Abstract
Immune dysregulation has been found to be related to a diagnosis of autism spectrum disorder (ASD). However, investigations in very early childhood examining immunological abnormalities such as altered neonatal cytokine/chemokine profiles in association with an aberrant developmental trajectory, are sparse. We assessed neonatal blood spots from 398 children, including 171 with ASD, which were subdivided according to severity (121 severe, 50 mild/moderate) and cognitive/adaptive levels (144 low-functioning, 27 typical to high-functioning). The remainder were 69 children with developmental delay (DD), and 158 with typical development (TD), who served as controls in the Childhood Autism Risks from Genetics and the Environment (CHARGE) study. Exploratory analysis suggested that, in comparisons with TD and DD, CTACK (CCL27) and MPIF-1 (CCL23), respectively, were independently associated with ASD. Higher neonatal levels of CTACK were associated with decreased odds of ASD compared to TD (odds ratio [OR]= 0.40, 95% confidence interval [Cl] 0.21, 0.77), whereas higher levels of MPIF-1 were associated with increased odds of ASD (OR= 2.38, 95% Cl 1.42, 3.98) compared to DD but not to TD. MPIF-1 was positively associated with better scores in several developmental domains. Dysregulation of chemokine levels in early life can impede normal immune and neurobehavioral development, which can lead to diagnosis of ASD or DD. This study collectively suggests that certain peripheral chemokines at birth are associated with ASD progression during childhood and that children with ASD and DD have distinct neonatal chemokine profiles that can differentiate their diagnoses.
Keywords: Autism spectrum disorder, delayed development, neonatal blood spot, neonatal cytokines, neonatal chemokines, neurodevelopment
Introduction
Autism spectrum disorders (ASD) is composed of a group of complex neurodevelopmental disorders that are characterized clinically by deficits in communication, social interactions, and restricted or stereotyped behaviors (Baio et al., 2018). The latest reported prevalence is as high as one out of every 54 children in the U.S. (Maenner et al., 2020). While ASD can sometimes be reliably diagnosed by the age of two, the ability to accurately diagnose ASD depends on behavioral assessments and developmental history (Hyman et al., 2020; Lord et al., 2006). Biological signatures or biomarkers could provide a method for early ASD risk assessment, advancing the identification of at-risk children earlier than current behavioral methods (Frye et al., 2019). Effective biomarkers could also facilitate classification of disease by severity and behavior, increasing the efficacy of monitoring response to therapeutic intervention. As reviewed by Hughes et al., numerous findings have supported links of the child’s immune dysfunction with their ASD diagnosis, as well as with the immune profile of their mothers (Hughes et al., 2018). Cytokines and chemokines have the potential to serve as biomarker candidates, as alterations in their profile provide an overview of immune system status. Growing evidence demonstrates that unique cytokine/chemokine profiles in individuals with ASD can be associated with symptom severity, aberrant behaviors, and impaired cognitive/adaptive function (Ashwood et al., 2011; Han et al., 2017; Heuer et al., 2019; Krakowiak et al., 2017; Masi et al., 2017; Zerbo et al., 2014). However, the heterogeneity in individuals’ demographics, cytokine/chemokine profiles, and prior study designs are hindering the determination of immune biomarkers as predictors of ASD. For example, some studies have demonstrated increased plasma levels of monocyte chemoattractant protein-1 (MCP-1) and RANTES in ASD patients (Ashwood et al., 2011; Han et al., 2017), whereas others observed decreased levels of RANTES in the newborn blood samples of children with ASD (Zerbo et al., 2014) or found comparable levels of MCP-1 in newborn bloodspots from control and ASD groups (Heuer et al., 2019). In the neonatal blood spot study by Krakowiak et al., IL-1β was positively associated with mild to moderate ASD (Krakowiak et al., 2017); however, in the study by Masi et al., severe ASD was associated with decreased level of IL-1β in females only (Masi et al., 2017). Possible explanations for these differences range from phenotypic heterogeneity, a polygenic etiology, a difference in time from birth to sample collection, and advances in technology. Although identifying a consistent postnatal biomarker for ASD remains challenging, it is promising that research on peripheral or neonatal cytokines and chemokines as predictors of ASD is growing.
Previous findings by our group demonstrated that neonatal IL-1β and IL-4 are independently associated with ASD, using newborn blood spots archived by Childhood Autism Risks from Genetics and the Environment (CHARGE) (Krakowiak et al., 2017). Children with severe ASD were more likely to have elevated IL-4 levels, whereas mild to moderate ASD symptoms were associated with increased levels of IL-1β. IL-4 was also a marker for the differentiation between children with severe ASD and those with mild to moderate ASD. Both cytokines were correlated with behavioral and developmental scores (Krakowiak et al., 2017). As an extension of this previous work, the current study utilized an expanded sample set of archived dried neonatal blood spot samples, as well as a broader array of cytokines and chemokines to investigate cytokine/chemokine levels from children later diagnosed with ASD or with developmental delay (DD) without autism, compared to children with typical development (TD). We further subdivided ASD individuals into groups based on symptom severity and developmental and adaptive functions to identify predictors of ASD.
Methods and materials
Participants
Archived neonatal blood spot samples corresponding to 398 children who enrolled in the Childhood Autism Risks from Genetics and the Environment (CHARGE) study (Hertz-Picciotto et al., 2006) between April 2003 and May 2009 with confirmed diagnoses (171 with ASD, 69 with DD, and 158 with TD) were used for cytokine/chemokine analysis. The CHARGE study is a population-based case-control study investigating risk factors for neurodevelopmental disorders, with participants from three groups: children with ASD, DD, and general population controls with TD. Eligible children were 2–5 years old, born in California, lived with a biological parent who spoke English or Spanish, and resided in selected regional center catchment areas at the time of recruitment. Consent was acquired from parents prior to participation. The CHARGE Study protocol was approved by the institutional review boards (IRB) at the University of California, Davis and Los Angeles, as well as the State of California Committee for the Protection of Human Subjects.
Diagnostic Confirmation
Diagnostic confirmation of the children (2–5 years of age) was performed at the University of California, Davis, MIND (Medical Investigation of Neurodevelopmental Disorders) Institute. Cognitive and adaptive functions were evaluated with Mullen Scales of Early Learning (MSEL) and Vineland Adaptive Behavior Scales (VABS), respectively. The Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS) confirmed a diagnosis of ASD. The Social Communication Questionnaire was used to screen controls for ASD; children with scores ≥15 were evaluated with ADI-R and ADOS. Children with ASD (n=171) were subdivided according to 2-subgroups by severity and cognitive/adaptive functions. Using ADOS comparison scores and DSM-IV criteria, children showing a score of ≥7 were grouped into severe ASD symptoms (ASDsev [n=121]) and children exhibiting a score of <7 were categorized as mild/moderate symptoms (ASDmild [n=50]). Controls with TD had no prior diagnosis of ASD or DD, and their composite scores on MSEL and VABS were ≥70. Controls with DD had composite scores <70 on MSEL and/or VABS. Children with ASD who were within the typical cognitive and adaptive developmental range (both MSEL and VABS composite standard scores of ≥70) were grouped as typical to high-functioning ASD (ASDhi [n=27]). Those with cognitive and/or adaptive delays were grouped as low-functioning ASD (ASDlo [n=144]). Children who met the criteria for ASD were reclassified to the ASD group.
Behavioral and Developmental Assessments
Aberrant Behavior Checklist
Maladaptive behavior assessment was performed using the Aberrant Behavior Checklist (ABC) whose subscales included irritability (15 items), lethargy/social withdrawal (16 items), stereotypy (7 items), and hyperactivity (16 items). Each item was rated on a 4-point Likert scale ranging from 0 (not at all a problem) to 3 (problem severe in degree).
Mullen Scales of Early Learning
MSEL is a standardized assessment used to determine cognitive development in young children. The scales include visual reception (nonverbal cognitive ability), fine motor, receptive language (language comprehension), and expressive language (language production). For each scale and composite, developmental quotients were calculated (by age equivalent/chronological age × 100) to overcome the floor effect.
Vineland Adaptive Behavior Scales
VABS is a standardized assessment to determine the level of personal and social skills needed for everyday living. The domains are communication, daily living skills, socialization, and motor skills. Developmental quotients were calculated as described above.
Blood Spot Specimen collection
Capillary blood was collected within 48 h of birth by heel stick method and spotted onto standardized filter paper for testing of various disorders as part of the Genetic Disease Screening Program. The remaining blood spots were stored at −20°C by the California Department of Public Health (CDPH).
Blood Spot Elution
Three 3 mm punches of dried blood spot specimen were put into a single well in a 96-well plate and stored at −80°C until elution. In each well, 200 μl of elution buffer (phosphate-buffered saline, 0.5% bovine serum albumin, and protease inhibitors [Complete Protease Inhibitor Cocktail, Roche Diagnostics Corporation, Indianapolis, Indiana]) was added. Plates were placed on a plate shaker overnight at 4°C and the eluates analyzed immediately following elution.
Total protein concentration
Following elution, a small 4μl aliquot was used for bicinchoninic acid assay (BCA) (Thermo Scientific, Rockford, IL) to determine total protein and to normalize cytokine/chemokine levels against blood sample quantity variation (Heuer et al., 2019).
Cytokine and Chemokine Measurement
Blood spot cytokine and chemokine levels were measured using Bio-Plex Luminex (Bio-Rad, Hercules, CA) assays. Using a 40-plex chemokine panel, cytokines IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-16, TNF-α and chemokines IL-8, 6Ckine (CCL21), BCA-1 (CXCL13), CTACK (CCL27), ENA78 (CXCL5), eotaxin (CCL11), eotaxin-2 (CCL24), eotaxin-3 (CCL26), fractalkine (CX3CL2), GCP-2 (CXCL6), GM-CSF, Gro-α (CXCL1), Gro-β (CXCL2), I-309 (CCL1), IP-10 (CXCL10), I-TAC (CXCL11), MCP-1 (CCL2), MCP-2 (CCL8), MCP-3 (CCL7), MCP-4 (CCL13), MDC (CCL22), MIF, MIG (CXCL9), MIP-1α (CCL3), MIP-1δ (CCL15), MIP-3α (CCL20), MIP-3β (CCL19), MPIF-1 (CCL23), SCYB16 (CXCL16), SDF-1α/β (CXCL12), TARC (CCL17), and TECK (CCL25) were measured according to the manufacturer’s directions. IL-12p70 and IL-13 (Bio-Rad) were also included as individual analytes to expand the 40-plex panel. Briefly, the blood spot eluate (50 μl) was incubated with fluorescent-labeled capture antibody-coated beads in a 96-well plate (shaking) for 1 h at RT. The sample-bead mix was removed, washed, and biotinylated detection antibodies added for 30 min at RT with shaking. The reaction mixture was then incubated with streptavidin-phycoerythrin at RT for 10 min (shaking). After washing, the beads were resuspended in sheath fluid on the plate shaker for 5 min. The plates were read on a Bio-Plex 200 system (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed using Bio-Plex Manager software (Bio-Rad Laboratories). A five-parameter curve was used to calculate final concentrations (pg/ml). Reference samples were run on each plate for assay consistency. All samples were run blinded to child developmental outcome.
Statistical Analysis
Statistical analyses were performed on cytokine/chemokine levels in blood spot eluates from 398 children. Each immune marker was normalized for sampling variation in blood collection by dividing total protein content in the eluate (as determined by BCA assay). Cytokine/chemokine concentrations that fell below the minimum level of detection (MLD) were assigned a value of MLD/√2, and all cytokines/chemokines were natural log-transformed to normalize the distribution. GM-CSF, IL-12p70 and IL-13 levels were not detectable for >25% of the samples and were excluded from further analysis. All remaining cytokines/chemokines were above the MLD for ≥97% of the samples (Supplementary Table 1). Analyses were carried out using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
Primary analyses examined the associations between individual cytokine/chemokine levels and child diagnosis using multinomial logistic regression. Mild and severe ASD cases were also examined separately in relation to DD and TD groups. Covariates in initial model fitting included mother’s education attainment, gestational age, child’s sex, age (hours) at blood spot collection, and years between blood spot collection and elution. Akaike information criterion (AIC) was used to select best model fit (Vreugdenhil et al., 2018).
The cytokines/chemokines was also modeled together as predictors and adjusted for covariates in a series of binary models (outcomes were ASD vs. TD, ASD vs. DD, DD vs. TD, ASDsev vs. ASDmild , ASDsev vs. TD, ASDmild vs. TD, ASDsev vs. DD, ASDmild vs. DD, ASDhi vs. ASDlo, ASDhi vs. TD, ASDlo vs. TD, ASDhi vs. DD and ASDlo vs. DD) using the Least Absolute Shrinkage and Selection Operator (LASSO) variable selection method (Tibshirani, 1996) to identify a parsimonious subset of cytokines and chemokines that was most strongly associated with the child’s diagnosis. For example, if several cytokines were highly correlated with one another and associated with a particular diagnosis, LASSO selected one of those cytokines for inclusion in a model. Adjustments for multiple comparisons were not performed for these exploratory analyses and to preclude failing to detect immune markers that show promise in their ability to differentiate between diagnostic groups.
Secondary analyses were stratified by child’s diagnosis and examined associations between the LASSO-selected cytokines/chemokines and developmental/behavioral domains measured by ABC, MSEL and VABS.
Results
Participant demographics
TD controls were frequency-matched to cases with ASD in 4:1 male-to-female ratio to ensure similar proportions of male/female participants in both groups (Table 1). This was not the case for the DD group where 70% were male, with 30% were female. In terms of birth season, fewer children with ASD and DD were born in the spring compared to children with TD (18% and 17% vs. 29%). Birth year was statistically different between populations, with ASD participants weighted toward the later years in the study (2003–2006), while DD and TD participants were distributed more toward the middle of the study, peaking in 2003. The average gestational age for all three groups was similar (TD 39.3 weeks, ASD 39.6 weeks, DD 39.1 weeks). However, newborn age at the time of blood spot collection was statistically different among the three groups. The maternal education status of those in the DD group demonstrated fewer mothers with a bachelor’s degree compared to mothers of children with TD and those in the ASD group (35% vs. 56% and 50%). There were no differences in regional catchment areas among the three groups as the TD controls were frequency-matched to a projected distribution of ASD cases for the regional center catchment area. In addition, no differences were found between cases with ASD and controls with TD or DD in terms of race/ethnicity, years between blood spot collection and elution, maternal age at delivery, or maternal allergies and asthma. The cases and controls were evenly distributed across the assay plates such that even representation of each study population was run on each plate.
Table 1:
Participant demographic and clinical characteristics, N=398
| ASD (n=171) |
DD (n=69) |
TD (n=158) |
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P-valueg | |
| Sexa | 0.03 | ||||||
| Male | 144 | 84 | 48 | 70 | 128 | 81 | |
| Female | 27 | 16 | 21 | 30 | 30 | 19 | |
| Race/Ethnicity | 0.22 | ||||||
| White | 90 | 53 | 29 | 42 | 81 | 51 | |
| Hispanic | 53 | 31 | 28 | 41 | 42 | 27 | |
| Otherb | 28 | 16 | 12 | 17 | 35 | 22 | |
| Season of birthc | 0.33 | ||||||
| Winter | 45 | 26 | 19 | 28 | 37 | 23 | |
| Spring | 30 | 18 | 12 | 17 | 45 | 29 | |
| Summer | 47 | 27 | 18 | 26 | 35 | 22 | |
| Fall | 49 | 29 | 20 | 29 | 41 | 26 | |
| Birth year | 0.02 | ||||||
| 2000–2001 | 18 | 10 | 2 | 3 | 9 | 6 | |
| 2002 | 24 | 14 | 17 | 25 | 36 | 23 | |
| 2003 | 44 | 26 | 22 | 32 | 54 | 34 | |
| 2004 | 49 | 29 | 20 | 29 | 43 | 27 | |
| 2005–2006 | 36 | 21 | 8 | 11 | 16 | 10 | |
| Maternal education | 0.004 | ||||||
| High school or less | 25 | 14 | 22 | 32 | 22 | 14 | |
| Some college/Vocational degree | 61 | 36 | 23 | 33 | 48 | 30 | |
| Bachelor’s degree | 85 | 50 | 24 | 35 | 88 | 56 | |
| Maternal allergies or asthmad | 103 | 61 | 39 | 57 | 92 | 58 | 0.82 |
| Regional Center catchment areaa | 0.45 | ||||||
| Alta, Far Northern, and Redwood Coast | 71 | 42 | 31 | 45 | 70 | 44 | |
| North Bay | 21 | 12 | 6 | 9 | 19 | 12 | |
| East Bay, San Andreas, and Golden Gate | 44 | 26 | 13 | 19 | 44 | 28 | |
| Valley Mountain, Central Valley, and selected Southern CA regionsf | 35 | 20 | 19 | 27 | 25 | 16 | |
| Mean | SD | Mean | SD | Mean | SD | P-valueg | |
| Maternal age at delivery | 31.3 | 5.3 | 30.9 | 6.4 | 31.0 | 5.4 | 0.81 |
| Gestational age (weeks)e | 39.6 | 2.1 | 39.1 | 2.0 | 39.3 | 1.6 | 0.11 |
| Age (hours) at blood spot collection | 29.1 | 8.8 | 33.1 | 8.4 | 28.7 | 8.9 | 0.001 |
| Age (months) at study enrollment for childa | 44.0 | 9.5 | 46.5 | 8.7 | 42.8 | 10.0 | 0.03 |
| Years between collection and elution | 12.1 | 1.3 | 12.2 | 1.1 | 12.3 | 1.1 | 0.13 |
TD controls were frequency-matched to a projected distribution of ASD cases on age, sex, and regional center catchment area
Includes Black/African American, American Indian/Alaska Native, Asian, Pacific Islander/Native Hawaiian, Multi-racial
Months grouped by season as follows: Winter = December to February, Spring = March to May, Summer = June to August, Fall = September to November
1 participant (ASD) was missing maternal allergies/asthma; allergies include the following types: environmental (e.g., seasonal, pet, mold), food, skin, medication, or other
2 participants were missing gestational age (1 ASD, 1 TD)
Southern California regions include Los Angeles, Kern, Orange, San Diego, Tri-counties, and Inland
P-values for categorical and continuous variables calculated with Chi-square test and one-way analysis of variance (ANOVA), respectively
Associations between neonatal cytokine and chemokine levels and child diagnosis
Comparison in total groups: ASD vs. TD, DD vs. TD and ASD vs. DD
We conducted multinomial logistic regression models adjusted for maternal education attainment, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution to determine the associations between individual cytokines/chemokines and ASD, regardless of symptom severity, compared to DD and TD. Overall, children with ASD and DD had significantly lower neonatal concentrations of nearly all cytokines and chemokines, and none were significantly higher, compared with concentrations in TD children (Fig. 1A, Table 2A). No sex differences were observed in cytokine/chemokine levels.
Fig. 1. Adjusted odds ratio plot comparing neonatal cytokine and chemokine concentrations.
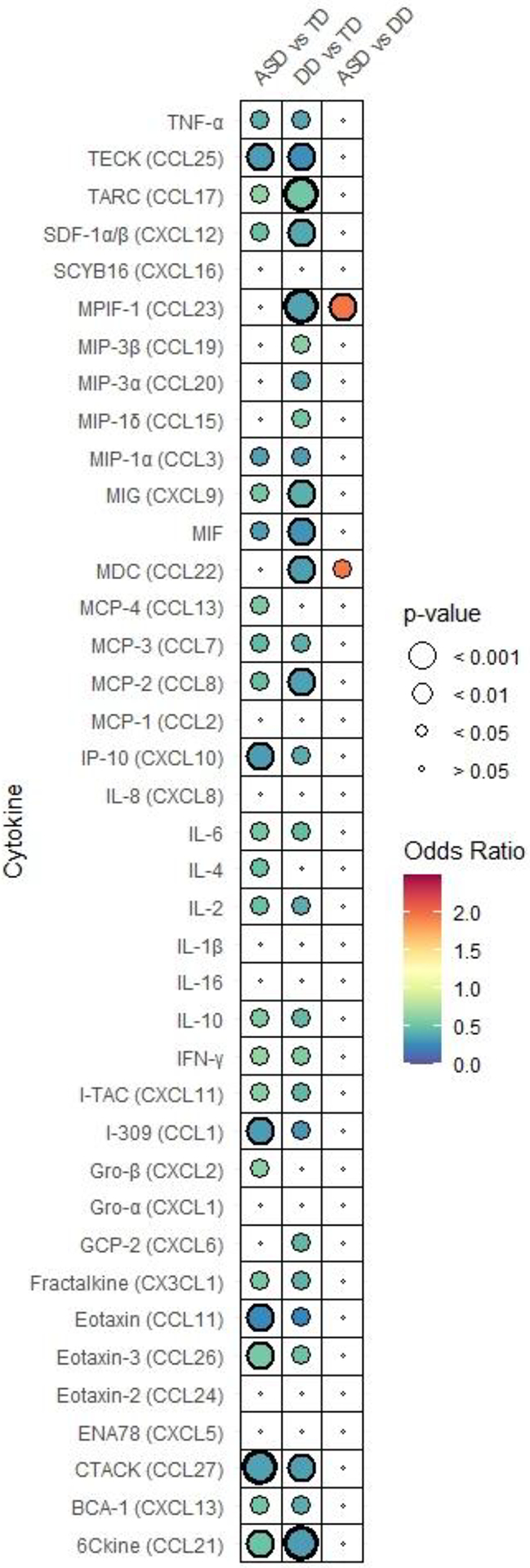
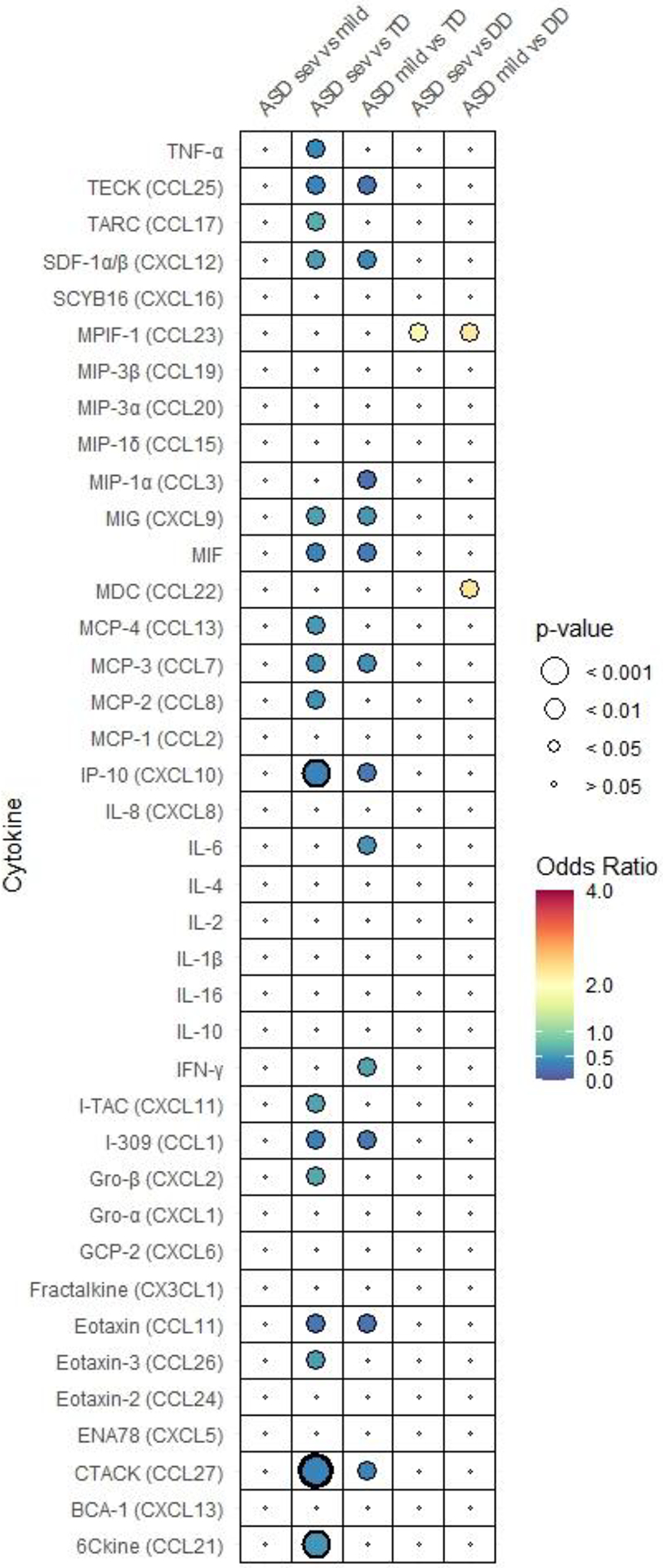
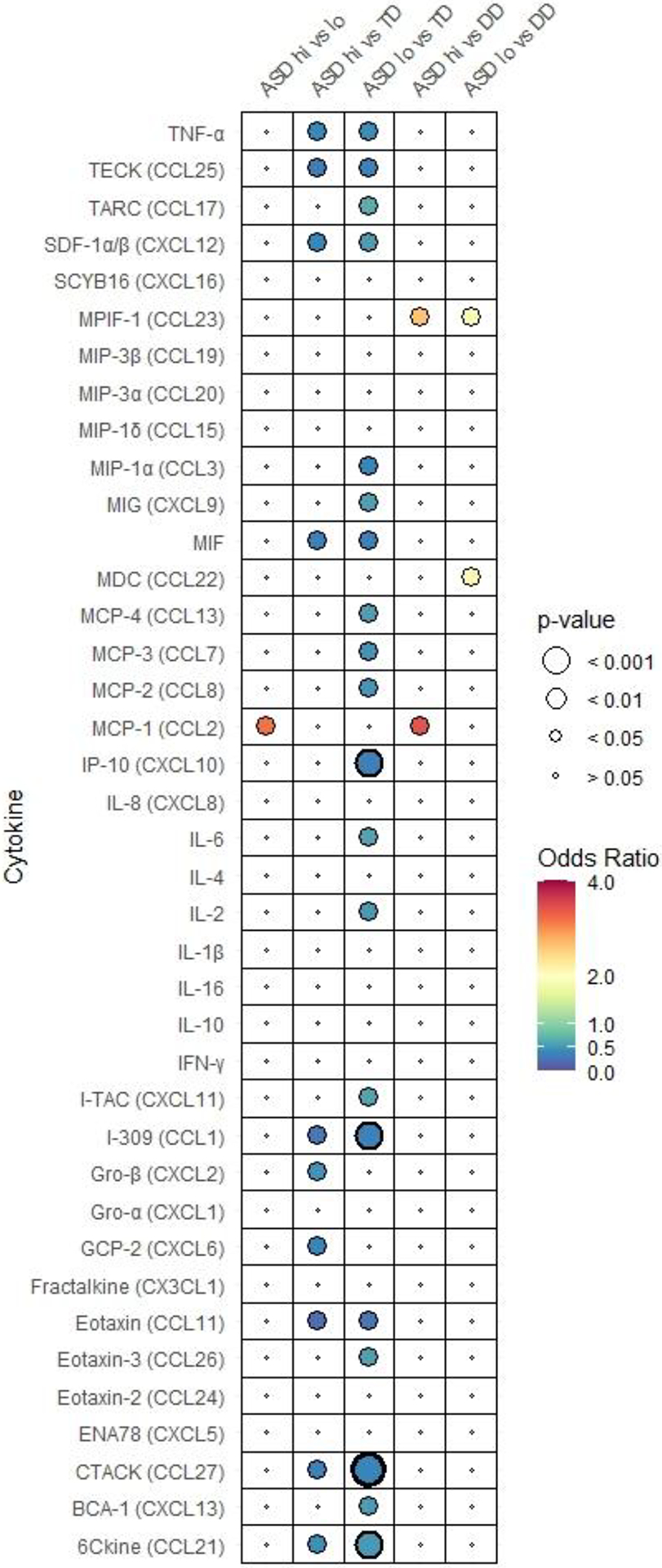
in (A) ASD, DD, and TD, (B) subgroups of ASD (ASDsev, ASDmild), DD, and TD, (C) subgroups of ASD (ASDhi, ASDlo) DD, and TD. Odds ratio is depicted by the heat map with highest ORs in red to lowest in blue. Each figure has its own heat map. Relative P-value is depicted by circle size. P-values that are below 0.001 are bolded.
Table 2A:
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD, DD, and TDa
| ASD vs. TD | DD vs. TD | ASD vs. DD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine/chemokine | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| 6Ckine (CCL21) | 0.51 | 0.33, 0.78 | 0.002 | 0.34 | 0.20, 0.59 | 0.0001 | 1.49 | 0.91, 2.44 | 0.12 |
| BCA-1 (CXCL13) | 0.54 | 0.31, 0.93 | 0.03 | 0.41 | 0.21, 0.82 | 0.01 | 1.30 | 0.68, 2.51 | 0.43 |
| CTACK (CCL27) | 0.36 | 0.21, 0.62 | 0.0002 | 0.35 | 0.18, 0.67 | 0.002 | 1.05 | 0.60, 1.81 | 0.88 |
| ENA78 (CXCL5) | 0.98 | 0.79, 1.21 | 0.84 | 0.82 | 0.64, 1.05 | 0.12 | 1.19 | 0.93, 1.52 | 0.16 |
| Eotaxin (CCL11) | 0.26 | 0.11, 0.63 | 0.003 | 0.25 | 0.09, 0.70 | 0.01 | 1.02 | 0.49, 2.12 | 0.95 |
| Eotaxin-2 (CCL24) | 0.88 | 0.69, 1.11 | 0.28 | 0.78 | 0.59, 1.04 | 0.09 | 1.13 | 0.86, 1.48 | 0.38 |
| Eotaxin-3 (CCL26) | 0.56 | 0.37, 0.83 | 0.005 | 0.51 | 0.31, 0.85 | 0.01 | 1.09 | 0.68, 1.75 | 0.71 |
| Fractalkine (CX3CL1) | 0.56 | 0.32, 0.99 | 0.04 | 0.44 | 0.22, 0.87 | 0.02 | 1.28 | 0.69, 2.35 | 0.43 |
| GCP-2 (CXCL6) | 0.60 | 0.34, 1.06 | 0.08 | 0.46 | 0.23, 0.93 | 0.03 | 1.25 | 0.69, 2.28 | 0.46 |
| Gro-α (CXCL1) | 0.66 | 0.38, 1.14 | 0.14 | 0.68 | 0.34, 1.36 | 0.27 | 0.97 | 0.50, 1.89 | 0.93 |
| Gro-β (CXCL2) | 0.63 | 0.42, 0.95 | 0.03 | 0.60 | 0.36, 1.01 | 0.05 | 1.04 | 0.65, 1.68 | 0.86 |
| I-309 (CCL1) | 0.34 | 0.17, 0.67 | 0.002 | 0.31 | 0.13, 0.72 | 0.01 | 1.11 | 0.49, 2.53 | 0.80 |
| IFN-γ | 0.67 | 0.46, 0.96 | 0.03 | 0.59 | 0.36, 0.95 | 0.03 | 1.14 | 0.71, 1.82 | 0.59 |
| IL-1β | 0.69 | 0.39, 1.23 | 0.21 | 0.50 | 0.25, 1.00 | 0.05 | 1.37 | 0.71, 2.65 | 0.34 |
| IL-2 | 0.51 | 0.28, 0.91 | 0.02 | 0.41 | 0.20, 0.84 | 0.01 | 1.25 | 0.64, 2.42 | 0.51 |
| IL-4 | 0.51 | 0.26, 0.99 | 0.048 | 0.45 | 0.20, 1.01 | 0.05 | 1.15 | 0.54, 2.45 | 0.72 |
| IL-6 | 0.56 | 0.34, 0.94 | 0.03 | 0.48 | 0.26, 0.90 | 0.02 | 1.17 | 0.67, 2.05 | 0.58 |
| IL-8 (CXCL8) | 0.91 | 0.59, 1.39 | 0.65 | 0.83 | 0.49, 1.40 | 0.48 | 1.10 | 0.64, 1.87 | 0.73 |
| IL-10 | 0.59 | 0.35, 0.99 | 0.046 | 0.47 | 0.26, 0.86 | 0.01 | 1.25 | 0.75, 2.10 | 0.39 |
| IL-16 | 0.74 | 0.40, 1.35 | 0.32 | 0.53 | 0.27, 1.01 | 0.07 | 1.38 | 0.77, 2.48 | 0.28 |
| IP-10 (CXCL10) | 0.34 | 0.18, 0.66 | 0.001 | 0.41 | 0.18, 0.92 | 0.03 | 0.84 | 0.39, 1.79 | 0.65 |
| I-TAC (CXCL11) | 0.62 | 0.41, 0.93 | 0.02 | 0.46 | 0.27, 0.79 | 0.01 | 1.33 | 0.80, 2.21 | 0.28 |
| MCP-1 (CCL2) | 0.88 | 0.58, 1.35 | 0.56 | 0.71 | 0.42, 1.21 | 0.20 | 1.22 | 0.73, 2.06 | 0.45 |
| MCP-2 (CCL8) | 0.49 | 0.28, 0.83 | 0.01 | 0.36 | 0.19, 0.71 | 0.003 | 1.33 | 0.75, 2.39 | 0.33 |
| MCP-3 (CCL7) | 0.47 | 0.27, 0.82 | 0.01 | 0.42 | 0.22, 0.80 | 0.01 | 1.13 | 0.66, 1.92 | 0.66 |
| MCP-4 (CCL13) | 0.57 | 0.36, 0.90 | 0.02 | 0.69 | 0.39, 1.23 | 0.21 | 0.82 | 0.48, 1.42 | 0.48 |
| MDC (CCL22) | 0.69 | 0.41, 1.14 | 0.15 | 0.35 | 0.19, 0.65 | 0.001 | 1.96 | 1.09, 3.53 | 0.03 |
| MIF | 0.35 | 0.16, 0.78 | 0.01 | 0.30 | 0.13, 0.69 | 0.005 | 1.19 | 0.79, 1.80 | 0.40 |
| MIG (CXCL9) | 0.55 | 0.35, 0.86 | 0.01 | 0.42 | 0.25, 0.73 | 0.002 | 1.30 | 0.80, 2.12 | 0.29 |
| MIP-1α (CCL3) | 0.36 | 0.15, 0.88 | 0.02 | 0.34 | 0.12, 0.99 | 0.047 | 1.04 | 0.38, 2.85 | 0.94 |
| MIP-1δ (CCL15) | 0.76 | 0.47, 1.23 | 0.27 | 0.54 | 0.31, 0.96 | 0.03 | 1.40 | 0.85, 2.30 | 0.19 |
| MIP-3α (CCL20) | 0.48 | 0.22, 1.09 | 0.08 | 0.39 | 0.16, 0.94 | 0.04 | 1.25 | 0.67, 2.37 | 0.48 |
| MIP-3β (CCL19) | 0.74 | 0.53, 1.03 | 0.07 | 0.61 | 0.41, 0.91 | 0.01 | 1.22 | 0.84, 1.75 | 0.29 |
| MPIF-1 (CCL23) | 0.72 | 0.49, 1.06 | 0.10 | 0.37 | 0.23, 0.59 | <.0001 | 1.97 | 1.26, 3.09 | 0.003 |
| SCYB16 (CXCL16) | 0.68 | 0.44, 1.04 | 0.07 | 0.69 | 0.41, 1.17 | 0.17 | 0.99 | 0.61, 1.61 | 0.96 |
| SDF-1α/β (CXCL12) | 0.51 | 0.31, 0.85 | 0.01 | 0.39 | 0.21, 0.71 | 0.002 | 1.31 | 0.80, 2.14 | 0.29 |
| TARC (CCL17) | 0.65 | 0.45, 0.93 | 0.02 | 0.54 | 0.34, 0.84 | 0.0004 | 1.21 | 0.80, 1.84 | 0.38 |
| TECK (CCL25) | 0.34 | 0.17, 0.71 | 0.004 | 0.28 | 0.12, 0.65 | 0.003 | 1.21 | 0.64, 2.28 | 0.56 |
| TNF-α | 0.43 | 0.22, 0.83 | 0.01 | 0.38 | 0.19, 0.79 | 0.01 | 1.12 | 0.64, 1.71 | 0.61 |
Multinomial logistic regression models were adjusted for maternal education attainment, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); OR represents the fold change in the odds of having one diagnosis relative to another diagnosis or no diagnosis for every 1-unit increase in the ln-transformed cytokine/chemokine (or for every e-fold increase in cytokine/chemokine levels); 398 participants comprised the following groups: 171 ASD, 69 DD and 158 TD; OR = adjusted odds ratio, CI = confidence interval, P = P-value
MDC (CCL22) and MPIF-1 (CCL23) emerged as the only chemokines whose concentrations at birth differed significantly between ASD and DD. Higher levels of MDC and MPIF-1 were each associated with a two-fold increased likelihood of having an ASD vs. DD diagnosis (MDC: OR=1.96, 95% Cl 1.09, 3.53; MPIF-1: OR=1.97, 95% Cl 1.26, 3.09) (Fig. 1A, Table 2A). MDC and MPIF-1 concentrations were also significantly higher in TD than DD but did not differentiate between ASD and TD.
Neonatal levels of Gro-β (CXCL2), IL-4 and MCP-4 (CCL13), were significantly decreased in ASD compared to TD but did not differ between DD and TD (Table 2A). Meanwhile, GCP-2 (CXCL6) was significantly decreased in DD compared to TD but not differ between ASD and TD (Table 2A). In addition, significant decreases in the levels of chemokines MIP-1δ (OR=0.54, 95% Cl 0.31, 0.96), MIP-3α (OR=0.39, 95% Cl 0.16, 0.94) and MIP-3β (OR=0.61, 95% Cl 0.41, 0.91) were noted only in DD compared to TD, while level of MIP-1α was significantly lower in both ASD vs TD (OR=0.36, 95% Cl 0.15, 0.88) and DD vs TD (OR=0.34, 95% Cl 0.12, 0.99) (Table 2A).
Comparison by symptom severity: ASDsev vs. ASDmild, ASDsev/ASDmild vs. TD and ASDsev/ASDmild vs. DD
We subdivided ASD cases into severe and mild/moderate groups based on symptom severity (ASDsev and ASDmild) to examine differences in ASD subgroups and to compare the subgroups with TD and DD groups in models adjusted for the same covariates as described earlier. None of the neonatal cytokines and chemokines differed significantly between ASDsev and ASDmild (Fig. 1B, Supplementary Table 2). Therefore, we observed similar trends in association between individual cytokine/chemokine levels and diagnosis for comparisons between subgroups ASDsev and ASDmild with TD and DD. Overall, regardless of the symptom severity, both ASD subgroups had lower level of cytokines and chemokines compared to TD (Table 2B-1). However, some cytokines/chemokines, namely, 6Ckine (CCL21), eotaxin-3 (CCL26), Gro-β (CXCL2), I-TAC (CXCL11), MCP-2 (CCL8; OR=0.47, 95% Cl 0.26), MCP-4 (CCL13), TARC (CCL17) and TNFα were significantly decreased in ASDsev compared to TD but did not differ when comparing ASDmild to TD (Table 2B-1). Meanwhile, IFN-γ, IL-6 and MIP-1α (CCL3) levels were significantly decreased in ASDmild compared to TD but did not differ between ASDsev and TD (Table 2B-1).
Table 2B-1:
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD (severe, mild to moderate symptoms) and TDa
| ASDsev vs. TD | ASDmild vs. TD | |||||
|---|---|---|---|---|---|---|
| Cytokine | OR | 95% CI | P | OR | 95% CI | P |
| 6Ckine (CCL21) | 0.49 | 0.30, 0.78 | 0.002 | 0.56 | 0.31, 1.02 | 0.06 |
| BCA-1 (CXCL13) | 0.56 | 0.30, 1.01 | 0.06 | 0.49 | 022, 1.09 | 0.08 |
| CTACK (CCL27) | 0.36 | 0.20, 0.63 | 0.0004 | 0.38 | 0.19, 0.79 | 0.01 |
| ENA78 (CXCL5) | 1.06 | 0.82, 1.36 | 0.67 | 0.86 | 0.65, 1.13 | 0.27 |
| Eotaxin (CCL11) | 0.26 | 0.10, 0.68 | 0.01 | 0.25 | 0.08, 0.74 | 0.01 |
| Eotaxin-2 (CCL24) | 0.88 | 0.68, 1.14 | 0.33 | 0.88 | 0.63, 1.22 | 0.44 |
| Eotaxin-3 (CCL26) | 0.55 | 0.35, 0.85 | 0.01 | 0.59 | 0.33, 1.04 | 0.07 |
| Fractalkine (CX3CL1) | 0.60 | 0.33, 1.11 | 0.10 | 0.49 | 0.23, 1.05 | 0.06 |
| GCP-2 (CXCL6) | 0.66 | 0.35, 1.23 | 0.19 | 0.51 | 0.24, 1.07 | 0.08 |
| Gro-α (CXCL1) | 0.68 | 0.37, 1.24 | 0.21 | 0.63 | 0.29, 1.33 | 0.22 |
| Gro-β (CXCL2) | 0.62 | 0.40, 0.97 | 0.04 | 0.66 | 0.37, 1.18 | 0.16 |
| I-309 (CCL1) | 0.36 | 0.17, 0.74 | 0.01 | 0.30 | 0.12, 0.80 | 0.02 |
| IFN-γ | 0.71 | 0.47, 1.05 | 0.09 | 0.58 | 0.34, 0.98 | 0.04 |
| IL-1β | 0.72 | 0.38, 1.36 | 0.31 | 0.63 | 0.28, 1.42 | 0.27 |
| IL-2 | 0.54 | 0.29, 1.02 | 0.06 | 0.45 | 0.20, 1.01 | 0.05 |
| IL-4 | 0.52 | 0.25, 1.07 | 0.08 | 0.49 | 0.19, 1.24 | 0.13 |
| IL-6 | 0.60 | 0.34, 1.05 | 0.07 | 0.49 | 0.24, 0.98 | 0.04 |
| IL-8 (CXCL8) | 0.87 | 0.54, 1.39 | 0.55 | 1.00 | 0.53, 1.86 | 0.99 |
| IL-10 | 0.59 | 0.34, 1.02 | 0.06 | 0.60 | 0.30, 1.21 | 0.16 |
| IL-16 | 0.72 | 0.37, 1.37 | 0.31 | 0.79 | 0.34, 1.86 | 0.59 |
| IP-10 (CXCL10) | 0.36 | 0.18, 0.74 | 0.005 | 0.30 | 0.12, 0.74 | 0.01 |
| I-TAC (CXCL11) | 0.58 | 0.36, 0.91 | 0.02 | 0.73 | 0.40, 1.34 | 0.31 |
| MCP-1 (CCL2) | 0.77 | 0.49, 1.23 | 0.27 | 1.25 | 0.64, 2.45 | 0.52 |
| MCP-2 (CCL8) | 0.47 | 0.26, 0.83 | 0.01 | 0.54 | 0.26, 1.13 | 0.10 |
| MCP-3 (CCL7) | 0.47 | 0.26, 0.85 | 0.01 | 0.46 | 0.22, 0.97 | 0.04 |
| MCP-4 (CCL13) | 0.52 | 0.31, 0.85 | 0.01 | 0.72 | 0.37, 1.37 | 0.31 |
| MDC (CCL22) | 0.64 | 0.37, 1.12 | 0.12 | 0.81 | 0.38, 1.69 | 0.57 |
| MIF | 0.37 | 0.16, 0.86 | 0.02 | 0.32 | 0.13, 0.77 | 0.01 |
| MIG (CXCL9) | 0.57 | 0.35, 0.93 | 0.02 | 0.51 | 0.28, 0.94 | 0.03 |
| MIP-1α (CCL3) | 0.42 | 0.16, 1.07 | 0.07 | 0.24 | 0.07, 0.85 | 0.03 |
| MIP-1δ (CCL15) | 0.76 | 0.45, 1.30 | 0.32 | 0.76 | 0.39, 1.48 | 0.42 |
| MIP-3α (CCL20) | 0.51 | 0.21, 1.20 | 0.12 | 0.44 | 0.16, 1.21 | 0.11 |
| MIP-3β (CCL19) | 0.74 | 0.52, 1.05 | 0.09 | 0.76 | 0.48, 1.19 | 0.23 |
| MPIF-1 (CCL23) | 0.69 | 0.45, 1.04 | 0.07 | 0.83 | 0.47, 1.45 | 0.51 |
| SCYB16 (CXCL16) | 0.64 | 0.41, 1.02 | 0.06 | 0.77 | 0.42, 1.42 | 0.41 |
| SDF-1α/β (CXCL12) | 0.55 | 0.32, 0.96 | 0.03 | 0.43 | 0.22, 0.85 | 0.01 |
| TARC (CCL17) | 0.65 | 0.44, 0.97 | 0.03 | 0.65 | 0.39, 1.08 | 0.10 |
| TECK (CCL25) | 0.38 | 0.17, 0.83 | 0.02 | 0.28 | 0.11, 0.70 | 0.01 |
| TNF-α | 0.42 | 0.21, 0.83 | 0.01 | 0.46 | 0.20, 1.06 | 0.07 |
Multinomial logistic regression models were adjusted for maternal education attainment, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); OR represents the fold change in the odds of having one diagnosis relative to another diagnosis or no diagnosis for every 1-unit increase in the ln-transformed cytokine/chemokine (or for every e-fold increase in cytokine/chemokine levels); 398 participants comprised the following groups: 121 ASD (severe), 50 ASD (mild), and 158 TD; ASD severity was defined using ADOS severity scores, where ≥7 indicated severe and <7 indicated mild to moderate symptoms; OR = adjusted odds ratio, CI = confidence interval, P = P-value
ASDsev vs ASDmild results are in the Supplementary Table 2.
In contrast, the levels of most cytokines and chemokines did not differ significantly when we compared the two subgroups of ASD with DD except for MDC (CCL22) and MPIF-1 (CCL23) (Table 2B-2). Higher levels of MPIF-1 were associated with a 1.9-fold higher likelihood of ASDsev (OR=1.87, 95% CI 1.16, 3.00) and a 2.3-fold higher likelihood of ASDmild relative to DD (OR=2.26, 95% CI 1.23, 4.16) (Table 2B-2). MDC was the only chemokine that differentiated between ASDsev and ASDmild compared to DD; higher neonatal levels of MDC were associated with a 2.3-fold higher likelihood of ASDmild relative to DD (OR=2.30, 95% CI 1.03, 5.16) but did not differ significantly between ASDsev and DD (Table 2B-2).
Table 2B-2:
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD (severe, mild to moderate symptoms) and DDa
| ASDsev vs. DD | ASDmild vs. DD | |||||
|---|---|---|---|---|---|---|
| Cytokine | OR | 95% CI | P | OR | 95% CI | P |
| 6Ckine (CCL21) | 1.43 | 0.84, 2.42 | 0.19 | 1.64 | 0.85, 3.14 | 0.14 |
| BCA-1 (CXCL13) | 1.35 | 0.67, 2.74 | 0.40 | 1.20 | 0.51, 2.83 | 0.68 |
| CTACK (CCL27) | 1.02 | 0.57, 1.83 | 0.94 | 1.10 | 0.54, 2.25 | 0.80 |
| ENA78 (CXCL5) | 1.29 | 0.98, 1.70 | 0.07 | 1.05 | 0.78, 1.40 | 0.77 |
| Eotaxin (CCL11) | 1.04 | 0.48, 2.26 | 0.92 | 0.97 | 0.37, 2.58 | 0.96 |
| Eotaxin-2 (CCL24) | 1.13 | 0.85, 1.51 | 0.41 | 1.13 | 0.79, 1.61 | 0.51 |
| Eotaxin-3 (CCL26) | 1.07 | 0.65, 1.76 | 0.78 | 1.15 | 0.62, 2.16 | 0.65 |
| Fractalkine (CX3CL1) | 1.37 | 0.70, 2.67 | 0.36 | 1.12 | 0.52, 2.42 | 0.78 |
| GCP-2 (CXCL6) | 1.39 | 0.70, 2.74 | 0.35 | 1.07 | 0.52, 2.23 | 0.85 |
| Gro-α (CXCL1) | 1.00 | 0.49, 2.05 | 0.995 | 0.92 | 0.40, 2.13 | 0.84 |
| Gro-β (CXCL2) | 1.02 | 0.61, 1.70 | 0.93 | 1.09 | 0.59, 2.03 | 0.78 |
| I-309 (CCL1) | 1.17 | 0.49, 2.80 | 0.73 | 1.00 | 0.34, 2.90 | 0.996 |
| IFN-γ | 1.21 | 0.74, 1.99 | 0.45 | 0.99 | 0.55, 1.81 | 0.98 |
| IL-1β | 1.44 | 0.70, 2.95 | 0.32 | 1.26 | 0.54, 2.95 | 0.59 |
| IL-2 | 1.33 | 0.65, 2.70 | 0.44 | 1.09 | 0.46, 2.61 | 0.84 |
| IL-4 | 1.17 | 0.52, 2.64 | 0.70 | 1.10 | 0.41, 2.99 | 0.85 |
| IL-6 | 1.25 | 0.68, 2.31 | 0.47 | 1.02 | 0.50, 2.08 | 0.97 |
| IL-8 (CXCL8) | 1.05 | 0.59, 1.86 | 0.87 | 1.21 | 0.60, 2.42 | 0.60 |
| IL-10 | 1.25 | 0.72, 2.17 | 0.43 | 1.27 | 0.63, 2.58 | 0.50 |
| IL-16 | 1.34 | 0.71, 2.53 | 0.37 | 1.48 | 0.64, 3.45 | 0.36 |
| IP-10 (CXCL10) | 0.89 | 0.40, 1.99 | 0.77 | 0.73 | 0.28, 1.94 | 0.53 |
| I-TAC (CXCL11) | 1.24 | 0.72, 2.12 | 0.44 | 1.57 | 0.80, 3.10 | 0.19 |
| MCP-1 (CCL2) | 1.07 | 0.62, 1.86 | 0.80 | 1.74 | 0.83, 3.65 | 0.15 |
| MCP-2 (CCL8) | 1.28 | 0.69, 2.38 | 0.43 | 1.47 | 0.67, 3.22 | 0.33 |
| MCP-3 (CCL7) | 1.14 | 0.64, 2.01 | 0.66 | 1.11 | 0.54, 2.28 | 0.77 |
| MCP-4 (CCL13) | 0.75 | 0.42, 1.33 | 0.33 | 1.03 | 0.50, 2.13 | 0.93 |
| MDC (CCL22) | 1.84 | 0.98, 3.43 | 0.06 | 2.30 | 1.03, 5.16 | 0.04 |
| MIF | 1.29 | 0.74, 2.26 | 0.37 | 1.10 | 0.68, 1.77 | 0.71 |
| MIG (CXCL9) | 1.36 | 0.80, 2.31 | 0.26 | 1.21 | 0.64, 2.27 | 0.56 |
| MIP-1α (CCL3) | 1.27 | 0.41, 3.91 | 0.68 | 0.73 | 0.21, 2.60 | 0.63 |
| MIP-1δ (CCL15) | 1.40 | 0.81, 2.44 | 0.23 | 1.39 | 0.71, 2.72 | 0.33 |
| MIP-3α (CCL20) | 1.31 | 0.64, 2.68 | 0.46 | 1.15 | 0.49, 2.67 | 0.75 |
| MIP-3β (CCL19) | 1.21 | 0.82, 1.78 | 0.34 | 1.24 | 0.77, 2.00 | 0.37 |
| MPIF-1 (CCL23) | 1.87 | 1.16, 3.00 | 0.01 | 2.26 | 1.23, 4.16 | 0.01 |
| SCYB16 (CXCL16) | 0.93 | 0.56, 1.56 | 0.79 | 1.13 | 0.59, 2.16 | 0.72 |
| SDF-1α/β (CXCL12) | 1.42 | 0.82, 2.47 | 0.22 | 1.12 | 0.60, 2.10 | 0.72 |
| TARC (CCL17) | 1.21 | 0.77, 1.90 | 0.40 | 1.21 | 0.70, 2.09 | 0.50 |
| TECK (CCL25) | 1.37 | 0.65, 2.86 | 0.41 | 1.01 | 0.47, 2.19 | 0.97 |
| TNF-α | 1.09 | 0.69, 1.71 | 0.71 | 1.21 | 0.62, 2.37 | 0.57 |
Multinomial logistic regression models were adjusted for maternal education attainment, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); OR represents the fold change in the odds of having one diagnosis relative to another diagnosis or no diagnosis for every 1-unit increase in the ln-transformed cytokine/chemokine (or for every e-fold increase in cytokine/chemokine levels); 398 participants comprised the following groups: 121 ASD (severe), 50 ASD (mild), and 69 DD; ASD severity was defined using ADOS severity scores, where ≥7 indicated severe and <7 indicated mild to moderate symptoms; OR = adjusted odds ratio, CI = confidence interval, P = P-value
Comparison by cognitive and adaptive development: ASDhi vs. ASDlo, ASDhi/ASDlo vs. TD and ASDhi/ASDlo vs. DD
Next, we subdivided the ASD group into typically-to-high- (ASDhi) and low-functioning (ASDlo) subgroups according to the children’s cognitive and adaptive development level based on the MSEL and VABS scores to examine associations between cytokine/chemokine levels and cognitive/adaptive function in children with ASD. Most neonatal cytokines and chemokines did not differ significantly between ASDhi and ASDlo except for MCP-1 (CCL2) (Fig. 1C, Table 2C-1). Higher levels of MCP-1 were associated with a 3.2-fold higher likelihood of ASDhi relative to ASDlo (OR=3.18, 95% CI 1.23, 8.26) (Table 2C-1).
Table 2C-1:
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD (typical to high-functioning, low-functioning) and TDa
| ASDhi vs. ASDlo | ASDhi vs. TD | ASDlo vs. TD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| 6Ckine (CCL21) | 0.86 | 0.42, 1.80 | 0.70 | 0.45 | 0.21, 0.95 | 0.04 | 0.52 | 0.34, 0.81 | 0.004 |
| BCA-1 (CXCL13) | 1.00 | 0.37, 2.72 | 0.99 | 0.54 | 0.20, 1.46 | 0.22 | 0.54 | 0.30, 0.95 | 0.03 |
| CTACK (CCL27) | 0.90 | 0.43, 1.92 | 0.79 | 0.34 | 0.15, 0.76 | 0.01 | 0.37 | 0.21, 0.64 | 0.0004 |
| ENA78 (CXCL5) | 0.85 | 0.60, 1.21 | 0.38 | 0.86 | 0.61, 1.22 | 0.39 | 1.01 | 0.80, 1.27 | 0.94 |
| Eotaxin (CCL11) | 0.78 | 0.27, 2.27 | 0.65 | 0.21 | 0.06, 0.75 | 0.02 | 0.27 | 0.11, 0.67 | 0.01 |
| Eotaxin-2 (CCL24) | 0.88 | 0.59, 1.34 | 0.56 | 0.79 | 0.52, 1.20 | 0.27 | 0.89 | 0.70, 1.15 | 0.38 |
| Eotaxin-3 (CCL26) | 0.93 | 0.46, 1.87 | 0.83 | 0.52 | 0.26, 1.07 | 0.08 | 0.57 | 0.37, 0.86 | 0.01 |
| Fractalkine (CX3CL1) | 0.74 | 0.31, 1.76 | 0.50 | 0.44 | 0.18, 1.08 | 0.07 | 0.60 | 0.33, 1.07 | 0.08 |
| GCP-2 (CXCL6) | 0.57 | 0.25, 1.32 | 0.19 | 0.39 | 0.16, 0.92 | 0.03 | 0.68 | 0.37, 1.25 | 0.21 |
| Gro-α (CXCL1) | 0.89 | 0.35, 2.26 | 0.80 | 0.60 | 0.23, 1.54 | 0.29 | 0.68 | 0.38, 1.20 | 0.18 |
| Gro-β (CXCL2) | 0.72 | 0.37, 1.40 | 0.33 | 0.48 | 0.24, 0.95 | 0.04 | 0.67 | 0.44, 1.03 | 0.07 |
| I-309 (CCL1) | 0.81 | 0.24, 2.74 | 0.74 | 0.28 | 0.08, 0.97 | 0.04 | 0.35 | 0.17, 0.71 | 0.004 |
| IFN-γ | 0.74 | 0.38, 1.45 | 0.38 | 0.52 | 0.26, 1.01 | 0.05 | 0.70 | 0.48, 1.02 | 0.07 |
| IL-1β | 1.19 | 0.40, 3.52 | 0.76 | 0.80 | 0.27, 2.38 | 0.69 | 0.68 | 0.37, 1.23 | 0.20 |
| IL-2 | 0.88 | 0.31, 2.49 | 0.81 | 0.46 | 0.16, 1.31 | 0.15 | 0.52 | 0.28, 0.95 | 0.03 |
| IL-4 | 0.75 | 0.24, 2.34 | 0.62 | 0.40 | 0.13, 1.28 | 0.12 | 0.54 | 0.27, 1.07 | 0.08 |
| IL-6 | 0.85 | 0.36, 2.02 | 0.71 | 0.49 | 0.20, 1.19 | 0.12 | 0.58 | 0.34, 0.98 | 0.04 |
| IL-8 (CXCL8) | 0.89 | 0.41, 1.93 | 0.77 | 0.82 | 0.38, 1.76 | 0.62 | 0.93 | 0.59, 1.46 | 0.74 |
| IL-10 | 0.95 | 0.42, 2.12 | 0.89 | 0.57 | 0.24, 1.32 | 0.19 | 0.60 | 0.35, 1.02 | 0.06 |
| IL-16 | 1.05 | 0.38, 2.90 | 0.93 | 0.77 | 0.27, 2.18 | 0.62 | 0.73 | 0.39, 1.37 | 0.33 |
| IP-10 (CXCL10) | 1.36 | 0.41, 4.50 | 0.61 | 0.45 | 0.13, 1.49 | 0.19 | 0.33 | 0.17, 0.65 | 0.001 |
| I-TAC (CXCL11) | 1.37 | 0.62, 3.00 | 0.44 | 0.80 | 0.37, 1.75 | 0.58 | 0.59 | 0.38, 0.91 | 0.02 |
| MCP-1 (CCL2) | 3.18 | 1.23, 8.26 | 0.02 | 2.43 | 0.94, 6.25 | 0.07 | 0.76 | 0.49, 1.19 | 0.23 |
| MCP-2 (CCL8) | 0.96 | 0.42, 2.19 | 0.91 | 0.47 | 0.20, 1.12 | 0.09 | 0.49 | 0.28, 0.86 | 0.01 |
| MCP-3 (CCL7) | 0.92 | 0.40, 2.14 | 0.84 | 0.44 | 0.18, 1.07 | 0.07 | 0.48 | 0.27, 0.84 | 0.01 |
| MCP-4 (CCL13) | 1.33 | 0.59, 3.03 | 0.49 | 0.73 | 0.32, 1.67 | 0.45 | 0.55 | 0.34, 0.87 | 0.01 |
| MDC (CCL22) | 0.69 | 0.30, 1.61 | 0.39 | 0.51 | 0.22, 1.18 | 0.12 | 0.73 | 0.43, 1.25 | 0.25 |
| MIF | 0.95 | 0.47, 1.92 | 0.89 | 0.34 | 0.13, 0.90 | 0.03 | 0.36 | 0.16, 0.80 | 0.01 |
| MIG (CXCL9) | 0.85 | 0.41, 1.77 | 0.66 | 0.48 | 0.23, 1.03 | 0.06 | 0.57 | 0.36, 0.90 | 0.02 |
| MIP-1α (CCL3) | 0.59 | 0.14, 2.43 | 0.46 | 0.23 | 0.05, 1.01 | 0.05 | 0.39 | 0.16, 0.98 | 0.045 |
| MIP-1δ (CCL15) | 1.17 | 0.47, 2.91 | 0.73 | 0.87 | 0.35, 2.17 | 0.76 | 0.74 | 0.45, 1.23 | 0.25 |
| MIP-3α (CCL20) | 0.84 | 0.30, 2.37 | 0.74 | 0.42 | 0.13, 1.35 | 0.15 | 0.50 | 0.22, 1.16 | 0.11 |
| MIP-3β (CCL19) | 0.97 | 0.58, 1.62 | 0.90 | 0.72 | 0.42, 1.23 | 0.23 | 0.75 | 0.53, 1.05 | 0.09 |
| MPIF-1 (CCL23) | 1.40 | 0.67, 2.93 | 0.38 | 0.96 | 0.46, 2.02 | 0.91 | 0.69 | 0.46, 1.03 | 0.07 |
| SCYB16 (CXCL16) | 1.12 | 0.55, 2.29 | 0.76 | 0.75 | 0.36, 1.56 | 0.44 | 0.67 | 0.43, 1.04 | 0.07 |
| SDF-1α/β (CXCL12) | 0.76 | 0.36, 1.62 | 0.48 | 0.41 | 0.18, 0.91 | 0.03 | 0.54 | 0.32, 0.91 | 0.02 |
| TARC (CCL17) | 0.92 | 0.49, 1.70 | 0.78 | 0.60 | 0.32, 1.14 | 0.12 | 0.66 | 0.45, 0.96 | 0.03 |
| TECK (CCL25) | 0.85 | 0.33, 2.19 | 0.73 | 0.30 | 0.10, 0.87 | 0.03 | 0.35 | 0.17, 0.75 | 0.01 |
| TNF-α | 0.94 | 0.46, 1.89 | 0.85 | 0.41 | 0.17, 0.98 | 0.046 | 0.43 | 0.22, 0.85 | 0.02 |
Multinomial logistic regression models were adjusted for maternal education attainment, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); OR represents the fold change in the odds of having one diagnosis relative to another diagnosis or no diagnosis for every 1-unit increase in the ln-transformed cytokine/chemokine (or for every e-fold increase in cytokine/chemokine levels); 398 participants comprised the following groups: 27 ASD (high), 144 ASD (low) and 158 TD; Mullen Scales of Early Learning (MSEL) and Vineland Adaptive Behavior Scales (VABS) composite standard scores were used to define high/low cognitive and adaptive development levels, where both MSEL and VABS scores of ≥70 indicated typical to high-function and a score of <70 on either MSEL or VABS indicated low-function; OR = adjusted odds ratio, CI = confidence interval, P = P-value
For comparisons of the ASD subgroups with TD, we observed significantly lower levels of cytokines/chemokines 6Ckine (CCL21), CTACK (CCL27), eotaxin (CCL11), I-309 (CCL1), MIF, SDF-1α/β (CXCL12), TECK (CCL25), TNFα, in both ASDhi and ASDlo relative to TD (Table 2C-1). Meanwhile, the levels of cytokines/chemokines BCA-1 (CXCL13), eotaxin-3 (CCL26), IL-2, IL-6, IP-10 (CXCL10), I-TAC (CXCL11), MCP-2 (CCL8), MCP-3 (CCL7), MCP-4 (CCL13), MIG (CXCL9), MIP-1α (CCL3), and TARC (CCL17) were significantly lower in ASDlo, but not ASDhi, relative to TD (Table 2C-1). The levels of two chemokines, GCP-2 (CXCL6) and Gro-β (CXCL2), were only significantly lower in ASDhi relative to TD (Table 2C-1).
Comparisons of ASDhi and ASDlo to the DD group revealed significantly higher neonatal levels of MDC (CCL22) and MPIF-1 (CCL23) in one or both ASD subgroups relative to DD that were not observed relative to TD (Fig. 1C, Table 2C-2). Higher levels of MPIF-1 were associated with 2.6-fold higher odds of ASDhi (OR= 2.63, 95% Cl 1.19, 5.79) and 1.9-fold higher odds of ASDlo (OR= 1.88, 95% Cl 1.19, 2.97) relative to DD (Table 2C-2). Higher levels of MDC were associated with a 2.1-fold higher likelihood of ASDlo (OR= 2.12, 95% Cl 1.14, 3.92), but MDC levels did not differ significantly between ASDhi and DD (Table 2C-2). Higher concentrations of MCP-1 were associated with an a 3.4-fold higher likelihood of ASDhi relative to DD (OR= 3.42, 95% Cl 1.25, 9.39), not observed between ASDlo and DD (Table 2C-2). MCP-1 concentrations differed significantly between ASDhi and ASDlo as described earlier. Interestingly, we did not observe significant differences in MCP-1 levels for either ASD subgroup relative to TD (Table 2C-1).
Table 2C-2:
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD (typical to high-functioning, low-functioning) and DDa
| ASDhi vs. DD | ASDlo vs. DD | |||||
|---|---|---|---|---|---|---|
| Cytokine | OR | 95% CI | P | OR | 95% CI | P |
| 6Ckine (CCL21) | 1.32 | 0.61, 2.86 | 0.48 | 1.53 | 0.92, 2.56 | 0.10 |
| BCA-1 (CXCL13) | 1.31 | 0.45, 3.79 | 0.62 | 1.30 | 0.66, 2.56 | 0.44 |
| CTACK (CCL27) | 0.97 | 0.43, 2.18 | 0.93 | 1.07 | 0.60, 1.89 | 0.82 |
| ENA78 (CXCL5) | 1.05 | 0.73, 1.51 | 0.81 | 1.23 | 0.95, 1.59 | 0.12 |
| Eotaxin (CCL11) | 0.83 | 0.27, 2.58 | 0.75 | 1.06 | 0.50, 2.26 | 0.87 |
| Eotaxin-2 (CCL24) | 1.02 | 0.66, 1.57 | 0.94 | 1.15 | 0.87, 1.52 | 0.33 |
| Eotaxin-3 (CCL26) | 1.03 | 0.48, 2.19 | 0.94 | 1.11 | 0.68, 1.80 | 0.68 |
| Fractalkine (CX3CL1) | 1.01 | 0.41, 2.50 | 0.98 | 1.36 | 0.72, 2.58 | 0.35 |
| GCP-2 (CXCL6) | 0.84 | 0.38, 1.88 | 0.67 | 1.47 | 0.75, 2.88 | 0.27 |
| Gro-α (CXCL1) | 0.88 | 0.32, 2.43 | 0.81 | 1.00 | 0.50, 1.98 | 0.99 |
| Gro-β (CXCL2) | 0.80 | 0.39, 1.63 | 0.53 | 1.11 | 0.67, 1.83 | 0.68 |
| I-309 (CCL1) | 0.93 | 0.25, 3.47 | 0.92 | 1.15 | 0.49, 2.67 | 0.75 |
| IFN-γ | 0.88 | 0.42, 1.84 | 0.74 | 1.19 | 0.74, 1.93 | 0.47 |
| IL-1β | 1.59 | 0.51, 4.98 | 0.42 | 1.34 | 0.68, 2.63 | 0.39 |
| IL-2 | 1.12 | 0.37, 3.38 | 0.85 | 1.27 | 0.64, 2.51 | 0.49 |
| IL-4 | 0.90 | 0.27, 3.04 | 0.86 | 1.21 | 0.55, 2.63 | 0.64 |
| IL-6 | 1.02 | 0.41, 2.54 | 0.96 | 1.20 | 0.68, 2.14 | 0.53 |
| IL-8 (CXCL8) | 1.00 | 0.44, 2.27 | 0.996 | 1.12 | 0.65, 1.95 | 0.68 |
| IL-10 | 1.20 | 0.52, 2.80 | 0.67 | 1.27 | 0.74, 2.17 | 0.38 |
| IL-16 | 1.44 | 0.52, 4.01 | 0.48 | 1.37 | 0.74, 2.55 | 0.31 |
| IP-10 (CXCL10) | 1.10 | 0.30, 3.95 | 0.89 | 0.81 | 0.37, 1.75 | 0.58 |
| I-TAC (CXCL11) | 1.74 | 0.74, 4.05 | 0.20 | 1.27 | 0.75, 2.14 | 0.37 |
| MCP-1 (CCL2) | 3.42 | 1.25, 9.39 | 0.02 | 1.08 | 0.63, 1.83 | 0.79 |
| MCP-2 (CCL8) | 1.29 | 0.53, 3.15 | 0.57 | 1.35 | 0.74, 2.48 | 0.33 |
| MCP-3 (CCL7) | 1.05 | 0.43, 2.55 | 0.91 | 1.14 | 0.66, 1.99 | 0.63 |
| MCP-4 (CCL13) | 1.05 | 0.43, 2.57 | 0.91 | 0.79 | 0.45, 1.38 | 0.41 |
| MDC (CCL22) | 1.46 | 0.62, 3.41 | 0.38 | 2.12 | 1.14, 3.92 | 0.02 |
| MIF | 1.15 | 0.60, 2.21 | 0.68 | 1.21 | 0.77, 1.91 | 0.41 |
| MIG (CXCL9) | 1.14 | 0.53, 2.45 | 0.74 | 1.34 | 0.81, 2.23 | 0.26 |
| MIP-1α (CCL3) | 0.68 | 0.15, 3.03 | 0.62 | 1.17 | 0.40, 3.41 | 0.78 |
| MIP-1δ (CCL15) | 1.60 | 0.63, 4.08 | 0.32 | 1.37 | 0.82, 2.29 | 0.23 |
| MIP-3α (CCL20) | 1.09 | 0.39, 3.07 | 0.87 | 1.30 | 0.66, 2.55 | 0.45 |
| MIP-3β (CCL19) | 1.19 | 0.68, 2.06 | 0.54 | 1.23 | 0.84, 1.79 | 0.29 |
| MPIF-1 (CCL23) | 2.63 | 1.19, 5.79 | 0.02 | 1.88 | 1.19, 2.97 | 0.01 |
| SCYB16 (CXCL16) | 1.09 | 0.50, 2.36 | 0.83 | 0.97 | 0.59, 1.60 | 0.91 |
| SDF-1α/β (CXCL12) | 1.05 | 0.49, 2.25 | 0.90 | 1.38 | 0.82, 2.32 | 0.23 |
| TARC (CCL17) | 1.12 | 0.58, 2.17 | 0.73 | 1.23 | 0.80, 1.90 | 0.35 |
| TECK (CCL25) | 1.06 | 0.41, 2.78 | 0.90 | 1.26 | 0.64, 2.47 | 0.51 |
| TNF-α | 1.06 | 0.52, 2.15 | 0.87 | 1.14 | 0.73, 1.78 | 0.58 |
Multinomial logistic regression models were adjusted for maternal education attainment, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); OR represents the fold change in the odds of having one diagnosis relative to another diagnosis or no diagnosis for every 1-unit increase in the ln-transformed cytokine/chemokine (or for every e-fold increase in cytokine/chemokine levels); 398 participants comprised the following groups: 27 ASD (high), 144 ASD (low), and 69 DD; Mullen Scales of Early Learning (MSEL) and Vineland Adaptive Behavior Scales (VABS) composite standard scores were used to define high/low cognitive and adaptive development levels, where both MSEL and VABS scores of ≥70 indicated typical to high-function and a score of <70 on either MSEL or VABS indicated low-function; OR = adjusted odds ratio, CI = confidence interval, P = P-value
Identifying the strongest subset of predictors associated with ASD and DD: LASSO variable selection
After observing the significant differences in numerous cytokine/chemokine concentrations among children with ASD, DD, and TD, we conducted an exploratory analysis using LASSO to identify the cytokines and chemokines that were most strongly associated with child diagnosis, taking the entire cytokine/chemokine profile into account. Chemokines CTACK (CCL27), MIF and MPIF-1 (CCL23) emerged as the strongest predictors of child diagnosis after conducting a series of binary models with outcomes: ASD vs. TD, ASD vs. DD, and DD vs. TD. No predictive immune markers were identified when comparing models of the following binary outcomes: ASDsev vs. ASDmild and ASDhi vs. ASDlo. Models that compared these ASD subgroups with DD and TD produced the same marker selection results as the models that included ASD as a combined group. In addition, no sex differences were observed in peripheral CTACK, MIF and MPIF-1 levels.
We conducted multinomial logistic regression with chemokines CTACK (CCL27), MIF and MPIF-1 (CCL23) as predictors and adjusted for maternal education attainment, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution to determine associations with child diagnosis. Higher neonatal levels of CTACK were independently associated with a 60% decrease in the odds of ASD relative to TD (OR 0.40; 95% Cl 0.21, 0.77) (Table 3A), indicating that lower CTACK levels at birth were associated with ASD. CTACK levels did not differentiate between ASD and DD or between DD and TD. Decreased newborn levels of CTACK were particularly associated with ASDsev, ASDhi and ASDlo compared with TD (Table 3B–C). In contrast, higher neonatal levels of MPIF-1 were associated with a 138% increase in the odds of ASD relative to DD (OR 2.38; 95% Cl 1.42, 3.98) and a 60% decrease in the odds of DD relative to TD (OR=0.40, 95% CI 0.24, 0.68) (Table 3A). However, MPIF-1 levels did not differ significantly between ASD and TD. We also observed similar significant associations between MPIF-1 levels and all ASD subgroups (ASDsev, ASDmild, ASDhi and ASDlo) when compared to DD (Table 3B–C). MIF no longer differed significantly between ASD and TD or between DD and TD, after adjusting for CTACK and MPIF-1 levels. Therefore, from the panel of 39 cytokines/chemokines examined, CTACK emerged as the strongest predictor of ASD relative to TD as well as MPIF-1 for DD relative to TD. No new associations emerged when ASD was divided into subsets based on symptom severity or cognitive/adaptive development level.
Table 3A:
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD, DD, and TD in one model, N=398a
| Cytokine or Chemokine | ASD vs. TD | ASD vs. DD | DD vs. TD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| CTACK | 0.40 | (0.21, 0.77) | 0.01 | 0.49 | (0.21, 1.14) | 0.10 | 0.82 | (0.35, 1.94) | 0.65 |
| MPIF-1 | 0.95 | (0.62, 1.45) | 0.81 | 2.38 | (1.42, 3.98) | 0.001 | 0.40 | (0.24, 0.68) | 0.001 |
| MIF | 0.59 | (0.24, 1.48) | 0.26 | 1.21 | (0.65, 2.25) | 0.56 | 0.49 | (0.18, 1.34) | 0.17 |
Multinomial logistic regression model was adjusted for maternal education, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); OR represents the fold change in the odds of having one diagnosis relative to another diagnosis or no diagnosis for every 1-unit increase in the ln-transformed cytokine/chemokine (or for every e-fold increase in cytokine/chemokine levels); 398 participants comprised the following groups: 171 ASD, 69 DD and 158 TD; OR = adjusted odds ratio, CI = confidence interval
Table 3B:
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD (severe, mild to moderate symptoms), DD, and TD in one model, N=398a
| Cytokine or Chemokine | ASDsev vs. ASDmild | ASDsev vs. TD | ASDmild vs. TD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| CTACK | 0.83 | 0.32, 2.12 | 0.70 | 0.38 | 0.49, 0.77 | 0.01 | 0.46 | 0.18, 1.15 | 0.10 |
| MPIF-1 | 0.79 | 0.42, 1.49 | 0.46 | 0.89 | 0.56, 1.41 | 0.61 | 1.13 | 0.60, 2.10 | 0.71 |
| MIF | 1.53 | 0.64, 3.66 | 0.34 | 0.69 | 0.26, 1.80 | 0.44 | 0.45 | 0.15, 1.30 | 0.14 |
| Cytokine or Chemokine | ASDsev vs. DD | ASDmild vs. DD | DD vs. TD | ||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| CTACK | 0.46 | 0.19, 1.11 | 0.08 | 0.55 | 0.19, 1.60 | 0.27 | 0.82 | (0.35, 1.94) | 0.65 |
| MPIF-1 | 2.23 | 1.29, 3.84 | 0.004 | 2.83 | 1.42, 5.64 | 0.003 | 0.40 | (0.24, 0.68) | 0.001 |
| MIF | 1.44 | 0.65, 3.21 | 0.37 | 0.94 | 0.45, 1.98 | 0.88 | 0.49 | (0.18, 1.34) | 0.17 |
Multinomial logistic regression model was adjusted for maternal education, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); OR represents the fold change in the odds of having one diagnosis relative to another diagnosis or no diagnosis for every 1-unit increase in the ln-transformed cytokine/chemokine (or for every e-fold increase in cytokine/chemokine levels); 398 participants comprised the following groups: 121 ASD-severe, 50 ASD-mild, 69 DD and 158 TD; OR = adjusted odds ratio, CI = confidence interval
Table 3C:
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD (high, low cognitive and adaptive functioning), DD, and TD in one model, N=398a
| Cytokine or Chemokine | ASDhi vs. ASDlo | ASDhi vs. TD | ASDlo vs. TD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| CTACK | 0.67 | 0.21, 2.09 | 0.49 | 0.29 | 0.09, 0.91 | 0.03 | 0.43 | 0.22, 0.84 | 0.01 |
| MPIF-1 | 1.64 | 0.72, 3.72 | 0.24 | 1.45 | 0.63, 3.32 | 0.38 | 0.89 | 0.57, 1.37 | 0.59 |
| MIF | 0.93 | 0.33, 2.60 | 0.89 | 0.56 | 0.16, 1.94 | 0.36 | 0.60 | 0.24, 1.52 | 0.28 |
| Cytokine or Chemokine | ASDhi vs. DD | ASDlo vs. DD | DD vs. TD | ||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| CTACK | 0.35 | 0.10, 1.26 | 0.11 | 0.52 | 0.22, 1.23 | 0.14 | 0.82 | (0.35, 1.94) | 0.65 |
| MPIF-1 | 3.64 | 1.50, 8.84 | 0.004 | 2.22 | 1.31, 3.76 | 0.003 | 0.40 | (0.24, 0.68) | 0.001 |
| MIF | 1.14 | 0.41, 3.16 | 0.80 | 1.22 | 0.62, 2.37 | 0.56 | 0.49 | (0.18, 1.34) | 0.17 |
Multinomial logistic regression model was adjusted for maternal education, gestational age, child’s age at blood spot collection, and years from blood spot collection to elution; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); OR represents the fold change in the odds of having one diagnosis relative to another diagnosis or no diagnosis for every 1-unit increase in the ln-transformed cytokine/chemokine (or for every e-fold increase in cytokine/chemokine levels); 398 participants comprised the following groups: 27 ASD-high, 144 ASD-low, 69 DD and 158 TD; OR = adjusted odds ratio, CI = confidence interval
Associations between CTACK and MPIF-1 levels and development among children with ASD
To assess whether neonatal CTACK and MPIF-1 concentrations were independently associated with behavioral or developmental patterns evaluated at age 2–5 years, the MSEL and VABS scores for each domain were individually modeled using linear regression adjusted for maternal education level. Increased MPIF-1 levels were associated with better scores on nearly all developmental domains examined (MSEL: fine motor, receptive language, expressive language, composite; VABS: communication, daily living skills, socialization, motor skills, and composite) among children with ASD, indicating less severe behaviors and impairments (Table 4). Meanwhile, CTACK was not significantly associated with any domains.
Table 4:
Developmental characteristics of 2–5-year-old children with ASD in relation to their neonatal CTACK and MPIF-1 concentrations, N=171a
| CTACK | MPIF-1 | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | |
| Mullen Scales of Early Learning | ||||||
| Visual Reception | −4.16 | −12.94, 4.61 | 0.35 | 6.03 | −1.16, 13.21 | 0.10 |
| Fine Motor | −3.96 | −10.91, 2.98 | 0.26 | 7.64 | 1.95, 13.33 | 0.01 |
| Receptive Language | −7.09 | −16.76, 2.57 | 0.15 | 11.48 | 3.56, 19.40 | 0.005 |
| Expressive Language | −4.60 | −13.19, 3.99 | 0.29 | 8.85 | 1.82, 15.89 | 0.01 |
| Composite | −4.95 | −12.65, 2.74 | 0.21 | 8.50 | 2.20, 14.80 | 0.01 |
| Vineland Adaptive Behavior Scales | ||||||
| Communication | −5.70 | −12.87, 1.47 | 0.12 | 7.26 | 1.39, 13.12 | 0.02 |
| Daily Living Skills | −2.97 | −7.68, 1.74 | 0.21 | 4.83 | 0.97, 8.68 | 0.01 |
| Socialization | −2.63 | −9.21, 3.95 | 0.43 | 6.74 | 1.36, 12.12 | 0.01 |
| Motor Skills | −5.66 | −12.91, 1.59 | 0.13 | 7.04 | 1.11, 12.96 | 0.02 |
| Composite | −4.34 | −9.64, 0.96 | 0.11 | 6.46 | 2.12, 10.80 | 0.004 |
Linear regression models were adjusted for maternal education (≤High school, Some college vs. ≥Bachelor degree) and CTACK or MPIF-1 (both were included in one model); β-coefficient (estimate) represents the change in developmental quotient (DQ) for a 1-unit increase in ln-transformed chemokine (pg/mg total protein), with a higher DQ indicating a better developmental outcome; DQ is defined as the developmental age divided by chronological age and multiplied by 100, with Mean = 100 and Standard Deviation = 15; CI = confidence interval
We also examined associations between neonatal CTACK and MPIF-1 levels and cognitive and adaptive development levels within the TD and DD groups; however, neither set of models revealed any significant associations between MPIF-1 or CTACK levels and the developmental domains (data not shown). ABC scales (irritability, lethargy, stereotypy, hyperactivity) were not associated with these chemokines within any diagnostic groups (data not shown).
Discussion
This study aimed to expand upon previously published work (Krakowiak et al., 2017) using a larger sample size, expanding the study population to include children with developmental delay without ASD, and an increased repertoire of analytes (42 vs.17) to better assess neonatal blood spots for additional immune predictors of risk for ASD and/or developmental delay. This study further examined a possible connection between potential early markers for cognitive and adaptive development levels of children diagnosed with ASD. Our findings suggest that children diagnosed with ASD or DD have lower overall neonatal cytokine/chemokines levels compared to those with TD, and the cytokine/chemokine profiles of children with ASD differ from those with DD. In addition, our exploratory analysis with immune markers identified by the LASSO variable selection method demonstrates that children with ASD were more likely to have decreased neonatal levels of CTACK relative to children with TD and higher levels of MPIF-1 relative to children with DD.
The previous study by Krakowiak et al. reported on IL-1β and IL-4 as early markers of ASD, where children with ASD had elevated levels of these cytokines depending upon their symptom intensity. Elevated IL-4 was associated with increased odds of severe ASD whereas IL-1β was associated with increased odds of mild/moderate ASD (Krakowiak et al., 2017). In the current study, although these two analytes were included in the study, neither IL-1β nor IL-4 were associated with an ASD diagnosis. Rather, lower levels of these cytokines were associated with children with DD compared to those with TD. Inconsistencies in the findings from this study and the previous one may be attributed to several notable methodological differences related to the measurement of immune markers, study population size and composition, and analytic approach. The two studies used cytokine/chemokine multiplex kits from different vendors, and the current panel had an expanded repertoire of 25 more analytes (compared to 17 analytes total in the earlier study) that included CTACK and MPIF-1, which were not previously measured. In addition, the immune markers in the current study had better detection rates compared to our prior study. For example, the current study had nearly all cytokines and chemokines detectable for ≥97% of the samples and only three analytes (GM-CSF, IL-12p70, IL-13) were below minimum detection levels, while previous study had one-third of the cytokines/chemokines (6 of 17) undetectable for >25% of the samples, with below-detection values imputed by multiple imputation methods. The current study was also larger with significantly more samples for the control groups, thus, providing more statistical power to detect differences among diagnostic groups and subgroups. Further, participant characteristics differed with regard to the geographic distribution (regional center catchment areas), racial/ethnic composition and other sociodemographic characteristics within diagnostic groups between the two study cohorts’ area was different. Additionally, the elapsed time before neonatal blood spot collection for the DD group was greater compared to that between the ASD and TD groups. Finally, the LASSO variable selection method was novel to the current study as we wished to expand our analytic approach. Thus, several notable differences in the quality and quantity of variables such as number of immune markers, study population size and demographic characteristics, and data analysis methods between the two related studies may have collectively contributed to contrasting results.
As previous studies have not examined CTACK levels in newborn blood samples (Ghassabian et al., 2018; Heuer et al., 2019; Krakowiak et al., 2017; Suzuki et al., 2011), the association between lower CTACK levels at birth and a higher likelihood of severe, low- and typical-to-high-functioning ASD relative to TD is a novel finding in this study. CTACK, an isoform of CCL27, is best known for its role in skin inflammation and lymphocyte trafficking, particularly the cutaneous lymphocyte-associated (CLA+) memory T cells (Morales et al., 1999). Numerous findings depict CTACK function in delayed-type hypersensitivity reactions and atopic dermatitis in both human and animal models (Chen et al., 2006; Kakinuma et al., 2003). Recent studies have suggested that the function of CTACK is not restricted to the skin, but rather this chemokine may play a pivotal role in homeostasis and immune surveillance in the brain. Serum CCL27 levels have been shown to be increased in individuals diagnosed with multiple sclerosis (MS) (Khaiboullina et al., 2015), while a follow-up study by Blatt et al. suggested that infiltrating T cells into the central nervous system (CNS) in MS patients may be of cutaneous origin (Blatt et al., 2016).
While these studies infer the possible role of T cells in the brain regarding CTACK production, secretion of CTACK may be independent of T cells but dependent on glial and non-neuronal cells that are present in the brain beginning in the early neurodevelopmental stages. For example, human astrocytes and neurons are capable of expressing CCL27 (Arimitsu et al., 2012) and its receptor, CCR10 (Flynn et al., 2003), suggesting both autocrine and paracrine effects of the chemokine within the CNS. In addition, expressions of CCL27 and CCR10 is abundant in the dentate gyrus (DG) of the hippocampus (Cartier et al., 2005; Gunsolly et al., 2010; Liu et al., 2007), cerebral cortex, and other limbic structures (Gunsolly et al., 2010) in the adult brain, indicating a critical role of CTACK in brain function throughout the lifespan. Immunologically, it is still possible that expression of CCL27 in the brain can trigger or modulate chemotaxis of memory T cells to these brain regions as the number of infiltrated T cells is at its peak during embryonic day 16 in the developing mouse brain (Tanabe and Yamashita, 2018). The holistic view of T cell involvement in early neurodevelopment is still unclear; however, increasing evidence using rodent models depicts the importance of T cell infiltration in maintaining and/or modulating CNS development (Clark et al., 2018; Derecki et al., 2010; Filiano et al., 2016; McGowan et al., 2011; Song et al., 2016). This emphasizes our observation that CTACK is indeed important in proper neurodevelopment, and deficient levels of CTACK could impede healthy neurodevelopment. Interestingly, neonatal levels of CTACK did not differ between children diagnosed with DD compared to those with TD, suggesting that the neurodevelopmental outcomes of DD and ASD are immunologically distinct. These findings provide support for the potential importance of sufficient levels CTACK in early life for healthy neurodevelopment, and thus the molecular basis of neuroimmune and neurobehavioral mechanisms involving cells that produce CTACK and express its cognate receptor in the CNS should be further examined.
The developmental characteristics of ASD and DD differ from each other in that each of the groups displays specific patterns of impairment in communication, cognition, and behaviors based on standard diagnostic tests. However, ASD and DD both lie under the spectrum of neurodevelopmental disorders, and a diagnosis of DD or ASD can change over time. As the behavioral intervention program is designed to address deficits specific to ASD, and because it is critical to identify these children as early as possible (Landa, 2018; Nahmias et al., 2019), it is important to find biomarkers with the potential to differentiate between ASD and DD cases. Here, we found that neonatal cytokine and chemokine profiles of children with ASD differ from those with DD, particularly that chemokines MDC and MPIF-1 were significantly lower in children with DD than those with ASD and TD. We did not see these differences when comparting ASD to TD subjects. This finding suggests that a significant reduction in these two chemokines in the early neonatal period. Specifically, our exploratory analysis determined that MPIF-1 was the strongest candidate to differentiate between the ASD and DD diagnostic groups, including the subgroups of ASD. Higher neonatal concentrations of peripheral MPIF-1 were associated with more than a two-fold higher likelihood of ASD compared to DD. This is of interest as a lower level of this chemokine at birth could indicate a potential role for MPIF-1 in development of executive and cognitive function, deficits in which are hallmark features of developmental delay. Of interest for future studies would be to design a study to compare neonatal samples from ASD with intellectual deficits to DD without ASD in the context of these differentiating chemokines.
With respect to function, immunologically, MPIF-1 (as indicated by its name myeloid progenitor inhibiting factor 1), inhibits colony formation of bone marrow myeloid immature progenitors and their activity. MPIF-1, also known as CCL23 and MIP-3, can modulate the immune response by promoting and directing the migration of mature immune cells such as activated T lymphocytes, macrophages, and granulocytes to local sites of injury (Arruda-Silva et al., 2017; Hwang et al., 2005; Kim et al., 2010), while simultaneously reducing the number of cells in the hematopoietic progenitor pool and inducing production of granulocytes and monocytes (Patel et al., 1997). This may suggest a supportive role for MPIF-1 during development. In addition, the interaction of MPIF-1 with its chemokine receptor, CCR1, can stimulate pro-inflammatory cytokine production, including IL-1β, TNFα, and MIP-1α (Hwang et al., 2005), although in the current study, the levels of these were not elevated in newborns later diagnosed with ASD or DD, but rather were lower or did not differ from children with TD. Interestingly, in the CNS, the CCL23-CCR1 interaction can induce angiogenesis by promoting the migration of endothelial cells through upregulation of matrix metalloproteinases in the endothelium (Hwang et al., 2005; Son et al., 2006). Blood vessel formation is critical in development and neuroplasticity, and either hypo- or hyper-angiogenesis can disrupt proper blood flow to the brain, which could ultimately affect neurodevelopmental outcome.
Recently, Azmitia et al. reported persistent angiogenesis in postmortem cortex, brainstem, and cerebellum of children and young adults with ASD and proposed that the heightened neuronal activity noted in some individuals with ASD was an outcome of sustained splitting angiogenesis (Azmitia et al., 2016). Mulligan and Trauner found that more than half of the ASD patients in their study exhibited abnormal epileptiform electroencephalogram (EEG) activity (Mulligan and Trauner, 2014), which has been considered as a method of early ASD diagnosis (Bosl et al., 2018; Gurau et al., 2017). Increased neuronal connectivity is closely related to pruning and myelination of axons, and heightened EEG activity could mean initial overgrowth and early maturation of brain white matter in ASD, possibly resulting in altered behavior (O’Reilly et al., 2017). In fact, children with ASD exhibit overconnectivity rather than underconnectivity as is shown by elevation of fractional anisotropy at age of six months, followed by a reduction below that of age-matched controls at 24 months (Wolff et al., 2012). Our observation of positive associations between MPIF-1 and MSEL cognitive and VABS adaptive scores in children with ASD may potentially support the modulation in angiogenesis thereby affecting neuroplasticity and neurodevelopment. The contribution of MPIF-1 in angiogenesis and neuronal activity in the ASD participants in the current study is still unknown; however, the fact that all subgroups of ASD (ASDsev, ASDmild, ASDhi, and ASDlo) had significantly higher levels of MPIF-1 than DD demonstrates the importance of MPIF-1 homeostasis during brain development and the potential of MPIF-1 as a checkpoint for ASD versus DD. In addition, numerous ligands for CCR1 other than MPIF-1 (i.e., MIP-1α [CCL3], RANTES [CCL5], MCP-3 [CCL7], MCP-4 [CCL13], MIP-1δ [CCL15]) (Murphy et al., 2000) and the global expression of CCR1 (immune/neuronal cells, tissues) (Patel et al., 1997; Simats et al., 2018; Stuart et al., 2015) should be taken into account when trying to better understand the mechanistic and functional role of MPIF-1 in ASD.
Conclusions
Our data collectively suggest that chemokine levels measured in children shortly after birth can serve as early predictors of abnormal immune and neuroimmune development associated with ASD and DD. Lower peripheral levels of select cytokines and chemokines in both ASD and DD groups compared to TD suggest the importance of homeostatic cytokine/chemokine levels in normal neurodevelopment. The differences in neonatal chemokine and cytokine profiles provide support for addressing the mechanisms between the immune and neuronal systems during gestation. Questions regarding the function of CTACK and MPIF-1 in brain development and their potential role in neuroplasticity should be further investigated, perhaps using a rodent model to elaborate on the effect of these select chemokines on neurodevelopment. Furthermore, continued investigation of very early immune molecule predictors of ASD and DD risk as well as understanding their functional role in neurodevelopment will be necessary to elucidate mechanistic pathways of immune dysregulation in these neurodevelopmental disorders.
Supplementary Material
Highlights.
Newborns later diagnosed with autism spectrum disorder (ASD) and delayed development (DD) have lower levels of cytokines and chemokines at birth compared to those with typical development (TD).
Our exploratory analysis suggests that CTACK (CCL27) and MPIF-1 (CCL23) are the strongest predictors of ASD compared to TD and DD, respectively.
Higher neonatal levels of CTACK were associated with a 60% decrease in the odds of ASD relative to TD while higher levels of MPIF-1 were associated with a 138% increase in the odds of ASD relative to DD.
Acknowledgements
This study was funded by the NIEHS Center for Children’s Environmental Health and Environmental Protection Agency (EPA) grants (2P01ES011269-11, 83543201, respectively), the NIEHS-funded CHARGE study (R01ES015359), and the NICHD funded IDDRC P50 (P50HD103526). We thank Dr. Kyuyoung Lee for his help on the figures.
Footnotes
Declaration of interests
Dr. Van de Water has a patent application involving the maternal autoantibody-related (MAR) ASD peptides described herein and has a UC Davis based startup company focusing on the development of the MAR-ASD autoantibody profile as a risk assessment for a child developing ASD. All other authors have no conflicts of interest to declare.
References
- Arimitsu N, Shimizu J, Fujiwara N, Takai K, Takada E, Kono T, Ueda Y, Suzuki T, Suzuki N, 2012. Role of SDF1/CXCR4 interaction in experimental hemiplegic models with neural cell transplantation. Int J Mol Sci 13, 2636–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Silva F, Bianchetto-Aguilera F, Gasperini S, Polletti S, Cosentino E, Tamassia N, Cassatella MA, 2017. Human Neutrophils Produce CCL23 in Response to Various TLR-Agonists and TNFalpha. Front Cell Infect Microbiol 7, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J, 2011. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol 232, 196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Saccomano ZT, Alzoobaee MF, Boldrini M, Whitaker-Azmitia PM, 2016. Persistent Angiogenesis in the Autism Brain: An Immunocytochemical Study of Postmortem Cortex, Brainstem and Cerebellum. J Autism Dev Disord 46, 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee LC, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Van Naarden Braun K, Dowling NF, 2018. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 67, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt NL, Khaiboullin TI, Lombardi VC, Rizvanov AA, Khaiboullina SF, 2016. The Skin-Brain Connection Hypothesis, Bringing Together CCL27-Mediated T-Cell Activation in the Skin and Neural Cell Damage in the Adult Brain. Front Immunol 7, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl WJ, Tager-Flusberg H, Nelson CA, 2018. EEG Analytics for Early Detection of Autism Spectrum Disorder: A data-driven approach. Sci Rep 8, 6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH, 2005. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev 48, 16–42. [DOI] [PubMed] [Google Scholar]
- Chen L, Lin SX, Agha-Majzoub R, Overbergh L, Mathieu C, Chan LS, 2006. CCL27 is a critical factor for the development of atopic dermatitis in the keratin-14 IL-4 transgenic mouse model. Int Immunol 18, 1233–1242. [DOI] [PubMed] [Google Scholar]
- Clark SM, Vaughn CN, Soroka JA, Li X, Tonelli LH, 2018. Neonatal adoptive transfer of lymphocytes rescues social behaviour during adolescence in immune-deficient mice. Eur J Neurosci 47, 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J, 2010. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 207, 1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, Peerzade SN, Chen H, Lee KS, Scott MM, Beenhakker MP, Litvak V, Kipnis J, 2016. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn G, Maru S, Loughlin J, Romero IA, Male D, 2003. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol 136, 84–93. [DOI] [PubMed] [Google Scholar]
- Frye RE, Vassall S, Kaur G, Lewis C, Karim M, Rossignol D, 2019. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med 7, 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, Sundaram R, Chahal N, McLain AC, Bell EM, Lawrence DA, Gilman SE, Yeung EH, 2018. Concentrations of immune marker in newborn dried blood spots and early childhood development: Results from the Upstate KIDS Study. Paediatr Perinat Epidemiol 32, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsolly C, Nicholson JD, Listwak SJ, Ledee D, Zelenka P, Verthelyi D, Chapoval S, Keegan A, Tonelli LH, 2010. Expression and regulation in the brain of the chemokine CCL27 gene locus. J Neuroimmunol 225, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurau O, Bosl WJ, Newton CR, 2017. How Useful Is Electroencephalography in the Diagnosis of Autism Spectrum Disorders and the Delineation of Subtypes: A Systematic Review. Front Psychiatry 8, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YM, Cheung WK, Wong CK, Sze SL, Cheng TW, Yeung MK, Chan AS, 2017. Distinct Cytokine and Chemokine Profiles in Autism Spectrum Disorders. Front Immunol 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN, 2006. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 114, 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer LS, Croen LA, Jones KL, Yoshida CK, Hansen RL, Yolken R, Zerbo O, DeLorenze G, Kharrazi M, Ashwood P, Van de Water J, 2019. An Exploratory Examination of Neonatal Cytokines and Chemokines as Predictors of Autism Risk: The Early Markers for Autism Study. Biol Psychiatry 86, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes HK, Mills Ko E, Rose D, Ashwood P, 2018. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front Cell Neurosci 12, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Son KN, Kim CW, Ko J, Na DS, Kwon BS, Gho YS, Kim J, 2005. Human CC chemokine CCL23, a ligand for CCR1, induces endothelial cell migration and promotes angiogenesis. Cytokine 30, 254–263. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Levy SE, Myers SM, Council On Children With Disabilities, S.O.D., Behavioral, P., 2020. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics 145. [DOI] [PubMed] [Google Scholar]
- Kakinuma T, Saeki H, Tsunemi Y, Fujita H, Asano N, Mitsui H, Tada Y, Wakugawa M, Watanabe T, Torii H, Komine M, Asahina A, Nakamura K, Tamaki K, 2003. Increased serum cutaneous T cell-attracting chemokine (CCL27) levels in patients with atopic dermatitis and psoriasis vulgaris. J Allergy Clin Immunol 111, 592–597. [DOI] [PubMed] [Google Scholar]
- Khaiboullina SF, Gumerova AR, Khafizova IF, Martynova EV, Lombardi VC, Bellusci S, Rizvanov AA, 2015. CCL27: Novel Cytokine with Potential Role in Pathogenesis of Multiple Sclerosis. Biomed Res Int 2015, 189638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim YS, Ko J, 2010. CK beta 8/CCL23 induces cell migration via the Gi/Go protein/PLC/PKC delta/NF-kappa B and is involved in inflammatory responses. Life Sci 86, 300–308. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, Van de Water J, 2017. Neonatal Cytokine Profiles Associated With Autism Spectrum Disorder. Biol Psychiatry 81, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, 2018. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry 30, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Cao X, Tang YC, Liu Y, Tang FR, 2007. CCR7, CCR8, CCR9 and CCR10 in the mouse hippocampal CA1 area and the dentate gyrus during and after pilocarpine-induced status epilepticus. J Neurochem 100, 1072–1088. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A, 2006. Autism from 2 to 9 years of age. Arch Gen Psychiatry 63, 694–701. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, EdS, Washington A, Patrick M, DiRienzo M, Christensen DL, Wiggins LD, Pettygrove S, Andrews JG, Lopez M, Hudson A, Baroud T, Schwenk Y, White T, Rosenberg CR, Lee LC, Harrington RA, Huston M, Hewitt A, PhD, Esler A, Hall-Lande J, Poynter JN, Hallas-Muchow L, Constantino JN, Fitzgerald RT, Zahorodny W, Shenouda J, Daniels JL, Warren Z, Vehorn A, Salinas A, Durkin MS, Dietz PM, 2020. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ 69, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Breen EJ, Alvares GA, Glozier N, Hickie IB, Hunt A, Hui J, Beilby J, Ravine D, Wray J, Whitehouse AJO, Guastella AJ, 2017. Cytokine levels and associations with symptom severity in male and female children with autism spectrum disorder. Mol Autism 8, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Hope TA, Meck WH, Kelsoe G, Williams CL, 2011. Impaired social recognition memory in recombination activating gene 1-deficient mice. Brain Res 1383, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A, 1999. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A 96, 14470–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan CK, Trauner DA, 2014. Incidence and behavioral correlates of epileptiform abnormalities in autism spectrum disorders. J Autism Dev Disord 44, 452–458. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA, 2000. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 52, 145–176. [PubMed] [Google Scholar]
- Nahmias AS, Pellecchia M, Stahmer AC, Mandell DS, 2019. Effectiveness of community-based early intervention for children with autism spectrum disorder: a meta-analysis. J Child Psychol Psychiatry 60, 1200–1209. [DOI] [PubMed] [Google Scholar]
- O’Reilly C, Lewis JD, Elsabbagh M, 2017. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS One 12, e0175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VP, Kreider BL, Li Y, Li H, Leung K, Salcedo T, Nardelli B, Pippalla V, Gentz S, Thotakura R, Parmelee D, Gentz R, Garotta G, 1997. Molecular and functional characterization of two novel human C-C chemokines as inhibitors of two distinct classes of myeloid progenitors. J Exp Med 185, 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simats A, Garcia-Berrocoso T, Penalba A, Giralt D, Llovera G, Jiang Y, Ramiro L, Bustamante A, Martinez-Saez E, Canals F, Wang X, Liesz A, Rosell A, Montaner J, 2018. CCL23: a new CC chemokine involved in human brain damage. J Intern Med 283, 461–475. [DOI] [PubMed] [Google Scholar]
- Son KN, Hwang J, Kwon BS, Kim J, 2006. Human CC chemokine CCL23 enhances expression of matrix metalloproteinase-2 and invasion of vascular endothelial cells. Biochem Biophys Res Commun 340, 498–504. [DOI] [PubMed] [Google Scholar]
- Song C, Nicholson JD, Clark SM, Li X, Keegan AD, Tonelli LH, 2016. Expansion of brain T cells in homeostatic conditions in lymphopenic Rag2(−/−) mice. Brain Behav Immun 57, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart MJ, Singhal G, Baune BT, 2015. Systematic Review of the Neurobiological Relevance of Chemokines to Psychiatric Disorders. Front Cell Neurosci 9, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, Yoshihara Y, Wakuda T, Takebayashi K, Takagai S, Matsumoto K, Tsuchiya KJ, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Mori N, 2011. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One 6, e20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Yamashita T, 2018. B-1a lymphocytes promote oligodendrogenesis during brain development. Nat Neurosci 21, 506–516. [DOI] [PubMed] [Google Scholar]
- Tibshirani R, 1996. Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society 58, 267–288. [Google Scholar]
- Vreugdenhil B, van der Velden W, Feuth T, Kox M, Pickkers P, van de Veerdonk FL, Blijlevens NMA, Bruggemann RJM, 2018. Moderate correlation between systemic IL-6 responses and CRP with trough concentrations of voriconazole. Br J Clin Pharmacol 84, 1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans AC, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J, Network I, 2012. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry 169, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Yoshida C, Grether JK, Van de Water J, Ashwood P, Delorenze GN, Hansen RL, Kharrazi M, Croen LA, 2014. Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: the Early Markers for Autism (EMA) study: a case-control study. J Neuroinflammation 11, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


