14:1413–8. https://dx.doi.org/10.1016%2Fj.dsx.2020.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stargardter G, Paraguassu L. Special report: Bolsonaro bets ‘miraculous cure’ for COVID‐19 can save Brazil – and his life. Reuters, 8 July 2020. www.reuters.com/article/us-health-coronavirus-brazil-hydroxychlo/special-report-bolsonaro-bets-miraculous-cure-for-covid-19-can-save-brazil-and-his-life-idUKKBN249396 [Google Scholar]
- Greene D. ‘Transcript: Full transcript with FDA Commissioner Stephen Hahn. NPR, 20 March 2020. www.npr.org/2020/03/20/818855649/transcript-full-transcript-with-fda-commissioner-stephen-hahn
- Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid‐19. BMJ 2020;369:m1432. https://doi.org/10.1136/bmj.m1432 [DOI] [PubMed] [Google Scholar]
- Else H. How a torrent of COVID science changed research publishing – in seven charts. Nature 2020;588(7839):553. http://doi.org/10.1038/d41586-020-03564-y [DOI] [PubMed] [Google Scholar]
- Davido B, Lansaman T, Lawrence C, Alvarez J‐C, Bouchand F, Moine P, et al. Hydroxychloroquine plus azithromycin: a potential interest in reducing in‐hospital morbidity due to COVID‐19 pneumonia (HI‐ZY‐COVID)? MedRxiv 2020.05.05.20088757. https://doi.org/10.1101/2020.05.05.20088757 (withdrawn) [Google Scholar]
- Mehra MR, Ruschitzka F, Patel AN. Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: a multinational registry analysis. Lancet 2020;395(10240):1820. http://doi.org/10.1016/S0140-6736(20)31324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oodendijk W, Rochoy M, Ruggeri V, Cova F, Lembrouille D, Trottinetta S, et al. Retracted: SARS‐CoV‐2 was unexpectedly deadlier than push‐scooters: could hydroxychloroquine be the unique solution? Asian Journal of Medicine and Health 2020;18(9):14–21. www.journalajmah.com/index.php/AJMAH/article/view/30232 [Google Scholar]
- Horby P, Landray M. No clinical benefit from use of hydroxychloroquine in hospitalized patients with COVID‐19: statement from the chief investigators of the Randomised Evaluation of COVid‐19 thERapY (RECOVERY) trial on hydroxychloroquine. 5 June 2020. Available at www.recoverytrial.net/news
- WHO Solidarity Trial Consortium, Pan H, Peto R, Karim QA, Alejandria M, Henao‐Restrepo AM, et al. Repurposed antiviral drugs for COVID‐19 interim WHO SOLIDARITY trial results. MedRxiv 2020;10.15.20209817. https://doi.org/10.1101/2020.10.15.20209817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM. These scientists are still studying the world's most controversial drug, but they can't find enough people to take it. Buzzfeed News, 28 August 2020. www.buzzfeednews.com/article/stephaniemlee/hydroxychloroquine-clinical-trials-prevention [Google Scholar]
- World Health Organization. “Solidarity” clinical trial for COVID‐19 treatments. www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (accessed 15 March 2021).
- Offord C. Surgisphere sows confusion about another COVID‐19 drug. The Scientist, 16 June 2020. www.the-scientist.com/news-opinion/surgisphere-sows-confusion-about-another-unproven-covid19-drug-67635 [Google Scholar]