Abstract
Background
Thromboelastogram (TEG) is an effective indicator that monitors the dynamic changes of blood coagulation in real-time. It still remains controversial about the performance and influence of coagulation at high altitude. The present study intends to describe comprehensively the clinical features of TEG in populations exposed to or transferring from high altitude.
Methods
Two groups were recruited in the present study. Group A included young males who worked at high-altitude (4888 m or 5418 m) areas for some time, while Group B included young males who had recently returned from high-altitude (4888 m or 5418 m) areas. Medical examinations were performed using portable devices. Spearman's test was used to evaluate the correlations between thromboelastogram (TEG) variables and other variables. Logistic regression analysis was used to analyze the factors affecting various abnormal TEG variables.
Results
A total of 51 adult males were included in the two groups. Significantly increased reaction time (R) and decreased maximum amplitude (MA) were found in group B (P < 0.05). No significant differences were observed in the comparisons of K and angle between the two groups. Various TEG variables were identified to be correlated with different coagulation and biochemical variables. Logistic regression analysis demonstrated that abnormal R was independently associated with direct bilirubin, and abnormal K was independently associated with the platelet count in Group A (P < 0.05). However, none of the factors were independently associated with abnormal TEG variables in Group B.
Conclusion
Populations exposed to or transferring from high altitudes are characterized by different TEG characteristics. Our findings give a comprehensive description of the complex interaction between TEG indexes, coagulation dynamics, and hematological parameters, which can help guide the development of appropriate medical approaches tailored to the unique needs of these populations.
Keywords: High altitude, Thromboelastogram, Coagulation, Liver function, Cardiac function
1. Introduction
Physiological challenges due to various environmental risk factors arise when humans are exposed to high altitudes, including low ambient temperatures, dehydration, and hypobaric hypoxia [1,2]. Studies have demonstrated significant differences in coagulation-related indices between people who stayed for extended periods at high altitudes and those in plain areas. Further, individuals exposed to high-altitude conditions have an increased occurrence of thrombotic disorders [1,3,4]. As previously described, compared with low-altitude regions, long-term stay at high altitudes is associated with a 30-times higher risk of thromboembolic events, along with a greater risk of stroke and related hospitalization [5,6]. Our previous work has demonstrated a significant association between altitudes and the overall incidence of venous thromboembolism, including the incidence at 30/90 days post-operation [7]. Therefore, routine monitoring of the coagulation function is important for the prevention of arterial or venous thrombosis. Moreover, changes in coagulation parameters for individuals before and after being posted at plateau areas are well documented. However, epidemiological data for individuals exposed to or transferring from high altitudes is limited.
Conventional coagulation tests have long been used to assess and diagnose coagulopathy, but they have many limitations. In these tests, platelets (PLTs) are removed from the plasma; hence, the critical role of PLTs in clotting is not measured. Further, thrombosis and fibrinolysis are not observed dynamically, so the coagulation status is not entirely reflected [8]. Thromboelastogram (TEG) measures coagulation function by collecting whole blood samples and detects the viscoelastic changes of blood clots during the coagulation process. TEG can record the entire coagulation factor activation, clot formation, and fibrinolysis process [8]. The TEG results curve indicates the functions of the coagulation factor, PLTs, and fibrinolysis system. Currently, TEG is mostly used to monitor the dynamic coagulation status. Some researchers recommend TEG over the conventional coagulation index for patients with abnormal coagulation function [9,10]. Patients with known acquired hypercoagulable states usually have abnormal TEG. TEG is especially useful in detecting post-operative hypercoagulable conditions and predicting the risk of venous thromboembolism development or bleeding tendency [11,12].
In the present study, we hypothesized that individuals who rapidly descended from high altitudes to low elevations may experience significant alterations in their coagulation function within a short timeframe. Thus, we aimed to investigate changes using the TEG in individuals exposed to or transferring from high altitudes. This was a retrospective cohort study including young male volunteers who resided at 4888 m or 5418 m, and provided evidence for the rational use of drugs in the event of an emergency (such as thrombosis or bleeding) occurring after the plateau-adapted population enters into the plain area.
2. Materials and methods
2.1. Study population
Two groups were recruited in the present study. Group A included young males who worked at high altitudes (4888 m or 5418 m) for a period of time, and Group B included young males who had just recently returned from high altitudes (4888 m or 5418 m). All participants were migrants from the plain areas and underwent medical examinations before entering the plateau area. Exclusion criteria were (1) natives of high altitudes; (2) those who were unhealthy before being exposed to high altitudes; (3) those with previously diagnosed hematological diseases; and (4) those with incomplete clinical data. This study was approved by the ethical committee of the General Hospital of Western Theater Command (approval No.2021EC2-30). All participants provided informed consent before their participation.
2.2. Data collection
Our medical team went to the plateau area to perform routine medical examinations for Group A using portable devices. On the first day after Group B returned from high altitude, they underwent a medical examination using the same devices. For each subject, 15 ml of whole blood were drawn into a ethylenediaminetetraacetic acid (EDTA) blood-collecting tube,a sodium citrate blood-collecting tube and a blood-collecting tube containing inert separator gel and procoagulant, and all tests were completed within 2 h after sample collection. A routine blood test was performed using the analyzer XN-2800 (Sysmex Corporation, Kobe, Japan), and data on variables were recorded, including white blood cell (WBC) counts, red blood cell (RBC) counts, hemoglobin (HGB), hematocrit (HCT), platelet (PLT), and RBC distribution width (RDW). Data were recorded for prothrombin time (PT), PT percent activity (PT%), international normalized ratio (INR), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB), D-dimer (D-D partate aminotransferase (AST), alanine), as transaminase (ALT), amylase (Amy), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), total protein (TP), albumin (ALB), globulin (GLO), urea nitrogen (UN), creatine, uric acid (UA), lactate dehydrogenase (LDH), creatine kinase (CK) and creatine kinase-MB (CK-MB). An electrocardiogram was performed using the analyzer AT-102 (SCHILLER, Bar, Switzerland), and data on variables including P–R intervals (PR), Q-R-S duration (QRS), Q-T intervals (QT), and heart rate (HR) were recorded. Echocardiogram was performed using the analyzer CX50 (Philips, Amsterdam, Netherlands), and data on variables including aortic dimension (AO), pulmonary artery dimension (PA), AO/PA, left ventricular end-diastolic dimension (LVEDD) and right ventricular outflow tract dimension (RVOT) were recorded. According to the instruction manual of the thromboelastography test kit (Lepu Technology, Beijing, China), whole blood and sodium citrate (109 mmol/L) were mixed in a ratio of 9:1, and then analyzed using a CFMS LEPU-8880 analyzer (Lepu Technology, Beijing, China). The normal ranges of each TEG parameter were set based on the kit instruction. Reaction time (R) refers to the time between the activation of the clotting system and the formation of fibrin clots (range: 2–8 min). K refers to the time from the onset of clotting until the amplitude of the TEG pattern reaches 20 mm (range: 1–3 min). The angle refers to the angle between the blood clot formation point and the maximum arc of the curve as the horizontal line and the tangent line (range: 55–78°). Maximum amplitude (MA) refers to the widest distance between the two sides of the curve (range: 51–75 min).
2.3. Statistical analysis
Statistical analysis was performed using SPSS 25.0 (IBM, Armonk, NY). A P value of <0.05 was considered statistically significant. Normal distribution was examined according to Kolmogorov-Smirnov test. Comparisons of TEG variables between two groups were carried out using t-test. Spearman test was used to evaluate the correlations between TEG variables and other variables, including coagulation, blood routine, liver function, renal function, myocardial enzymes, electrocardiogram and echocardiogram. Logistic regression analysis was used to calculate odds ratio (OR) values respectively with each variable of TEG as independent variable. Variables with statistical differences in spearman analysis were taken as covariates.
3. Results
3.1. Comparisons of TEG variables between populations exposed to and transferring from high altitudes
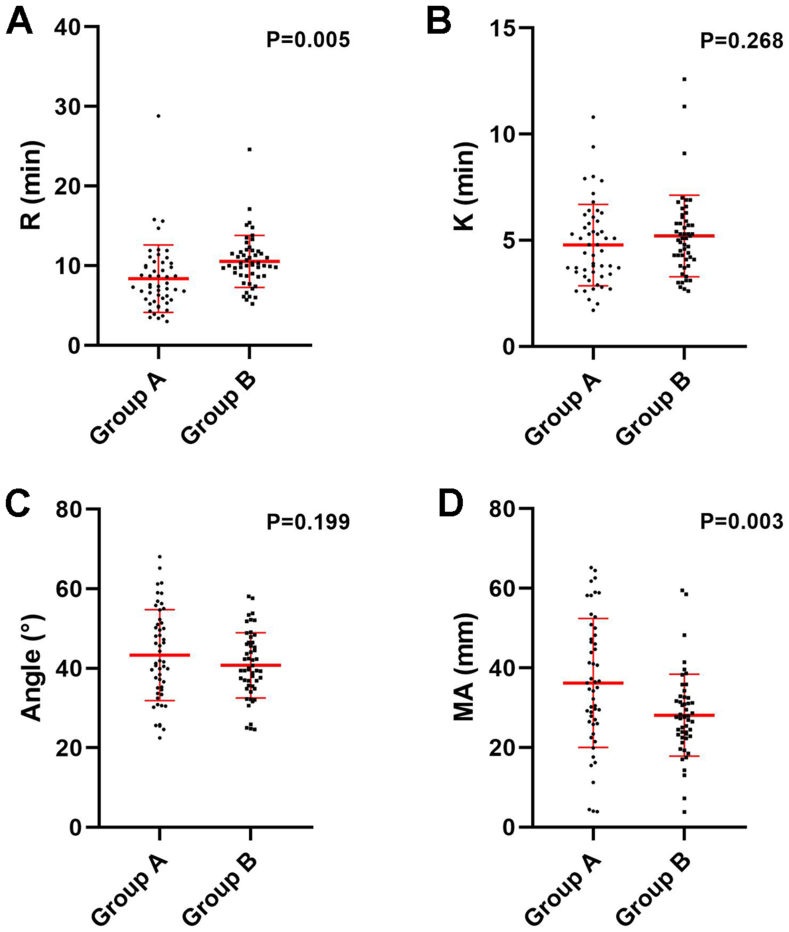
Group A included 51 adult males, with a mean age of 23.5 ± 2.8 years. Twenty-seven worked at an altitude of 4888 m and the others worked at 5418 m. The median exposure duration to high altitude was 7 months (from 3 to 11 months). Group B included 51 adult males, with a mean age of 22.9 ± 3.0 years, of whom 30 adults transferred from an altitude of 4888 m, and the others from 5418 m, with a median duration of exposure of 6.5 months (from 1 to 12 months). Significantly increased R (Fig. 1A, P < 0.05) and decreased MA (Fig. 1D, P < 0.05) were found in group B. No significant differences were identified in the comparisons of K (Fig. 1B, P < 0.05) and the angle (Fig. 1C, P < 0.05) between the two groups. These results indicated that exposure to high altitude would bring a hypercoagulable tendency, which faded away once returning to the plain areas.
Fig. 1.
Comparisons of (A) R, (B) K, (C) Angle and (D) MA between populations exposed to (Group A) and transferring from high altitude (Group B).
3.2. Correlation between TEG and coagulation variables
As displayed in Table 1, Table 2, in Group A, R was significantly correlated with PT (r = 0.551, P < 0.05), PT% (r = −0.414, P < 0.05), INR (r = 0.418, P < 0.05), and FIB (r = 0.323, P < 0.05). In contrast, in Group B, R was not associated with any coagulation variables. K had a significant relationship with PT% (r = −0.325, P < 0.05), INR (r = 0.340, P < 0.05) in Group A, and with FIB (r = −0.335, P < 0.05) in Group B. The angle between the groups was positively correlated with PT and INR in both groups, while negatively correlated with PT% in both groups (P < 0.05). Additionally, the angle was negatively correlated with APTT in Group B (r = −0.341, P < 0.05). The MA value significantly indicated the level of PT% and INR in both groups. Moreover, the MA value significantly indicated PT and APTT in Group B only (P < 0.05). All these findings suggested that TEG parameters could reflect the coagulation level in both two populations.
Table 1.
Correlation between TEG and other variables in population exposed to high altitude.
| R | K | Angle | MA | ||
|---|---|---|---|---|---|
| Coagulation | |||||
| PT | r | 0.551 | 0.230 | −0.343 | −0.122 |
| P | 0.000a | 0.112 | 0.016a | 0.402 | |
| PT% | r | −0.414 | −0.325 | 0.410 | 0.288 |
| P | 0.003a | 0.023a | 0.003a | 0.045a | |
| INR | r | 0.418 | 0.340 | −0.418 | −0.297 |
| P | 0.003a | 0.017a | 0.003a | 0.038a | |
| APTT | r | −0.085 | 0.076 | −0.031 | −0.160 |
| P | 0.563 | 0.602 | 0.832 | 0.271 | |
| TT | r | −0.131 | −0.024 | 0.167 | 0.261 |
| P | 0.369 | 0.872 | 0.252 | 0.070 | |
| FIB | r | 0.323 | −0.243 | 0.056 | 0.081 |
| P | 0.024a | 0.093 | 0.704 | 0.579 | |
| D-D | r | −0.064 | 0.154 | −0.077 | −0.186 |
| P | 0.661 | 0.292 | 0.601 | 0.201 | |
| Blood Routine | |||||
| WBC | r | −0.012 | 0.033 | −0.067 | −0.365 |
| P | 0.932 | 0.819 | 0.646 | 0.009a | |
| RBC | r | 0.213 | 0.460 | −0.507 | −0.592 |
| P | 0.138 | 0.001a | 0.000a | 0.000a | |
| HGB | r | 0.162 | 0.470 | −0.518 | −0.633 |
| P | 0.261 | 0.001a | 0.000a | 0.000a | |
| HCT | r | 0.181 | 0.416 | −0.466 | −0.534 |
| P | 0.207 | 0.003a | 0.001a | 0.000a | |
| RDW | r | 0.079 | 0.234 | −0.230 | −0.171 |
| P | 0.587 | 0.102 | 0.108 | 0.234 | |
| PLT | r | −0.064 | −0.520 | 0.462 | 0.426 |
| P | 0.659 | 0.000a | 0.001a | 0.002 | |
| PDW | r | −0.017 | 0.094 | −0.098 | 0.047 |
| P | 0.909 | 0.518 | 0.497 | 0.746 | |
| Liver Function | |||||
| AST | r | −0.022 | 0.200 | −0.156 | −0.253 |
| P | 0.877 | 0.164 | 0.278 | 0.076 | |
| ALT | r | −0.055 | 0.000 | 0.067 | −0.052 |
| P | 0.704 | 0.999 | 0.646 | 0.722 | |
| AMY | r | −0.262 | 0.160 | −0.078 | −0.199 |
| P | 0.066 | 0.266 | 0.590 | 0.167 | |
| Total bilirubin | r | 0.001 | −0.104 | 0.013 | 0.065 |
| P | 0.994 | 0.471 | 0.928 | 0.653 | |
| Direct bilirubin | r | −0.392 | −0.048 | 0.103 | −0.060 |
| P | 0.005a | 0.742 | 0.476 | 0.678 | |
| Indirect bilirubin | r | 0.255 | −0.146 | 0.023 | 0.126 |
| P | 0.074 | 0.310 | 0.874 | 0.383 | |
| TP | r | −0.235 | −0.003 | 0.081 | 0.018 |
| P | 0.100 | 0.982 | 0.574 | 0.904 | |
| ALB | r | −0.074 | −0.073 | 0.111 | 0.225 |
| P | 0.610 | 0.617 | 0.442 | 0.116 | |
| GLO | r | −0.208 | 0.040 | 0.040 | −0.105 |
| P | 0.147 | 0.784 | 0.785 | 0.470 | |
| Renel Function | |||||
| UN | r | −0.023 | 0.176 | −0.110 | −0.139 |
| P | 0.872 | 0.223 | 0.448 | 0.335 | |
| Creatine | r | −0.164 | 0.222 | −0.141 | −0.290 |
| P | 0.254 | 0.121 | 0.330 | 0.041a | |
| UA | r | 0.000 | 0.085 | −0.061 | −0.121 |
| P | 0.999 | 0.555 | 0.674 | 0.401 | |
| Myocardial Enzymes | |||||
| LDH | r | −0.004 | 0.286 | −0.252 | −0.388 |
| P | 0.977 | 0.044a | 0.077 | 0.005a | |
| CK | r | −0.161 | −0.045 | 0.124 | 0.181 |
| P | 0.263 | 0.758 | 0.389 | 0.208 | |
| CK-MB | r | −0.100 | 0.257 | −0.232 | −0.299 |
| P | 0.490 | 0.072 | 0.104 | 0.035a | |
| Electrocardiogram | |||||
| PR | r | 0.147 | −0.167 | 0.047 | 0.242 |
| P | 0.303 | 0.242 | 0.746 | 0.088 | |
| QRS | r | −0.015 | −0.180 | 0.143 | 0.110 |
| P | 0.919 | 0.206 | 0.317 | 0.443 | |
| Q-T | r | 0.019 | 0.019 | −0.101 | −0.228 |
| P | 0.896 | 0.893 | 0.482 | 0.107 | |
| HR | r | −0.090 | −0.023 | 0.125 | 0.140 |
| P | 0.530 | 0.930 | 0.382 | 0.326 | |
| Echocardiogram | |||||
| AO | r | −0.165 | 0.013 | 0.155 | 0.052 |
| P | 0.274 | 0.930 | 0.303 | 0.731 | |
| PA | r | −0.356 | −0.075 | 0.216 | 0.170 |
| P | 0.015a | 0.622 | 0.150 | 0.260 | |
| AO/PA | r | 0.093 | 0.168 | −0.066 | −0.125 |
| P | 0.538 | 0.265 | 0.665 | 0.410 | |
| LVEDD | r | 0.140 | −0.199 | −0.001 | 0.078 |
| P | 0.353 | 0.185 | 0.996 | 0.606 | |
| RVOT | r | 0.036 | 0.090 | −0.079 | 0.074 |
| P | 0.814 | 0.552 | 0.602 | 0.624 | |
PT = prothrombin time, PT% = PT percent activity, INR = international normalized ratio, APTT = activated partial thromboplastin time, TT = thrombin time, FIB = fibrinogen, D-D = D-dimer, WBC = white blood cells counting, RBC = red blood cells counting, HGB = hemoglobin, HCT = hematocrit, RDW = red blood cell distribution width, PLT = platelet, PDW = platelet distribution width, AST = aspartate amino transferase, ALT = alanine transaminase, Amy = amylase, TP = total protein, ALB = albumin, GLO = globulin, UN = urea nitrogen, UA = uric acid, LDH = lactate dehydrogenase, CK = creatine kinase, CK-MB = creatine kinase-MB, PR P–R intervals, QRS = Q-R-S duration, QT = Q-T intervals, HR = heart rate, AO = aortic dimension, PA = pulmonary artery dimension, LVEDD = left ventricular end-diastolic dimension, RVOT = right ventricular outflow tract dimension.
Indicates difference is significant.
Table 2.
Correlation between TEG and other variables in population transferring from high altitude.
| R | K | Angle | MA | ||
|---|---|---|---|---|---|
| Coagulation | |||||
| PT | r | 0.276 | 0.210 | −0.426 | −0.309 |
| P | 0.066 | 0.166 | 0.004a | 0.039a | |
| PT% | r | −0.248 | −0.192 | 0.402 | 0.325 |
| P | 0.100 | 0.207 | 0.006a | 0.029a | |
| INR | r | 0.226 | 0.178 | −0.394 | −0.327 |
| P | 0.135 | 0.242 | 0.007a | 0.028a | |
| APTT | r | 0.178 | 0.156 | −0.341 | −0.322 |
| P | 0.243 | 0.307 | 0.022a | 0.031a | |
| TT | r | 0.004 | 0.073 | −0.075 | 0.058 |
| P | 0.981 | 0.633 | 0.623 | 0.705 | |
| FIB | r | 0.022 | −0.335 | 0.277 | −0.078 |
| P | 0.884 | 0.024a | 0.066 | 0.612 | |
| D-D | r | −0.160 | 0.230 | −0.074 | 0.064 |
| P | 0.294 | 0.128 | 0.628 | 0.676 | |
| Blood Routine | |||||
| WBC | r | 0.032 | 0.147 | −0.058 | 0.026 |
| P | 0.839 | 0.346 | 0.714 | 0.871 | |
| RBC | r | 0.013 | 0.352 | −0.359 | −0.041 |
| P | 0.936 | 0.021a | 0.018a | 0.793 | |
| HGB | r | 0.078 | 0.346 | −0.447 | −0.114 |
| P | 0.619 | 0.023a | 0.003a | 0.469 | |
| HCT | r | 0.075 | 0.379 | −0.452 | −0.102 |
| P | 0.634 | 0.012a | 0.002a | 0.516 | |
| RDW | r | 0.080 | 0.186 | −0.217 | 0.096 |
| P | 0.609 | 0.232 | 0.161 | 0.541 | |
| PLT | r | 0.225 | −0.381 | 0.260 | −0.053 |
| P | 0.147 | 0.012a | 0.161 | 0.737 | |
| PDW | r | 0.000 | −0.088 | −0.001 | 0.124 |
| P | 0.998 | 0.576 | 0.997 | 0.427 | |
| Liver Function | |||||
| AST | r | −0.258 | 0.194 | −0.138 | 0.035 |
| P | 0.083 | 0.196 | 0.360 | 0.815 | |
| ALT | r | −0.190 | 0.022 | −0.065 | −0.006 |
| P | 0.206 | 0.885 | 0.669 | 0.968 | |
| AMY | r | −0.371 | −0.275 | 0.345 | 0.176 |
| P | 0.011a | 0.064 | 0.019a | 0.241 | |
| Total bilirubin | r | −0.313 | 0.022 | 0.015 | −0.029 |
| P | 0.034a | 0.882 | 0.924 | 0.846 | |
| Direct bilirubin | r | −0.019 | 0.242 | −0.147 | 0.038 |
| P | 0.899 | 0.105 | 0.330 | 0.802 | |
| Indirect bilirubin | r | −0.033 | 0.257 | −0.200 | 0.058 |
| P | 0.828 | 0.084 | 0.183 | 0.702 | |
| TP | r | −0.029 | 0.256 | −0.160 | 0.050 |
| P | 0.847 | 0.086 | 0.289 | 0.742 | |
| ALB | r | −0.071 | 0.024 | 0.037 | 0.084 |
| P | 0.639 | 0.873 | 0.807 | 0.581 | |
| GLO | r | −0.021 | 0.223 | −0.160 | 0.024 |
| P | 0.892 | 0.136 | 0.288 | 0.875 | |
| Renel Function | |||||
| UN | r | −0.077 | 0.009 | −0.116 | −0.207 |
| P | 0.612 | 0.952 | 0.442 | 0.167 | |
| Creatine | r | 0.192 | 0.090 | −0.137 | −0.241 |
| P | 0.200 | 0.550 | 0.365 | 0.107 | |
| UA | r | −0.059 | −0.064 | 0.067 | 0.025 |
| P | 0.698 | 0.673 | 0.656 | 0.869 | |
| Myocardial Enzymes | |||||
| LDH | r | −0.247 | −0.010 | 0.153 | 0.208 |
| P | 0.097 | 0.949 | 0.309 | 0.166 | |
| CK | r | −0.121 | 0.372 | −0.314 | −0.187 |
| P | 0.424 | 0.011a | 0.033a | 0.213 | |
| CK-MB | r | −0.070 | −0.028 | 0.134 | 0.116 |
| P | 0.643 | 0.853 | 0.374 | 0.444 | |
| Electrocardiogram | |||||
| PR | r | 0.318 | 0.270 | −0.249 | −0.042 |
| P | 0.067 | 0.123 | 0.155 | 0.812 | |
| QRS | r | −0.020 | 0.108 | 0.020 | 0.183 |
| P | 0.912 | 0.542 | 0.912 | 0.301 | |
| Q-T | r | −0.114 | 0.204 | −0.136 | −0.161 |
| P | 0.520 | 0.247 | 0.443 | 0.362 | |
| HR | r | −0.002 | −0.182 | 0.148 | 0.196 |
| P | 0.989 | 0.304 | 0.405 | 0.267 | |
| Echocardiogram | |||||
| AO | r | 0.079 | 0.044 | −0.187 | −0.205 |
| P | 0.701 | 0.831 | 0.359 | 0.316 | |
| PA | r | −0.060 | 0.173 | 0.069 | 0.027 |
| P | 0.771 | 0.399 | 0.739 | 0.894 | |
| AO/PA | r | 0.090 | −0.111 | −0.200 | −0.162 |
| P | 0.661 | 0.591 | 0.327 | 0.431 | |
| LVEDD | r | 0.355 | 0.033 | −0.358 | −0.551 |
| P | 0.064 | 0.867 | 0.061 | 0.002a | |
| RVOT | r | 0.191 | 0.268 | −0.302 | −0.140 |
| P | 0.349 | 0.185 | 0.321 | 0.494 | |
PT = prothrombin time, PT% = PT percent activity, INR = international normalized ratio, APTT = activated partial thromboplastin time, TT = thrombin time, FIB = fibrinogen, D-D = D-dimer, WBC = white blood cells counting, RBC = red blood cells counting, HGB = hemoglobin, HCT = hematocrit, RDW = red blood cell distribution width, PLT = platelet, PDW = platelet distribution width, AST = aspartate amino transferase, ALT = alanine transaminase, Amy = amylase, TP = total protein, ALB = albumin, GLO = globulin, UN = urea nitrogen, UA = uric acid, LDH = lactate dehydrogenase, CK = creatine kinase, CK-MB = creatine kinase-MB, PR P–R intervals, QRS = Q-R-S duration, QT = Q-T intervals, HR = heart rate, AO = aortic dimension, PA = pulmonary artery dimension, LVEDD = left ventricular end-diastolic dimension, RVOT = right ventricular outflow tract dimension.
Indicates difference is significant.
3.3. Correlations between TEG and other variables
In Group A (Table 1), R was negatively correlated with DBIL (r = −0.392, P < 0.05) and PA (r = −0.356, P < 0.05). However, K was positively correlated with RBCs (r = 0.460, P < 0.05), HGB (r = 0.470, P < 0.05), HCT (r = 0.416, P < 0.05), LDH (r = 0.286, P < 0.05), and negatively correlated with PLT (r = −0.520, P < 0.05). In contrast, the angle was negatively correlated with RBCs (r = −0.507, P < 0.05), HGB (r = −0.518, P < 0.05), HCT (r = −0.466, P < 0.05), and positively correlated with PLT (r = 0.462, P < 0.05). MA was negatively correlated with WBCs (r = −0.365, P < 0.05), RBCs (r = −0.592, P < 0.05), HGB (r = −0.633, P < 0.05), HCT (r = −0.534, P < 0.05), creatine (r = −0.290, P < 0.05), LDH (r = −0.388, P < 0.05) and CK-MB (r = −0.299, P < 0.05).
In Group B (Table 2), R was negatively correlated with AMY (r = −0.371, P < 0.05) and TBIL (r = −0.313, P < 0.05). Additionally, K was positively correlated with RBCs (r = 0.352, P < 0.05), HGB (r = 0.346, P < 0.05), HCT (r = 0.379, P < 0.05), CK (r = 0.372, P < 0.05), and negatively correlated with PLT (r = −0.381, P < 0.05). The angle was negatively correlated with RBCs (r = −0.359, P < 0.05), HGB (r = −0.447, P < 0.05), HCT (r = −0.452, P < 0.05), CK (r = −0.314, P < 0.05), and positively correlated with AMY (r = 0.345, P < 0.05). MA was significantly correlated with LVEDD only (r = −0.551, P < 0.05).
3.4. Logistic regression analysis of various abnormal TEG variables
In Group A, 24 participants were present with abnormal R, 42 with abnormal K, 41 with abnormal angle, and 41 participants with abnormal MA. Table 3 displays that abnormal R was independently associated with DBIL (odds ratio (OR) = 0.798, P < 0.05), and abnormal K was independently associated with PLT (OR = 0.974, P < 0.05). In Group B, 41 participants exhibited abnormal R, 44 displayed abnormal K, 49 had an abnormal angle, and 49 displayed abnormal MA. As displayed in Table 4, no factors were independently associated with various abnormal TEG variables.
Table 3.
Logistic regression analysis of various abnormal TEG variables in population exposed to high altitude.
| B | SE | Wals | P | OR | |
|---|---|---|---|---|---|
| R | |||||
| Direct bilirubin | −0.225 | 0.107 | 4.441 | 0.035a | 0.798 |
| PA | −0.095 | 0.214 | 0.199 | 0.655 | 0.909 |
| K | |||||
| RBC | 1.606 | 2.008 | 0.640 | 0.424 | 4.983 |
| HGB | −0.055 | 0.100 | 0.301 | 0.583 | 0.947 |
| HCT | 0.063 | 0.352 | 0.032 | 0.857 | 1.065 |
| PLT | −0.027 | 0.013 | 4.600 | 0.032a | 0.974 |
| LDH | 0.013 | 0.011 | 1.334 | 0.248 | 1.013 |
| Angle | |||||
| RBC | 1.775 | 1.866 | 0.905 | 0.342 | 5.900 |
| HGB | −0.011 | 0.085 | 0.016 | 0.900 | 0.989 |
| HCT | 0.024 | 0.313 | 0.006 | 0.939 | 1.024 |
| PLT | −0.016 | 0.011 | 2.104 | 0.147 | 0.984 |
| MA | |||||
| WBC | 0.054 | 0.304 | 0.032 | 0.858 | 1.056 |
| RBC | |||||
| HGB | 0.127 | 0.111 | 1.316 | 0.251 | 1.135 |
| HCT | −0.274 | 0.404 | 0.460 | 0.498 | 0.760 |
| Creatine | 0.074 | 0.056 | 1.718 | 0.190 | 1.077 |
| LDH | 0.018 | 0.011 | 2.528 | 0.112 | 1.018 |
| CK-MB | −0.026 | 0.104 | 0.063 | 0.802 | 0.974 |
PA = pulmonary artery dimension, RBC = red blood cells counting, HGB = hemoglobin, HCT = hematocrit, PLT = platelet, LDH = lactate dehydrogenase, WBC = white blood cells counting, CK-MB = creatine kinase-MB.
Indicates difference is significant.
Table 4.
Logistic regression analysis of various abnormal TEG variables in population transferring from high altitude.
| B | SE | Wals | P | OR | |
|---|---|---|---|---|---|
| R | |||||
| AMY | −0.036 | 0.047 | 0.599 | 0.439 | 0.965 |
| Total bilirubin | 0.010 | 0.025 | 0.164 | 0.686 | 1.010 |
| K | |||||
| RBC | −2.201 | 2.429 | 0.821 | 0.365 | 0.111 |
| HGB | −0.163 | 0.195 | 0.696 | 0.404 | 0.850 |
| HCT | 0.658 | 0.938 | 0.493 | 0.483 | 1.932 |
| PLT | −0.022 | 0.015 | 2.147 | 0.143 | 0.978 |
| CK | 0.002 | 0.015 | 0.023 | 0.880 | 1.002 |
| Angle | |||||
| RBC | 5.793 | 7.502 | 0.596 | 0.440 | 328.040 |
| HGB | 0.209 | 0.498 | 0.176 | 0.674 | 1.233 |
| HCT | −1.586 | 2.440 | 0.423 | 0.516 | 0.205 |
| AMY | −0.125 | 0.162 | 0.593 | 0.441 | 0.883 |
| CK | 0.015 | 0.036 | 0.177 | 0.674 | 1.015 |
| MA | |||||
| LVEDD | 1.386 | 0.979 | 2.006 | 0.157 | 3.999 |
Amy = amylase, RBC = red blood cells counting, HGB = hemoglobin, HCT = hematocrit, PLT = platelet, CK = creatine kinase, LVEDD = left ventricular end-diastolic dimension.
4. Discussion
High-altitude areas are characterized by hypoxia, low pressure, and other environmental factors. This can cause a compensatory increase in RBCs [13], aggregation of blood cells, increased blood viscosity, and a significant decrease in blood flow velocity [14,15]. Additionally, hypoxia can cause damage to the capillary walls, leading to increased PLT aggregation and adhesion [16] and a hypercoagulable state in the body. Upon return from a high to a low altitude area, the hypoxia radially transforms to a relative oxygen enrichment state, during which the body can sustain hypoxia-reoxygenation injury [17]. Alterations in hematological indices, mainly in RBC counts, HGB, erythrocyte pressure, and PLT counts, are observed during altitude deacclimatization, and it can take about a year for some of these indices to return to normal levels. In some cases, the indices can take more than 4 years to achieve normal levels [18]. Conventional coagulation function tests are commonly used to evaluate and diagnose coagulopathy in patients at high altitudes. However, these tests do not consider the role of PLTs in the coagulation system. TEG is an indicator that monitors the dynamic changes of blood coagulation in real time, providing a comprehensive view of the coagulation process, excluding vascular endothelial factors from coagulation to fibrinolysis [19]. Integrating TEG with coagulation function and routine blood tests enables an accurate and comprehensive assessment of patients' coagulation status. This makes the assessment a more suitable examination method for individuals with high-altitude-related diseases [4,20]. Recently, TEG emerges as a valuable tool for providing a dynamic graphical representation of clot formation, development, and lysis, allowing quantitative measurements for diagnostic purposes [21].
This study examined individuals who were either exposed to high altitudes or had transferred from high altitudes. We identified noteworthy changes in the mean R and mean MA values within the population transferring from high altitudes (as assessed by TEG). Specifically, a notable reduction was observed in coagulation factor activity (an increase in mean R) and reduced PLT function (a significant decrease in mean MA). These changes could be related to the hypercoagulable tendency of blood due to prolonged exposure to high-altitude environments. Additionally, the aforementioned changes could be related to hypoxia-reoxygenation-induced vascular re-regulation, endothelial cell damage, and functional changes. The endothelium balances vasodilation and vasoconstriction in response to physical and biochemical stimuli [22]. In studies related to obstructive sleep apnea, repeated hypoxia-reoxygenation has been identified to independently impair endothelial function by altering the vasorelaxation regulation and repair capacity of endothelial cells while promoting vascular inflammation and oxidative stress [23]. According to reports, the vascular endothelium is a major site of reactive oxygen species (ROS) production and injury during hypoxia and reperfusion. The accumulation of ROS induces mitochondrial damage and apoptosis in endothelial cells [24], which is closely related to the imbalance of the coagulation system [25]. Furthermore, excessive PLT activation is also reported in some hypoxia-reoxygenation and reperfusion studies [26,27]. The findings expand our knowledge of the coagulation dynamics during high-altitude exposure and its transition, and the potential impact of the altitude on coagulation factor activity and PLT function.
Moreover, we conducted a correlation analysis of TEG indices, coagulation function indices, and PLT counts in both the high-altitude exposure group and the group that transferred from high altitude to the plains. Compared to the high-altitude exposure group, we observed different patterns in the population transitioning from high altitudes to the plain. The phenomenon may be related to the hypercoagulable tendency caused by hypoxia at high altitudes and the possible damage to vascular endothelial function after transferring to the plain. To sustain oxygen supply to vital organs during prolonged exposure to high altitudes, the body compensates by increasing the oxygen-carrying capacity of the blood, increasing the RBC count, and elevating blood viscosity [28]. Hypoxia and other factors induce damage to vascular endothelial cells, triggering the activation of coagulation pathways and subsequent consumption of coagulation factors [29,30]. Consequently, the blood gradually shifts toward a hypercoagulable tendency. Excessive consumption of coagulation factors and damage to vascular endothelial cells leads to the production of plasminogen activators, which can disrupt coagulation regulation and fibrinolysis, culminating in disorders [31]. Furthermore, short-term exposure to high altitudes often increases PLT count, whereas long-term exposure typically results in a decline in the PLT count [4,32]. The aforementioned process might explain the correlation between TEG-related indicators and other indicators. Considering the variations in the correlations of TEG measures with other hemostatic factors between the two groups, our study suggests an intricate interplay of different coagulation elements during exposure to high altitudes and the return to plain terrestrial areas. This indicates that TEG might be valuable in obtaining information on clot formation and thrombus stability [33] and can offer a unique perspective on coagulation processes, which are not always related to conventional blood tests like PT, APTT, and PLT count.
In our study, we discovered a negative correlation between R in TEG and DBIL levels in the group exposed to high altitudes, and a negative correlation between R and TBIL levels in the group that transferred from high altitudes. Additionally, TEG variables were independently associated with IBIL or PLT. Given the liver's pivotal role in coagulation regulation and bilirubin metabolism [34], the study emphasizes the significance of assessing liver function and its potential influence on coagulation parameters during high-altitude exposure and its reversal. By producing circulating coagulation proteins necessary for the complex hemostatic process in vivo, the liver plays a key role in preventing unwanted thrombin generation and fibrin deposition to stop bleeding effectively [35]. During the cell death or eryptosis of RBCs, HGB is released and broken down into heme, which enters the bloodstream along with the RBCs [36]. Subsequently, heme undergoes enzymatic processes, leading to the generation of IBIL. The liver cells then take up this IBIL, which undergoes enzymatic reactions with Y and Z proteins to form DBIL, which is eventually excreted. DBIL is considered a more sensitive indicator of endogenous hepatocyte changes and liver function impairment compared to TBIL [37]. The utilization of TEG and the investigation of bilirubin levels may aid in the assessment of coagulation and liver function impairment, offering valuable information for managing health risks in such environments.
Besides, our results demonstrated significant correlations between K and α with RBC, HGB, and HCT in both groups. These results align with existent reports on a gradual decrease in whole blood viscosity, plasma viscosity, RBC, HGB, and HCT levels during a transfer from high altitudes [3]. Despite this gradual decline, the measured parameters remained relatively high, and a significant decrease was not observed until approximately 2 weeks later. The identified correlations between K, and α with RBC, HGB, and HCT provide further insights into the dynamic changes in hematological parameters during transitioning from high altitudes. These alterations can indicate the body's adaptive response to high-altitude environments where blood composition and viscosity adjustments are crucial for maintaining sufficient oxygen delivery and circulation. This study exhibited intriguing correlations between the MA and certain biochemical markers in different groups. In the high-altitude exposure group, MA was negatively correlated with LDH, creatine, and CK-MB. In the group that transferred from high altitudes, MA exhibited a negative correlation with LVEDD. These findings align with previous research indicating that severe hypoxia can initiate pulmonary artery constriction, subsequently impacting cardiac structural function [38,39]. This phenomenon, known as hypoxic pulmonary vasoconstriction showcases a sophisticated interplay between a mitochondrial-redox sensor and an effector system. This effector system encompasses redox-sensitive ion channels, enzymes, and transcription factors contributing to the intricate modulation of pulmonary vascular resistance [40]. Furthermore, the detrimental effects of hypoxia are not limited to vascular dynamics alone. Oxidative stress induced by hypoxia can inflict injury upon cardiomyocytes. The observed morphological changes in cardiac structure often accompany the transition from high altitudes to low levels. On the other hand, this adaptation process may also entail the risk of hypoxia-reoxygenation injury. This particular form of injury has the potential to inflict harm upon myocardial fibers, cardiomyocytes, and endothelial cells, contributing to overall cardiac dysfunction. In the context of oxidative stress, significant contributors include the mitochondrial respiratory chain and nicotinamide adenine dinucleotide phosphate hydrogen oxidases from the NOX family [41,42]. These entities are prominent sources of ROS within cardiomyocytes. During reoxygenation, the mitochondrial electron transport chain generates superoxides, leading to an accumulation of ROS. Notably, this accumulation has notable effects on the opening of the mitochondrial permeability transition pore (mPTP). The mPTP is a non-selective pore in the inner mitochondrial membrane, and its opening is associated with irreversible cardiac damage. The observed correlations between MA and LDH, CK-MB, and LVEDD provide valuable insights into the potential impact of hypoxia on cardiac function during exposure to high altitudes and return. These correlations improve our understanding of the interplay between coagulation dynamics, cardiac health, and hypoxic conditions.
In conclusion, this study contributes to our understanding of the complex interaction between coagulation dynamics, PLT function, liver function, and hematological parameters during exposure to high altitudes and return from them. The study has certain limitations, such as the two groups not comprising the same population and the potential impact of the low oxygen and low-pressure environment at high altitudes on TEG testing. Furthermore, the smoking status is not taken in consideration, which has a negative impact on the coagulation system. The findings from this study can guide the development of appropriate medical approaches tailored to the unique needs of these populations.
Ethical statement
Ethical approval to conduct the study was obtained from The Ethical Committee of the General Hospital of Western Theater Command (approval No.2021EC2-30) in accordance with the Declaration of Helsinki.
Consent for publication
Informed consent was given by all participants.
Data availability statement
The dataset used is available upon reasonable request from the corresponding author.
Funding
The research reported was supported by grants from The General Hospital of Western Theater Command (2021-XZYG-C30, 2021-XZYG- B31, C222021-XZYG-C21), Natural Science Foundation of Sichuan Province (No. 2022NSFSC1295).
CRediT authorship contribution statement
Zhu Huang: Writing – original draft, Methodology, Formal analysis, Data curation. Dong-xin Huang: Writing – original draft, Formal analysis, Data curation. Yan-yan Wang: Writing – original draft, Formal analysis, Data curation. Li-juan Jiang: Writing – original draft, Formal analysis, Data curation. Yong-hua Wang: Validation, Methodology. Jing Dai: Validation. Xia Kang: Writing – review & editing, Supervision, Formal analysis, Conceptualization. Yi Wen: Writing – review & editing, Data curation. Si-yi He: Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We sincerely thank for all the staff participating in collection of samples.
Contributor Information
Xia Kang, Email: kxpaper@sina.com.
Yi Wen, Email: 13980881194@163.com.
Si-yi He, Email: hesiyi@vip.163.com.
References
- 1.Bendz B., Rostrup M., Sevre K., Andersen T.O., Sandset P.M. Association between acute hypobaric hypoxia and activation of coagulation in human beings. Lancet. 2000;356:1657–1658. doi: 10.1016/S0140-6736(00)03165-2. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y., Zhang J.H., Gao X.B., Wu X.J., Yu J., Chen J.F., Bian S.Z., Ding X.H., Huang L. Correlation between blood pressure changes and AMS, sleeping quality and exercise upon high-altitude exposure in young Chinese men. Mil Med Res. 2014;1:19. doi: 10.1186/2054-9369-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong R., Liu H., Wang H., Li X., He Z., Gangla M., Zhang J., Han D., Liu J. Adaption to high altitude: an evaluation of the storage quality of suspended red blood cells prepared from the whole blood of Tibetan plateau migrants. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocke A.S., Paterson G.G., Barber M.T., Jackson A.I.R., Main S.E., Stannett C., Schnopp M.F., MacInnis M., Baillie J.K., Horn E.H., Moores C., Harrison P., Nimmo A.F., Thompson A.A.R. Thromboelastometry and platelet function during acclimatization to high altitude. Thromb. Haemostasis. 2018;118:63–71. doi: 10.1160/TH17-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalyva N., Mousafeiris V.K., Giannakopoulos A. Sigmoid sinus thrombosis as complication of otitis media in a 3-year-old boy: case report and review of the literature. Cureus. 2022;14 doi: 10.7759/cureus.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jha S.K., Anand A.C., Sharma V., Kumar N., Adya C.M. Stroke at high altitude: Indian experience. High Alt. Med. Biol. 2002;3:21–27. doi: 10.1089/152702902753639513. [DOI] [PubMed] [Google Scholar]
- 7.Yang H., Yang G., Xu M., Zhao Y., He S., Wang Q., Wen Y., Huang C., Wu J., Ren C., Yang Y., He S. Impact of high altitude on the incidence of postoperative venous thromboembolism and its genetic susceptibility: a meta-analysis and systematic review. Sci. Total Environ. 2022;838 doi: 10.1016/j.scitotenv.2022.156632. [DOI] [PubMed] [Google Scholar]
- 8.Othman M., Kaur H. Thromboelastography (TEG) Methods Mol. Biol. 2017;1646:533–543. doi: 10.1007/978-1-4939-7196-1_39. [DOI] [PubMed] [Google Scholar]
- 9.Walsh M., Thomas S.G., Howard J.C., Evans E., Guyer K., Medvecz A., Swearingen A., Navari R.M., Ploplis V., Castellino F.J. Blood component therapy in trauma guided with the utilization of the perfusionist and thromboelastography. J. Extra Corpor. Technol. 2011;43:162–167. [PMC free article] [PubMed] [Google Scholar]
- 10.Burton A.G., Jandrey K.E. Use of thromboelastography in clinical practice. Vet Clin North Am Small Anim Pract. 2020;50:1397–1409. doi: 10.1016/j.cvsm.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Sumislawski J.J., Christie S.A., Kornblith L.Z., Stettler G.R., Nunns G.R., Moore H.B., Moore E.E., Silliman C.C., Sauaia A., Callcut R.A., Cohen M.J. Discrepancies between conventional and viscoelastic assays in identifying trauma-induced coagulopathy. Am. J. Surg. 2019;217:1037–1041. doi: 10.1016/j.amjsurg.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan D., Ouyang Z., Ying Y., Huang S., Tao P., Pan X., Lu S., Pan Q. Thromboelastography for the prevention of perioperative venous thromboembolism in orthopedics. Clin. Appl. Thromb. Hemost. 2022;28 doi: 10.1177/10760296221077975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villafuerte F.C., Simonson T.S., Bermudez D., Leon-Velarde F. High-altitude erythrocytosis: mechanisms of adaptive and maladaptive responses. Physiology. 2022;37:1. doi: 10.1152/physiol.00029.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stauffer E., Loyrion E., Hancco I., Waltz X., Ulliel-Roche M., Oberholzer L., Robach P., Pichon A., Brugniaux J.V., Bouzat P., Doutreleau S., Connes P., Verges S. Blood viscosity and its determinants in the highest city in the world. J. Physiol. 2020;598:4121–4130. doi: 10.1113/JP279694. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay J.C., Hoiland R.L., Howe C.A., Coombs G.B., Vizcardo-Galindo G.A., Figueroa-Mujica R.J., Bermudez D., Gibbons T.D., Stacey B.S., Bailey D.M., Tymko M.M., MacLeod D.B., Gasho C., Villafuerte F.C., Pyke K.E., Ainslie P.N. Global REACH 2018: high blood viscosity and hemoglobin concentration contribute to reduced flow-mediated dilation in high-altitude excessive erythrocytosis. Hypertension. 2019;73:1327–1335. doi: 10.1161/HYPERTENSIONAHA.119.12780. [DOI] [PubMed] [Google Scholar]
- 16.Paterson G.G., Young J.M., Willson J.A., Graham C.J., Dru R.C., Lee E.W., Torpey G.S., Walmsley S.R., Chan M.V., Warner T.D., Baillie J.K., Thompson A.A.R. Hypoxia modulates platelet purinergic signalling pathways. Thromb. Haemostasis. 2020;120:253–261. doi: 10.1055/s-0039-3400305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He B., Hu M., Liang Z., Ma Q., Zi Y., Dong Z., Li Q., Luo Y., Qian G., Guo L., Lin K., Liu Z., Wang G. Efficacy of shenqi pollen capsules for high-altitude deacclimatization syndrome via suppression of the reoxygenation injury and inflammatory response. J Immunol Res. 2019;2019 doi: 10.1155/2019/4521231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He B., Wang J., Qian G., Hu M., Qu X., Wei Z., Li J., Chen Y., Chen H., Zhou Q., Wang G. Analysis of high-altitude de-acclimatization syndrome after exposure to high altitudes: a cluster-randomized controlled trial. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting D., DiNardo J.A. TEG and ROTEM: technology and clinical applications. Am. J. Hematol. 2014;89:228–232. doi: 10.1002/ajh.23599. [DOI] [PubMed] [Google Scholar]
- 20.Qu Z., Wang G., Xu C., Zhang D., Qu X., Zhou H., Ma J. The effects of platelet apheresis on blood saving and coagulation in bilateral total hip replacement: a prospective study on 60 patients. Int. J. Surg. 2016;34:58–63. doi: 10.1016/j.ijsu.2016.08.233. [DOI] [PubMed] [Google Scholar]
- 21.Da Luz L.T., Nascimento B., Shankarakutty A.K., Rizoli S., Adhikari N.K. Effect of thromboelastography (TEG(R)) and rotational thromboelastometry (ROTEM(R)) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit. Care. 2014;18:518. doi: 10.1186/s13054-014-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aird W.C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 23.Atkeson A., Yeh S.Y., Malhotra A., Jelic S. Endothelial function in obstructive sleep apnea. Prog. Cardiovasc. Dis. 2009;51:351–362. doi: 10.1016/j.pcad.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhar-Mascareno M., Carcamo J.M., Golde D.W. Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radic. Biol. Med. 2005;38:1311–1322. doi: 10.1016/j.freeradbiomed.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 25.von Kanel R., Dimsdale J.E. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–1967. doi: 10.1378/chest.124.5.1956. [DOI] [PubMed] [Google Scholar]
- 26.Davies M.J. The contribution of thrombosis to the clinical expression of coronary atherosclerosis. Thromb. Res. 1996;82:1–32. doi: 10.1016/0049-3848(96)00035-7. [DOI] [PubMed] [Google Scholar]
- 27.Leo R., Pratico D., Iuliano L., Pulcinelli F.M., Ghiselli A., Pignatelli P., Colavita A.R., FitzGerald G.A., Violi F. Platelet activation by superoxide anion and hydroxyl radicals intrinsically generated by platelets that had undergone anoxia and then reoxygenated. Circulation. 1997;95:885–891. doi: 10.1161/01.cir.95.4.885. [DOI] [PubMed] [Google Scholar]
- 28.Savourey G., Garcia N., Besnard Y., Guinet A., Hanniquet A.M., Bittel J. Pre-adaptation, adaptation and de-adaptation to high altitude in humans: cardio-ventilatory and haematological changes. Eur. J. Appl. Physiol. Occup. Physiol. 1996;73:529–535. doi: 10.1007/BF00357675. [DOI] [PubMed] [Google Scholar]
- 29.Norooznezhad A.H., Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19) Microvasc. Res. 2021;137 doi: 10.1016/j.mvr.2021.104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M., Tang X., Liao Z., Shen C., Cheng R., Fang M., Wang G., Li Y., Tang S., Xie L., Zhang Z., Kamau P.M., Mwangi J., Lu Q., Li Y., Wang Y., MacKeigan D.T., Cerenzia E.G., Ni H., Lai R. Hypoxia and low temperature upregulate transferrin to induce hypercoagulability at high altitude. Blood. 2022;140:2063–2075. doi: 10.1182/blood.2022016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cralley A.L., Moore E.E., Sauaia A., Carani P.H., Schaid T.R., Jr., DeBot M., Fragoso M., Ghasabyan A., Hansen K., Cohen M.J., Silliman C.C., Fox C.J. REBOA for the treatment of blast polytrauma: zone 3 provides cerebral perfusion, attenuates organ dysfunction and reperfusion coagulopathy compared to zone 1 in a swine model. J. Trauma Acute Care Surg. 2023;94:718–724. doi: 10.1097/TA.0000000000003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantysaari M., Joutsi-Korhonen L., Siimes M.A., Siitonen S., Parkkola K., Lemponen M., Lassila R. Unaltered blood coagulation and platelet function in healthy subjects exposed to acute hypoxia. Aviat Space Environ. Med. 2011;82:699–703. doi: 10.3357/asem.3012.2011. [DOI] [PubMed] [Google Scholar]
- 33.Walsh M., Moore E.E., Moore H., Thomas S., Lune S.V., Zimmer D., Dynako J., Hake D., Crowell Z., McCauley R., Larson E.E., Miller M., Pohlman T., Achneck H.E., Martin P., Nielsen N., Shariff F., Ploplis V.A., Castellino F.J. Use of viscoelastography in malignancy-associated coagulopathy and thrombosis: a review. Semin. Thromb. Hemost. 2019;45:354–372. doi: 10.1055/s-0039-1688497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thaler J., Lisman T., Quehenberger P., Hell L., Schwabl P., Scheiner B., Bucsics T., Nieuwland R., Ay C., Trauner M., Pabinger I., Reiberger T., Mandorfer M. Intraperitoneal activation of coagulation and fibrinolysis in patients with cirrhosis and ascites. Thromb. Haemostasis. 2022;122:353–362. doi: 10.1055/a-1515-9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisman T., Caldwell S.H., Burroughs A.K., Northup P.G., Senzolo M., Stravitz R.T., Tripodi A., Trotter J.F., Valla D.C., Porte R.J. & Coagulation in Liver Disease Study, G. Hemostasis and thrombosis in patients with liver disease: the ups and downs. J. Hepatol. 2010;53:362–371. doi: 10.1016/j.jhep.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Vitek L., Tiribelli C. Bilirubin. The yellow hormone? J. Hepatol. 2021;75:1485–1490. doi: 10.1016/j.jhep.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Fevery J. Bilirubin in clinical practice: a review. Liver Int. 2008;28:592–605. doi: 10.1111/j.1478-3231.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 38.Luks A.M., Swenson E.R., Bartsch P. Acute high-altitude sickness. Eur. Respir. Rev. 2017;26 doi: 10.1183/16000617.0096-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sylvester J.T., Shimoda L.A., Aaronson P.I., Ward J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012;92:367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunham-Snary K.J., Wu D., Sykes E.A., Thakrar A., Parlow L.R.G., Mewburn J.D., Parlow J.L., Archer S.L. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest. 2017;151:181–192. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahles T., Brandes R.P. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxidants Redox Signal. 2013;18:1400–1417. doi: 10.1089/ars.2012.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used is available upon reasonable request from the corresponding author.