Abstract
COVID-19 infection is a multi-system clinical disorder that was associated with increased morbidity and mortality. Even though antiviral therapies such as Remdesvir offered modest efficacy in reducing the mortality and morbidity, they were not efficacious in reducing the risk of future infections. So, FDA approved COVID-19 vaccines which are widely administered in the general population worldwide. These COVID-19 vaccines offered a safety net against future infections and re-infections. Most of these vaccines contain inactivated virus or spike protein mRNA that are primarily responsible for inducing innate and adaptive immunity. These vaccines were also formulated to contain supplementary adjuvants that are beneficial in boosting the immune response. During the pandemic, clinicians all over the world witnessed an uprise in the incidence and prevalence of cardiovascular diseases (COVID-Heart Syndrome) in patients with and without cardiovascular risk factors. Clinical researchers were not certain about the underlying reason for the upsurge of cardiovascular disorders with some blaming them on COVID-19 infections while others blaming them on COVID-19 vaccines. Based on the literature review, we hypothesize that adjuvants included in the COVID-19 vaccines are the real culprits for causation of cardiovascular disorders. Operation of various pathological signaling events under the influence of these adjuvants including autoimmunity, bystander effect, direct toxicity, anti-phospholipid syndrome (APS), anaphylaxis, hypersensitivity, genetic susceptibility, epitope spreading, and anti-idiotypic antibodies were partially responsible for stirring up the onset of cardiovascular disorders. With these mechanisms in place, a minor contribution from COVID-19 virus itself cannot be ruled out. With that being said, we strongly advocate for careful selection of vaccine adjuvants included in COVID-19 vaccines so that future adverse cardiac disorders can be averted.
Keywords: adjuvants, auto-antibodies, autoimmunity, cardiomyopathy, cardiovascular disorders, COVID-heart syndrome, COVID-19 vaccine, myocarditis
Plain language summary
Role of COVID-19 vaccines in casuation of cardiovascular diseases
COVID-19 infection is a multi-system clinical disorder that was associated with increased morbidity and mortality. Even though antiviral therapies such as Remdesvir offered modest efficacy in reducing the mortality and morbidity, they were not efficacious in reducing the risk of future infections. So, FDA approved COVID-19 vaccines which are widely administered in the general population worldwide. These COVID-19 vaccines offered safety net against future infections and re-infections. Most of these vaccines contain inactivated virus or spike protein mRNA that are primary responsible for inducing innate and adaptive immunity. These vaccines were also formulated to contain supplementary adjuvants that are beneficial in boosting the immune response. During the pandemic, clinicians all over the world witnessed an uprise in the incidence and prevalence of cardiovascular diseases (COVID-Heart Syndrome) in patients with and without cardiovascular risk factors. Clinical researchers were not certain the underlying reason for upsurge of cardiovascular disorder with some blaming them on COVID-19 infections while others blaming them on COVID-19 vaccines. Based on the literature review, we hypothesize that adjuvants included in the COVID-19 vaccines the real culprits for causation of cardiovascular disorders. Operation of various pathological signaling events under the influence of these adjuvants including autoimmunity, bystander effect, direct toxicity, anti-phospholipid syndrome (APS), anaphylaxis, hypersensitivity, genetic susceptibility, epitope spreading, and anti-idiotypic antibodies were partially responsible for stirring up the onset of cardiovascular disorders. With these mechanisms in place, a minor contribution from COVID-19 virus itself cannot be ruled out. With that being said, we strongly advocate for careful selection of vaccine adjuvants included in COVID-19 vaccines so that future adverse cardiac disorders can be averted.
Introduction
Since its origin in China, COVID-19 infection has become a global pandemic involving almost 185 countries worldwide with more than 3000,000 cases worldwide and approximately 210,000 deaths. In the United States alone, COVID-19 virus accounted for 30.6 million cases and 550,000 deaths. 1 In a recent study involving US veteran affairs of national healthcare databases, a cohort of 153,760 US veterans who lived through COVID-19 infection experienced heightened risk and 12-month liability for developing future cardiovascular diseases. 2 Furthermore, this study guesstimated that the increased risk was directly proportional to the disease severity and was more rampant in non-hospitalized COVID-19 patients. 2 That being the case, the incidence and prevalence of cardiovascular disorders have shown an escalating trend during the COVID-19 pandemic.
The spectrum of cardiovascular disorders encountered during the recently concluded pandemic included myocarditis, arrythmias, thromboembolism, coronary artery disease, acute heart failure and Takotsubo cardiomyopathy.3,4 Keeping in mind very propitious reports regarding the increased vaccine effectiveness of COVID-19 mRNA vaccines (25% and 68%) following second and third doses, full compliance with the vaccination protocol was advocated in the United States and the rest of the world. 5 Henceforth, approximately 230,637,468 or 70% of the US population were fully protected with two doses of CDC-recommended vaccines (Pfizer-BioNTech, Moderna, Johnson& Johnson and Novavax).6,7 Whole population health study in the United Kingdom that scrutinized the electronic health records uncovered that these vaccines dwindle the risk of developing myocardial infarction in older age groups (>70 years and <70 years). 8 On the contrary these vaccines are also alleged to instigate post vaccine cardiovascular complications including myocarditis [87 (131.7)], venous thromboembolism [626 (951.9)], DIC [87 (45.4)], pulmonary embolism [503 (762.8)], and acute myocardial infarction [613 (935.3)] events per million person-years. 9
Discussion
Culpability of vaccine adjuvants in COVID-19 HEART SYNDROME: Master regulator for triggering the pathological switch
According to CDC, COVID-19 vaccines primarily are composed of COVID-19 spike mRNA and vaccine adjuvants or immune boosters.
Given the scope of cardiovascular disorders, it would be logical to interpret that COVID-19 spike mRNA might be the likely culprit behind inception of these above-mentioned cardiovascular disorders. In fact, there are few reports that speculate the deployment of pathological signaling events post COVID-19 vaccination that keeps the ball rolling for materialization of various cardiovascular disorders. It is likely that an amalgamation of malignant aberrations ranging from COVID-19 spike mRNA auto-reactivity, molecular mimicry, autoimmunity, to abnormal immune responses were partially incriminated in causation of these cardiac disorders. 10 On the other hand, COVID-19 virus due to its inherent baneful potency can provoke these cardiovascular abnormalities via cytokine storm, sepsis, autoimmunity, molecular mimicry, and direct cardiomyocyte invasion.10–13
Most of the current evidence as of now inculpates COVID-19 spike mRNA as the principal and supereminent ingredient for instigating these cardiac abnormalities. However relative contribution of immune adjuvants which are innocent bystanders in the vaccine cocktail cannot be completely disregarded. As a matter of fact, these immune boosters can possibly be equally vicious by infuriating nefarious signaling pathways that can possibly engender COVID-19 heart syndrome. A foreseeable scenario is that COVID-19 spike mRNA and vaccine adjuvants can work like hand in glove for giving rise to these cardiac abnormalities. Although the physiological efficacy of these vaccine adjuvants is well known, their relative role in triggering cardiac pathology is unexplored. With this review, we would like to bring to light some vital information regarding the types, physiological functioning, and mechanism of commonly used vaccine adjuvants in COVID-19 vaccines. Taking a step further, we would like to discuss their pernicious role in fomenting virulent signaling as well as maladaptive immune responses which in close collusion might incite COVID-HEART syndrome.
We strongly advocate that this small step would be a future steppingstone for launching future research studies with these vaccine adjuvants. This would lend a helping hand to thoroughly scrutinize their potential role in perturbing the protective immune response and maneuvering it into a deleterious counterstrike that is sooner or later targeted toward cardiac tissues. Even though it would be a herculean task to assess their virulent effect separately from COVID-19 mRNA, at least a thorough comprehension of their malignant role would be benevolent in making small strides for crafting future COVID-19 vaccines less cardiotoxic for the general population.
Working strategy of Spike mRNA in COVID-19 vaccines
The fundamental and primary component of COVID-19 vaccines is spike mRNA which is primarily responsible for conjuring anti-viral immune response as well as arousing immunological memory. Once COVID-19 spike mRNA enters the systemic circulation, it executes its effect by virtue of invoking dendritic cell maturation as well as engendering resilient T-cell and B-cell response. 14 Upon entering the cells, it will be spotted by an array of endosomal (TLR3, TLR7, and TLR8) and cytosolic (PKR, OAS, MDA5 &RIG-1) antennae or sensing agents. 14 This is a cardinal and pre-requisite step which can be the harbinger for subsequent downstream signaling events and upping of hardy anti-viral immune response. As the spike mRNAs are perceived by these sensing molecules, the next series of steps are dedicated in begetting of type-1 interferons (IFNs) which eventually decamps cellular milieu into the blood stream. In the systemic circulation these IFNs engineer through energizing CD80 and CD dendritic cells and stimulating MHC (Major Histocompatability Complex) class I and II molecules. Stimulation of these molecules accomplishes their task of maneuvering the immune response toward COVID-19 virus.
Why do the COVID-19 mRNA vaccines need adjuvants? What is the premise behind inclusion of these adjuvants
In the past, whole virus inactivated vaccines were administered for vaccination among all the age groups. Unfortunately, despite their decent efficacy in kindling reasonable immune response, they are associated with deplorable side effects particularly in the elderly population.15,16 To circumvent this issue, researchers started to incorporate fractioned viruses or purified antigens in the vaccine formulations hoping that this mitigates side effects experienced while retaining their potency.15,17 Unfortunately, decreased toxicity resulting from these vaccine structural alterations were offset by waning vaccine productiveness.15,18 In the hope of finding a working solution to this problem, scientists blended vaccine adjuvants with fractionated viral proteins to ensure that vaccine efficaciousness is retained along with minimal side effect profile.
The two components of the COVID-19 vaccine namely spike mRNA (antigen) and adjuvants (accessory) work like hand in glove where antigen component to a greater degree is responsible for stimulating adaptive immunity while accessory component is essentially dedicated to rejuvenating innate immunity.19,20 These adjuvants are comprised of pathogen-recognized molecular patterns that are recognized by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) present on the membrane of dendritic cells.20,21 Appropriate meshing of adjuvants with PRRs on the dendritic cells results in their activation and their subsequent immigration to the neighboring lymph nodes (LNs). 19 Emigration of these dendritic cells in the LNs enables interaction as well as collaboration with resident lymphocytes. 19 Specifically, T-cell receptor of lymphocytes entangles with peptide MHC class II molecule receptor on these dendritic cells. 20 This leads to positive co-stimulation and eventual activation of T-lymphocytes. Subsequently, these T-lymphocytes are metamorphosed into three classes namely Th1, Th2, and regulatory lymphocytes.20,21 One important thing to underscore here is that viral infection dictates the preponderance of Th1 lymphocyte lineage whereas parasitic infections mandate the proliferation of Th2 lymphocyte family descendants. 20 In the next series of steps necessary revamping is enacted so that these proliferated lymphocytes reach and take refuge in the appropriate target tissue for shielding against forthcoming infections. This task is skillfully consummated by intertwining target-specific homing receptor on their plasma membrane. 20 Post amalgamation of homing receptor, these newly generated lymphocytes will depart LN and cruise through the blood vessels to reach their pre-destined target tissue. 20 After taking sanctuary in the target tissue, these lymphocytes are encumbered with the responsibility of defending the tissue against future microbial infections. 20
Types of vaccine adjuvants used in COVID-19 vaccines
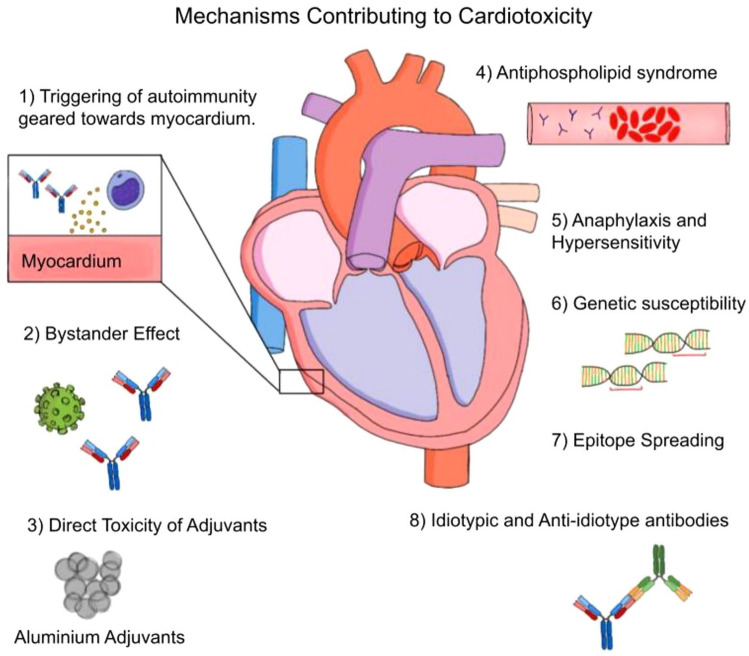
The adjuvants used in COVID-19 vaccines can be categorized into five classes namely Aluminum salt-based, Emulsion-based, TLR agonists, Metabolic, Cell death, and Epigenetic.15,22 Each of these adjuvants instigate different mechanisms that are ultimately responsible for onset of spectrum of cardiovascular diseases seen in COVID-19 patients and those receiving vaccination (Figure 1). This review provides a brief overview of different mechanisms that can be arising out of these adjuvants, and each might be contributing at least partially to instigate cardiomyocyte damage.
Figure 1.
Pathological mechanism operating under the influence of vaccine adjuvants. Adjuvants included in the COVID-19 vaccine stir up a wide range of pathological events such as direct toxicity, autoimmunity, bystander effect, anti-phospholipid syndrome, hypersensitivity, genetic susceptibility, epitope spreading, and anti-idiotype antibodies. These processes are set to operate in the background and can wholly or partially be responsible for whipping of cardiovascular disorders in patients receiving COVID-19 vaccines. A minor contribution from previous COVID-19 infection can also be implicated in aiding the cardiotoxicity of these adjuvants. So, we strongly advocate for careful screening of any adjuvants included in the cocktail of COVID-19 vaccines.
Modus operandi for plotting cardiotoxicity by these adjuvants
Triggering of autoimmunity geared toward myocardium
Coxsackie virus-induced myocarditis animal models were previously utilized for exploring and understanding the relationship between autoimmunity and myocarditis. 23 It appears that there is an element of molecular mimicry involved which literally means that there is overlapping of sequences between viral epitopes and self-antigens expressed on myocardial cells. 24 Due to this cross-sharing of antigen-epitopes, neo-antibodies provoked by coxsackie viral antigens traverse along circulation and make their way toward myocardium. 21
In autoimmune myocarditis and dilated cardiomyopathy, one of most prominent targets for inception of myocardial injury is cardiac-specific α-myosin isoform.25,26 Some of the patients with autoimmune dilated cardiomyopathy have been found to have autoantibodies devised against β-1 adrenergic receptor. 27 Most specifically, these antibodies that are mass-produced during autoimmune phenomenon principally belong to IgG3 subclass. 28 It has been documented that mice hearts which have a propensity to develop autoimmune myocarditis are bound to express myosin in the extracellular matrix over and above its expression within the cardiomyocytes. 29 It is logical to assume that this supernumerary extracellular expression of myosin is considered a liability as auto-antibodies floating around in the circulation recognize and interblend with them. Accordingly, prior mice studies reveal that overproduced IgG antibodies are prone to bind to myosin or myosin-like protein which is predominantly expressed in the extracellular matrix of cardiomyocytes resulting in formation of antigen–antibody complexes. 29 Accumulation of antigen–antibody complexes within the extracellular matrix of myocardium can be detrimental as it paves the way for incipiency of myocarditis.
Vacccines harboring oil emulsion adjuvants and TLR agonist adjuvants were shown to instigate a spectrum of autoimmune diseases ranging from autoimmune diseases ranging from arthritis, lupus, hypothyroidism, vitiligo, and hepatitis.30–33 The underlying premise based on which induction of autoimmune disease transpires is via eroding self-tolerance, exuberant synthesis of auto-reactive T-cells and massive production of IL-1 and IL-17.34–36 It is possible that precedent COVID-19 infection might have engendered subclinical myocardial injury thereby sparking exudation of cardiac self-antigens into the circulation. In such a scenario, autoantibodies brought into existence against these spilled self-antigens along with circulating self-reactive T-cells and cytokine storm can rouse the onset of autoimmune myocarditis in genetically susceptible individuals. Plausibly, some of the adjuvants can induce the generation of autoantibodies even without the exposure of self-antigens. In rodents and salmon fish, administration of oil adjuvants in vaccine were associated with formation of autoantibodies along with Systemic Lupus Erythematosus (SLE)-like syndrome, immune complex glomerulonephritis, arthritis and granulomatous hepatitis. 37
Bystander effect
An alternative mechanism called bystander effect has been described in the literature where viral infection can potentially unmask, and release hidden self-antigens from the body tissues there launching a chain reaction for subsequent development of autoimmunity.38–40 In Coxsackie-induced diabetes, it was revealed that that viral infection resulted in local pancreatic tissue damage and subsequent decamping of concealed islet antigens into the systemic circulation. 39 Breaking loose of these segregated self-antigens from their habitat results in budding of re-energized auto-reactive T-cells. 39 These over-reactive and motivated T-cells can be a driving force for autoimmune-mediated destruction of pancreatic tissue thereby instigating the onset of pancreatitis in the setting of coxsackie viral infections. 39 This bystander effect can be a potential underlying mechanism for occurrence of myocarditis in COVID-vaccinated individuals.
Adjuvants that are currently incorporated in the COVID vaccines might conceivably incite subclinical myocardial inflammation and thereby boost the extracellular expression of myosin or instigate extravasation of myosin from within cardiomyocytes. Exposing of self-antigens such as myosin initiates a chain of events that will ultimately culminate in myocarditis and cardiomyopathy. Autoimmune myocarditis is putatively seen more frequently in patients in other existing diseases such as Grave’s disease, Celiac Disease, and SLE.29,41 As compared to the general population, these high-risk patients are inherently more fated to develop auto-antibodies if there is unmasking of self-antigens in the body tissues. That being the case, this unmasking of self-antigens can enkindle the generation of autoantibodies which eventually resettle in myocardial tissues. Once they transmigrate into the myocardium it will also bring to existence a potential binding domain for these circulating autoimmune antibodies in the blood to interact and couple with these unshielded self-antigens. Once these auto-antibodies transmigrate to the myocardium, it will sow the seeds for creation of a scaffolding where circulating autoimmune antibodies can bind with unmasked myocardial self-antigens. This lead to formation of antigen-autoantibody complexes in the myocardium with the resultant un forrseen consequences. On top of that, shackling of IgG-myosin complexes in the extracellular milieu of cardiomyocytes might plausibly become a launch pad for kickstarting pathogenic signaling pathways that ultimately effectuate and abet myocarditis as well as dilated cardiomyopathy in the susceptible patients.
Over and above that, these exposed self-antigens such as myosin can be the driving force for replication of cardiac-specific auto-reactive T-cells, which can subsequently attack the myocardium thereby causing myocarditis. 42
Direct toxicity of adjuvants
Some of the adjuvants used in the vaccines can be directly toxic to the myocardial tissues. For example, aluminum which is a commonly used adjuvant is known for its nefarious features such as oxidative stress, inflammatory gene expression, apoptotic gene expression, immunotoxicity as well as disruption of physiological cellular pathways.43–46 Previous reports even unearthed the proposition that aluminum-based adjuvants are blameworthy for inciting NLRP3 inflammasome activation in dendritic cells as well as B&T lymphocytes. 47 The resulting after-effects from this NLRP3 activation in the immune cells can lay the foundation for kickstarting disastrous consequences in the myocardial tissues. Furthermore, aluminum adjuvants were also notorious to trigger Th2 immune bias which is principally characterized by elevated levels of IgE and eosinophils.48,49 Vaccination with aluminum adjuvant SARS COVID-19 vaccines in mice resulted in Th2-type lung immunopathology with predominant eosinophilia bringing to light paramount presumption of prevailing hypersensitivity against these adjuvants.49,50 Pragmatically, substitution of these aluminum-based adjuvants with delta inulin-based adjuvants in the COVID-19 vaccines was associated with decreased risk of developing Th2-based lung immunopathology in mice. 49 Full-fledged engagement of this phenomenon entails massive production of IL-4, IL-5, and IL-13 cytokines which plausibly have deleterious consequences in lung as well as neighboring heart tissues due to their anatomical proximity. 51 On top of that, there is also a high probability that this Th2-mediated hypersensitivity and inflammation can spill over from lung to neighboring myocardial tissues thereby inciting pathological consequences such as pericarditis, myocarditis, and arrhythmias.
Antiphosphospholipid syndrome
Mice studies have revealed that vaccines containing aluminum adjuvants were previously associated with development of Antiphosphospholipid syndrome (APS) syndrome.48,52,53 The mice developed fetal resorption and decreased fecundity secondary to superlative T-lymphocyte activation and polyclonal B-cell stimulation. 52 Aluminum’s inherent cytotoxicity along with amassment of uric acid and release of intracellular DNA can putatively trigger NLRP3 inflammasome in dendritic cell and T-lymphocyte populations.54–57 These cellular alterations can kickstart revamped adaptive immune responses leading to augmented manufacture of anti-phospholipid antibodies.58,59 These autoantibodies can subsequently bind to negatively charged phospholipids, platelets and endothelial cells giving rise to APS syndrome.58–60 Once APS syndrome materializes, secondary inflammation and hyper thrombotic tendency can fast-track the emergence of cardiovascular manifestations such as coronary artery disease, valvular disease, pulmonary hypertension, intra-cardiac thrombi and cardiomyopathy. 61 Some of the vaccines that are currently in clinical and preclinical trials have incorporated TLR agonists (TLR3, TLR4, TLR5, TLR7/8, and TLR9) which have a propensity to instigate APS syndrome by a combination of bizarre immune counterattack, molecular mimicry and bystander effect.54,62
Anaphylaxis and hypersensitivity
Anaphylaxis can be one of the important mechanisms that might be plausibly responsible for onset of cardiovascular diseases particularly with incorporation of specific types of adjuvants. In such a scenario, adjuvants such as metal ions tend to act as haptens and tether with MHC class II complex of αβ T cells. 63 Once this interaction is consummated, there will be substantial alteration of binding structure of MHC class II molecule on T-cells which ultimately becomes the basis for launching a hypersensitivity response. 63 This type of mechanism holds true particularly when metal adjuvants such as aluminum, nickel, gold, and mercury are incorporated in vaccines.43,63 With the polysaccharide adjuvants, activation of complement system, spilling of anaphylatoxins, mast cell stimulation, and anaphylactoid shock might be the lurking manifestations in all likelihood inciting indirect adverse repercussions upon the myocardium. 48
Genetic susceptibility
Autoimmune syndrome induced by adjuvants (ASIA) is a clinical spectrum characterized by myopathy, arthralgias, neurological impairments, gastrointestinal symptoms, respiratory signs, skin involvement, and auto-antibodies.64,65 The prevalence of this disorder is specifically seen in the individuals with HLA DRB1 and HLA DQB1 genes.64,65 Practically speaking, it is not feasible to assess the genetic profile of all the vaccinated individuals to assess their risk of autoimmune syndrome. But these reports bring to light genetic susceptibility as a lurking risk factor and this presumption needs to be taken into account particularly in individuals with prior history of autoimmune diseases.
Epitope spreading
According to Powell and Black, A.M. epitope spreading literally means immune cells and antibodies develop a proclivity to strike not only foreign antigens but also non-specific and non-reactive self-antigens during immune surveillance. 66 In other words, reactive T-cells develop broad specificity so that they are free to carry on their crusade against a wide variety of self-antigens that become unmasked secondary to tissue damage. Generally, this epitope spreading is bound to be protective and offered recovery in animal model of acute adjuvant arthritis. Unfortunately in other disease models such as multiple sclerosis and type I diabetes, haphazard and uncontrolled epitope spreading had been instrumental in sparking tissue injury and effectuated in disease progression and tissue injury. 66 Furthermore, epitope spreading has been implicated in the pathogenesis of autoimmune skin diseases, periodontitis, autoimmune bullous diseases, multiple sclerosis, and autoimmune encephalomyelitis.67–70 In multiple sclerosis and autoimmune encephalomyelitis, propelling of disease progression is primarily contingent upon transition of autoreactivity from primary antigen epitopes to secondary antigen epitopes. 67 Epitope spreading is speculated to be occurring in two phases. 70 In the first phase, primary antigen epitope is processed by APC (antigen presenting cells) and presented to T-helper cells via MHC class II proteins. 70 Subsequently, activated T-helper cells collude with local macrophages and hereafter trigger tissue damage through secretion of mediators such as IFN -γ (Interferon gamma), TNFβ (tumor necrosis factor alpha) and LT (Leukotrienes). 70 In the secondary phase this preceding tissue damage instigates the spilling of secondary antigen that is eventually handled by APCs. 70 Sooner or later, this secondary antigen is presented to naïve T-cells, and this breeds cell- or antibody-mediated autoimmunity against secondary antigen. 70 This adjuvant mediated epitope spreading can be the underlying premise based on which cardiac damage emanates thereby prompting the onset of autoimmune-mediated myocarditis, pericarditis, arrhythmias, and cardiomyopathy in vaccinated individuals. Although this phenomenon cannot happen alone, it can be presumed to proceed parallelly along with other mechanisms set in motion by entry of toxic adjuvants into the body.
Idiotypic and anti-idiotype antibodies
This is one of the predominant and plausible mechanisms through which adjuvants might sooner or later affect myocardial tissues. It is already well established that adjuvants by themselves or via uncovering of self-antigens might be influential in eliciting the generation of autoantibodies. In this hypothesis, these autoantibodies can conceivably act as self-antigens and trigger the generation of anti-idiotypic antibodies that at some point inflict myocardial damage. 71 Previous reports theorize that autoantibodies were witnessed in 50% of the hospitalized COVID-19 patients. 72 These antibodies in all likelihood can target self-antigens thereby enkindling the onset of autoimmune diseases such as multiple sclerosis, myositis, and others in COVID-19 patients. 72 In addition to targeting self-antigens, these COVID-19 autoantibodies can restructure themselves to display novel antigen epitopes and operate as self-antigens.71–73 With that being said, autoantibodies assuming a new role as self-antigens can spawn the breeding of anti-idiotype antibodies which will belatedly target myocardial tissues to trigger inflammation and cardiomyopathy.71–73 The occurrence of this phenomenon is widely documented in diseases such as type I diabetes, Sjogren’s syndrome, Systemic lupus erythematosus (SLE) and neonatal lupus syndrome.71,74 We presume that breeding of anti-idiotype antibodies might at least have a fair share of culpability in impelling the pathogenic signaling pathways that ultimately culminate into myocardial inflammation, cardiomyopathy and arrhythmias in COVID-19 vaccinated individuals.
COVID-19 restrictions and the impacts on cardiovascular risk factors
The prevalence of cardiovascular comorbidities and the association of such conditions with COVID-19 infection results in increased mortality and morbidity. Their findings underscore the importance of considering cardiovascular health in the context of COVID-19. 75 Studies emphasize the significance of understanding the interplay between comorbidities and COVID-19 outcomes, particularly among the athletes. 76 The consequences of quarantine measures on increasing cardiovascular risk among COVID-19 patients has been demonstrated in few prior studies so far. There are adverse effects of lifestyle changes during quarantine, including unhealthy dietary habits and reduced physical activity, and their potential long-term implications for cardiovascular health. 77 Collectively, these studies underscore the complex relationship between COVID-19, cardiovascular comorbidities, and the broader public health implications of pandemic-related restrictions on lifestyle. The pandemic has emphasized the importance of vitamin D, physical activity, and managing obesity to reduce cardiovascular risk. 78 The COVID-19 restrictions introduced many complex factors, including changes in lifestyle, social isolation, psychological stress, and economic challenges. These factors collectively contributed to an increased risk of cardiovascular events. Adjuvants, in this context, are viewed as potential catalysts that exacerbate the underlying mechanisms linking these restrictions to cardiovascular risks. This creates a direct link between the restrictions, adjuvants, and the heightened cardiovascular risk among COVID-19 patients.
Studies about the COVID-19 pandemic and its public health responses, including the impact on CV risk factors, healthcare access, and mental health, highly emphasize the importance of telehealth interventions in mitigating these risks. Telehealth has played a critical role in providing healthcare services during the COVID-19 pandemic, allowing patients to manage CV risk factors remotely. 79 Studies discuss several challenges faced in cardiovascular disease prevention and management during the COVID-19 pandemic, including disruptions in routine healthcare services, delays in elective procedures, reduced access to healthcare facilities, and altered patient behaviors due to fear and restrictions.80,81 These challenges have the potential to impact cardiovascular disease outcomes and risk factor management, highlighting the need for solutions to maintain and enhance cardiovascular health during such pandemic crises.
Which adjuvants are not safe to use in COVID Vaccines
Aluminum adjuvants
Research suggests that these adjuvants are associated with serious immunological disorders with high propensity to develop autoimmunity, neurological inflammation, and other associated widespread systemic complications including cardiovascular disorders.82,83 Aluminum is associated with autoimmunity-associated adjuvants syndrome (ASIA) as well as autism due to hyperactive immune system.84,85 A recent study showed that aluminum adjuvants are associated with impairment of social impairments if administered in early postnatal development 86 . Rare cases of antiphospholipid syndrome (APS) have also been reported with usage of aluminum adjuvants in tetanus vaccines. 53
Based on these studies, it would be prudent to use some caution and discourage the incorporation of aluminum adjuvants in COVID-vaccines to reduce the risk of autoimmune and inflammatory conditions including cardiovascular disorders.
Oil emulsion Adjuvants (CFA, MF59, AS02, and AS03)
Oil emulsion adjuvants are known to trigger the production of pro-inflammatory cytokines (IL-1, IL-17).35,48,87 These cytokines are implicated in collapse of self-tolerance.34,48 This will permit the percolation of self-reactive T-cell across the blood-brain barrier leading to increased propensity to develop autoimmune diseases including multiple sclerosis, experimental allergic encephalitis, and narcolepsy.48,87–89 Due to their inherent nature to disrupt the self-tolerance, these adjuvants might possibly also increase the risk of inciting autoimmune cardiovascular disorders.
TLR agonist adjuvants
TLR9 agonists tagged to unmethylated CpG has been used in a wide variety of vaccines including disease (hepatitis B, influenza, malaria, anthrax, human immunodeficiency virus [HIV]), cancer (melanoma, non-small cell lung cancer) and allergic rhinitis.90,91 TLR9 agonist used in hepatitis-B vaccination was associated with development of autoimmune Wegener’s granulomatosis, autoimmunity, Vitiligo, and hypothyroidism.33,48,92
Caution is needed regarding the selection of adjuvants in COVID-19 vaccines
Keeping in mind the proclivity of some of the adjuvants in the causation autoimmune diseases, appropriate caution is necessary when future COVID-19 vaccines are formulated. Furthermore, the susceptibility to develop these autoimmune diseases is also dependent on their genetic background. For example, severe autoimmune reactions were noticed particularly in the individuals with DRB01*01 genetic background. 93 Also, patients with 20%–25% of type-1 diabetes mellitus were more prone to develop anti-thyroid antibodies as well as clinical autoimmune thyroid disease. 94 In these susceptible individuals, the interaction between self and microbial antigens will most likely transmute a protective immune response into a self-destructive pathological onslaught toward tissues thereby paving the way for developing autoimmune diseases.95,96 The need of the hour is careful selection of adjuvants and validation of their safety by conducting basic scientific and clinical research studies. This will helpfully eliminate or reduce the possibility of having untoward side effects and improve the safety of newly crafted COVID-19 vaccines.
Summary and conclusions
This review explored the potential role of vaccine adjuvants in contributing to developing COVID-19-related heart syndrome. While the primary focus of concern has been the COVID-19 spike mRNA as a possible culprit, there is also an argument to be made that vaccine adjuvants, which are included to enhance immune responses, may play a role in triggering cardiac abnormalities. Adjuvants work in tandem with antigens to stimulate adaptive and innate immune responses, making them a crucial component of vaccines. However, their role in potentially triggering adverse effects in the cardiovascular system needs to be better understood. Several mechanisms have been proposed through which adjuvants could lead to cardiovascular disorders. This review highlights that adjuvants could lead to induced autoimmunity, which could involve molecular mimicry, where vaccine components resemble self-antigens, leading to a misguided immune response against the body’s tissues (Figure 1). Another proposed mechanism is the ‘bystander effect’, the immune system could unmask hidden self-antigens in body tissues, leading to an autoimmune reaction (Figure 1). Direct toxicity, general susceptibility, Epitope spreading, and Idiotypic and Anti-idiotype Antibodies are other possible mechanisms that could result in cardiovascular disorders due to the vaccine adjuvants (Figure 1).
While the primary focus of vaccine safety research has been on the efficacy of adjuvants and their immune-boosting capabilities, their potential role in adverse reactions should not be disregarded. This review calls for further research to thoroughly investigate the impact of adjuvants on immune responses and potential adverse effects, especially in the context of COVID-19 heart syndrome. This review suggests that a more comprehensive understanding of the interaction between adjuvants, immune responses, and cardiac tissues could lead to the development of safer and more effective COVID-19 vaccines for the general population.
Limitations of the study
Since this is a non-systematic review based on the extensive literature search, the risk of bias in interpreting the casual relationship between onset of cardiovascular diseases and adjuvants in COVID-19 vaccines is moderately high. With this being a review based on the current scientific literature and based upon author’s subjective intuition, appropriate studies should be designed based on these hypotheses to ratify the toxicity of current adjuvants used in COVID-19 vaccines. 97 Another limitation is that this review did not include a qualitative analysis of study findings of previous studies and these postulations should be proved and tested before these can be applied for crafting future COVID-19 vaccines. Additionally, since these reviews are not based upon predefined protocol, there is every possibility of inclusion of subjective selection bias. 97
Although we speculated some most probable propositions that would link the onset of spectrum of diseases included in the COVID-Heart syndrome to the adjuvants incorporated in the COVID-19 vaccines, the actual underlying mechanism is still ambiguous, and thus a matter of future investigation. As the level of scientific evidence for association of the inception of cardiovascular diseases to the adjuvants in the COVID-19 vaccines in low, we propound that multi-center clinical cohort studies should be carried out to unravel whether adjuvants are indeed responsible for the cardiovascular events in the susceptible populations. Furthermore, most toxic, and culpable adjuvants should be further studied in animal models to ascertain the pathogenetic mechanisms that come in play for provoking cardiovascular diseases. This might shed light on probable downstream events and molecular targets involved in materialization of COVID-Heart syndrome under the influence of these adjuvants. By gleaning this information, researchers might be extra-cautious during the selection of adjuvants so that future COVID-19 vaccines formulated can be deemed safer for reducing or even preventing the onset of cardiovascular diseases. Further, we also propose future basic scientific research studies so that all the currently used adjuvants should be classified based on their level of toxicity and autoimmune propensity. This information should be standardized and available to the pharmaceutical and biotech companies so that less toxic adjuvants are selected for manufacturing RNA-virus vaccines. Lastly, we also recommend for future studies that would craft novel non-toxic and potent adjuvants that can be deemed to be safely incorporated into future COVID-19 vaccine formulations. Taken together, steps taken toward understanding the pathogenesis of cardiovascular diseases might be beneficial in reducing the morbidity and mortality in the COVID-19 vaccine recipients.
Acknowledgments
None.
Footnotes
ORCID iD: Sri Harsha Kanuri  https://orcid.org/0000-0003-0442-1489
https://orcid.org/0000-0003-0442-1489
Contributor Information
Sri Harsha Kanuri, Research Fellow, Stark Neurosciences Institute, Indiana University School of Medicine, 320 W 15 ST, Indianapolis, IN 46202, USA.
Prapthi Jayesh Sirrkay, University of Minnesota, Minneapolis, MN, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Sri Harsha Kanuri: Conceptualization; Supervision; Visualization; Figures; Writing – original draft; Writing – review & editing.
Prapthi Jayesh Sirrkay: Figures; Supervision; Visualization; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Chung MK, Zidar DA, Bristow MR, et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res 2021; 128: 1214–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022; 28: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vidal-Perez R, Brandão M, Pazdernik M, et al. Cardiovascular disease and COVID-19, a deadly combination: a review about direct and indirect impact of a pandemic. World J Clin Cases 2022; 10: 9556–9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishiga M, Wang DW, Han Y, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020; 17: 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Link-Gelles R, Levy ME, Natarajan K, et al. Estimation of COVID-19 mRNA vaccine effectiveness and COVID-19 illness and severity by vaccination status during omicron BA.4 and BA.5 sublineage periods. JAMA Netw Open 2023; 6: e232598–e232598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. USAFACTSORG. https://usafacts.org/visualizations/covid-vaccine-tracker-states/ (accessed 10 May 2023). [Google Scholar]
- 7. CDC. Stay up to date with COVID-19 vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html (accessed 18 January 2024). [Google Scholar]
- 8. Whiteley WN, Ip S, Cooper JA, et al. CVD-COVID-UK Consortium. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: a population-based cohort study of 46 million adults in England. PLoS Med 2022; 19: e1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021; 326: 1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ulucay AS, Singh G, Kanuri SH. Do COVID-19 viral infection and its mRNA vaccine carry an equivalent risk of myocarditis? Review of the current evidence, insights, and future directions. Indian Heart J 2023; 75: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patone M, Mei XW, Handunnetthi L, et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation 2022; 146: 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schreiber A, Elango K, Soussu C, et al. COVID-19 induced cardiomyopathy successfully treated with tocilizumab. Case Rep Cardiol 2022; 2022: 9943937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abbasi J. The COVID Heart-one year after SARS-CoV-2 infection, patients have an array of increased cardiovascular risks. JAMA 2022; 327: 1113–1114. [DOI] [PubMed] [Google Scholar]
- 14. Pardi N, Hogan MJ, Porter FW, et al. MRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov 2018; 17: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castrodeza-Sanz J, Sanz-Muñoz I, Eiros JM. Adjuvants for COVID-19 vaccines. Vaccines 2023; 11: 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geeraedts F, Goutagny N, Hornung V, et al. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog 2008; 4: e1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Pasquale A, Preiss S, Tavares Da Silva F, et al. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines 2015; 3: 320–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19: 1597–1608. [DOI] [PubMed] [Google Scholar]
- 19. Iwasaki A, Omer SB. Why and how vaccines work. Cell 2020; 183: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark R, Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Investig Dermatol 2005; 125: 629–637. [DOI] [PubMed] [Google Scholar]
- 21. Fairweather D, Frisancho-Kiss S, Rose NR. Viruses as adjuvants for autoimmunity: evidence from Coxsackievirus-induced myocarditis. Rev Med Virol 2005; 15: 17–27. [DOI] [PubMed] [Google Scholar]
- 22. Pulendran B, S., Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov 2021; 20: 454–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fairweather D, Kaya Z, Shellam GR, et al. From infection to autoimmunity. J Autoimmun 2001; 16: 175–186. [DOI] [PubMed] [Google Scholar]
- 24. Rose NR, Mackay IR. Molecular mimicry: a critical look at exemplary instances in human diseases. Cell Mol Life Sci 2000; 57: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caforio AL, Mahon NJ, Mckenna WJ. Cardiac autoantibodies to myosin and other heart-specific autoantigens in myocarditis and dilated cardiomyopathy. Autoimmun 2001; 34: 199–204. [DOI] [PubMed] [Google Scholar]
- 26. Massilamany C, Gangaplara A, Steffen D, et al. Identification of novel mimicry epitopes for cardiac myosin heavy chain-α that induce autoimmune myocarditis in A/J mice. Cell Immunol 2011; 271: 438–449. [DOI] [PubMed] [Google Scholar]
- 27. Warraich RS, Dunn MJ, Yacoub MH. Subclass specificity of autoantibodies against myosin in patients with idiopathic dilated cardiomyopathy: pro-inflammatory antibodies in DCM patients. Biochem Biophys Res Commun 1999; 259: 255–261. [DOI] [PubMed] [Google Scholar]
- 28. Limas CJ, Goldenberg IF, Limas C. Influence of anti-beta-receptor antibodies on cardiac adenylate cyclase in patients with idiopathic dilated cardiomyopathy. Am Heart J 1990; 119: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 29. Liao L, Sindhwani R, Rojkind M, et al. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J Exp Med 1995; 181: 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Svelander L, Erlandsson Harris H, Lorentzen JC, et al. Oligodeoxynucleotides containing CpG motifs can induce T cell-dependent arthritis in rats. Arthritis Rheum 2004; 50: 297–304. [DOI] [PubMed] [Google Scholar]
- 31. Holm BC, Lorentzen JC, Bucht A. Adjuvant oil induces waves of arthritogenic lymph node cells prior to arthritis onset. Clin Exp Immunol 2004; 137: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naik SR, Wala SM. Arthritis, a complex connective and synovial joint destructive autoimmune disease: animal models of arthritis with varied etiopathology and their significance. J Postgrad Med 2014; 60: 309–317. [DOI] [PubMed] [Google Scholar]
- 33. Heyward WL, Kyle M, Blumenau J, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine 2013; 31: 5300-5305 . [DOI] [PubMed] [Google Scholar]
- 34. Cosmi L, Liotta F, Maggi E, et al. Th17 and non-classic Th1 cells in chronic inflammatory disorders: two sides of the same coin. Int Arch Allergy Immunol 2014; 164: 171–177 . [DOI] [PubMed] [Google Scholar]
- 35. Vitoriano-Souza J, Moreira ND, Teixeira-Carvalho A, et al. Cell recruitment and cytokines in skin mice sensitized with the vaccine adjuvants: saponin, incomplete Freund’s adjuvant, and monophosphoryl lipid A. PLoS One 2012; 7: e40745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maroof A, Yorgensen YM, Li Y, et al. Intranasal vaccination promotes detrimental T h17-mediated immunity against influenza infection. PLoS Pathog 2014; 10: e1003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koppang EO, Bjerkås I, Haugarvoll E, et al. Vaccination-induced systemic autoimmunity in farmed Atlantic salmon. J Immunol 2008; 181: 4807–4814. [DOI] [PubMed] [Google Scholar]
- 38. Neu N, Craig SW, Rose NR, et al. Coxsackievirus induced myocarditis in mice: cardiac myosin autoantibodies do not cross-react with the virus. Clin Exp Immunol 1987; 69: 566–574. [PMC free article] [PubMed] [Google Scholar]
- 39. Horwitz MS, Bradley LM, Harbertson J, et al. Diabetes induced by Coxsackie virus: Initiation by bystander damage and not molecular mimicry. Nat Med 1998; 4: 781–785. [DOI] [PubMed] [Google Scholar]
- 40. Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 1996; 272: 1947–1950. [DOI] [PubMed] [Google Scholar]
- 41. Tincani A, Rebaioli CB, Taglietti M, et al. Heart involvement in systemic lupus erythematosus, anti-phospholipid syndrome and neonatal lupus. Rheumatology 2006; 45 Suppl 4: (suppl_4 iv8–iv13. [DOI] [PubMed] [Google Scholar]
- 42. Bracamonte-Baran W, Čiháková D. Cardiac autoimmunity: myocarditis. Adv Exp Med Biol 2017; 1003: 187–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guimarães LE, Baker B, Perricone C, et al. Vaccines, adjuvants and autoimmunity. Pharmacol Res 2015; 100: 190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Exley C. The pro-oxidant activity of aluminum. Free Radic Biol Med 2004; 36: 380–387. [DOI] [PubMed] [Google Scholar]
- 45. Lukiw WJ, Percy ME, Kruck TP. Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J Inorg Biochem 2005; 99: 1895–1898. [DOI] [PubMed] [Google Scholar]
- 46. Israeli E, Agmon-Levin N, Blank M, et al. Adjuvants and autoimmunity. Lupus 2009; 18: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 47. Exley C, Siesjö P, Eriksson H. The immunobiology of aluminium adjuvants: how do they really work? Trends Immunol 2010; 31: 103–109. [DOI] [PubMed] [Google Scholar]
- 48. Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf 2015; 38: 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Honda-Okubo Y, Barnard D, Ong CH, et al. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol 2015; 89: 2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One 2012; 7: e35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Power UF, Huss T, Michaud V, et al. Differential histopathology and chemokine gene expression in lung tissues following respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV- or BBG2Na-immunized mice. J Virol 2001; 75: 12421–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dimitrijević L, Živković I, Stojanović M, et al. Vaccine model of antiphospholipid syndrome induced by tetanus vaccine. Lupus 2012; 21: 195–202. [DOI] [PubMed] [Google Scholar]
- 53. Zivkovic I, Petrusic V, Stojanovic M, et al. Induction of decreased fecundity by tetanus toxoid hyper-immunization in C57BL/6 mice depends on the applied adjuvant. Innate Immun 2012; 18: 333–342. [DOI] [PubMed] [Google Scholar]
- 54. Blank M, Israeli E, Shoenfeld Y. When APS (Hughes syndrome) met the autoimmune/inflammatory syndrome induced by adjuvants (ASIA). Lupus 2012; 21: 711–714. [DOI] [PubMed] [Google Scholar]
- 55. Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008; 9: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008; 8: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takeshita F, Ishii KJ. Intracellular DNA sensors in immunity. Curr Opin Immunol 2008; 20: 383–388. [DOI] [PubMed] [Google Scholar]
- 58. Cervera R, Piette J, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002; 46: 1019–1027. [DOI] [PubMed] [Google Scholar]
- 59. Schreiber K, Sciascia S, de Groot PG, et al. Antiphospholipid syndrome. Nat Rev Dis Primers 2018; 4: 18005. [DOI] [PubMed] [Google Scholar]
- 60. Mezhov V, Segan JD, Tran H, et al. Antiphospholipid syndrome: a clinical review. Med J Aust 2019; 211: 184–188. [DOI] [PubMed] [Google Scholar]
- 61. Kolitz T, Shiber S, Sharabi I, et al. Cardiac manifestations of antiphospholipid syndrome with focus on its primary form. Front Immunol 2019; 10: 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mbow ML, De Gregorio E, Valiante NM, et al. New adjuvants for human vaccines. Curr Opin Immunol 2010; 22: 411–416. [DOI] [PubMed] [Google Scholar]
- 63. Wang Y, Dai S. Structural basis of metal hypersensitivity. Immunol Res 2013; 55: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shoenfeld Y, Agmon-Levin N. Asia’ – autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun 2011; 36: 4–8. [DOI] [PubMed] [Google Scholar]
- 65. Perricone C, Colafrancesco S, Mazor RD, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: unveiling the pathogenic, clinical and diagnostic aspects. J Autoimmun 2013; 47: 1–16. [DOI] [PubMed] [Google Scholar]
- 66. Powell AM, Black MM. Epitope spreading: protection from pathogens, but propagation of autoimmunity? Clin Exp Dermatol 2001; 26: 427–433. [DOI] [PubMed] [Google Scholar]
- 67. Tuohy VK, Yu M, Yin L, et al. Spontaneous regression of primary autoreactivity during chronic progression of experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med 1999; 189: 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Didona D, Di Zenzo G. Humoral epitope spreading in autoimmune bullous diseases. Front Immunol 2018; 9: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kwon EY, Cha GS, Jeong E, et al. Pep19 drives epitope spreading in periodontitis and periodontitis-associated autoimmune diseases. J Periodontal Res 2016; 51: 381–394. [DOI] [PubMed] [Google Scholar]
- 70. Chan LS, Vanderlugt CJ, Hashimoto T, et al. Epitope spreading: lessons from autoimmune skin diseases. J Investig Dermatol 1998; 110: 103–109. [DOI] [PubMed] [Google Scholar]
- 71. Tzioufas AG, Routsias JG. Idiotype, anti-idiotype network of autoantibodies: Pathogenetic considerations and clinical application. Autoimmun Rev 2010; 9: 631–633. [DOI] [PubMed] [Google Scholar]
- 72. Chang SE, Feng A, Meng W, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun 2021; 12: 5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Plotz P. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet 1983; 322: 824–826. [DOI] [PubMed] [Google Scholar]
- 74. Routsias JG, Dotsika E, Touloupi E, et al. Idiotype-anti-idiotype circuit in non-autoimmune mice after immunization with the epitope and complementary epitope 289-308aa of La/SSB: implications for the maintenance and perpetuation of the anti-La/SSB response. J Autoimmun 2003; 21: 17–26. [DOI] [PubMed] [Google Scholar]
- 75. Muhammad DG, Abubakar IA. COVID-19 lockdown may increase cardiovascular disease risk factors. Egypt Heart J 2021; 73: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kirsch M, Vitiello D. The COVID-19 pandemic lowers active behavior of patients with cardiovascular diseases, healthy peoples and athletes. Int J Environ Res Public Health 2022; 19: 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mattioli AV, Ballerini Puviani M, Nasi M, et al. COVID-19 pandemic: the effects of quarantine on cardiovascular risk. Eur J Clin Nutr 2020; 74: 852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. van Bakel BMA, Bakker EA, de Vries F, et al. Impact of COVID-19 lockdown on physical activity and sedentary behaviour in Dutch cardiovascular disease patients. Neth Heart J 2021; 29: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lau D, McAlister FA. Implications of the COVID-19 pandemic for cardiovascular disease and risk-factor management. Can J Cardiol 2021; 37: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dale CE, Takhar R, Carragher R, et al.; CVD-COVID-UK Consortium. The impact of the COVID-19 pandemic on cardiovascular disease prevention and management. Nat Med 2023; 29: 219–225. [DOI] [PubMed] [Google Scholar]
- 81. Zahmatkeshan N, Khademian Z, Zarshenas L, et al. Experience of adherence to treatment among patients with coronary artery disease during the COVID-19 pandemic: A qualitative study. Health Promot Perspect 2021; 11: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tomljenovic L, A. Shaw C. Aluminum vaccine adjuvants: are they safe? Curr Med Chem 2011; 18: 2630–2637. [DOI] [PubMed] [Google Scholar]
- 83. Shaw CA, Li D, Tomljenovic L. Are there negative CNS impacts of aluminum adjuvants used in vaccines and immunotherapy? Immunotherapy 2014; 6: 1055–1071. [DOI] [PubMed] [Google Scholar]
- 84. Tomljenovic L, Shaw CA. Mechanisms of aluminum adjuvant toxicity and autoimmunity in pediatric populations. Lupus 2012; 21: 223–230. [DOI] [PubMed] [Google Scholar]
- 85. Tomljenovic L, Shaw CA. Do aluminum vaccine adjuvants contribute to the rising prevalence of autism? J Inorg Biochem 2011; 105: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 86. Sheth SKS, Li Y, Shaw CA. Is exposure to aluminium adjuvants associated with social impairments in mice? A pilot study. J Inorg Biochem 2018; 181: 96–103. [DOI] [PubMed] [Google Scholar]
- 87. Sutton C, Brereton C, Keogh B, et al. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 2006; 203: 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One 2012; 7: e33723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One 2012; 7: e33536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hartmann G, Weeratna RD, Ballas ZK, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol 2000; 164: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 91. Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014; 32: 6377–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Halperin SA, Ward B, Cooper C, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine 2012; 30: 2556–2563. [DOI] [PubMed] [Google Scholar]
- 93. Vera-Lastra O, Medina G, Cruz-Dominguez Mdel P, et al. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): clinical and immunological spectrum. Expert Rev Clin Immunol 2013; 9: 361–373. [DOI] [PubMed] [Google Scholar]
- 94. Kahaly GJ, Hansen MP. Type 1 diabetes associated autoimmunity. Autoimmun Rev 2016; 15: 644–648. [DOI] [PubMed] [Google Scholar]
- 95. Oldstone MB. Molecular mimicry and autoimmune disease. Cell 1987; 50: 819–820. [DOI] [PubMed] [Google Scholar]
- 96. Watad A, David P, Brown S, et al. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol 2016; 7: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pae CU. Why systematic review rather than narrative review? Psychiatry Investig 2015; 12: 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]