Abstract
We have investigated changes in gene expression in mouse peritoneal macrophages following infection with virulent Mycobacterium tuberculosis. Using differential-display reverse transcription-PCR (RT-PCR), we have identified a gene that was markedly down-regulated within 6 h of infection and remained so for the duration of the experiment (5 days). On sequencing, this gene was found to encode the murine cytochrome c oxidase subunit VIIc (COX VIIc). Down-regulation of COX VIIc during M. tuberculosis infection was confirmed by three independent techniques: limiting-dilution RT-PCR, RNase protection assay, and Northern analysis. Limiting-dilution RT-PCR and Northern analysis were also used to analyze the specificity of this regulation; heat-killed M. tuberculosis, Mycobacterium bovis BCG, and latex beads had no effect on expression of COX VIIc. Down-regulation of this enzyme was also confirmed by using adherent cells isolated from spleens of M. tuberculosis-infected mice. These ex vivo macrophages showed apoptotic features, suggesting a possible involvement of cytochrome c oxidase in the programmed cell death of the host cells.
Pathogenic mycobacteria such as Mycobacterium tuberculosis and Mycobacterium leprae can survive and grow within host cells, particularly macrophages. In addition to these pathogenic species, the genus Mycobacterium includes many nonpathogenic species and also species which are pathogenic under certain circumstances, such as in immunocompromised hosts. The ability to withstand the hostile macrophage environment is crucial to mycobacterial pathogenicity; for example, M. tuberculosis is capable of remaining dormant for many years within alveolar macrophages but may start to divide when conditions are suitable. In addition, the initial response to the macrophage at the site of invasion is likely to play a key role in determining the outcome of the interaction and the overall regulation of the ensuing acquired response.
The ability to survive within macrophages is likely to be multifactorial. The organisms themselves have evolved mechanisms for surviving exposure to antimicrobial agents produced by activated macrophages. For example, the leprosy bacillus produces a specific phenolic glycolipid capsule which can protect it against reactive oxygen intermediates (8). Mycobacteria have also evolved mechanisms for regulating their own environment within a host cell. For example, the mycobacterial phagosomal environment does not become acidic due to exclusion of the vesicular proton-ATPase (31), and mycobacterial components are able to down-regulate the induction of an immune response by macrophages (21, 23). Thus, the interaction between the host cell and the bacterium represents a balance between antimicrobial activity of the macrophage and evasion mechanisms of the mycobacterium. The demonstration of trafficking of mycobacterial constituents, particularly lipoarabinomannan, a molecule with diverse regulatory activities on host cells, within macrophages (34) emphasizes the cross-talk which can occur between the host cell and the intracellular parasite.
In order to gain additional insights into the mycobacterium-macrophage interaction, we have investigated changes in macrophage gene expression following invasion by and growth of M. tuberculosis. Using differential-display reverse transcription-PCR (DD RT-PCR), we have identified one particular macrophage gene which is rapidly and persistently down-regulated during infection and have demonstrated that this change in expression is specific to infection with M. tuberculosis. Furthermore, expression of this gene was down-regulated in adherent cells isolated from the spleens of M. tuberculosis-infected mice. Such cells showed phenotypic and transcriptional changes which were consistent with programmed cell death, suggesting that down-regulation of the enzyme could be an early event in the commitment to apoptosis.
(Part of this work was published in the proceedings of the 13th European Immunology Meeting [23a].)
MATERIALS AND METHODS
Bacterial culture.
M. tuberculosis H37Rv was grown in Middlebrook medium, and stock cultures of mid-log-phase bacilli were stored in 1-ml aliquots of Dulbecco modified Eagle medium (DMEM) containing 20% heat-inactivated fetal calf serum (FCS) (Advanced Protein Products, Brierly Hill, United Kingdom) in liquid nitrogen. Viable counts of the stock cultures were determined by performing 10-fold serial dilutions in saline and plating onto Middlebrook agar medium. Plates were incubated at 37°C for 3 weeks, and CFU were counted. Mycobacterium bovis BCG was obtained as a lyophilized suspension from Evans Medical Ltd. (Langhurst, England).
Macrophage culture and infection.
Peritoneal macrophages were collected from 6- to 8-week-old female BALB/c mice and cultured in DMEM (Flow Laboratories, High Wycombe, United Kingdom) plus 20% FCS without addition of antibiotics. The cells were aliquoted into six-well culture plates (Nunc, Roskilde, Denmark) at a concentration of approximately 106 cells per well. After 2 days, the medium was replaced with medium containing approximately 106 CFU of live M. tuberculosis (strain H37Rv) or equivalent concentrations of heat-killed (85°C for 30 min) M. tuberculosis, live BCG, or 6.4-μm-diameter latex beads (Sigma). Ziehl-Neelsen staining was carried out 24 h later to confirm that the M. tuberculosis had been phagocytosed.
DD RT-PCR.
Total RNA was extracted from noninfected and infected macrophages at 6 h and 5 days after addition of M. tuberculosis, as described previously (33). To minimize the risk of amplification of contaminating DNA, total RNAs were digested with 10 U of DNase I (Boehringer Mannheim, Lewes, United Kingdom) for 15 min at 37°C in accordance with the manufacturer’s instructions, followed by phenol-chloroform extraction and reprecipitation in 2 volumes of 100% ethanol and 0.5 volume of 3 M sodium acetate (33). cDNAs for DD RT-PCR were synthesized from 300 ng of total RNA by using 1 μM oligo(dT12)CV anchored primers (DD1, T12CA; DD2, T12CG; and DD3, T12CC), where V may be A, G, or C, at 65°C for 10 min. The RT mix, consisting of 1× buffer, 200 U of Moloney murine leukemia virus reverse transcriptase (Life Technology, Paisley, United Kingdom), 20 μM deoxynucleoside triphosphates (dNTP) (Pharmacia Biotech, Herts, United Kingdom), and 10 mM dithiothreitol (Life Technology), was added, and the reaction mixture was incubated at 37°C for 1 h.
PCR (40 cycles of 94°C for 30 s, 40°C for 2 min, and 72°C for 30 s and 1 cycle at 72°C for 5 min) was carried out in an Omnigene thermocycler (Hybaid, Middlesex, United Kingdom) with 1 μM anchored primers (DD1, DD2, and DD3), 0.2 μM random 10-mer primers (OPA 14, TCT GTG CTG G; OPA 18, AGG TGA CCG T; and OPA 20, GTT GCG ATC C), 2 μM dNTP, 1× PCR buffer I (1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl [pH 8.3], 0.001% gelatin), 1 U of Taq polymerase (Amplitaq; Perkin-Elmer, London, United Kingdom), and 0.37 MBq of α-35S-dATP (Amersham, Buckingamshire, United Kingdom). DD RT-PCR products were electrophoresed on a denaturing 6% polyacrylamide gel with urea and visualized by autoradiography with X-ray film exposed overnight.
Reamplification of DD RT-PCR bands.
Differentially expressed bands were cut out from the dried gels and eluted in water. Samples were boiled for 15 min and ethanol precipitated. One microliter of isolated cDNAs was PCR reamplified (40 cycles of 94°C for 30 s, 42°C for 1 min, and 72°C for 30 s and 1 cycle at 72°C for 5 min) with the same anchored and random primers used for DD RT-PCR but under the following conditions: 2 μM anchored primers, 0.5 μM random 10-mer primers, 20 μM dNTP, 4 mM MgCl2, 1× PCR buffer II (50 mM KCl, 10 mM Tris-HCl [pH 8.3]), 2.5 U of Taq polymerase (Amplitaq; Perkin-Elmer), and sterile distilled water to 40 μl.
Cloning of reamplified bands.
Fresh PCR products of isolated DNA bands were ligated into pCRII or pCR2.1 and transformed by using One Shot Escherichia coli cells from a TA cloning kit (InVitrogen, Abingdon, United Kingdom) according to the manufacturer’s instructions. White colonies on Luria agar containing 50 μg of ampicillin per ml and 40 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) per ml were screened for the presence of the appropriate insert by restriction enzyme digestion and by PCR.
DNA sequencing.
Recombinant plasmids were purified by using the Wizard Miniprep DNA purification system (Promega, Southampton, United Kingdom), and sequencing of double-stranded plasmid DNA was carried out with T7 and SP6/M13 primers by the dideoxy termination procedure (26) with a Sequenase 2.0 kit (Amersham) or an ABI-PRISM DNA sequencing kit (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom).
RPA.
To carry out RNase protection assays (RPAs), isolated cDNAs were reamplified by using 35-mer synthetic primers consisting of the appropriate anchored primer linked to the T7 RNA polymerase binding site (5′-TAA TAC GAC TCA CTA TAG GG-DD1, -DD2, or -DD3). PCR solutions consisted of 0.2 μM T7-anchored primers, 0.2 μM random 10-mer primers, 50 μM dNTP, 2.5 mM MgCl2, 1× PCR buffer II, 2.5 U of Taq polymerase (Amplitaq; Perkin-Elmer), and sterile distilled water to 50 μl. The PCR was carried out under the following conditions: 40 cycles of 94°C for 30 s, 42°C for 1 min, and 72°C for 30 s and 1 cycle at 72°C for 5 min.
In vitro transcription was carried out with 2 μl of T7-amplified PCR product in accordance with the manufacturer’s instruction (RiboprobeR In Vitro Transcription Systems; Promega). The riboprobe was loaded onto a 6% polyacrylamide gel. After visualization on an X-ray film, the probe was excised from the dried gel and eluted overnight at 37°C in 400 μl of elution buffer (0.5 M ammonium acetate, 1 mM EDTA, 0.2% sodium dodecyl sulfate [SDS]). After precipitation with 100% ethanol, the riboprobe was resuspended in 20 μl of diethylpyrocarbonate water. Hybridization was carried out with 2 × 105 cpm of the riboprobe with 2 μg of total RNA at 50°C overnight in hybridization buffer {80% formamide, 40 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] [pH 6.7], 0.4 M NaCl, 1 mM EDTA}. The hybrid (riboprobe and target total RNA) was digested with a cocktail of 1 μg of RNase A and 20 U of RNase T1 (Ambion, Oxon, United Kingdom) for 30 min at 37°C in digestion buffer (10 mM Tris-HCl [pH 7.5], 5 mM EDTA, 200 mM sodium acetate). The reaction was stopped by adding 400 μl of phenol-chloroform. Samples were resuspended in gel loading buffer (80% deionized formamide, 1 mM EDTA, 0.1% bromophenol blue, 0.1% xylene cyanol, 0.1% SDS), electrophoresed with a 6% polyacrylamide gel, and exposed overnight at −70°C.
RT-PCR.
PCR was carried out on cDNAs from 1 μg of total RNA by using specific primers for cytochrome c oxidase subunit VIIc (COX VIIc) (forward, 5′-CCA GAG TAT CCG GAG GTT CA; reverse, 5′-GAA AGG TGC GGC AAA CC; expected product size, 151 bp), β-actin (forward, 5′-ATG GAT GAC GAT ATC GCT; reverse, 5′-ATG AGG TAG TCT GTC AGG T; expected product size, 540 bp), COX I (forward, 5′-TCG CAG TCA TAG CCA CAG CAT TTA; reverse, 5′-GGG TGG GGT GTT TAG TGG ATT AGC; expected product size, 437 bp), bcl-2 (forward, 5′-TCG CTA CCG TCG TGA CTT; reverse, 5′-CAT TCA ACC AGA CAT GCA CC; expected product size, 291 bp); bax (forward, 5′-GAG CAG CCG CCC CAG GAT G; reverse, 5′-GGT GAG CGA GGC GGT GAG GAC; expected product, 417 bp). The PCR mixture contained 5 μl of 10× PCR buffer, 5 μl of 25 mM MgCl2, 1 μl of 10 mM dNTP, 0.5 μl of 20 μM primers, 0.5 μl of Taq polymerase (5 U/μl), and 5 μl of cDNA. Samples were electrophoresed on a 2% agarose gel and visualized with ethidium bromide. For limiting-dilution PCR, cDNAs were serially fourfold diluted.
Northern blot hybridization.
Ten micrograms of total RNA per lane was electrophoresed on a 1% denaturing formaldehyde-agarose gel. The RNAs were transferred to a nylon membrane (Hybond N+; Amersham) by capillary elution overnight. The probes for Northern blot hybridizations were the RT-PCR products prepared as described above and labelled with an oligolabelling kit according to the instructions of the manufacturer (Pharmacia Biotech). Unincorporated nucleotides were removed by using a Chroma Spin-100 column (Clontech, Cambridge, United Kingdom), and 106 cpm of probe per ml and 1 mg of salmon sperm DNA (Sigma) were added to the hybridization solution. The membranes were prehybridized for 1 h in QuikHyb Solution (Stratagene, Cambridge, United Kingdom) at 65°C in a Mini 10 hybridization oven (Hybaid). Hybridization was carried out at 65°C for 1 h. Filters were washed twice at room temperature in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS and once at 60°C for 30 min with 0.1× SSC–0.1% SDS. The filters were exposed to an X-ray film with an intensifying screen at −70°C for 24 to 50 h. Filters were stripped of radioactivity by using boiled 0.1× SSC–0.1% SDS, and the RNA was reprobed with a labelled β-actin PCR product prepared as described above. Reprobed membranes were processed as described above. To determine quantitative differences between bands, filters were analyzed by phosphorimaging (model 400S PhosphorImager; Molecular Dynamics, Sunnyvale, Calif.).
Preparation of adherent cells from spleen cells.
Locally bred athymic (nude) mice were infected by intraperitoneal injection with the virulent M. tuberculosis H37Rv or with saline as a control. Three weeks after infection, the animals were sacrificed, spleens were removed and either 5 × 107 single-cell suspensions in DMEM plus 20% FCS were plated into six-well culture plates or 5 × 106 single-cell suspensions were plated into four-well Permanox slides (LabTek Chamber Slide; Nunc, Naperville, Ill.). Nonadherent cells were removed after 3 days of culture, and adherent cells were subjected to RNA extraction and TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labelling) assay.
TUNEL assay.
Cells were fixed in 2% paraformaldehyde for 30 min. TUNEL staining was performed according to the instructions of the manufacturer (Oncor, Gaithersburg, Md.).
RESULTS
Differential display of mRNA expression in M. tuberculosis-infected macrophages.
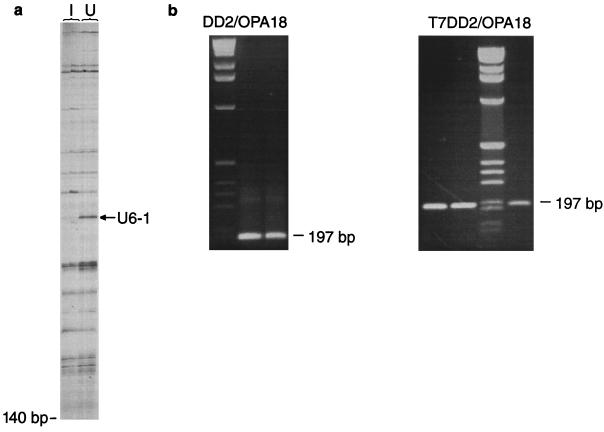
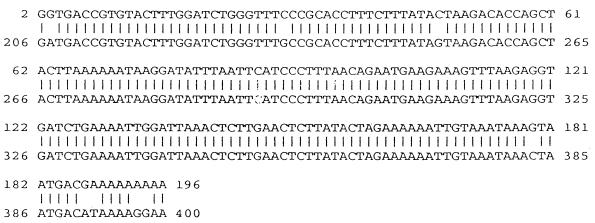
In order to identify genes which may be up- or down-regulated during infection of macrophages with M. tuberculosis, we used DD RT-PCR. Total RNA was prepared from uninfected and infected murine peritoneal macrophages and reverse transcribed into cDNAs. The resultant cDNAs were amplified with nine combinations of arbitrary and oligo(dT)-anchored primers, and the resultant products were visualized by electrophoresis and autoradiography. Products from infected and uninfected macrophages were compared at 6 h and 5 days after infection in order to identify genes which were differentially expressed during infection. Figure 1a shows a representative differential-display analysis in which M. tuberculosis-infected macrophages and uninfected control macrophages are compared at 5 days after infection. cDNAs were run in duplicate for every DD RT-PCR amplification. One band, designated U61, was found to be down-regulated rapidly (within 6 h of infection) and remained down-regulated throughout the infection. This differentially regulated band was eluted and reamplified (Fig. 1b) for cloning and sequencing. Sequencing revealed that band U61 showed a high degree of identity with the murine mitochondrial enzyme subunit COX VIIc (Fig. 2).
FIG. 1.
(a) Example of DD RT-PCR polyacrylamide gel analysis after overnight exposure. The arrow indicates band U61, which is down-regulated in M. tuberculosis-infected cells 5 days after infection. The primer combination in this case was DD2-OPA 18. Lanes: I, infected macrophages; U, uninfected macrophages. (b) Reamplification of U61. The left panel shows a 197-bp PCR product obtained following elution of the band and amplification with primers DD2 and OPA 18, run in duplicate on a 2% agarose gel. The right panel shows the same PCR product amplified with primers T7-DD2 and OPA 18; this product was used for the RPA.
FIG. 2.
Sequence alignment showing sequence identity (95%) between U61 and the murine COX VIIc (accession no. X52940).
Confirmation of down-regulation of COX VIIc following infection.
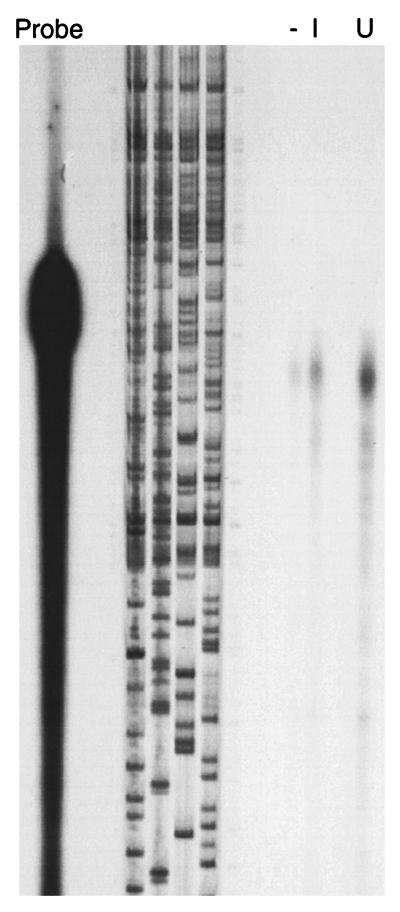
Since DD RT-PCR is known to produce a high rate of false-positive results (7), it was necessary to confirm differential expression of COX VIIc. We used an RPA to confirm mRNA levels in uninfected and infected macrophages. The results indicated that there was approximately a 50% decrease in mRNA in 5-day-infected macrophages compared to uninfected cells (Fig. 3).
FIG. 3.
RPA to confirm down-regulation of U61. The product obtained from the M. tuberculosis-infected macrophages (lane I) shows a reduction in intensity of approximately 50% in comparison with that from uninfected cells (lane U). Lane −, negative control consisting of the probe plus an irrelevant RNA.
Specificity of COX VIIc down-regulation determined by limiting-dilution RT-PCR and Northern analysis.
Because phagocytosis alone can lead to changes in the host cell, we investigated the extent to which the down-regulation of COX VIIc was a specific response to infection with M. tuberculosis. RNA from macrophage cultures infected with live, virulent M. tuberculosis (5 days) was compared to that from macrophages which had phagocytosed latex beads or heat-killed M. tuberculosis or had been infected with live BCG, using limiting-dilution RT-PCR and Northern hybridization.
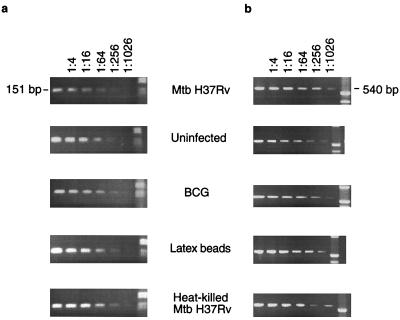
For limiting-dilution RT-PCR analysis, specific primers based on the published sequence for COX VIIc were used; these primers gave an expected product size of 151 bp. cDNAs from infected macrophages and control cells were diluted in serial fourfold steps prior to the PCR. The results (Fig. 4a) show that at a dilution of 1:256, the message for COX VIIc disappears from the M. tuberculosis-infected macrophages but is still detectable in uninfected and BCG infected cells and in cells which have phagocytosed latex particles or heat-killed M. tuberculosis. Similar experiments were carried out with primers for β-actin in order to confirm that the amounts of cDNAs used were similar and that the signal disappeared at the same dilution in each group (Fig. 4b).
FIG. 4.
Limiting-dilution RT-PCR analysis of cDNAs from uninfected, live M. tuberculosis (Mtb)-infected, and BCG-infected macrophages and from macrophages treated with latex beads and heat-killed M. tuberculosis 5 days after infection or phagocytosis. Specific primers for murine COX VIIc (a) or for beta-actin (as a loading control) (b) were used. PCR products were electrophoresed on a 2% agarose gel.
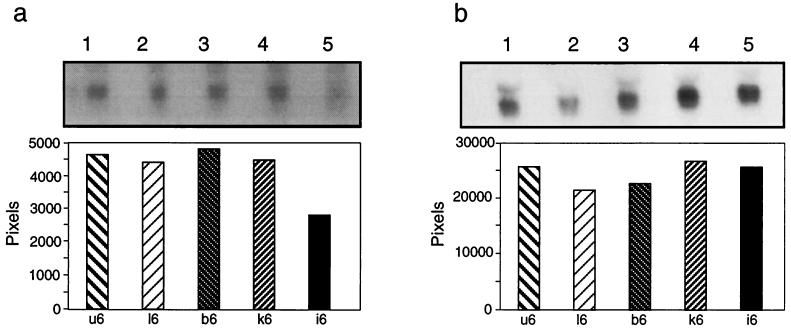
Further confirmation of the specificity of the down-regulation of COX VIIc was obtained by Northern analysis. The hybridization probe was a 32P-labelled COX VIIc cDNA product giving a transcript of approximately 0.4 kb. Figure 5a (lane 5) shows that COX VIIc was down-regulated only in live M. tubercuosis-infected macrophages. The radioactivity of the bands was integrated over selected areas of the scanned image (ImageQuant software). The histograms show quantitative differences between the amounts of hybridization products: M. tuberculosis-infected macrophages (Fig. 5a, lane 5) gave a value of 2,809 U, while killed M. tuberculosis-treated macrophages (lane 4) (4,450 U), BCG-infected macrophages (lane 3) (4,809 U), and macrophages treated with latex beads (lane 2) (4,249 U) showed no significant difference from the uninfected control cells (lane 1) (4,615 U). For loading controls, filters were stripped and reprobed with a labelled β-actin probe; Fig. 5b shows that the amounts of RNAs in all five lanes were equal. Thus, the 50% reduction in COX VIIc RNA demonstrated by the RPA was confirmed by Northern analysis, and this down-regulation appeared to be specific for macrophages infected with M. tuberculosis.
FIG. 5.
Northern analysis of RNAs from uninfected (lanes 1), live M. tuberculosis-infected (lanes 5), and BCG-infected (lanes 3) macrophages and from macrophages treated with latex beads (lanes 2) and heat-killed M. tuberculosis (lanes 4) 5 days after infection or phagocytosis. (a) Expression of COX VIIc; (b) expression of beta-actin. In each case the top panel shows an autoradiogram of the hybridization membrane, and the bottom panel shows quantification of the hybridization signal by phosphorimaging analysis.
Down-regulation of COX VIIc also occurs in splenic macrophages of mice infected with M. tuberculosis and is associated with apoptosis.
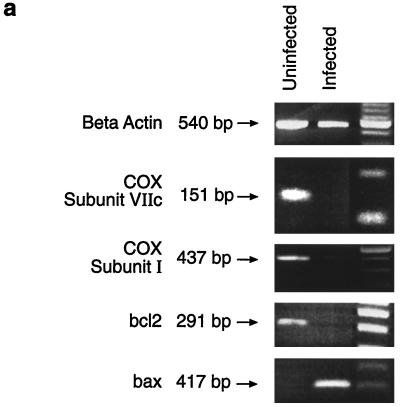
In order to investigate whether down-regulation of COX VIIc is a common feature of M. tuberculosis-infected cells, we used an ex vivo model of infection in which splenic macrophages from infected mice are cultured in vitro for 3 days. Such cells are 90% positive for the macrophage marker F4/80 by immunohistochemical staining (data not shown). RNA was extracted from these cells, and RT-PCR was carried out to detect COX VIIc mRNA. As can be seen in Fig. 6a, there was once again a clear down-regulation of COX VIIc mRNA in infected compared to uninfected cells. Because COX VIIc is a subunit of the holoenzyme COX, we also probed for changes in expression of COX I, which forms the core of the whole oxidase complex; again there was a clear down-regulation of mRNA (Fig. 6a).
FIG. 6.
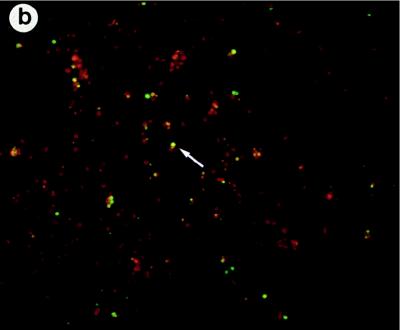
(a) RT-PCR analysis of cDNAs from adherent spleen cells of M. tuberculosis-infected mice and uninfected control mice. Specific primers for murine COX VIIc, COX I, Bcl2, and Bax or for beta-actin (as a loading control) were used. PCR products were electrophoresed on a 2% agarose gel. (b) TUNEL staining of adherent spleen cells from M. tuberculosis-infected mice. The arrow indicates apoptotic cells in green.
COX is associated with the membranes of mitochondria (10). Since perturbation of mitochondrial membranes is an early event in apoptosis (14, 16, 35) and since apoptosis of mycobacterium-infected cells has been reported (15, 20), we investigated whether changes in COX VIIc and COX I mRNAs could be correlated with changes in the expression of genes associated with apoptosis. We therefore compared expression of the gene encoding the prosurvival molecule Bcl-2 (16, 22, 30, 32, 35) with that of the gene encoding the proapoptotic protein Bax (22). As is evident from Fig. 6, there is a striking down-regulation of bcl-2 and a corresponding up-regulation of bax in the infected ex vivo cells. The fact that these ex vivo cells were apoptotic was confirmed by using a TUNEL assay to demonstrate the presence of cells undergoing programmed cell death (Fig. 6b).
DISCUSSION
In order to be able to survive and grow within macrophages, pathogenic bacteria, such as mycobacteria, have to avoid or resist exposure to the microbicidal potential of those cells. In order to investigate mechanisms by which this might be achieved, we have used DD RT-PCR to investigate the influence of phagocytosed M. tuberculosis on macrophage gene expression. DD RT-PCR allows identification of mRNAs that are uniquely expressed by a cell or tissue type, by cells at various stages of development, or by cells responding to different stimuli (2, 18, 19). The value of DD RT-PCR lies in the possibility of isolating and identifying new genes involved in a particular cellular response rather than monitoring expression of known genes. The nine combinations of arbitrary and anchored primers which were used in this study allow analysis of only a small fraction of the macrophage mRNA population to be investigated. Nevertheless, several differentially expressed mRNAs were identified. We were particularly interested in one of these, designated U61, because it was rapidly down-regulated following infection and remained so throughout the course of the infection. Sequencing of this band revealed it to encode COX VIIc; subsequent analysis of expression of the gene encoding COX VIIc, carried out by using probes and primers based on the COX VIIc sequence (rather than the U61, sequence), confirmed that this gene is down-regulated.
Three techniques, i.e., RPA, limiting-dilution RT-PCR, and Northern analysis, were used to confirm and quantify the degree of down-regulation of the COX VIIc gene and to investigate the specificity of the response. These three techniques were all used with independently generated material, and hence the findings were confirmed by using the three different techniques. The results indicate that there is approximately a 50% reduction in COX VIIc mRNA in macrophages infected with live M. tuberculosis compared to macrophages infected with BCG, untreated macrophages, or macrophages which had phagocytosed latex particles or heat-killed M. tuberculosis. Since M. tuberculosis and BCG are closely related organisms, this implies that down-regulation of COX VIIc is a very specific response and hence may be important in the infection process. The fact that both COX VIIc and COX I are significantly down-regulated in ex vivo cells cultured from M. tuberculosis-infected mice (Fig. 6a) suggests that this response is genuinely associated with the infection process.
COX is the terminal enzyme of the electron transport chain (10). It catalyzes the reduction of oxygen to water. The electron donor is cytochrome c, and the reaction results in translocation of protons across the organelle membrane (6). COX is located in the mitochondrial membrane, and some of its subunits are encoded in mitochondrial DNA, while others are encoded in the nucleus. The subunit COX VIIc is encoded in the nucleus (11, 27), and the protein is translocated to the mitochondria, where it is incorporated into the membrane-bound COX complex. Several possible physiological effects of down-regulation of COX VIIc by M. tuberculosis might be envisaged. First, it could reflect a general interference with oxidative metabolism and in particular the generation of reactive oxygen species. Reactive oxygen species are known to be important components of the antimicrobial defense system of macrophages, and M. tuberculosis is thought to inhibit the macrophage oxidative burst (5, 24, 28) as a survival mechanism; this could be achieved by transcriptional regulation of host cell genes involved in oxidative metabolism. Alternatively, the response could reflect the importance of apoptosis in the macrophage-mycobacterium interaction. A number of intracellular pathogens have been shown to induce apoptosis in infected host cells (1, 9, 20, 38), and this has been shown to be the case for macrophages infected with mycobacteria (15, 20). Perturbation of the mitochondrial outer membrane is a key feature of apoptotic death (14, 17, 35, 36). Since COX is a mitochondrial membrane-embedded protein, down-regulation of COX could represent either an early apopotic signalling event or a downstream consequence of apoptotic signalling.
In order to investigate the association between changes in expression of COX-encoding genes and genes known to be involved in apoptosis, we used the ex vivo model to look at changes in expression of bcl-2 and bax. The bcl-2 oncogene promotes hemopoietic survival and cooperates with c-myc to immortalize cells (32); thus, up-regulation of bcl-2 is associated with cell survival (35). Bax, on the other hand is a proapoptotic protein; up-regulation of bax is associated with accelerated programmed cell death (22). In our ex vivo model system the down-regulation of bcl-2 and up-regulation of bax (Fig. 6a) indicate that the host cells were committed to apoptosis, a fact confirmed by TUNEL staining. Proapoptotic changes in expression of bcl-2 and bax, along with the down-regulation of the genes encoding COX I and COX VIIc, were also shown to occur within 6 h of infection of peritoneal macrophages in vitro, before apoptotic cells could be detected by TUNEL staining (data not shown); this suggests that changes in COX gene expression are part of an early commitment to programmed cell death.
The regulation of cytokine-encoding genes in macrophages infected with mycobacteria has been widely investigated (3, 4, 12, 13, 24, 25, 29, 37). In this study we demonstrate that mycobacterium-macrophage interactions are likely to involve the cross-regulation of many genes other than those encoding cytokines. Of course, many of these changes are likely to be the result of phagocytosis or nonspecific interactions between intracellular bacteria and macrophages. However, the demonstration of at least one gene which is transcriptionally regulated in a manner which is relatively specific to infection with M. tuberculosis emphasizes that both the pathogen and the host have evolved specific mechanisms for coping with the interaction. Understanding of these mechanisms should shed new light on the pathogenesis of tuberculosis.
ACKNOWLEDGMENT
I.E.-G. was supported by a fellowship from the EDD/COFFA/SNI.
REFERENCES
- 1.Baran J, Guzik K, Hryniewicz W, Ernst M, Flad H-D, Pryjma J. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect Immun. 1996;64:4242–4248. doi: 10.1128/iai.64.10.4242-4248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer D, Muller H, Reich J, Riedel H, Arenkiel V, Warthoe P, Strauss M. Identification of differentially expressed mRNA species by an improved display technique (DDRT-PCR) Nucleic Acids Res. 1993;21:4272–4280. doi: 10.1093/nar/21.18.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradbury M G, Moreno C. Effect of lipoarabinomannan and mycobacteria on tumour necrosis factor production by different populations of murine macrophages. Clin Exp Immunol. 1993;94:57–63. doi: 10.1111/j.1365-2249.1993.tb05977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown M C, Taffet S M. Lipoarabinomanans derived from different strains of Mycobacterium tuberculosis:differentially stimulate the activation of NF-κB and KBF1 in murine macrophages. Infect Immun. 1995;63:1960–1968. doi: 10.1128/iai.63.5.1960-1968.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brozna J P, Horan M, Rademacher J M, Pabst K M, Pabst M J. Monocyte responses to sulfatide from Mycobacterium tuberculosis:: inhibition of priming for enhanced release of superoxide, associated with increased secretion of interleukin-1 and tumor necrosis factor alpha, and altered protein phosphorylation. Infect Immun. 1991;59:2542–2548. doi: 10.1128/iai.59.8.2542-2548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunori M, Wilson M T. Electron transfer and proton pumping in cytochrome oxidase. Biochimie. 1995;77:668–676. doi: 10.1016/0300-9084(96)88182-x. [DOI] [PubMed] [Google Scholar]
- 7.Callard D, Lescure B, Mazzolini L. A method for the elimination of false positives generated by the mRNA differential display techniques. BioTechniques. 1994;16:1096. [PubMed] [Google Scholar]
- 8.Chan J, Fujiwara T, Brennan P, McNeil M, Turco S J, Sibille J C, Snapper M, Aisen P, Bloom B R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proct Natl Acad Sci USA. 1989;86:2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper C E, Nicholls P, Freedman J A. Cytochrome c oxidase: structure, function, and membrane topology of the polypeptide subunits. Biochem Cell Biol. 1990;69:586–607. doi: 10.1139/o91-089. [DOI] [PubMed] [Google Scholar]
- 11.Cumsky M G, McEwen J E, Ko C, Poyton R O. Nuclear genes for mitochondrial proteins. J Biol Chem. 1983;258:13418–13421. [PubMed] [Google Scholar]
- 12.Dahl K E, Shiratsuchi H, Hamilton B D, Ellner J J, Toossi Z. Selective induction of transforming growth factor β in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis: Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedland J S, Shattock R J, Griffin G E. Phagocytosis of Mycobacterium tuberculosis or particulate stimuli by human monocytic cells induces equivalent monocyte chemotactic protein-1 gene expression. Cytokine. 1993;5:150–156. doi: 10.1016/1043-4666(93)90054-9. [DOI] [PubMed] [Google Scholar]
- 14.Henkart P A, Grinstein S. Apoptosis: mitochondria resurected. J Exp Med. 1996;183:1293–1295. doi: 10.1084/jem.183.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klingler K, Tchou-Wong K-M, Brandli O, Aston C, Kim R, Chi C, Rom M N. Effects of mycobacteria on regulation of apoptosis in mononuclear phagocytes. Infect Immun. 1997;65:5272–5278. doi: 10.1128/iai.65.12.5272-5278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Zamzami N, Susin S A. Mitochondrial control of apoptosis. Immunol Today. 1997;1:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 18.Liang P, Averboukh L, Pardee A B. Distribution and cloning of eukaroytic mRNAs by means of differential display: refinements and optimisation. Nucleic Acids Res. 1993;21:3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang P, Pardee A B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 20.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus calmette-guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno C, Mehlert A, Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988;74:206–210. [PMC free article] [PubMed] [Google Scholar]
- 22.Oltavi Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 23.Pabst M J, Gross J M, Bronzna J P, Goren M B. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988;140:634–640. [PubMed] [Google Scholar]
- 23a.Ragno S, Estrada I, Butler R, Colston M J. Regulation of macrophage gene expression following invasion by Mycobacterium tuberculosis. Immunol Lett. 1997;57:143–146. doi: 10.1016/s0165-2478(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez G M L, Rom W N, Ciotoli C, Talbot A, Martinuik F, Cronstein B, Reibman J. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect Immun. 1994;62:2515–2520. doi: 10.1128/iai.62.6.2515-2520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roach T I A, Barton C H, Chatterjee D, Blackwell J M. Macrophage activation: lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, JE, and tumor necrosis factor. J Immunol. 1993;150:1886–1896. [PubMed] [Google Scholar]
- 26.Sanger F, Nicklen S, Coulsen A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schatz G, Mason T L. Biosynthesis of mitochondrial proteins. Annu Rev Biochem. 1974;43:51–87. [Google Scholar]
- 28.Schlesinger L S, Bellinger-Hawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 29.Schuller-Levis G B, Levis W R, Ammazzalorso M, Nosrati A, Park E. Mycobacterial lipoarabinomannan induces nitric oxide and tumor necrosis factor alpha production in a macrophage cell line: down regulation by taurine chloramine. Infect Immun. 1994;62:4671–4674. doi: 10.1128/iai.62.10.4671-4674.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda M, Tsujimoto Y. Bcl-2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene. 1996;13:21–29. [PubMed] [Google Scholar]
- 31.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 32.Vaux D L, Cory S, Adams J M. Bcl-2 oncogene promotes haemopoietic survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 33.Walker K B, Butler R, Colston M J. Role of Th-1 lymphocytes in the development of protective immunity against Mycobacterium leprae. J Immunol. 1992;148:1885–1889. [PubMed] [Google Scholar]
- 34.Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]
- 35.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 36.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom W N. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95:586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–168. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]