Abstract
Background:
Breast cancer (BC) patients have a higher chance of survival if it is diagnosed at an early stage, which is essential for efficient treatment of the condition. The results of an elevated risk of cancer, including BC, previously associated with the ins/del polymorphism rs145204276 in the promoter region of growth arrest-specific 5 (GAS5) are still up for debate. Thus, this study aimed to appraise the frequency of the GAS5 rs145204276 variant with BC risk and demonstrate the potential impact of the sirtuin 1 (SIRT-1), transforming growth factor-beta (TGF-β), and microRNA-182 (miR-182) expression and their diagnostic value in BC.
Methods:
Blood samples of 155 patients with BC and fibroadenoma and 80 healthy controls were analyzed for GAS5 rs145204276 single nucleotide polymorphism (SNP), SIRT-1, TGF-β, and miRNA-182 expression levels.
Results:
Ins/ins genotype and ins allele frequencies for GAS5 rs145204276 were considerably higher in BC patients compared with controls. Patients with BC had significantly greater serum levels of TGF-β, miR-182, and SIRT-1 expression.
Conclusions:
The SIRT-1, TGF-β, and miR-182 genes provide novel, noninvasive diagnostic biomarkers for BC.
Keywords: Breast cancer, polymorphism, GAS5, SIRT-1, TGF-β, miR-182
Introduction
Breast cancer (BC) has been implicated as the cornerstone cancer among women worldwide, with roughly 1 million new cases of diagnosis each year. 1 Breast cancer survivors make up about 38.8% of all newly diagnosed cancer patients among Egyptian women. 2 Age, hormone levels, lifestyle choices, environmental factors, genetic factors, and ethnicity are all factors that affect the chance of developing BC. 3
The most significant prognostic indicators for BC continue to be the conventional prognostic parameters including tumor size, grade, and lymph node metastatic status. However, the prediction incorporated just a small amount of genetic information interpretations. Single nucleotide polymorphisms (SNPs) can be a useful tool for examining various diseases or disease characteristics since they are a relatively small allele variable, have a higher availability, and show linkage disequilibrium across populations and races. 4 As genome-wide association study (GWAS) technology has advanced, a growing number of genetic factors have been confirmed to be linked to BC.
The designation “growth arrest-specific 5” (GAS5) refers to long noncoding RNA in growth-arrested cells that overexpressed it. 5 The association between GAS5 and a variety of malignancies was examined because of GAS5 capacity to trigger apoptosis and inhibit tumor growth. 6 On the contrary, there are numerous ways in which the genetic polymorphism of GAS5 can impact carcinogenesis; the SNP rs145204276 del/del genotype of GAS5 was linked to a higher risk of cancer.7,8
Sirtuin 1 (SIRT-1) is a class III histone deacetylase that requires NAD+ (Nicotinamide Adenine Dinucleotide) and emerged as a potent mediator of various cellular processes, including DNA repair, cell survival, proliferation, apoptosis, aging, and cancer. 9 Based on the tumor types’ microenvironment, upstream regulators, and downstream target genes, the function of SIRT-1 is still controversial. 10 Transforming growth factor-beta (TGF-β) is a cytokine that controls how cells behave. It has numerous ways it affects epithelial cells and cell clusters that develop into carcinomas both in vitro and in vivo. Transforming growth factor-beta has been demonstrated to have dual impacts on the development and spread of cancer. 11 Therefore, it is crucial to research SIRT-1 and TGF-β regulation and function in BC.
Multiple forms of cancers have been linked to dysregulation of miRNA expression; thus, miRNA expression profiles may be diagnostic and/or prognostically significant for human malignancies, even if the molecular mechanisms of miRNA-mediated gene regulation are still being studied. MicroRNA-182 is a part of the miR-183 cluster, which has significant roles in the development of many cancer types and is involved in drug resistance, migration, and epithelial-mesenchymal transition (EMT). 12 Moreover, it appears to have a significant effect on the metastasis of cancer cells. 13
According to our knowledge, the correlation between genetic polymorphism of the GAS5 rs145204276 ins/del variant and SIRT-1, TGF-β, and miR-182 expression in patients with BC poorly understood; thus, the purpose of this study was to appraise the potential impact of GAS5 rs145204276 ins/del variant on the SIRT-1, TGF-β, and miR-182 expression and their diagnostic value in BC.
Materials and Methods
This study was conducted on 235 Egyptian participants during the period from March 2022 to October 2022, and all participants in the study provided their written, informed consent, which the BUC-Institutional Ethical Committee endorsed (BUC-IACUC-230122-13). Study participants were clinically categorized according to the participants’ histories and clinical examinations. The enrolled individuals’ diagnoses were verified using mammography. Consequently, they were categorized into 3 main groups. Group I comprises the control group that was recruited during the routine checkup (80 age-matched healthy women with no history of BC, fibroadenoma (FA), or palpable breast lumps in the family and no hypertension or diabetes mellitus). Group II included 40 patients with FA. Group 3 includes patients with BC. The clinical information was obtained from the reports of the patients including age, family history, and the state of menstrual cycles. None of the patients had had radiation, chemotherapy, or any form of antihormonal medication before taking part in the study.
Peripheral blood samples were divided into 2 tubes. One tube was used for the separation of serum for the estimation of SIRT-1, TGF-β, and miR-182 expression. DNA was extracted for GAS genotyping using the other tube, which included EDTA (Ethylenediaminetetraacetic acid). All samples were handled at 4°C until they were stored for final analysis at −80°C. DNA extractions were performed simultaneously for all samples.
An enzyme-linked immunosorbent assay (ELISA) kit provided by Shanghai SunRed Biological Technology (Shanghai, China) was used to calculate the serum SIRT-1 level. A kit provided by BioSource Europe SA (Nivelles, Belgium) was used to quantify serum TGF-β1 using the ELISA technique. 14
Extraction and genotyping of growth arrest-specific rs145204276 (ins/del)
As instructed by the manufacturer, DNA was extracted from whole blood samples using the Qiagen-amplification extraction kit (Qiagen, Venlo, Limburg, Netherlands). The NanoDrop1-1000 spectrophotometer (NanoDrop Technologies, Inc, Wilmington, USA) was used to evaluate the DNA content. Then, genotyping was performed via TaqMan allelic discrimination assay with predesigned primer/probe sets for rs2067079C/T (Assay ID: C_166593916_10) (Thermo Fisher), as previously described, 15 using real-time polymerase chain reaction (PCR). Briefly, the amplification of DNA was carried out in a 25 μL volume, which contained 12.5 μL of TaqMan master mix, 1.25 μL of primer/probe, 1 μL of DNA, and 10.25 μL of H2O, using the Rotor-Gene Q System (Qiagen). The following conditions were applied: 95°C for 10 minutes, then 45 cycles at 92°C for 15 seconds, and 60°C for 90 seconds.
Quantitative real-time polymerase chain reaction of microRNA-182
Following the manufacturer’s instructions, total RNA extraction was performed using the miRNeasy extraction kit (Qiagen, Valencia, CA). For gene expression assay, reverse transcription (RT) and PCR steps were carried out in the reaction well using the miScript II RT kit, miR-182-miScript primer assay (Catalog number # 218300, Qiagen), and the Maxima SYBR Green PCR kit (Qiagen, Valencia, CA) as instructed by the manufacturer. The cycle threshold method 2−ΔΔCt 16 was used to assay the relative miR-182 expression levels normalized to miRNA SNORD 68 as an internal reference.
Statistical analysis
Data were analyzed with SPSS (statistical package for the social sciences) version 22 for statistical analysis using descriptive statistics. The normality assumptions were verified for each variable by the Shapiro test. Group means were compared via 1-way analysis of variance with post hoc Tukey multiple range tests for pair-wise comparisons. The chi-square test was used to compare GAS rs145204276 (ins/del) frequency using the Hardy-Weinberg equation, and P < .05 was regarded as statistically significant. Using a receiver operating characteristic (ROC) curve, the diagnostic efficacy of serum miR-182 was visualized.
Results
The clinical and histopathologic data of the study participants are displayed in Table 1. The ages of the study’s participants ranged from 39 to 67. Breast cancer patients and healthy controls did not exhibit any age-related differences (P > .05). As shown in Table 1, we observed that 73% of the tumor size less than 5 cm, 79.10% of the patients displayed negative estrogen receptor (ER)/progesterone receptor (PR) status, and 28.70% of the breast patients exhibited M1 metastasis.
Table 1.
Baseline characteristics of the patients and the healthy control.
| Features | Healthy control (n = 80) | FA (n = 40) | BC (n = 115) | P | |
|---|---|---|---|---|---|
| Age (mean ± SD) | 51.26 ± 7.53 ab | 48.55 ± 6.66 a | 51.74 ± 7.65 b | .064 | |
| Menstrual status | Premenopausal | 48 (60%) | 34 (85%) | 48 (41.70%) | <.05 |
| Postmenopausal | 32 (40%) | 6 (15%) | 67 (58.30%) | ||
| Family history for BC | Yes | — | 11 (27.5%) | 68 (59.10%) | <.05 |
| No | 80 (100%) | 29 (72.5%) | 47 (40.90%) | ||
| Diabetes mellitus | Yes | — | 40 (100%) | 26 (22.60%) | <.05 |
| No | 80 (100%) | — | 89 (77.40%) | ||
| Tumor size | ⩽5 cm | — | — | 84 (73%) | N/A |
| >5 cm | — | — | 31 (27%) | ||
| ER/PR status | −ve | — | — | 91 (79.10%) | N/A |
| +ve | — | — | 24 (20.90%) | ||
| Metastasis | M0 | — | — | 82 (71.30%) | N/A |
| M1 | — | — | 33 (28.70%) | ||
Abbreviations: −ve, negative; +ve, positive; BC, breast cancer; ER, estrogen receptor; FA, fibroadenoma; N/A, not applicable; PR, progesterone receptor; SD, standard deviation.
Significance at a P < .05.
Groups with different letters show significant difference, and those with similar letters show no significant difference.
On comparing the distribution of genotypic frequencies for GAS5 lncRNA genotypes of study participants (Table 2), a statistically significant difference was found (P = .04) concerning the GAS5 polymorphism. The distribution of the genotype ins/ins, del/ins, and del/del were 47%, 51.30%, and 1.70% and 42.50%, 46.30%, and 11.30% in the BC patients and healthy controls, respectively. In addition, BC patients with an ER/PR-positive status, the ins/ins genotype was more prevalent (Table 3).
Table 2.
Genotype frequency of GAS rs145204276 gene SNP among breast cancer patient participants.
| Genotype | Healthy control (n = 80) | FA (n = 40) | BC (n = 115) | ||
|---|---|---|---|---|---|
| Frequency (%) | Frequency (%) | P | Frequency (%) | P | |
| ins/ins | 34 (42.50) | 21 (52.50) | 1 | 54 (47) | 1 |
| ins/del | 37 (46.30) | 18 (45) | .55 | 59 (51.30) | .989 |
| del/del | 9 (11.30) | 1 (2.50) | .116 | 2 (1.70) | .015 |
Abbreviations: BC, breast cancer; FA, fibroadenoma.
Results are expressed as number and in parentheses percentage. P < .05 is considered statistically significant. Chi-squared (χ2) test was performed to compare categorical data.
Table 3.
Genotype frequency of GAS rs145204276 gene SNP among breast cancer patients.
| Genotype | ER/PR-negative | ER/PR-positive | |
|---|---|---|---|
| Frequency (%) | Frequency (%) | P | |
| ins/ins | 36 (39.60) | 18 (75) | 1 |
| ins/del | 53 (58.20) | 6 (25) | .004 |
| del/del | 2 (2.20) | — | — |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
P value of difference in genotypes between ER/PR-negative subgroup and ER/PR-positive subgroup of BC patients group. Results are expressed as number and in parentheses percentage. P < .05 is considered statistically significant. Chi-squared (χ2) test was performed to compare categorical data.
Table 4 shows the levels of miR-182, TGF-β, and SIRT-1 expression in the serum of all recruited participants. Compared with matched healthy women, BC patients had a higher mean in concentration levels of serum TGF-β and SIRT-1 (Table 4). In addition, serum miR-182 expression level showed a noticeably (P < .05) upregulation in the BC patients versus the healthy participants. Moreover, miR-182, TGF-β, and SIRT-1 expression demonstrated a noticeable elevation in its differential expression in BC with M1 metastasis participants (Table 5).
Table 4.
Expression levels of SIRT-1, TGF-β, and miR-182 among healthy, FA, and BC study participants.
| Parameters | Healthy control | FA | BC | ||
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | P | Mean ± SEM | P | |
| SIRT-1 (ng/mL) | 4.27 ± 0.066a | 4.66 ± 0.105b | .007 | 8.22 ± 0.080c | .001 |
| TGF-β (ng/mL) | 1.74 ± 0.034a | 6.26 ± 0.474b | .001 | 10.46 ± 0.352c | .001 |
| miR-182 | 0.97 ± 0.006a | 1.31 ± 0.034a | .556 | 6.13 ± 0.40b | .001 |
Abbreviations: BC, breast cancer; FA, fibroadenoma; miR-182, microRNA-182; SIRT-1, sirtuin 1; TGF-β, transforming growth factor-beta.
Data were expressed as mean ± SEM (standard error mean); according to the Tukey multiple range test, the different letters indicate statistical significance different means.
Table 5.
Expression levels of SIRT-1, TGF-β, and miR-182 among BC study participants.
| Study participants | SIRT-1 (ng/mL) | TGF-β (ng/mL) | miR-182 |
|---|---|---|---|
| BC (M0) | 8.09 ± 0.084 | 10.22 ± 0.417 | 5.25 ± 0.515 |
| BC (M1) | 8.54 ± 0.168 | 10.55 ± 0.663 | 8.32 ± 0.249 |
| F value | 6.869 | 0.188 | 13.702 |
| P | <.05 | >.05 | <.001 |
Abbreviations: BC, breast cancer; miR-182, microRNA-182; SIRT-1, sirtuin 1; TGF-β, transforming growth factor-beta.
Data were expressed as mean ± SEM.
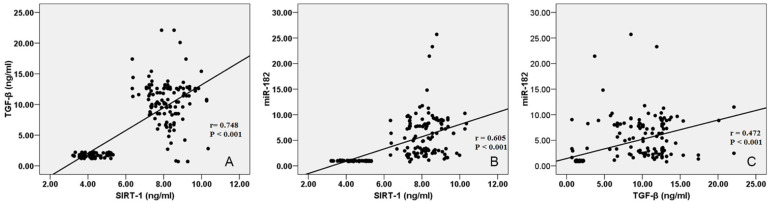
The concentration expression level of TGF-β and concentration levels of SIRT-1 revealed a strong positive correlation among BC patients (Figure 1A). In addition, a positive relationship between the SIRT-1 and the miR-182 differential expression was also noticed (Figure 1B). Moreover, a positive relationship between the TGF-β and the miR-182 differential expression was also noticed (Figure 1C).
Figure 1.
(A) Correlations between TGF-β and SIRT-1 expression levels of study participants. (B) Correlations between miR-182 and SIRT-1 expression levels of study participants. (C) Correlations between miR-182 and TGF-β expression levels of study participants. miR-182 indicates microRNA-182; SIRT-1, sirtuin 1; TGF-β, transforming growth factor-beta.
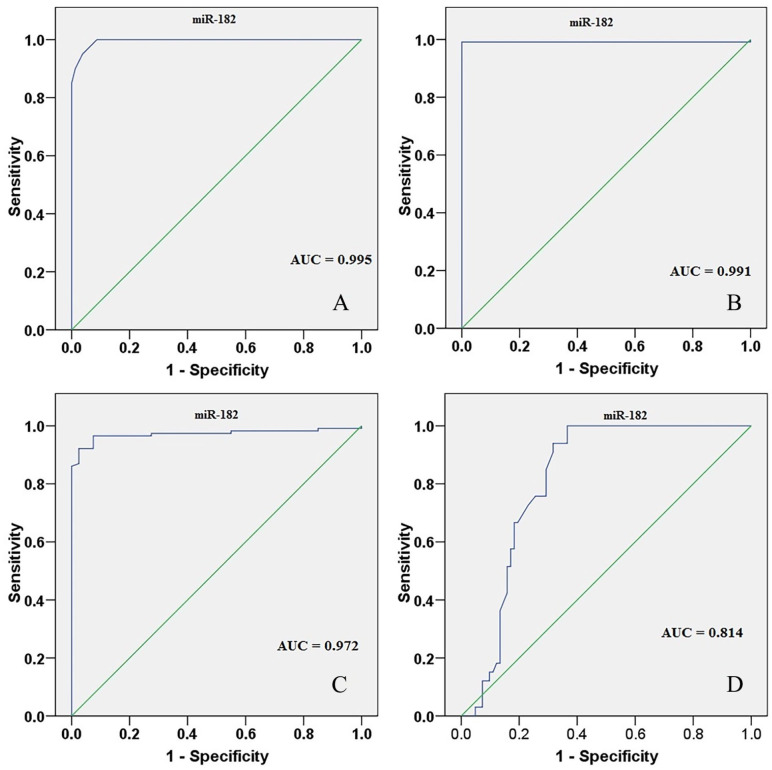
A ROC curve revealed that miR-182 distinguishes between participants with FA and healthy controls with 95% sensitivity and 96.25% specificity, in addition, differentiates BC patients from healthy controls with a sensitivity and specificity of 99.13% and 100%, respectively (Figure 2A and B). Serum miR-182 also separated FA participants from BC patients, the area under the curve (AUC) = 0.972, and 95% confidence interval (CI) = 0.947-0.998 with a sensitivity and specificity of 97.17% and 92.17%, respectively (Figure 2C). Breast cancer patients with M1 metastasis were distinguished from those with M0 metastasis by serum miR-182, AUC = 0.814, 95% CI = 0.737-0.890, a sensitivity and specificity of 63.41% and 100%, respectively (Figure 2D).
Figure 2.
(A) ROC analysis regarding miR-182 between FA and healthy controls. (B) ROC analysis regarding serum miR-182 BC and healthy controls. (C) ROC analysis regarding serum miR-182 FA participants from BC patients. (D) ROC analysis regarding serum miR-182 BC patients with M1 metastasis from M0 BC patients. AUC indicates area under the curve; FA, fibroadenoma; BC, breast cancer; miR-182, microRNA-182; ROC, receiver operating characteristic.
Discussion
According to published studies, the incidence rate of BC varies significantly due to the change in risk factors, which includes both modifiable and nonmodifiable factors, making the development and identification of biomarkers or approaches for early detection of malignancy look very promising.17,18
Anomalies in lncRNA levels can serve as crucial cancer-specific diagnostic indicators as lncRNAs have emerged as important regulators of cellular functions.19,20 The contradictory and divergent findings in the literature pose a considerable obstacle to practical use. Given the previous observations, our study aimed to explore the relationship between rs145204276 in the promoter of lncRNA GAS5 and BC risk. Moreover, our study aimed to assess the expression of miR-182 and its diagnostic value in patients with BC and correlates its expression level with the SIRT-1 and TGF-β levels.
We predicted that rs145204276 may be able to predict the risk of BC in this investigation. This idea was supported by our findings. We identified a link between the ins/ins genotype of rs145204276 and a greater risk of BC. Furthermore, in the current investigation, a highly significant association was observed between GAS5 rs145204276 and BC as evidenced by significantly higher ins/ins genotype distribution and ins allele frequency in BC patients compared with normal control subjects. These results imply the potential involvement of the ins allele of GAS5 rs145204276 SNP as a risk factor for BC.
The promoter region GAS5 rs145204276 may influence the methylation status at the seventh CpG site, which in turn may impact GAS5 expression. 21 Tang et al 22 demonstrated that the deletion allele in GAS5 rs145204276 may protect against the tendency for BC via binding to transcriptional factor specificity protein 1, which in turn increased GAS5 expression. Furthermore, Xiang et al 23 showed that patients with renal cell carcinoma had a statistically reduced prevalence of the genotype del/del in GAS5 rs145204276. According to Li et al, 24 both the genotypes ins/del and del/del showed a functional role in susceptibility to colorectal cancer.
Class III histone deacetylase (SIRT-1) plays a role in several biological processes, including DNA repair, cancer, and cell survival and proliferation. 10 This study demonstrated that serum SIRT-1 levels in BC patients were considerably higher than in healthy subjects. Multiple human cancers, including prostate cancer, colon cancer, and hepatocellular carcinoma, have been linked to increased SIRT-1 expression. 25 Our findings on elevated expression levels of SIRT-1 in BC patients are consistent with the work by Sung et al. 26 In addition, BC tissues exhibit significant SIRT-1 expression, as noted by Jin et al, 27 and Kuo et al. 28
Upregulation of SIRT-1 leads to deacetylated p53 inactivation, which makes it possible for cells to grow in the presence of DNA damage. 9 As a result, mutations build up, including those in p53 itself, which impair cell cycle regulation and hasten tumor progression.29,30 Despite the debate around SIRT-1’s role in carcinogenesis, Lin and Fang 31 noted that SIRT-1 plays a consistent role in mediating cancer cell survival.
Regarding the relative expression of miR-182, current research has shown that miR-182 plays a critical regulatory role and functions as an oncogene in several malignancies. 32 It has been widely noted as one of the most highly upregulated miRNAs in tumor tissues that promote the development and spread of BC, lung cancer, glioma, melanoma, and ovarian cancer tumors. 33 According to the findings of our study, there is a considerable increase in the expression of miR-182 and TGF-β in BC patients, and the M1 subgroup of BC patients experienced the highest expression in miR-182 and TGF-β levels. Recent research has shown that persistent TGF-β expression in BC is primarily linked to advanced stages of the disease and is a risk factor for substantial metastases. 34
The miR-182 expression was shown to be upregulated in BC patients, as previously reported, along with SIRT-1 and TGF-β expression increase. The SIRT-1/TGF-β axis is thought to be one of the crucial regulators of the genes involved in the development of cancer and metastasis. In addition, our results were consistent with the work by Zhao et al 35 and Farcas et al. 36
To explore the correlation underlying the signaling efficacy of SIRT-1/TGF-β and its modulatory effect on the miR-182 expression. Herein, Pearson’s statistical analysis revealed a noticeable positive correlation between the signaling of SIRT-1 and the differential expression of miR-182 and TGF-β. The miR-182 diagnostic performance was constructed using ROC curve analysis, which had a 95% CI: 0.989 to 1.002, P < .001, 95% sensitivity, and 96.25% specificity in the discrimination of patients with BC from study participants. In concordance with our results, Wang et al, 37 have identified miR-182 expression as a discriminator biomarker between BC and healthy controls, providing a potential diagnostic candidate. As far as we know, this is the first investigation pointed to the relationship between rs145204276 lncRNA GAS5 and BC risk with the differential expression of miR-182, and their correlation with the SIRT-1/TGF-β expression. However, the small sample size and lack of information on the status of Her2/Neu can be considered the main limitation of this study due to this study having no financial support and not being funded. Consequently, further large-scale clinical studies are required to clarify the relationship between miR-182, SIRT-1, and TGF-β with the SNP of lncRNA GAS5 in clinical trials. There are highlights that much work was still needed to clarify the connection between chemotherapy and ER/PR and human epidermal growth factor receptor-2 (HER2) status. In future work, we look forward to illuminating the HER2 status and its impact on noncoding expression and crosstalk in BC prognosis.
Conclusions
The study highlighted the susceptibility of BC was associated with the frequency of the ins/ins genotype of the lncRNA GAS5 polymorphism. Moreover, our study clarified the crucial functions of SIRT-1 and TGF-β as viable candidates for therapeutic targets for BC and demonstrated the potential diagnostic use of miR-182 as a biomarker for BC.
Footnotes
Author Contributions: OGS and NAH contributed to the study’s conception and design. GA, OZ, and ME contributed to material preparation, data collection, and analysis. All authors read and approved the final article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate: This study complied with the Declaration of Helsinki, and the BUC-Institutional Ethical Committee provided its approval (grant no. BUC-IACUC-230122-13). All participants in the study provided written informed consent to participate.
ORCID iD: Nabil A Hasona  https://orcid.org/0000-0003-1034-0458
https://orcid.org/0000-0003-1034-0458
References
- 1. Harbeck N, Cortes J, Gnant M, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:1-31. [DOI] [PubMed] [Google Scholar]
- 2. Abdelaziz AH, Shawki MA, Shaaban AM, et al. Breast cancer awareness among Egyptian women and the impact of caring for patients with breast cancer on family caregivers’ knowledge and behavior. Res Oncol. 2021;17:1-8. [Google Scholar]
- 3. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer. 2019;11:151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hettiarachchi G, Komar AA. GWAS to identify SNPs associated with common diseases and individual risk: Genome Wide Association Studies (GWAS) to identify SNPs associated with common diseases and individual risk. In: Sauna ZE, Kimchi-Sarfaty C, eds. Single Nucleotide Polymorphisms. Cham, Switzerland: Springer; 2022:51-76. [Google Scholar]
- 5. Zhou Z, Chen J, Huang Y, Liu D, Chen S, Qin S. Long noncoding RNA GAS5: a new factor involved in bone diseases. Front Cell Dev Biol. 2022;9:807419. doi: 10.3389/fcell.2021.807419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaur J, Salehen N, Norazit A, et al. Tumor suppressive effects of GAS5 in cancer cells. Noncoding RNA. 2022;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsieh MH, Lu HJ, Lin CW, et al. Genetic variants of lncRNA GAS5 are associated with the clinicopathologic development of oral cancer. J Pers Med. 2021;11:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsao TCY, Huang YW, Lin JC, Lee CY, Hsieh MJ, Yang SF. Impact of LncRNA GAS5 genetic variants and the epidermal growth factor receptor phenotypes on the clinicopathological characteristics of lung adenocarcinoma patients. Int J Environ Res Public Health. 2022;19:9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alves-Fernandes DK, Jasiulionis MG. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int J Mol Sci. 2019;20:3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onyiba CI, Scarlett CJ, Weidenhofer J. The mechanistic roles of sirtuins in breast and prostate cancer. Cancers. 2022;14:5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang M, Zhang YY, Chen Y, Wang J, Wang Q, Lu H. TGF-β signaling and resistance to cancer therapy. Front Cell Dev Biol. 2021;9:786728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spitschak A, Meier C, Kowtharapu B, Engelmann D, Pützer BM. MiR-182 promotes cancer invasion by linking RET oncogene activated NF-κB to loss of the HES1/Notch1 regulatory circuit. Mol Cancer. 2017;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang W, Yin Y, Bi L, et al. MiR-182-5p promotes the metastasis and epithelial-mesenchymal transition in non-small cell lung cancer by targeting EPAS1. J Cancer. 2021;12:7120-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massague J, Andres J, Boyd FT, Cheifertz S. Mediators of TGF-β actions, TGF-β receptors, and TGF-β binding proteoglycans. Ann NY Acad Sci. 1990;593:59-72. [DOI] [PubMed] [Google Scholar]
- 15. Khalil EH, Shaker OG, Hasona NA. lncRNA H-19 and miR-200a implication and frequency of lncRNA H-19 rs2170425 SNP in ulcerative colitis and Crohn’s disease. Comp Clin Pathol. 2023;32:565-571. [Google Scholar]
- 16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2021;25:402-408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 17. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers. 2021;13:4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdel-Samed SA, Hozyen WG, Shaaban SM, Hasona NA. Biochemical significance of miR-155 and miR-375 as diagnostic biomarkers and their correlation with the NF-κβ/TNF-α axis in breast cancer (published online ahead of print November 27, 2022). Ind J Clin Biochem. doi: 10.1007/s12291-022-01101-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khalil EH, Shaker OG, Hasona NA. Impact of rs2107425 polymorphism and expression of lncH19 and miR-200a on the susceptibility of colorectal cancer. Ind J Clin Biochem. 2023;38:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdel Hameed NA, Shaker OG, Hasona NA. Significance of LINC00641 and miR-378 as a potential biomarker for colorectal cancer. Comp Clin Pathol. 2022;31:807-814. [Google Scholar]
- 21. Lu S, Su Z, Fu W, Cui Z, Jiang X, Tai S. Altered expression of long non-coding RNA GAS5 in digestive tumors. Biosci Rep. 2019;39:BSR20180789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang Y, Wang Y, Wang X, Liu Y, Zheng K. A genetic variant of rs145204276 in the promoter region of long noncoding RNA GAS5 is associated with a reduced risk of breast cancer. Clin Breast Cancer. 2019;19:e415-e421. [DOI] [PubMed] [Google Scholar]
- 23. Xiang X, Chen L, He J, Ma G, Li Y. LncRNA GAS5 rs145204276 polymorphism reduces renal cell carcinoma susceptibility in Southern Chinese population. J Inflamm Res. 2022;15:1147-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C, Feng L, Niu L, et al. An insertion/deletion polymorphism within the promoter of EGLN2 is associated with susceptibility to colorectal cancer. Int J Biol Markers. 2017;32:274-277. [DOI] [PubMed] [Google Scholar]
- 25. Yousafzai NA, Jin H, Ullah M, Wang X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am J Cancer Res. 2021;11:5233-5248. [PMC free article] [PubMed] [Google Scholar]
- 26. Sung JY, Kim R, Kim JE, Lee J. Balance between SIRT1 and DBC1 expression is lost in breast cancer. Cancer Sci. 2010;101:1738-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin X, Wei Y, Xu F, et al. SIRT1 promotes formation of breast cancer through modulating Akt activity. J Cancer. 2018;9:2012-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuo S, Lin H, Chien S, Chen D. SIRT1 suppresses breast cancer growth through downregulation of the Bcl-2 protein. Oncol Rep. 2013;30:125-130. [DOI] [PubMed] [Google Scholar]
- 29. Feroz W, Sheikh AMA. Exploring the multiple roles of guardian of the genome: P53. Egypt J Med Hum Genet. 2020;21:49. [Google Scholar]
- 30. Marei HE, Althani A, Afifi N, et al. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021;21:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin Z, Fang D. The roles of SIRT1 in cancer. Genes Cancer. 2013;4:97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu X, Wang W, Wu G, Peng C, Liu J. miR-182-5p serves as an oncogene in lung adenocarcinoma through binding to STARD13. Comput Math Methods Med. 2021;2021:7074343. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Li G, Li M, Hu J, et al. The microRNA-182-PDK4 axis regulates lung tumorigenesis by modulating pyruvate dehydrogenase and lipogenesis. Oncogene. 2017;36:989-998. [DOI] [PubMed] [Google Scholar]
- 34. Suriyamurthy S, Baker D, Ten Dijke P, Iyengar PV. Epigenetic reprogramming of TGF-β signaling in breast cancer. Cancers. 2019;11:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao E, Hou J, Ke X, et al. The roles of sirtuin family proteins in cancer progression. Cancers. 2019;11:1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farcas M, Gavrea AA, Gulei D, et al. SIRT1 in the development and treatment of hepatocellular carcinoma. Front Nutr. 2019;6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang PY, Gong HT, Li BF, et al. Higher expression of circulating miR-182 as a novel biomarker for breast cancer. Oncol Lett. 2013;6:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]